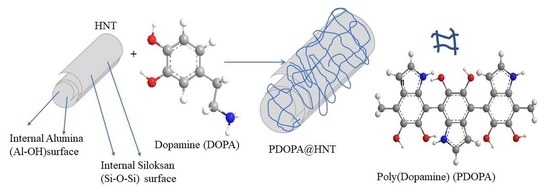
Enhanced Bioactive Properties of Halloysite Nanotubes via Polydopamine Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
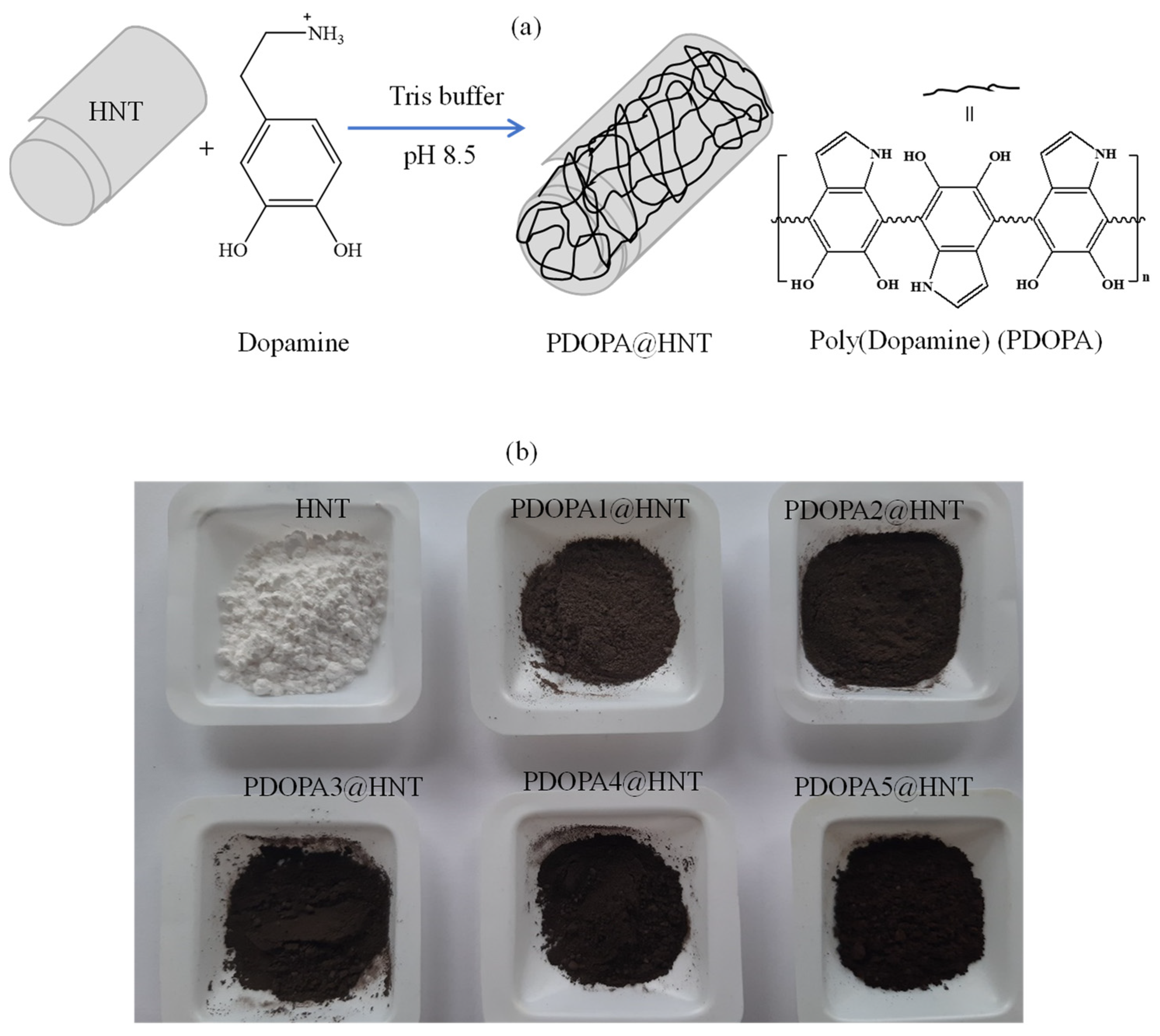
2.2. Coating of HNTs with PDOPA
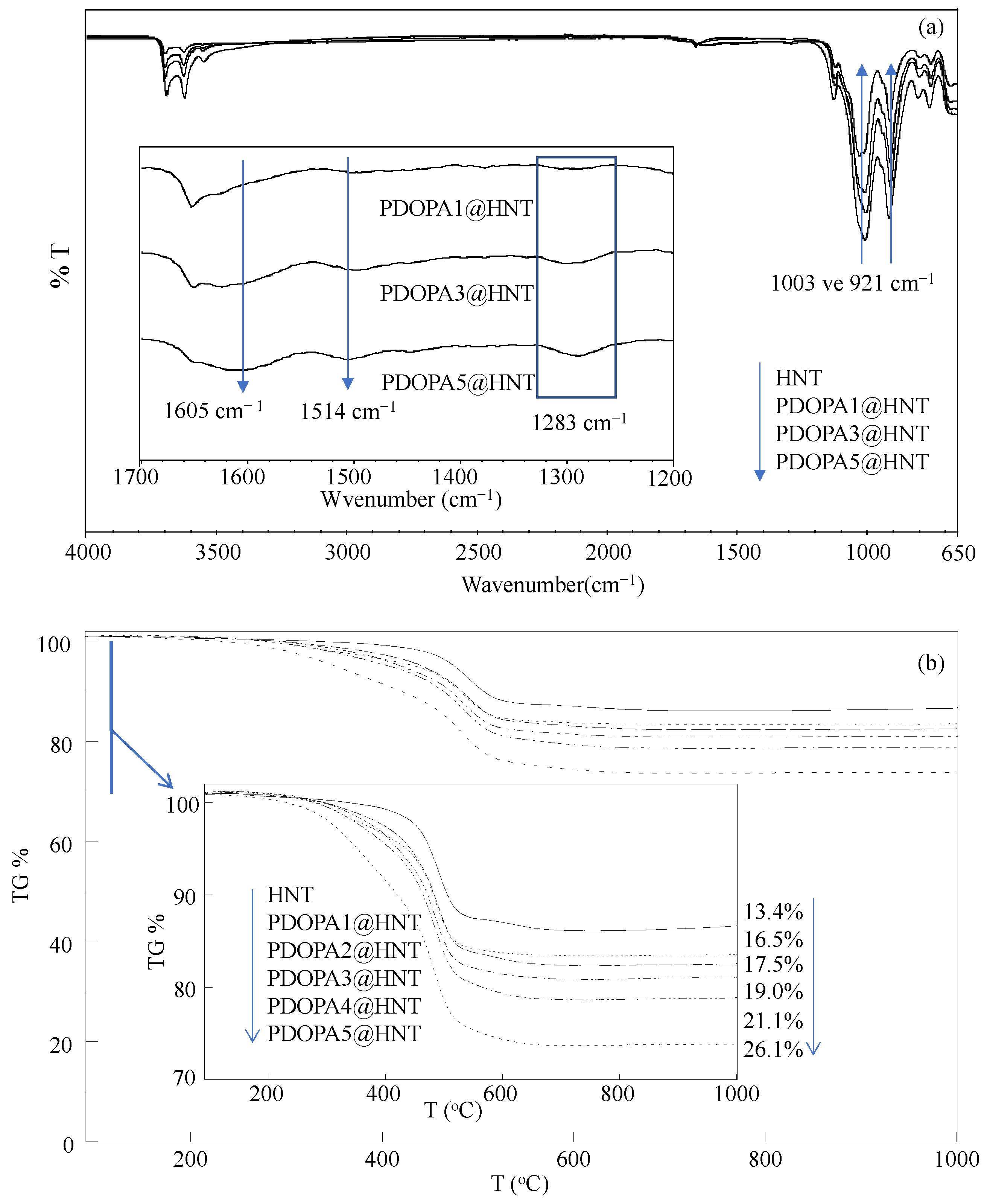
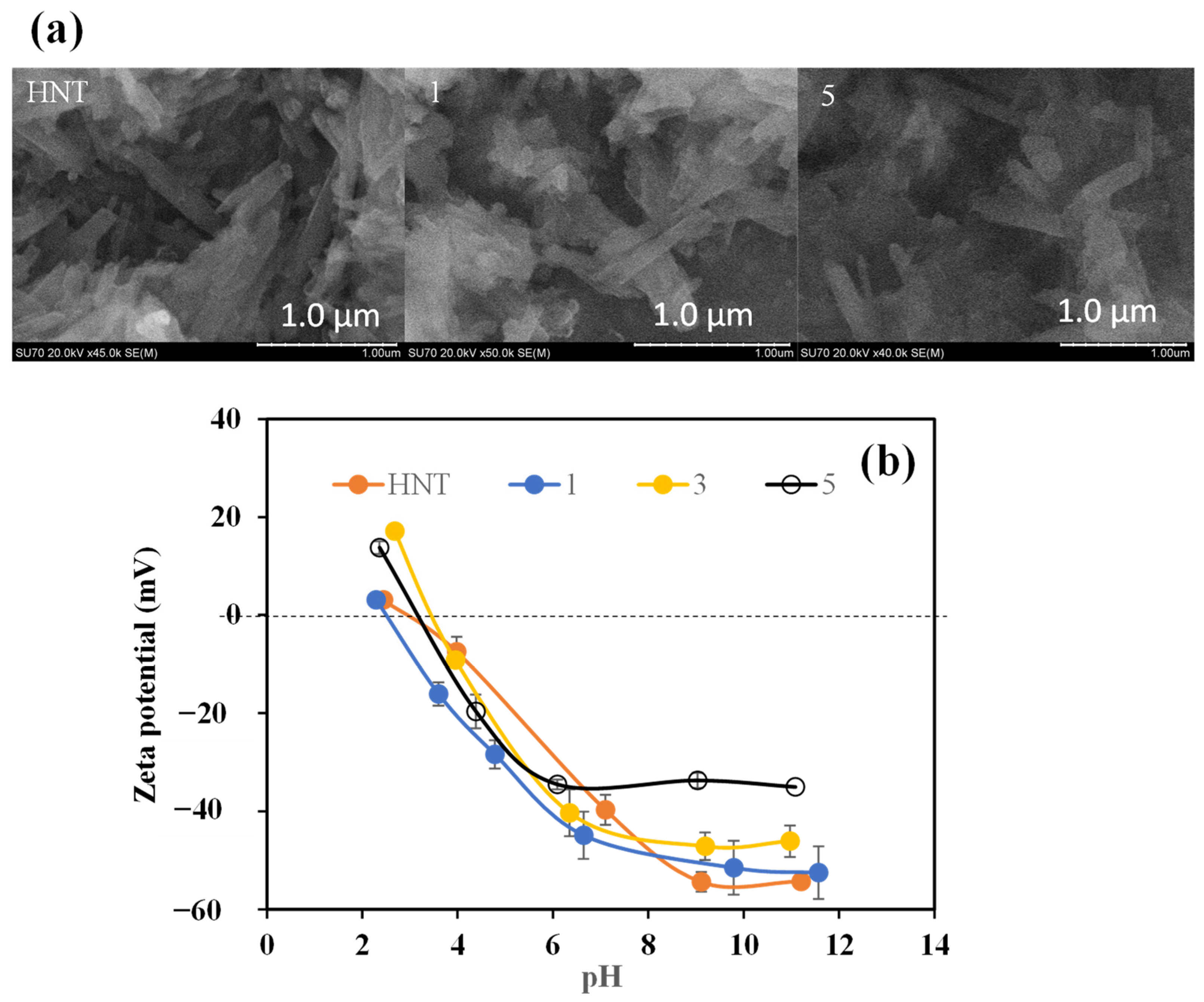
2.3. Characterization of PDOPA@HNTs
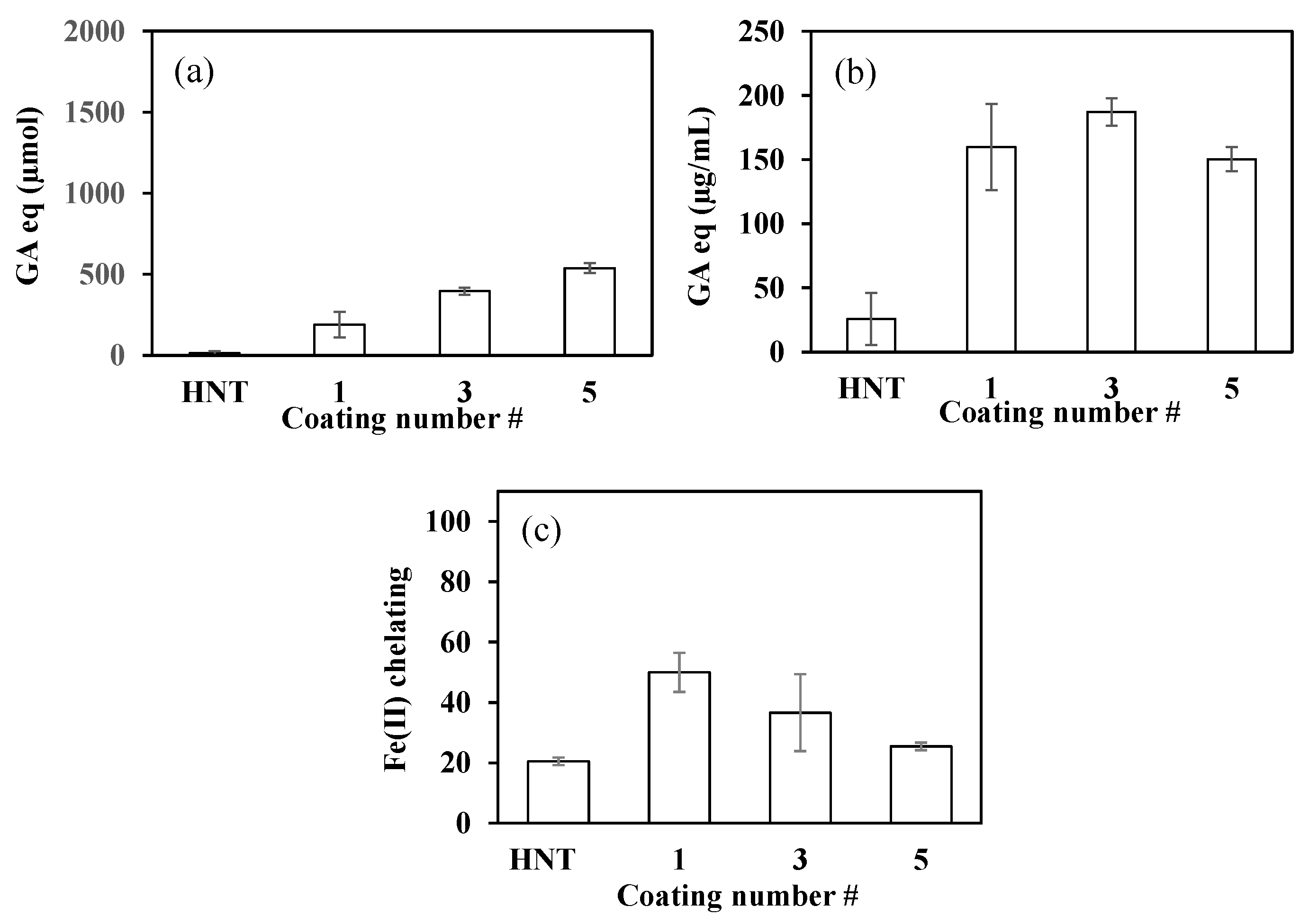
2.4. Antioxidant Properties of PDOPA@HNTs
2.5. Inhibition of AChE and AG Enzymes by PDOPA@HNTs
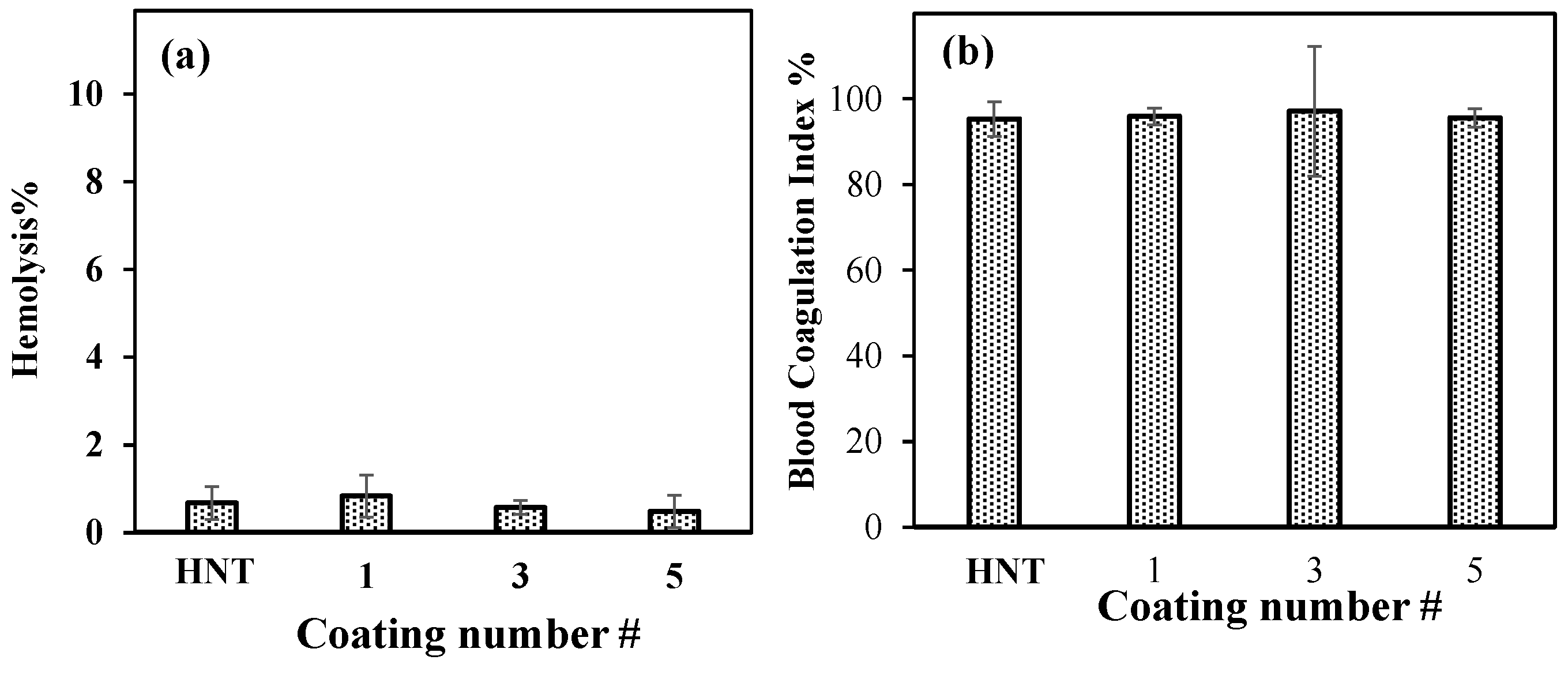
2.6. Blood Compatibility of HNT with Multiple PDOPAs
3. Results and Discussion
Coating and Characterization of HNT with Multiple PDOPA Layers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Pereira, I.; Saleh, M.; Nunes, C.; Reis, S.; Veiga, F.; Paiva-Santos, A.C. Preclinical developments of natural-occurring halloysite clay nanotubes in cancer therapeutics. Adv. Colloid Interface Sci. 2021, 291, 102406. [Google Scholar] [CrossRef] [PubMed]
- Same, S.; Nakhjavani, S.A.; Samee, G.; Navidi, G.; Jahanbani, Y.; Davaran, S. Halloysite clay nanotube in regenerative medicine for tissue and wound healing. Ceram. Int. 2022, 48, 31065–31079. [Google Scholar] [CrossRef]
- Guo, W.; Xu, L.; Feng, P.; Gu, Y.; Shuai, C. In-situ growth of silica nano-protrusions on halloysite nanotubes for interfacial reinforcement in polymer/halloysite scaffolds. Appl. Surf. Sci. 2020, 513, 145772. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K. Development of high performance electroless Ni–P–HNT composite coatings. Appl. Surf. Sci. 2012, 263, 149–156. [Google Scholar] [CrossRef]
- Yah, W.O.; Takahara, A.; Lvov, Y.M. Selective Modification of Halloysite Lumen with Octadecylphosphonic Acid: New Inorganic Tubular Micelle. J. Am. Chem. Soc. 2012, 134, 1853–1859. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of Halloysite Clay Nanotubes by Grafting with γ-Aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef]
- Churchman, G.J.; Davy, T.J.; Aylmore, L.A.G.; Gilkes, R.J.; Self, P.G. Characteristics of fine pores in some halloysites. Clay Miner. 1995, 30, 89–98. [Google Scholar] [CrossRef]
- Koosha, M.; Raoufi, M.; Moravvej, H. One-pot reactive electrospinning of chitosan/PVA hydrogel nanofibers reinforced by halloysite nanotubes with enhanced fibroblast cell attachment for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 179, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Abbas, G.; Hanif, M.; Bernkop-Schnürch, A.; Jalil, A.; Yaqoob, M. Mucoadhesive micro-composites: Chitosan coated halloysite nanotubes for sustained drug delivery. Colloids Surf. B Biointerfaces 2019, 184, 110527. [Google Scholar] [CrossRef] [PubMed]
- Pietraszek, A.; Karewicz, A.; Widnic, M.; Lachowicz, D.; Gajewska, M.; Bernasik, A.; Nowakowska, M. Halloysite-alkaline phosphatase system—A potential bioactive component of scaffold for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 173, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goda, E.S.; Gab-Allah, M.A.; Singu, B.S.; Yoon, K.R. Halloysite nanotubes based electrochemical sensors: A review. Microchem. J. 2019, 147, 1083–1096. [Google Scholar] [CrossRef]
- Tharmavaram, M.; Pandey, G.; Rawtani, D. Surface modified halloysite nanotubes: A flexible interface for biological, environmental and catalytic applications. Adv. Colloid Interface Sci. 2018, 261, 82–101. [Google Scholar] [CrossRef]
- Gkouma, E.; Gianni, E.; Avgoustakis, K.; Papoulis, D. Applications of halloysite in tissue engineering. Appl. Clay Sci. 2021, 214, 106291. [Google Scholar] [CrossRef]
- Doustdar, F.; Olad, A.; Ghorbani, M. Development of a novel reinforced scaffold based on chitosan/cellulose nanocrystals/halloysite nanotubes for curcumin delivery. Carbohydr. Polym. 2022, 282, 119127. [Google Scholar] [CrossRef]
- Fizir, M.; Dramou, P.; Dahiru, N.S.; Ruya, W.; Huang, T.; He, H. Halloysite nanotubes in analytical sciences and in drug delivery: A review. Microchim. Acta 2018, 185, 389. [Google Scholar] [CrossRef]
- Paul, A.; Augustine, R.; Hasan, A.; Zahid, A.A.; Thomas, S.; Agatemor, C.; Ghosal, K. Halloysite nanotube and chitosan polymer composites: Physicochemical and drug delivery properties. J. Drug Deliv. Sci. Technol. 2022, 72, 103380. [Google Scholar] [CrossRef]
- Kubade, P.R.; Senanayake, R. Thermo-mechanical properties of polyethyleneimine (PEI) modified HNTs filled ABS/PVC composites. Mater. Today Proc. 2022, 59, 852–857. [Google Scholar] [CrossRef]
- Taroni, T.; Meroni, D.; Fidecka, K.; Maggioni, D.; Longhi, M.; Ardizzone, S. Halloysite nanotubes functionalization with phosphonic acids: Role of surface charge on molecule localization and reversibility. Appl. Surf. Sci. 2019, 486, 466–473. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, K.; Feng, Z.; Hou, Y.; Li, H.; Zhao, J.; Tian, Y.; Song, H. High performance liquid crystalline bionanocomposite ionogels prepared by in situ crosslinking of cellulose/halloysite nanotubes/ionic liquid dispersions and its application in supercapacitors. Appl. Surf. Sci. 2018, 455, 599–607. [Google Scholar] [CrossRef]
- Ahmad, Y.H.; Mohamed, A.T.; Hassan, W.M.I.; Soliman, A.; Mahmoud, K.A.; Aljaber, A.S.; Al-Qaradawi, S.Y. Bimetallic palladium-supported halloysite nanotubes for low temperature CO oxidation: Experimental and DFT insights. Appl. Surf. Sci. 2019, 493, 70–80. [Google Scholar] [CrossRef]
- Wedemeyer, C.; Goutman, J.D.; Avale, M.E.; Franchini, L.F.; Rubinstein, M.; Calvo, D.J. Functional activation by central monoamines of human dopamine D4 receptor polymorphic variants coupled to GIRK channels in Xenopus oocytes. Eur. J. Pharmacol. 2007, 562, 165–173. [Google Scholar] [CrossRef]
- Ercan, H.; Elçin, A.E.; Elçin, Y.M. Assessment of dopamine-conjugated decellularized bovine tendon extracellular matrix as a bioadhesive. Mater. Today Commun. 2022, 31, 103634. [Google Scholar] [CrossRef]
- Tran, N.A.T.; Phuoc, N.M.; Khoi, T.M.; Jung, H.B.; Cho, N.; Lee, Y.-W.; Jung, E.; Kang, B.-G.; Park, K.; Hong, J.; et al. Electroactive self-polymerized dopamine with improved desalination performance for flow- and fixed- electrodes capacitive deionization. Appl. Surf. Sci. 2022, 579, 152154. [Google Scholar] [CrossRef]
- Wu, L.; Dang, Y.; Liang, L.-X.; Gong, Y.-C.; Zeeshan, M.; Qian, Z.; Geiger, S.D.; Vaughn, M.G.; Zhou, Y.; Li, Q.-Q.; et al. Perfluorooctane sulfonates induces neurobehavioral changes and increases dopamine neurotransmitter levels in zebrafish larvae. Chemosphere 2022, 297, 134234. [Google Scholar] [CrossRef]
- Trapani, A.; De Giglio, E.; Cometa, S.; Bonifacio, M.A.; Dazzi, L.; Di Gioia, S.; Hossain, M.N.; Pellitteri, R.; Antimisiaris, S.G.; Conese, M. Dopamine-loaded lipid based nanocarriers for intranasal administration of the neurotransmitter: A comparative study. Eur. J. Pharm. Biopharm. 2021, 167, 189–200. [Google Scholar] [CrossRef]
- Sohal, P.; Kaur, R.; Singh, M.; Banipal, T.S.; Banipal, P.K.; Kang, T.S. Binding studies of dopamine HCl drug with the mixed {sodium bis(2-ethylhexyl) sulfosuccinate + sodium dodecylsulfate} micelles: Physicochemical, spectroscopic, calorimetric and computational approach. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128386. [Google Scholar] [CrossRef]
- Osugo, M.; Whitehurst, T.; Shatalina, E.; Townsend, L.; O’Brien, O.; Mak, T.L.A.; McCutcheon, R.; Howes, O. Dopamine partial agonists and prodopaminergic drugs for schizophrenia: Systematic review and meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 2022, 135, 104568. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, W.; Wang, P.; Zhou, M.; Cui, L.; Wang, Q.; Yu, Y. Multifunctional coating of cotton fabric via the assembly of amino-quinone networks with polyamine biomacromolecules and dopamine quinone. Int. J. Biol. Macromol. 2022, 213, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Wei, Z.-H.; Jiao, Y.-J.; An, D.-Y.; Huang, Y.-P.; Liu, Z.-S.; Yan, C. Dual-function monolithic enzyme reactor based on dopamine/graphene oxide coating for simultaneous protein enzymatic hydrolysis and glycopeptide enrichment. J. Chromatogr. A 2022, 1666, 462848. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Zhang, Y.; Lin, Y. Controlled release of dopamine coatings on titanium bidirectionally regulate osteoclastic and osteogenic response behaviors. Mater. Sci. Eng. C 2021, 129, 112376. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; Van Der Westen, R.; Postma, A.; Städler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Ma, Y.; Zhou, F.; Hong, S.; Lee, H. Material-Independent Surface Chemistry beyond Polydopamine Coating. Acc. Chem. Res. 2019, 52, 704–713. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Saha, A.; Noked, M.; Gedanken, A.; Margel, S. Mussel-Inspired Polynorepinephrine/MXene-Based Magnetic Nanohybrid for Electromagnetic Interference Shielding in X-Band and Strain-Sensing Performance. Langmuir 2022, 38, 3936–3950. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Yuan, X.; Cui, Z.; Yang, X. Fabrication of dopamine-modified hyaluronic acid/chitosan multilayers on titanium alloy by layer-by-layer self-assembly for promoting osteoblast growth. Appl. Surf. Sci. 2013, 284, 732–737. [Google Scholar] [CrossRef]
- Xia, L.; Hao, Z.; Vemuri, B.; Zhao, S.; Gadhamshetty, V.; Kilduff, J.E. Improving antifouling properties of poly (ether sulfone) UF membranes with hydrophilic coatings of dopamine and poly(2-dimethylamino) ethyl methacrylate salt to enable water reuse. Sep. Purif. Technol. 2022, 285, 120300. [Google Scholar] [CrossRef]
- Zavala-Ocampo, L.M.; Aguirre-Hernández, E.; López-Camacho, P.Y.; Cárdenas-Vázquez, R.; Dorazco-González, A.; Basurto-Islas, G. Acetylcholinesterase inhibition and antioxidant activity properties of Petiveria alliacea L. J. Ethnopharmacol. 2022, 292, 115239. [Google Scholar] [CrossRef]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
- Sahiner, M.; Blake, D.A.; Fullerton, M.L.; Suner, S.S.; Sunol, A.K.; Sahiner, N. Enhancement of biocompatibility and carbohydrate absorption control potential of rosmarinic acid through crosslinking into microparticles. Int. J. Biol. Macromol. 2019, 137, 836–843. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure–activity relationship study. J. Enzyme Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Fontana Pereira, D.; Cazarolli, L.H.; Lavado, C.; Mengatto, V.; Figueiredo, M.S.R.B.; Guedes, A.; Pizzolatti, M.G.; Silva, F.R.M.B. Effects of flavonoids on α-glucosidase activity: Potential targets for glucose homeostasis. Nutrition 2011, 27, 1161–1167. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, M.; Suner, S.S.; Sahiner, N. Improved Biomedical Properties of Polydopamine-Coated Carbon Nanotubes. Micromachines 2021, 12, 1280. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Blake, D.A.; Reed, W.F. Polydopamine particles as nontoxic, blood compatible, antioxidant and drug delivery materials. Colloids Surf. B Biointerfaces 2018, 172, 618–626. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Sun, D.; Wang, C.; Zhang, J.; Xie, Y.; Guo, T. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015, 3, 328–334. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, M.; Ayyala, R.S.; Bhethanabotla, V.R.; Sahiner, N. Nitrogen-Doped Arginine Carbon Dots and Its Metal Nanoparticle Composites as Antibacterial Agent. C—J. Carbon Res. 2020, 6, 58. [Google Scholar] [CrossRef]
- Ferreres, F.; Taveira, M.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Tomato (Lycopersicon esculentum) Seeds: New Flavonols and Cytotoxic Effect. J. Agric. Food Chem. 2010, 58, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, T.K.; Mondal, S.; Das, N.C. Synthesis of polydopamine-coated halloysite nanotube-based hydrogel for controlled release of a calcium channel blocker. RSC Adv. 2016, 6, 105350–105362. [Google Scholar] [CrossRef]
- Nyankson, E.; Agyei-Tuffour, B.; Annan, E.; Yaya, A.; Mensah, B.; Onwona-Agyeman, B.; Amedalor, R.; Kwaku-Frimpong, B.; Efavi, J.K. Ag2CO3-halloysite nanotubes composite with enhanced removal efficiency for water soluble dyes. Heliyon 2019, 5, e01969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Wang, S.; Rao, Z.; Yang, Y. A luminescent Terbium-Succinate MOF thin film fabricated by electrodeposition for sensing of Cu2+ in aqueous environment. Sensors Actuators B Chem. 2015, 220, 779–787. [Google Scholar] [CrossRef]
- Tas, C.E.; Sevinis Ozbulut, E.B.; Ceven, O.F.; Tas, B.A.; Unal, S.; Unal, H. Purification and Sorting of Halloysite Nanotubes into Homogeneous, Agglomeration-Free Fractions by Polydopamine Functionalization. ACS Omega 2020, 5, 17962–17972. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Sahiner, M.; Ari, B.; Sunol, A.K.; Sahiner, N. Chondroitin Sulfate-Based Cryogels for Biomedical Applications. Gels 2021, 7, 127. [Google Scholar] [CrossRef] [PubMed]


| Materials | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| HNT | 57.9 | 4.1 | 31.3 |
| PDOPA1@HNT | 55.9 ± 0.3 | 4.2 | 34.2 |
| PDOPA2@HNT | 53.4 | 4.5 | 31.5 |
| PDOPA3@HNT | 53.3 | 4.3 | 32.9 |
| PDOPA4@HNT | 47.4 | 4.1 | 24.6 |
| PDOPA5@HNT | 46.4 | 3.9 | 18.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahiner, M.; Demirci, S.; Sahiner, N. Enhanced Bioactive Properties of Halloysite Nanotubes via Polydopamine Coating. Polymers 2022, 14, 4346. https://doi.org/10.3390/polym14204346
Sahiner M, Demirci S, Sahiner N. Enhanced Bioactive Properties of Halloysite Nanotubes via Polydopamine Coating. Polymers. 2022; 14(20):4346. https://doi.org/10.3390/polym14204346
Chicago/Turabian StyleSahiner, Mehtap, Sahin Demirci, and Nurettin Sahiner. 2022. "Enhanced Bioactive Properties of Halloysite Nanotubes via Polydopamine Coating" Polymers 14, no. 20: 4346. https://doi.org/10.3390/polym14204346
APA StyleSahiner, M., Demirci, S., & Sahiner, N. (2022). Enhanced Bioactive Properties of Halloysite Nanotubes via Polydopamine Coating. Polymers, 14(20), 4346. https://doi.org/10.3390/polym14204346