Effects of Chitosan on Loading and Releasing for Doxorubicin Loaded Porous Hydroxyapatite–Gelatin Composite Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Porous HAp–Gelatin Composite Microspheres
2.3. Characterization of HAp–Gelatin
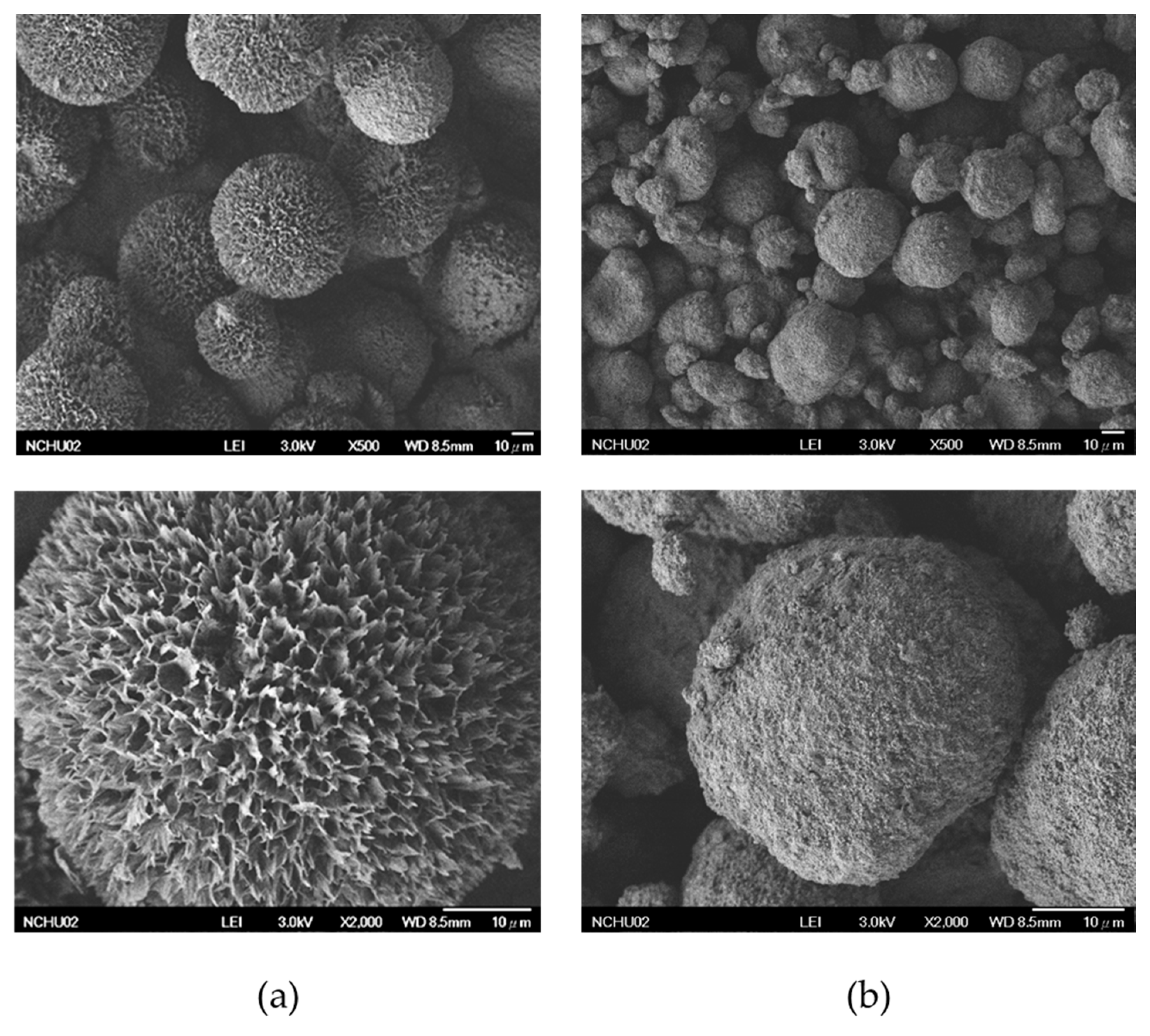
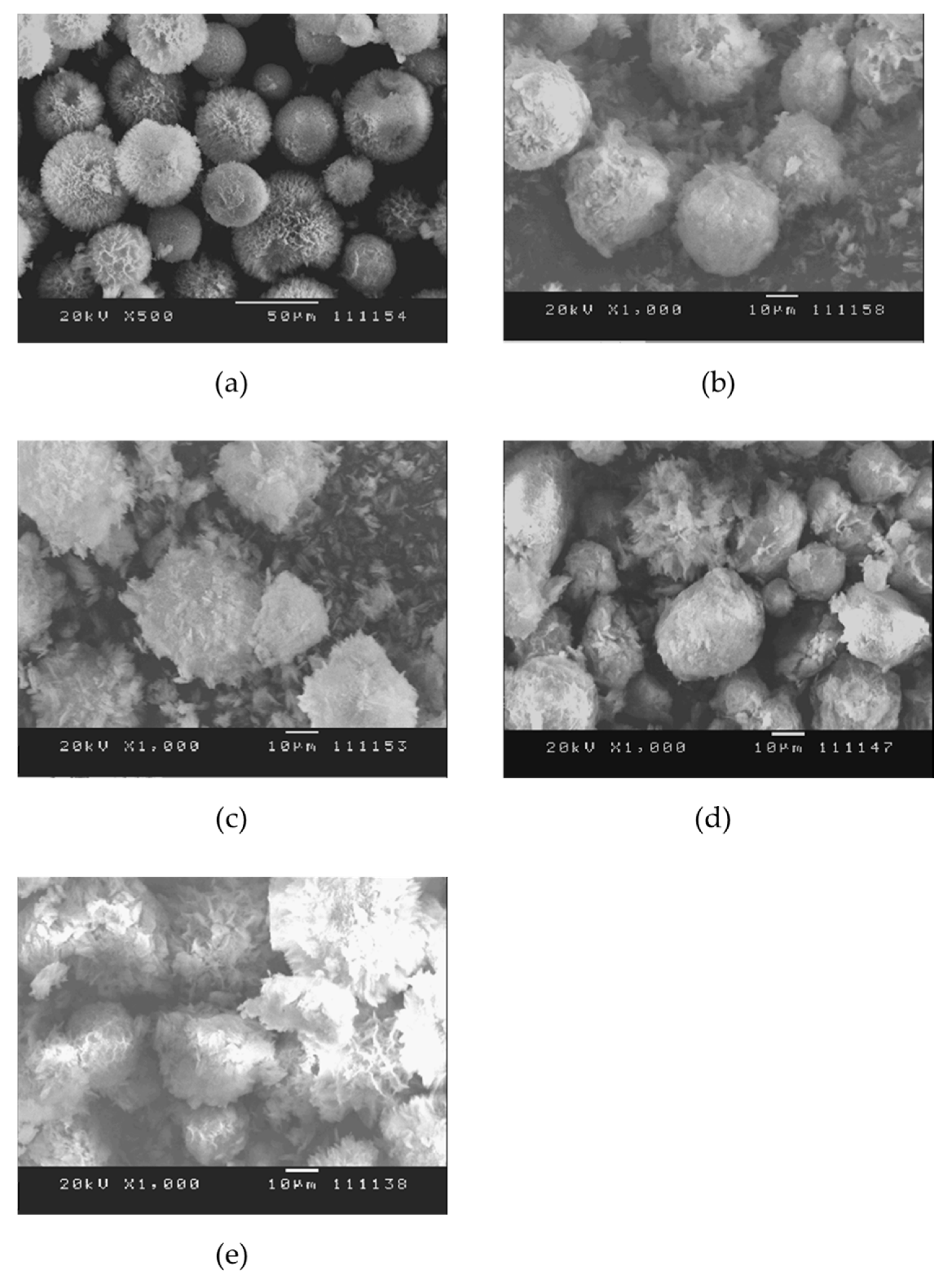
2.3.1. SEM
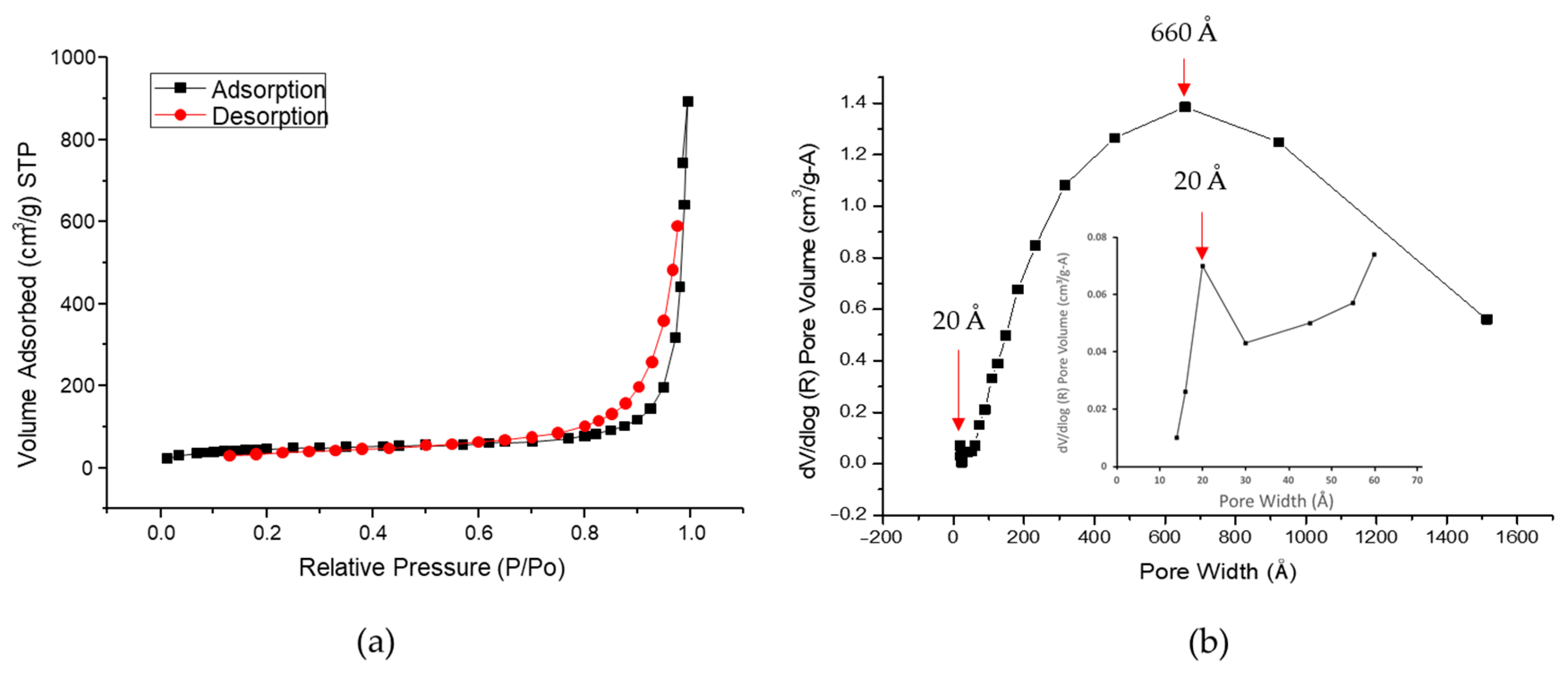
2.3.2. Specific Surface Area Porosimetry and Chemisorption Analysis
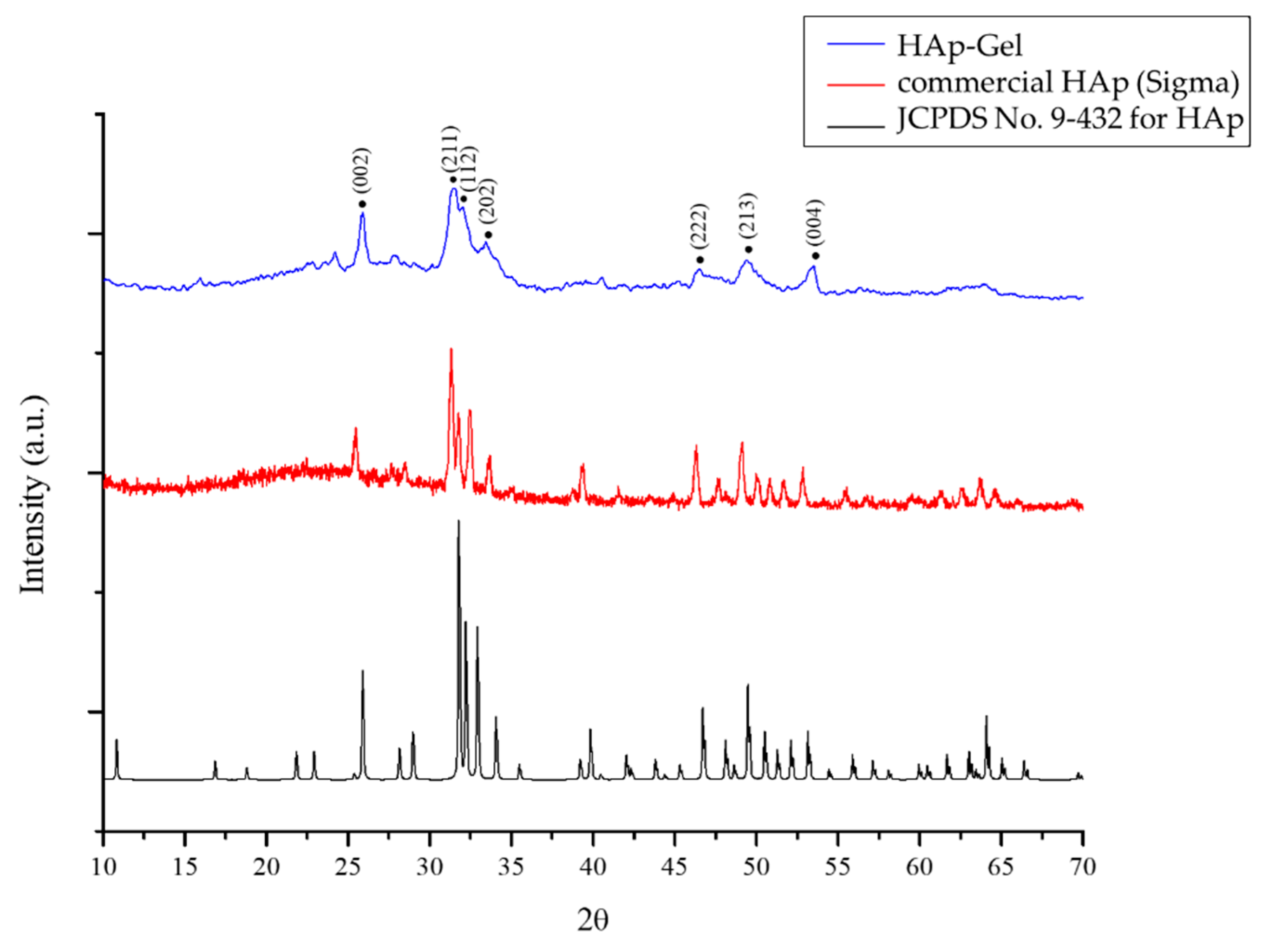
2.3.3. XRD
2.3.4. ICP-MS
2.4. Drug Load and Release Kinetics
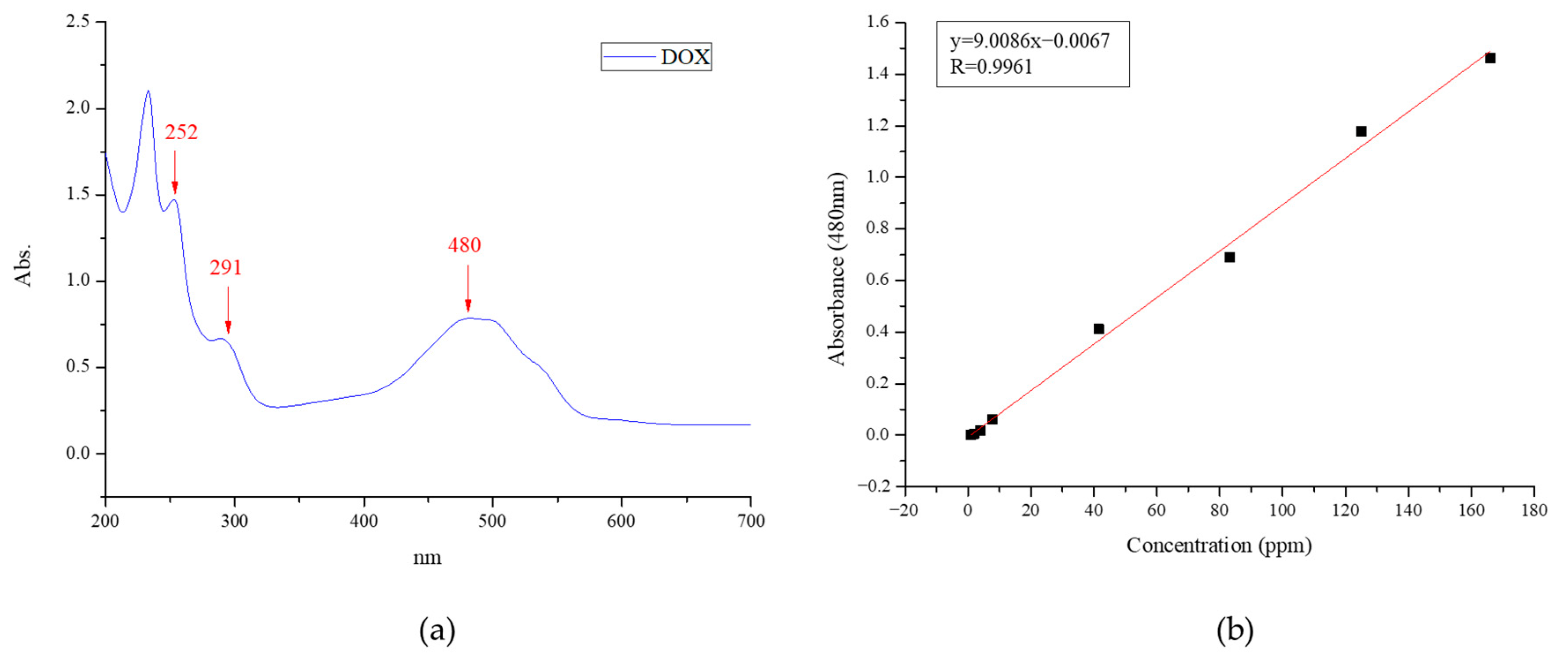
2.4.1. DOX Calibration Curves
2.4.2. DOX Coupled with Chitosan Loading and Content Determination
2.4.3. In Vitro DOX Releasing from DOX-Loaded Composite Microspheres
2.5. Cell Experiment
2.5.1. Cell Culture
2.5.2. Medium Extracted from Immersion Test
2.5.3. Cytotoxicity Tests
2.5.4. MTT Assay
3. Results and Discussion
3.1. Characterization of HAp–Gelatin Porous Composite Microspheres
3.1.1. Specific Surface Area and Pore Volume of HAp–Gelatin Composite Microspheres
3.1.2. XRD Patterns
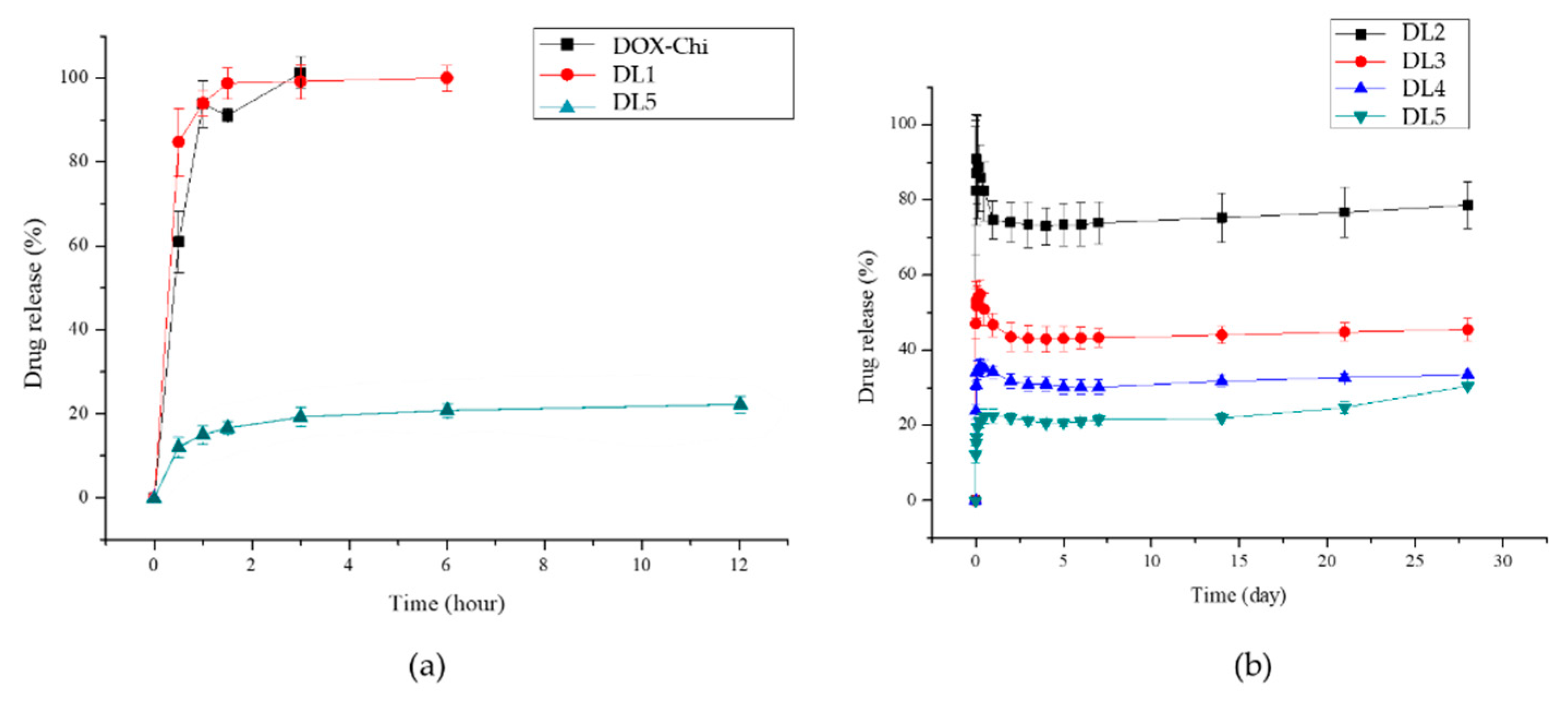
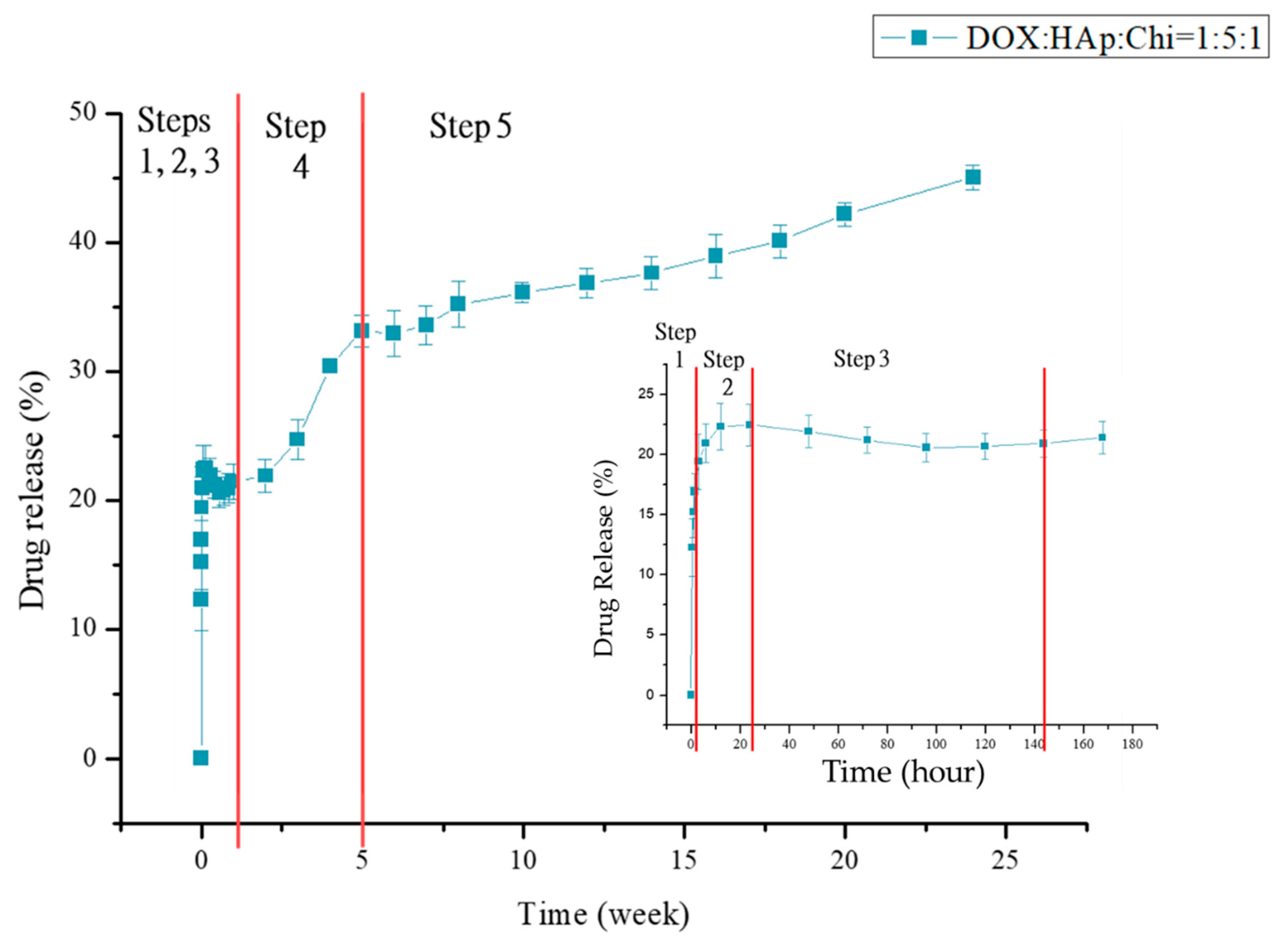
3.2. Drug Loading and Releasing
3.2.1. UV–VIS Spectrum for DOX Concentrations
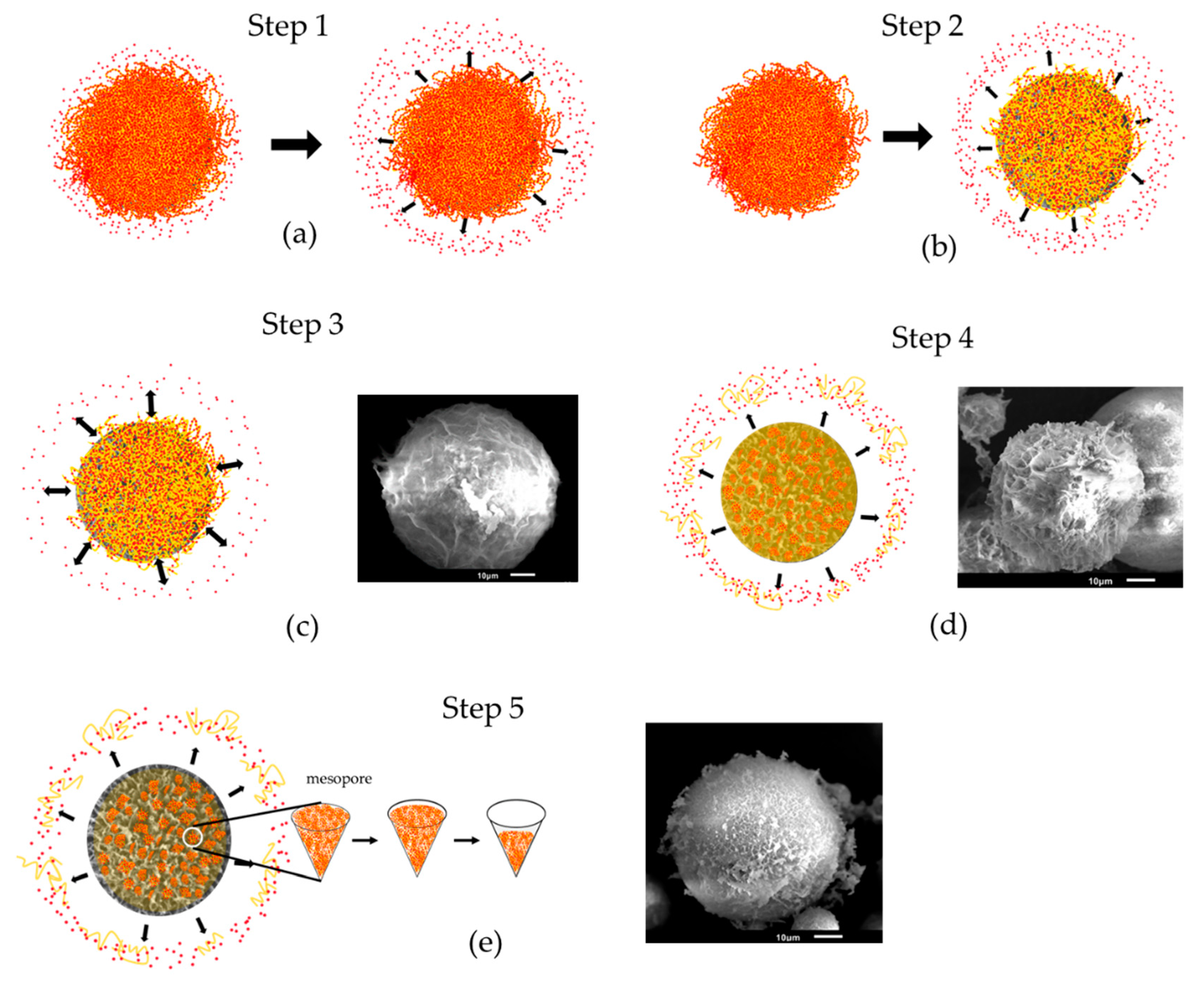
3.2.2. Mechanisms of DOX Loading and Releasing
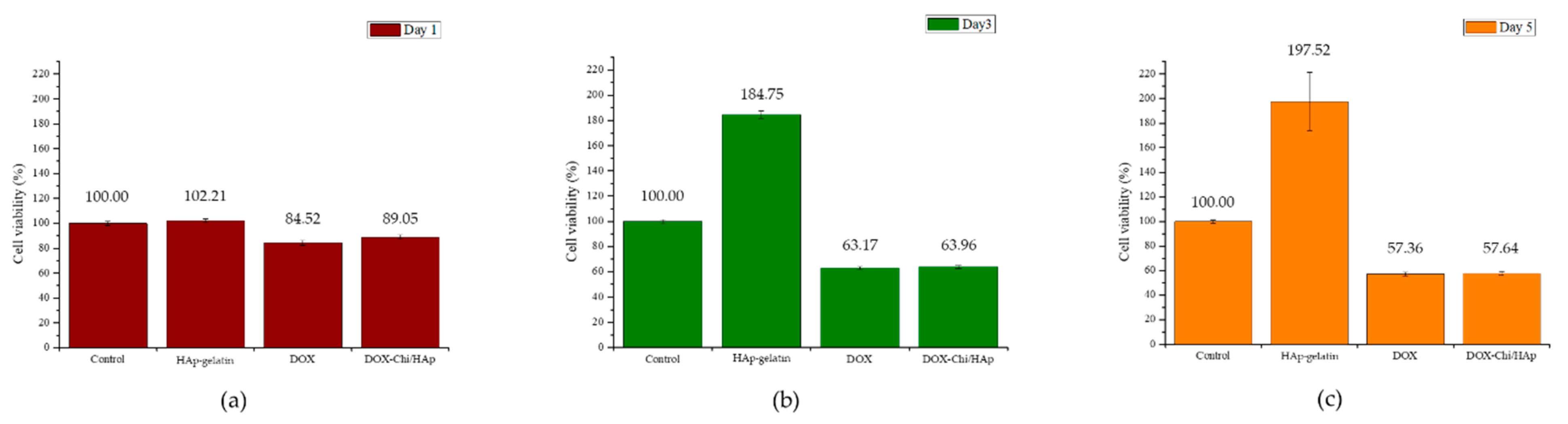
3.3. MTT Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Giessen, W.J.; Sorop, O.; Serruys, P.W.; Peters-Krabbendam, I.; van Beusekom, H.M. Lowering the dose of sirolimus, released from a nonpolymeric hydroxyapatite coated coronary stent, reduces signs of delayed healing. JACC Cardiovasc. Interv. 2009, 2, 284–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pezzatini, S.; Solito, R.; Morbidelli, L.; Lamponi, S.; Boanini, E.; Bigi, A.; Ziche, M. The effect of hydroxyapatite nanocrystals on microvascular endothelial cell viability and functions. J. Biomed. Mater. Res. Part A 2006, 76A, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Wang, M.-J.; Pai, N.-S.; Yen, S.-K. Preparation and characterization of gelatin–hydroxyapatite composite micro-spheres for hard tissue repair. Mater. Sci. Eng. C 2015, 57, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef] [PubMed]
- Aval, N.A.; Islamian, J.P.; Hatamian, M.; Arabfirouzjaei, M.; Javadpour, J.; Rashidi, M.-R. Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment. Int. J. Pharm. 2016, 509, 159–167. [Google Scholar] [CrossRef]
- Lai, W.; Chen, C.; Ren, X.; Lee, I.-S.; Jiang, G.; Kong, X. Hydrothermal fabrication of porous hollow hydroxyapatite microspheres for a drug delivery system. Mater. Sci. Eng. C 2016, 62, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-L.; Cheng, Y.-M.; Yena, S.-K. Doxorubicin—Chitosan—Hydroxyapatite composite coatings on titanium alloy for localized cancer therapy. Mater. Sci. Eng. C 2019, 104, 109953. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-F.; Yang, C.-K.; Ou, Y.-C. Urologic cancer in Taiwan. Jpn. J. Clin. Oncol. 2016, 46, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, D.; Yu, J.; Dong, H.; Zhang, J.; Yang, S. Targeting autophagy in cancer stem cells as an anticancer therapy. Cancer Lett. 2017, 393, 33–39. [Google Scholar] [CrossRef]
- Hauner, K.; Maisch, P.; Retz, M. Side effects of chemotherapy. Urologe A 2017, 56, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.K.; Needham, D. Targeted drug delivery. Expert Opin. Ther. Pat. 1999, 9, 1499–1513. [Google Scholar] [CrossRef]
- Cui, X.; Guan, X.; Zhong, S.; Chen, J.; Zhu, H.; Li, Z.; Xu, F.; Chen, P.; Wang, H. Multi-stimuli responsive smart chitosan-based microcapsules for targeted drug delivery and triggered drug release. Ultrason. Sonochem. 2017, 38, 145–153. [Google Scholar] [CrossRef]
- Dong, F.; Dong, X.; Zhou, L.; Xiao, H.; Ho, P.-Y.; Wong, M.-S.; Wang, Y. Doxorubicin-loaded biodegradable self-assembly zein nanoparticleand its anti-cancer effect: Preparation, in vitro evaluation, and cellularuptake. Colloids Surf. B Biointerfaces 2016, 140, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-S.; Chien, S. Chemotherapeutic engineering: Application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003, 58, 4087–4114. [Google Scholar] [CrossRef]
- DOXORUBICIN HYDROCHLORIDE—Doxorubicin Hydrochloride Injection, Solution, Pfizer Laboratories Div Pfizer Inc. Available online: https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=51500 (accessed on 14 September 2022).
- Ajish, J.K.; Ajish Kumar, K.S.; Chattopadhyay, S.; Kumar, M. Glycopolymeric gel stabilized N-succinyl chitosan beads for controlled doxorubicin delivery. Carbohydr. Polym. 2016, 144, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Nicastri, A.; Coluccio, M.L.; Gentile, F.; Candeloro, P.; Cojoc, G.; Liberale, C.; De Angelis, F.; Di Fabrizio, E. FT-IR, Raman, RRS measurements and DFT calculation for doxorubicin. Microsc. Res. Tech. 2010, 73, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Chitosan-based nanoparticles as drug de-livery systems for doxorubicin: Optimization and modeling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef]
- Wells, P.G.; Boerth, R.C.; Oates, J.A.; Harbison, R.D. Toxicologic enhancement by a combination of drugs which deplete hepatic glutathione: Acetaminophen and doxorubicin (adriamycin). Toxicol. Appl. Pharmacol. 1980, 54, 197–209. [Google Scholar] [CrossRef]
- DNA Structure, Replication, and Technology. Available online: http://www.shmoop.com/dna/dna-replication.html (accessed on 14 September 2022).
- Yaylaoǧlu, M.B.; Korkusuz, P.; Örs, Ü.; Korkusuz, F.; Hasirci, V. Development of a calcium phosphate–gelatin composite as a bone substitute and its use in drug release. Biomaterials 1999, 20, 711–719. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.K.; Wang, M.J. Method for Preparing Hydroxyapatite—Gelatin Microspheres. Taiwan Patent No. I407979, 11 September 2013. [Google Scholar]
- Pai, N.-S.; Yen, S.-K. Preparation and characterization of platinum/iron contained hydroxyapatite/carbon black composites. Int. J. Hydrogen Energy 2013, 38, 13249–13259. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Sing, K.S.W.; Evertt, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Huang, T.-H.; Hsu, S.-H.; Chang, S.-W. Molecular interaction mechanisms of glycol chitosan self-healing hydrogel as a drug delivery system for gemcitabine and doxorubicin. Comput. Struct. Biotechnol. J. 2022, 20, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Lin, C.-C.; Yen, S.-K. Electrochemical Deposition of Vancomycin/Chitosan Composite on Ti Alloy. J. Electrochem. Soc. 2011, 158, E152–E158. [Google Scholar] [CrossRef]
- Jennings, J.A. 7—Controlling chitosan degradation properties in vitro and in vivo. In Chitosan Based Biomaterials; Woodhead Publishing: Cambridge, UK, 2017; Volume 1, pp. 159–182. [Google Scholar]
- Mocanu, A.; Cadar, O.; Frangopol, P.T.; Petean, I.; Tomoaia, G.; Paltinean, G.-A.; Racz, C.P.; Horovitz, O.; Tomoaia-Cotisel, M. Ion release from hydroxyapatite and substituted hydroxyapatites in different immersion liquids: In vitro experiments and theoretical modelling study. R. Soc. Open Sci. 2021, 8, 201785. [Google Scholar] [CrossRef] [PubMed]

| Ca (wt%) | Ca (mol) | P (wt%) | P (mol) | Ca/P | |
|---|---|---|---|---|---|
| Commercial HAp (sigma) | 37.7 | 0.95 | 18.6 | 0.6 | 1.57 |
| HAp–gelatin microsphere | 28.1 | 0.7 | 14.3 | 0.46 | 1.52 |
| BET Adsorption Theory | BJH Adsorption | BJH Desorption | |
|---|---|---|---|
| Specific surface area | 158.64 m2/g | 123.92 m2/g | 172.97 m2/g |
| Pore volume | 0.4915 cm3/g | ||
| Average pore size | 123.93 Å | 442.58 Å | 319.24 Å |
| DOX (mg) | HAp–Gel (mg) | Chitosan (mg) | DL (%) | EE (%) | |
|---|---|---|---|---|---|
| DL1 | 5 | 25 | 0 | 13.95 ± 0.29 | 69.76 ± 5.81 |
| DL2 | 5 | 25 | 0.625 | 13.79 ± 0.53 | 68.93 ± 10.69 |
| DL3 | 5 | 25 | 1.25 | 17.53 ± 0.17 | 87.67 ± 3.44 |
| DL4 | 5 | 25 | 2.5 | 19.55 ± 0.06 | 97.75 ± 1.28 |
| DL5 | 5 | 25 | 5 | 19.88 ± 0.01 | 99.39 ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-Y.; Liang, Y.-H.; Yen, S.-K. Effects of Chitosan on Loading and Releasing for Doxorubicin Loaded Porous Hydroxyapatite–Gelatin Composite Microspheres. Polymers 2022, 14, 4276. https://doi.org/10.3390/polym14204276
Wu M-Y, Liang Y-H, Yen S-K. Effects of Chitosan on Loading and Releasing for Doxorubicin Loaded Porous Hydroxyapatite–Gelatin Composite Microspheres. Polymers. 2022; 14(20):4276. https://doi.org/10.3390/polym14204276
Chicago/Turabian StyleWu, Meng-Ying, Yu-Hsin Liang, and Shiow-Kang Yen. 2022. "Effects of Chitosan on Loading and Releasing for Doxorubicin Loaded Porous Hydroxyapatite–Gelatin Composite Microspheres" Polymers 14, no. 20: 4276. https://doi.org/10.3390/polym14204276
APA StyleWu, M.-Y., Liang, Y.-H., & Yen, S.-K. (2022). Effects of Chitosan on Loading and Releasing for Doxorubicin Loaded Porous Hydroxyapatite–Gelatin Composite Microspheres. Polymers, 14(20), 4276. https://doi.org/10.3390/polym14204276








