Approaches for Extracting Nanofibrillated Cellulose from Oat Bran and Its Emulsion Capacity and Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
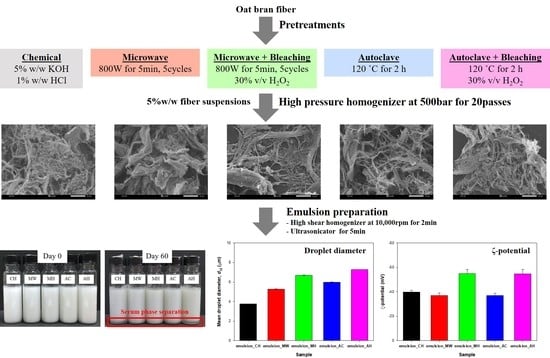
2.2. Oat Nanofibers Extraction
2.2.1. Chemical Pretreatment
2.2.2. Hydrothermal Pretreatment
2.2.3. Bleaching Process
2.2.4. Mechanical Process
2.3. Oil-in-Water (O/W) NFC-Stabilized Emulsion Preparation
2.4. Scanning Electron Microscope (SEM)
2.5. Particle Size and Size Distribution
2.6. ζ-Potential
2.7. Color
2.8. Rheological Properties
2.9. Creaming Stability
2.10. Statistical Analysis
3. Results and Discussion
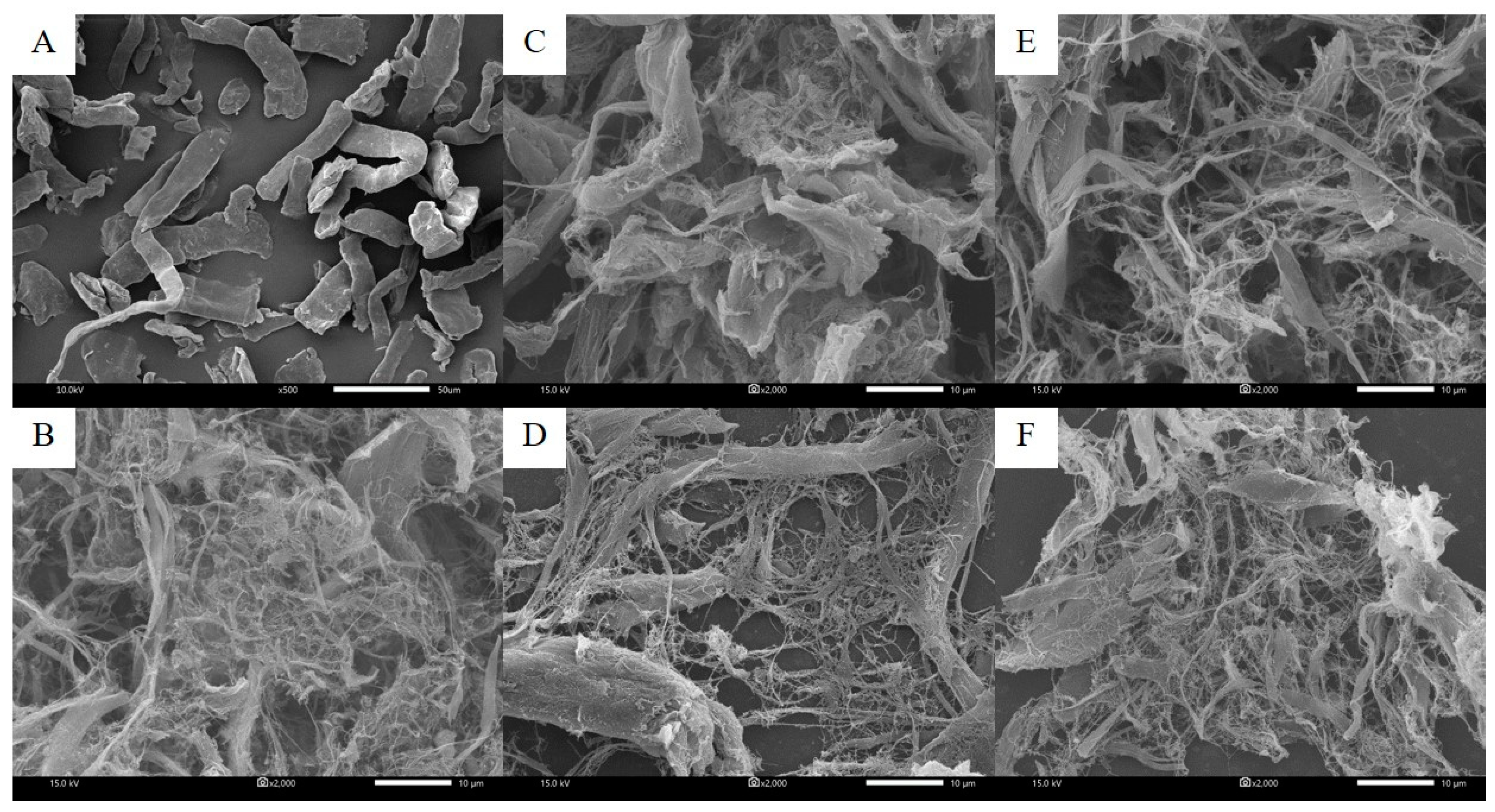
3.1. Microstructure of Oat Fibers and NFC
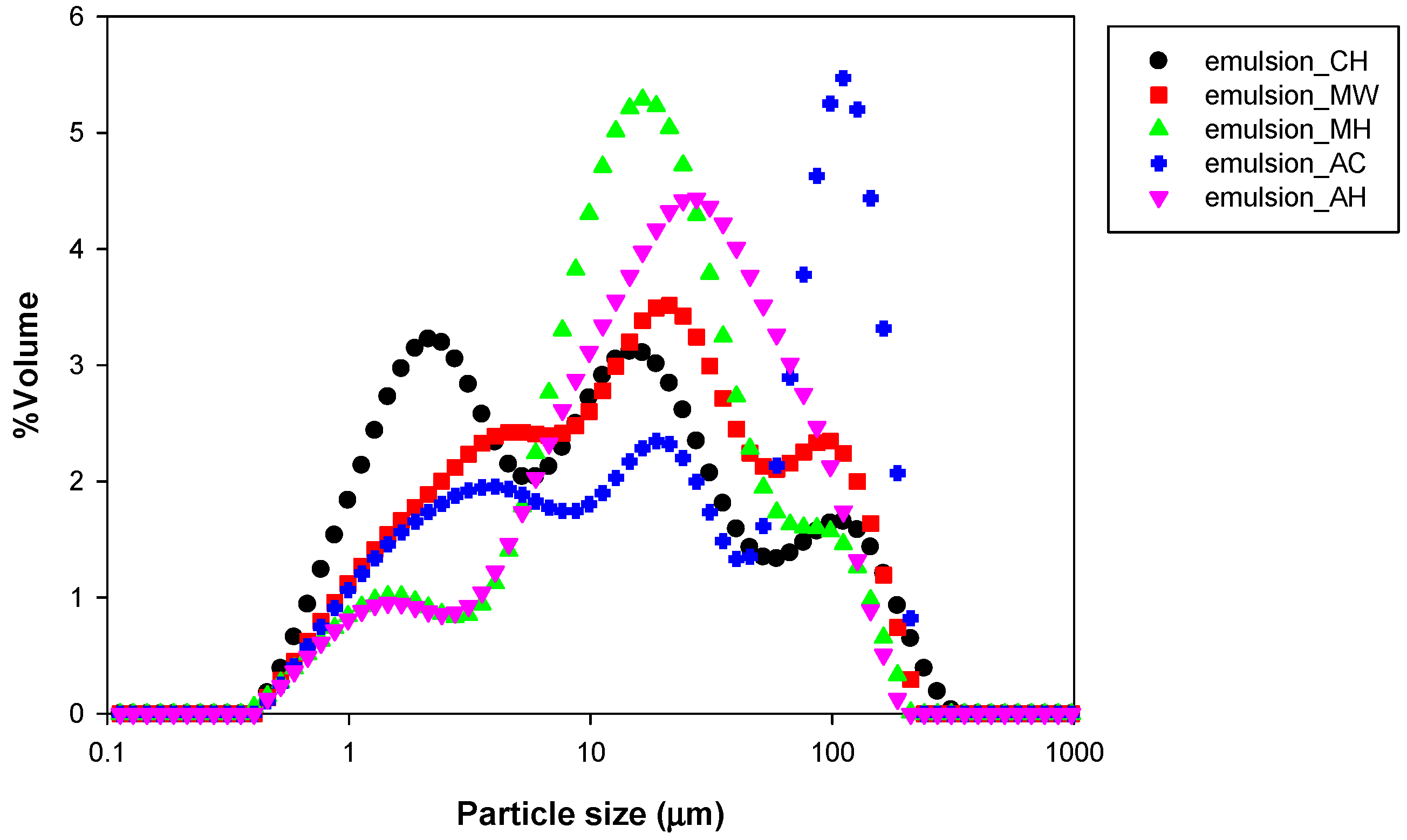
3.2. Particle Size and Distribution
3.3. ζ-Potential
3.4. Color
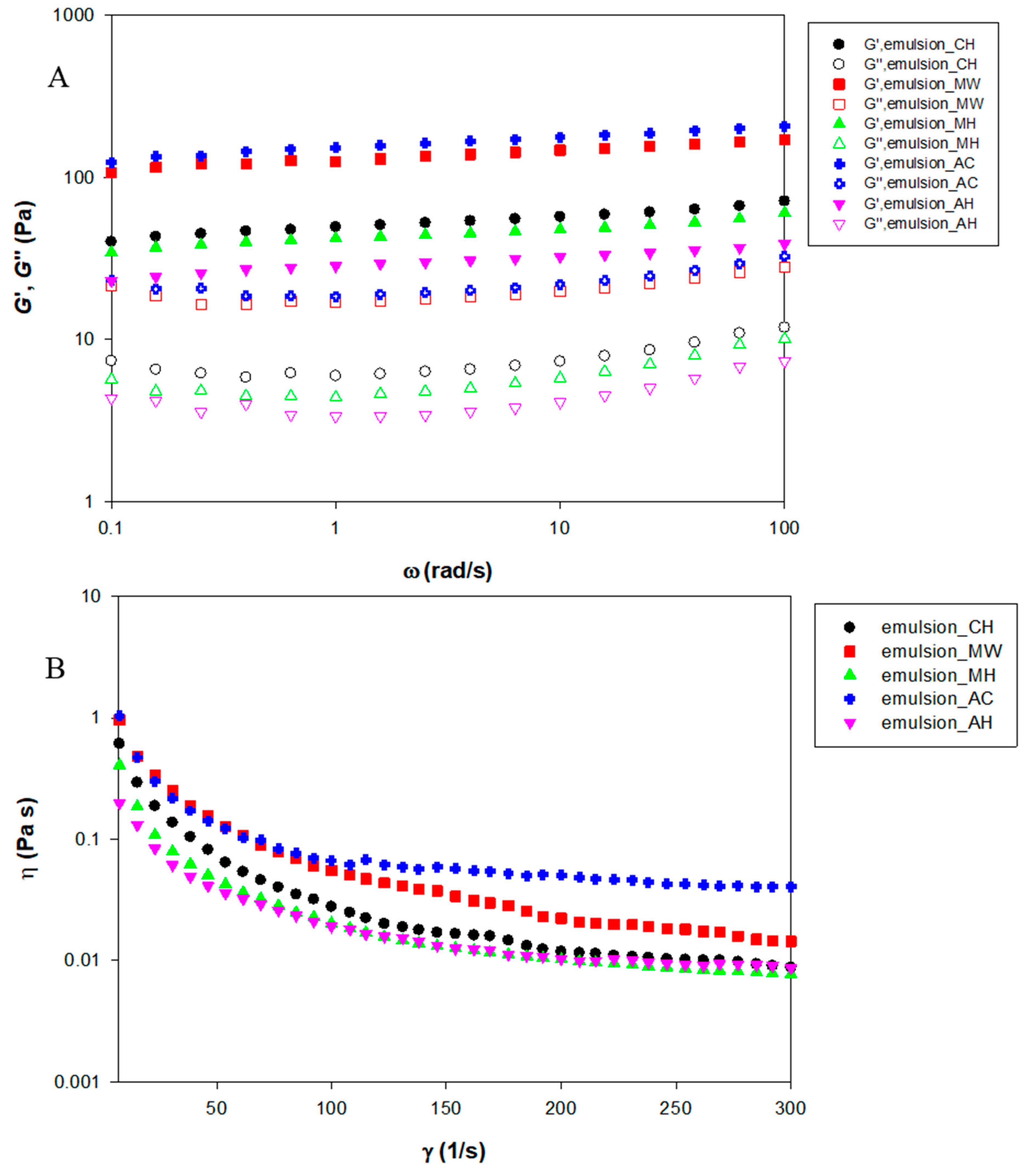
3.5. Rheological Properties
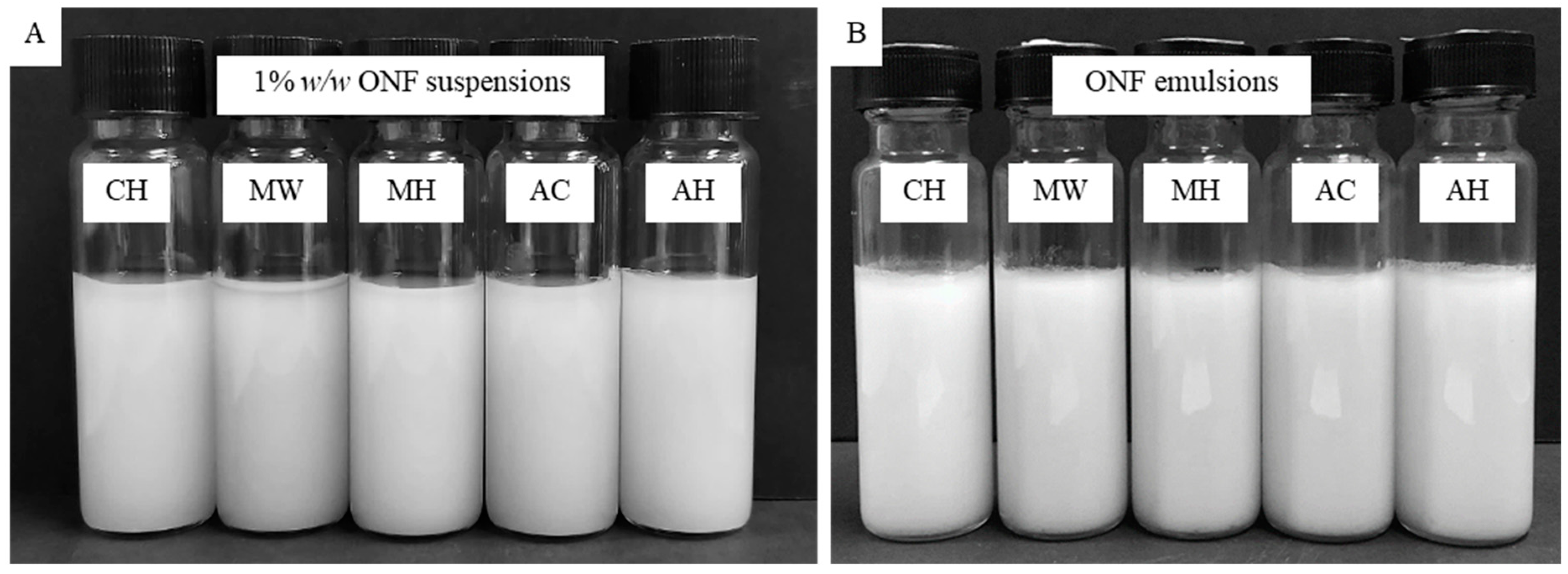
3.6. Creaming Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CH | Chemical pretreatment |
| MW | Microwave without bleaching pretreatment |
| MH | Microwave with bleaching pretreatment |
| AC | Autoclave without bleaching pretreatment |
| AH | Autoclave with bleaching pretreatment |
References
- Li, J.; Song, Z.; Li, D.; Shang, S.; Guo, Y. Cotton cellulose nanofiber-reinforced high density polyethylene composites prepared with two different pretreatment methods. Ind. Crops Prod. 2014, 59, 318–328. [Google Scholar] [CrossRef]
- Tian, C.; Yi, J.; Wu, Y.; Wu, Q.; Qing, Y.; Wang, L. Preparation of highly charged cellulose nanofibrils using high-pressure homogenization coupled with strong acid hydrolysis pretreatments. Carbohydr. Polym. 2016, 136, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Gao, R.; Lu, Y.; Li, J.; Sun, Q. Fabrication and characterization of nanofibrillated cellulose and its aerogels from natural pine needles. Carbohydr. Polym. 2015, 119, 202–209. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Song, Y.S. Viscoelastic characteristics of all cellulose suspension and nanocomposite. Carbohydr. Polym. 2016, 151, 119–129. [Google Scholar] [CrossRef]
- Wang, W.; Liang, T.; Bai, H.; Dong, W.; Liu, X. All cellulose composites based on cellulose diacetate and nanofibrillated cellulose prepared by alkali treatment. Carbohydr. Polym. 2018, 179, 297–304. [Google Scholar] [CrossRef]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyoung, A.; McClements, D.J. Encapsulation of vitamin D 3 in pickering emulsions stabilized by nano fi brillated mangosteen cellulose: Impact on in vitro digestion and bioaccessibility. Food Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Jain, S.; Winuprasith, T. Properties and stability of Pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact of oil type and emulsifier concentration. Songklanakarin J. Sci. Technol. 2020, 42, 468–476. [Google Scholar]
- Maliha, M.; Herdman, M.; Brammananth, R.; McDonald, M.; Coppel, R.; Werrett, M.; Andrews, P.; Batchelor, W. Bismuth phosphinate incorporated nanocellulose sheet with antimicrobial and barrier properties for packaging applications. J. Clean. Prod. 2020, 246, 119016. [Google Scholar] [CrossRef]
- Bongao, H.C.; Gabatino, R.R.A.; Arias, C.F.H.; Magdaluyo Jr., E.R. Micro/nanocellulose from waste Pili (Canarium ovatum) pulp as a potential anti-aging ingredient for cosmetic formulations. Mater. Today Proc. 2020, 22, 275–280. [Google Scholar] [CrossRef]
- Beluns, S.; Platnieks, O.; Gaidukovs, S.; Starkova, O.; Sabalina, A.; Grase, L.; Thakur, V.K.; Gaidukova, G. Lignin and Xylan as interface engineering additives for improved environmental durability of sustainable cellulose nanopapers. Int. J. Mol. Sci. 2021, 22, 12939. [Google Scholar] [CrossRef]
- Jose, J.; Thomas, V.; Vinod, V.; Abraham, R.; Abraham, S. Nanocellulose based functional materials for supercapacitor applications. J. Sci. Adv. Mater. Devices 2019, 4, 333–340. [Google Scholar] [CrossRef]
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2019, 27, 1149–1194. [Google Scholar] [CrossRef]
- Beluns, S.; Gaidukovs, S.; Platnieks, O.; Gaidukova, G.; Mierina, I.; Grase, L.; Starkova, O.; Brazdausks, P.; Thakur, V.K. From Wood and Hemp Biomass Wastes to Sustainable Nanocellulose Foams. Ind. Crops Prod. 2021, 170, 113780. [Google Scholar] [CrossRef]
- Auxenfans, T.; Buchoux, S.; Djellab, K.; Avondo, C.; Husson, E.; Sarazin, C. Mild pretreatment and enzymatic saccharification of cellulose with recycled ionic liquids towards one-batch process. Carbohydr. Polym. 2012, 90, 805–813. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Zhang, Y.; Yu, G.; Si, C.; Li, B. Preparation and characterization of functional cellulose nanofibrils via formic acid hydrolysis pretreatment and the followed high-pressure homogenization. Ind. Crops Prod. 2016, 94, 736–745. [Google Scholar] [CrossRef]
- Sun, B.; Peng, G.; Duan, L.; Xu, A.; Li, X. Pretreatment by NaOH swelling and then HCl regeneration to enhance the acid hydrolysis of cellulose to glucose. Bioresour. Technol. 2015, 196, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhou, Y.; Li, Y. Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour. Technol. 2011, 102, 6254–6259. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Zakaria, S.; Chia, C.H.; Padzil, F.N.M.; Ng, P. Effect of hydrothermal pretreatment on solubility and formation of kenaf cellulose membrane and hydrogel. Carbohydr. Polym. 2015, 115, 62–68. [Google Scholar] [CrossRef]
- Kouteu Nanssou, P.A.; Jiokap Nono, Y.; Kapseu, C. Pretreatment of cassava stems and peelings by thermohydrolysis to enhance hydrolysis yield of cellulose in bioethanol production process. Renew. Energy 2016, 97, 252–265. [Google Scholar] [CrossRef]
- Tuzzin, G.; Godinho, M.; Dettmer, A.; Zattera, A.J. Nanofibrillated cellulose from tobacco industry wastes. Carbohydr. Polym. 2016, 148, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2018, 123, 379–388. [Google Scholar] [CrossRef]
- Zhang, J.; Song, H.; Lin, L.; Zhuang, J.; Pang, C.; Liu, S. Microfibrillated cellulose from bamboo pulp and its properties. 2010, 9, 78–83. Biomass Bioenergy 2010, 9, 78–83. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. Nanofibrillated cellulose obtained from soybean hull using simple and eco-friendly processes based on reactive extrusion. Cellulose 2020, 27, 1975–1988. [Google Scholar] [CrossRef]
- Hassan, E.A.; Hassan, M.L. Rice straw nanofibrillated cellulose films with antimicrobial properties via supramolecular route. Ind. Crops Prod. 2016, 93, 142–151. [Google Scholar] [CrossRef]
- Harini, K.; Ramya, K.; Sukumar, M. Extraction of nano cellulose fibers from the banana peel and bract for production of acetyl and lauroyl cellulose. Carbohydr. Polym. 2018, 201, 329–339. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Ralla, T.; Salminen, H.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Oat bran extract (Avena sativa L.) from food by-product streams as new natural emulsifier. Food Hydrocoll. 2018, 81, 253–262. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Zhang, Z. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J. Cereal Sci. 2011, 54, 98–103. [Google Scholar] [CrossRef]
- Chen, W.; Lickfield, G.C.; Yang, C.Q. Molecular modeling of cellulose in amorphous state. Part I: Model building and plastic deformation study. Polymer 2004, 45, 1063–1071. [Google Scholar] [CrossRef]
- Igarashi, K.; Uchihashi, T.; Koivula, A.; Wada, M.; Kimura, S.; Okamoto, T.; Penttilä, M.; Ando, T.; Samejima, M. Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface. Science 2011, 333, 1279–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, M.; Alexandridis, P.; Tsianou, M. Cellulose dissolution: Insights on the contributions of solvent-induced decrystallization and chain disentanglement. Cellulose 2017, 24, 571–590. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose structure and property changes indicated via wetting-drying cycles. Polym. Degrad. Stab. 2019, 167, 33–43. [Google Scholar] [CrossRef]
- Ramsden, W. Separation of solids in the surface-layers of solutions and “suspensions”. Proc. R. Soc. Lond. 1903, 72, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Pickering, S.U. CXCVI.-Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Gould, J.; Garcia-garcia, G.; Wolf, B. Pickering Particles Prepared from Food Waste. Materials 2016, 9, 791. [Google Scholar] [CrossRef]
- Calabrese, V.; Courtenay, J.C.; Edler, K.J.; Scott, J.L. Pickering emulsions stabilized by naturally derived or biodegradable particles. Curr. Opin. Green Sustain. Chem. 2018, 12, 83–90. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Tibolla, H.; Menegalli, F.C.; Cunha, R.L. Cellulose nanofibers from banana peels as a Pickering emulsifier: High-energy emulsification processes. Carbohydr. Polym. 2018, 194, 122–131. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Kuang, Y.; Guo, S.; Mo, L.; Ni, Y. Stabilization of Pickering emulsions with cellulose nanofibers derived from oil palm fruit bunch. Cellulose 2020, 27, 839–851. [Google Scholar] [CrossRef]
- Gestranius, M.; Stenius, P.; Kontturi, E.; Sjöblom, J.; Tammelin, T. Phase behaviour and droplet size of oil-in-water Pickering emulsions stabilised with plant-derived nanocellulosic materials. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 60–70. [Google Scholar] [CrossRef]
- Jongaroontaprangsee, S.; Chiewchan, N.; Devahastin, S. Production of nanofibrillated cellulose with superior water redispersibility from lime residues via a chemical-free process. Carbohydr. Polym. 2018, 193, 249–258. [Google Scholar] [CrossRef]
- Winuprasith, T.; Suphantharika, M. Properties and stability of oil-in-water emulsions stabilized by micro fi brillated cellulose from mangosteen rind. Food Hydrocoll. 2015, 43, 690–699. [Google Scholar] [CrossRef]
- Ali, N.; Giwa, A.S.; Abdalla, M.; Liu, X. Alkaline hydrogen peroxide pretreatment of bamboo culm for improved enzymatic release of reducing sugars using recombinant cellulases. Cellulose 2019, 27, 769–779. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Yang, Y.; Xie, G. Alkali-based pretreatments distinctively extract lignin and pectin for enhancing biomass saccharification by altering cellulose features in sugar-rich Jerusalem artichoke stem. Bioresour. Technol. 2016, 208, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, T.; Pöhler, E.; Geiger, T. Cellulose fibrils for polymer reinforcement. Adv. Eng. Mater. 2004, 6, 754–761. [Google Scholar] [CrossRef]
- Paschoal, G.B.; Muller, C.M.O.; Carvalho, G.M.; Tischer, C.A.; Mali, S. Isolation and characterization of nanofibrillated cellulose from oat hulls. Quim. Nov. 2015, 38, 478–482. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Lee, H.; Sundaram, J.; Zhu, L.; Zhao, Y.; Mani, S. Improved thermal stability of cellulose nanofibrils using low-concentration alkaline pretreatment. Carbohydr. Polym. 2018, 181, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Grumezescu, A.M. (Ed.) Emulsions; Academic Press: Cambridge, MA, USA, 2016; ISBN 978-012-804306-6. [Google Scholar]
- Taheri, H.; Samyn, P. Effect of homogenization (microfluidization) process parameters in mechanical production of micro- and nanofibrillated cellulose on its rheological and morphological properties. Cellulose 2016, 23, 1221–1238. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Tayeb, P.; Joyce, M.; Tyagi, P.; Kehoe, M.; Misic, K.D.; Pla, L. Rheology of Nanocellulose-rich Aqueous Suspensions: A Review. BioResourcesesources 2017, 12, 9556–9661. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Winuprasith, T.; Suphantharika, M. Microfibrillated cellulose from mangosteen (Garcinia mangostana L.) rind: Preparation, characterization and evaluation as an emulsion stabilizer. Food Hydrocoll. 2013, 32, 383–394. [Google Scholar] [CrossRef]


| Emulsions | d32 (μm) | ζ-Potential (mV) | L* | a* | b* | ηγ=300 (Pa·s) |
|---|---|---|---|---|---|---|
| emulsion_CH | 3.76 ± 0.006 e | −39.82 ± 1.51 b | 51.56 ± 0.22 ab | −0.11 ± 0.01 a | −0.04 ± 0.03 e | 0.0088 ± 0.0003 a |
| emulsion_MW | 5.28 ± 0.015 d | −37.00 ± 2.06 b | 52.04 ± 0.01 a | −0.09 ± 0.00 b | 0.43 ± 0.02 c | 0.0144 ± 0.0006 a |
| emulsion_MH | 6.70 ± 0.010 b | −54.90 ± 3.34 a | 49.23 ± 0.67 c | −0.05 ± 0.01 c | 0.85 ± 0.08 a | 0.0077 ± 0.0001 a |
| emulsion_AC | 6.00 ± 0.012 c | −37.05 ± 1.77 b | 51.35 ± 0.11 b | −0.12 ± 0.01 a | 0.30 ± 0.05 d | 0.0407 ± 0.0109 a |
| emulsion_AH | 7.28 ± 0.015 a | −54.85 ± 3.40 a | 49.25 ± 0.22 c | −0.09 ± 0.01 b | 0.65 ± 0.04 b | 0.0087 ± 0.0023 a |
| Nanofibrillated Cellulose (NFC) | ζ-Potential (mV) | L* | a* | b* | ηγ=300 (Pa·s) |
|---|---|---|---|---|---|
| NFC_CH | −20.12 ± 5.28 cd | 36.76 ± 0.13 c | −0.76 ± 0.01 a | −4.11 ± 0.02 a | 0.2413 ± 0.1227 a |
| NFC_MW | −26.73 ± 1.41 ab | 38.56 ± 0.51 b | −0.71 ± 0.01 b | −3.38 ± 0.06 c | 0.2413 ± 0.1963 a |
| NFC_MH | −23.08 ± 3.72 bc | 40.09 ± 0.20 a | −0.70 ± 0.01 b | −3.53 ± 0.02 b | 0.0250 ± 0.0045 b |
| NFC_AC | −27.95 ± 3.58 a | 39.29 ± 0.56 ab | −0.78 ± 0.02 a | −3.56 ± 0.02 b | 0.2453 ± 0.0412 a |
| NFC_AH | −17.05 ± 1.94 d | 39.80 ± 0.56 a | −0.72 ± 0.01 b | −3.52 ± 0.04 b | 0.0288 ± 0.0134 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitbumrung, W.; Rungraung, N.; Muangpracha, N.; Akanitkul, P.; Winuprasith, T. Approaches for Extracting Nanofibrillated Cellulose from Oat Bran and Its Emulsion Capacity and Stability. Polymers 2022, 14, 327. https://doi.org/10.3390/polym14020327
Mitbumrung W, Rungraung N, Muangpracha N, Akanitkul P, Winuprasith T. Approaches for Extracting Nanofibrillated Cellulose from Oat Bran and Its Emulsion Capacity and Stability. Polymers. 2022; 14(2):327. https://doi.org/10.3390/polym14020327
Chicago/Turabian StyleMitbumrung, Wiphada, Numphung Rungraung, Niramol Muangpracha, Ploypailin Akanitkul, and Thunnalin Winuprasith. 2022. "Approaches for Extracting Nanofibrillated Cellulose from Oat Bran and Its Emulsion Capacity and Stability" Polymers 14, no. 2: 327. https://doi.org/10.3390/polym14020327
APA StyleMitbumrung, W., Rungraung, N., Muangpracha, N., Akanitkul, P., & Winuprasith, T. (2022). Approaches for Extracting Nanofibrillated Cellulose from Oat Bran and Its Emulsion Capacity and Stability. Polymers, 14(2), 327. https://doi.org/10.3390/polym14020327