The Unique Carboxymethyl Fenugreek Gum Gel Loaded Itraconazole Self-Emulsifying Nanovesicles for Topical Onychomycosis Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. High-Performance Liquid Chromatography (HPLC)
2.2. Preparation of ITZ-nPEVs
2.3. Drug Content and % EE
2.4. PS, PDI and ZP
2.5. Elasticity
2.6. Viscosity
2.7. ITZ- nPEVs Shape
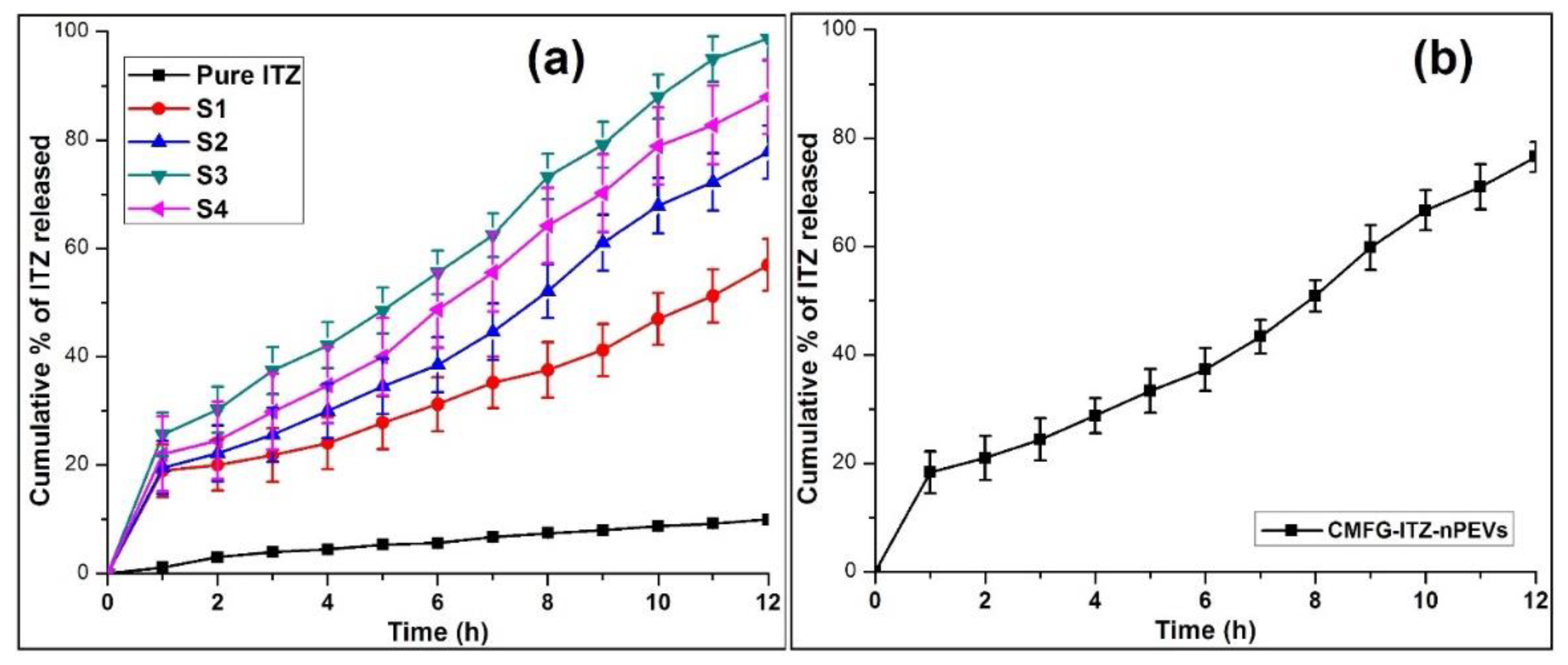
2.8. In Vitro ITZ Release
2.9. Nail Hydration/Transungual Drug Uptake of ITZ-nPEVs
2.10. The Efficacy of ITZ-nPEVs for the Treatment of Onychomycosis
2.11. Stability Study
3. Materials and Methods
3.1. Reagents
3.2. HPLC Analysis
3.3. Preparation of ITZ-nPEVs
3.4. Elimination of Unentrapped ITZ from ITZ-nPEVs
3.5. Preparation of ITZ Self- Emulsifying Nanovesicles Gel (CMFG-ITZ- nPEVs)
3.5.1. Extraction and Purification of Fenugreek Gum (FG)
3.5.2. Synthesis of Carboxymethyl Fenugreek Gum (CMFG)
3.5.3. Preparation of CMFG-ITZ-nPEVs Gel
3.6. Characterization
3.6.1. Drug Content (DC)
3.6.2. % EE
3.6.3. Deformability Index
- D is the deformability index (mL·s−1),
- j is the amount of vesicular dispersion extruded in mL,
- t is the time of extrusion in second,
- rv is the size of vesicles after extrusion (nm), and
- rp is the pore size of the filter (nm).
3.6.4. Viscosity of nPEVs
3.6.5. DLS
3.6.6. Morphology
3.6.7. In Vitro Drug Release and Kinetics
3.6.8. Nail Hydration Study
3.6.9. Transungual Drug Uptake Study
3.6.10. Anti-Microbiological Efficacy
3.6.11. Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nouripour-Sisakht, S.; Mirhendi, H.; Shidfar, M.R.; Ahmadi, B.; Rezaei-Matehkolaei, A.; Geramishoar, M.; Zarei, F.; Jalalizand, N. Aspergillus species as emerging causative agents of onychomycosis. J. Mycol. Med. 2015, 25, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, J.A.; Tilakaratne, W.M.; Panagoda, G.J. Candidal onychomycosis: A mini-review. Mycopathologia 2009, 168, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Sigurgeirsson, B.; Ghannoum, M. Therapeutic potential of TDT 067 (terbinafine in Transfersome): A carrier-based dosage form of terbinafine for onychomycosis. Expert Opin. Investig. Drugs 2012, 21, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Bsieso, E.A.; Nasr, M.; Moftah, N.H.; Sammour, O.A.; Abd-El-Gawad, N.A. Could nanovesicles containing a penetration enhancer clinically improve the therapeutic outcome in skin fungal diseases? Nanomedicine 2015, 10, 2017–2031. [Google Scholar] [CrossRef]
- Tiwary, A.K.; Sapra, B. High failure rate of transungal drug delivery: Need for new strategies. Ther. Deliv. 2017, 8, 239–242. [Google Scholar] [CrossRef]
- Dhamoon, R.K.; Harvinder Popli, H.; Madhu Gupta, M. Novel Drug Delivery Strategies for the Treatment of Onychomycosis. Pharm. Nanotechnol. 2019, 7, 24–38. [Google Scholar] [CrossRef]
- Wang, F.; Yang, P.; Choi, J.S.; Antovski, P.; Zhu, Y.; Xu, X.; Kuo, T.H.; Lin, L.E.; Kim, D.N.H.; Huang, P.C.; et al. Cross-Linked Fluorescent Supramolecular Nanoparticles for Intradermal Controlled Release of Antifungal Drug-A Therapeutic Approach for Onychomycosis. ACS Nano. 2018, 24, 6851–6859. [Google Scholar] [CrossRef]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine hydrochloride trans-ungual delivery via nanovesicular systems: In vitro characterization and ex vivo evaluation. AAPS PharmSciTech. 2017, 18, 551–562. [Google Scholar] [CrossRef]
- Palliyil, B.; Lebo, D.B.; Patel, P.R. A preformulation strategy for the selection of penetration enhancers for a transungual formulation. AAPS PharmSciTech. 2013, 14, 682–691. [Google Scholar] [CrossRef]
- Miron, D.; Cornelio, R.; Troleis, J.; Mariath, J.; Zimmer, A.R.; Mayorga, P.; Schapoval, E.E. Influence of penetration enhancers and molecular weight in antifungals permeation through bovine hoof membranes and prediction of efficacy in human nails. Eur. J. Pharm. Sci. 2014, 51, 20–25. [Google Scholar] [CrossRef]
- Hao, J.; Li, S.K. Mechanistic study of electroosmotic transport across hydrated nail plates: Effects of pH and ionic strength. J. Pharm. Sci. 2008, 97, 5186–5197. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef]
- Muthu, M.J.M.; Kavitha, K.; Ruckmani, K.; Shanmuganathan, S. Skimmed milk powder and pectin decorated solid lipid nanoparticle containing soluble curcumin used for the treatment of colorectal cancer. J. Food Process Eng. 2019, 43, 1–15. [Google Scholar]
- Mohamed, J.M.M.; Alqahtani, A.; Al Fatease, A.; Alqahtani, T.; Khan, B.A.; Ashmitha, B.; Vijaya, R. Human Hair Keratin Composite Scaffold: Characterisation and Biocompatibility Study on NIH 3T3 Fibroblast Cells. Pharmaceuticals 2021, 14, 781. [Google Scholar] [CrossRef]
- Mohamed, J.M.M.; Alqahtani, A.; Khan, B.A.; Al Fatease, A.; Alqahtani, T.; Venkatesan, K.; Ahmad, F.; Alzghoul, B.I.; Alamri, A. Preparation of Soluble Complex of Curcumin for the Potential Antagonistic Effects on Human Colorectal Adenocarcinoma Cells. Pharmaceuticals 2021, 14, 939. [Google Scholar] [CrossRef]
- Fagir, W.; Hathout, R.M.; Sammour, O.A.; ElShafeey, A.H. Self-microemulsifying systems of Finasteride with enhanced oral bioavailability: Multivariate statistical evaluation, characterization, spray-drying and in vivo studies in human volunteers. Nanomedicine 2015, 10, 3373–3389. [Google Scholar] [CrossRef]
- Yusuf, M.; Sharma, V.; Pathak, K. Nanovesicles for transdermal delivery of felodipine: Development, characterization, and pharmacokinetics. Int. J. Pharm. Investig. 2014, 4, 119–130. [Google Scholar]
- Almahfood, M.; Bai, B. Characterization and oil recovery enhancement by a polymeric nanogel combined with surfactant for sandstone reservoirs. Pet. Sci. 2021, 18, 123–135. [Google Scholar] [CrossRef]
- Mohamed, J.M.; Alqahtani, A.; Ahmad, F.; Krishnaraju, V.; Kalpana, K. Stoichiometrically Governed Curcumin Solid Dispersion and Its Cytotoxic Evaluation on Colorectal Adenocarcinoma Cells. Drug Des. Devel. Ther. 2020, 14, 4639–4658. [Google Scholar] [CrossRef]
- Neeraj Kumar, N.; Goindi, S.; Bansal, G. Physicochemical evaluation and in vitro release studies on itraconazolium sulfate salt. Asian J. Pharm. Sci. 2014, 9, 8–16. [Google Scholar] [CrossRef][Green Version]
- Mohamed, J.M.; Kavitha, K.; Chitra Karthikeyini, S.C.; Nanthineeswari, S. Soluble curcumin prepared using four different carriers by solid dispersions: Phase solubility, molecular modelling and physicochemical characterization. Trop. J. Pharm. Res. 2019, 18, 1581–1588. [Google Scholar]
- Hafeez, F.; Hui, X.; Chiang, A.; Hornby, S.; Maibach, H. Transungual delivery of ketoconazole using novel lacquer formulation. Int. J. Pharm. 2013, 456, 357–361. [Google Scholar] [CrossRef]
- Shahin, M.; Hady, S.A.; Hammad, M.; Mortada, N. Novel jojoba oilbased emulsion gel formulations for clotrimazole delivery. AAPS PharmSciTech. 2011, 12, 239–247. [Google Scholar] [CrossRef]
- Abobakr, F.E.; Fayez, S.M.; Elwazzan, V.S.; Sakran, W. Effect of Different Nail Penetration Enhancers in Solid Lipid Nanoparticles Containing Terbinafine Hydrochloride for Treatment of Onychomycosis. AAPS PharmSciTech. 2021, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.; Kumar, S.M.; Mahadevan, N. Amphotericin-B loaded vesicular systems for the treatment of topical fungal infection. Int. J. Rec. Adv. Pharm. Res. 2011, 4, 37–46. [Google Scholar]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef]
- Sudan, P.; Goswami, M.; Singh, J. Antifungal potential of Fenugreek seeds (Trigonella foenum-graecum) crude extracts against Microsporum gypseum. Int. J. Pharm. Sci. Res. 2020, 11, 646–649. [Google Scholar] [CrossRef]
- Tanrıverdi, S.T.; Özer, Ö. Novel topical formulations of Terbinafine-HCl for treatment of onychomycosis. Eur. J. Pharm. Sci. 2013, 48, 628–636. [Google Scholar] [CrossRef]
- Mohamed, J.M.; Ahmad, F.; Al-Subaie, A.M. Soluble 1:1 stoichiometry curcumin binary complex for potential apoptosis in human colorectal adenocarcinoma cells (SW480 and Caco-2 cells). Res. J. Pharm. Tech. 2021, 14, 21–29. [Google Scholar]
- Shivakumar, H.N.; Juluri, A.; Desai, B.G.; Murthy, S.N. Ungual and transungual drug delivery. Drug Dev. Ind. Pharm. 2012, 38, 901–911. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, A.; Zalewski, T.; Elvang, P.A.; Nowaczyk, G.; Czajkowski, M.; Piotrowska-Kempisty, H. Insight into theranostic nanovesicles prepared by thin lipid hydration and microfluidic method. Colloids Surf. B Biointer. 2021, 205, 111871. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Mansour, S.; Mortada, N.D.; El Shamy, A.A. Lipospheres as carriers for topical delivery of aceclofenac: Preparation, characterization and in vivo evaluation. AAPS PharmSciTech. 2008, 9, 154–162. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, A.R. Preliminary studies on the mucilages extracted from Okra fruits, Taro tubers, Jew’s mellow leaves and Fenugreek seeds. Food Chem. 1984, 14, 237–249. [Google Scholar] [CrossRef]
- Parvathy, K.S.; Susheelamma, N.S.; Tharanathan, R.N.; Gaonkar, A.K. A simple non-aqueous method for carboxymethylation of galactomannans. Carbohydr. Polym. 2005, 62, 137–141. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Xu, W.; Sun, T.; Xiao, H.; Zhuang, X.; Chen, X. Receptor and microenvironment dual-recognizable nanogel for targeted chemotherapy of highly metastatic malignancy. Nano Lett. 2017, 17, 4526–4533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, L.; Wu, M.; Ou, N.A. validated LC-MS/MS method for determination of sertaconazole nitrate in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 4047–4050. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.M.; Alqahtani, A.; Ahmad, F.; Krishnaraju, V.; Kalpana, K. Pectin co-functionalized dual layered solid lipid na-noparticle made by soluble curcumin for the targeted potential treatment of colorectal cancer. Carbohydr. Polym. 2020, 252, 117180. [Google Scholar] [CrossRef]
- Moideen, M.M.J.; Alqahtani, A.; Venkatesan, K.; Ahmad, F.; Krisharaju, K.; Gayasuddin, M.; Shaik, R.A.; Ibraheem, K.M.M.; Salama, M.E.M.; Abed, S.Y. Application of the Box–Behnken design for the production of soluble curcumin: Skimmed milk powder inclusion complex for improving the treatment of colorectal cancer. Food Sci. Nutr. 2020, 8, 6643–6659. [Google Scholar] [CrossRef]
- Pal, P.; Thakur, R.S.; Ray, S.; Mazumder, B. Design and development of a safer non-invasive transungual drug delivery system for topical treatment of onychomycosis. Drug Dev. Ind. Pharm. 2015, 41, 1095–1099. [Google Scholar] [CrossRef]
- Chouhan, P.; Saini, T.R. Hydration of nail plate: A novel screening model for transungual drug permeation enhancers. Int. J. Pharm. 2012, 436, 179–182. [Google Scholar] [CrossRef]
- Farhana, A.R.N.; Amin, I.; Hassan, A.S.S.; Shuhaimi, M. Gel formation of pectin from okra (Abelmoschus esculentus L. Moench) leaves, pulp and seeds. Int. Food Res. J. 2017, 24, 2161–2169. [Google Scholar]
- Sahoo, S.; Pani, N.R.; Sahoo, S.K. Effect of microemulsion in topical sertaconazole hydrogel: In vitro and in vivo study. Drug Deliv. 2016, 23, 338–345. [Google Scholar] [CrossRef]
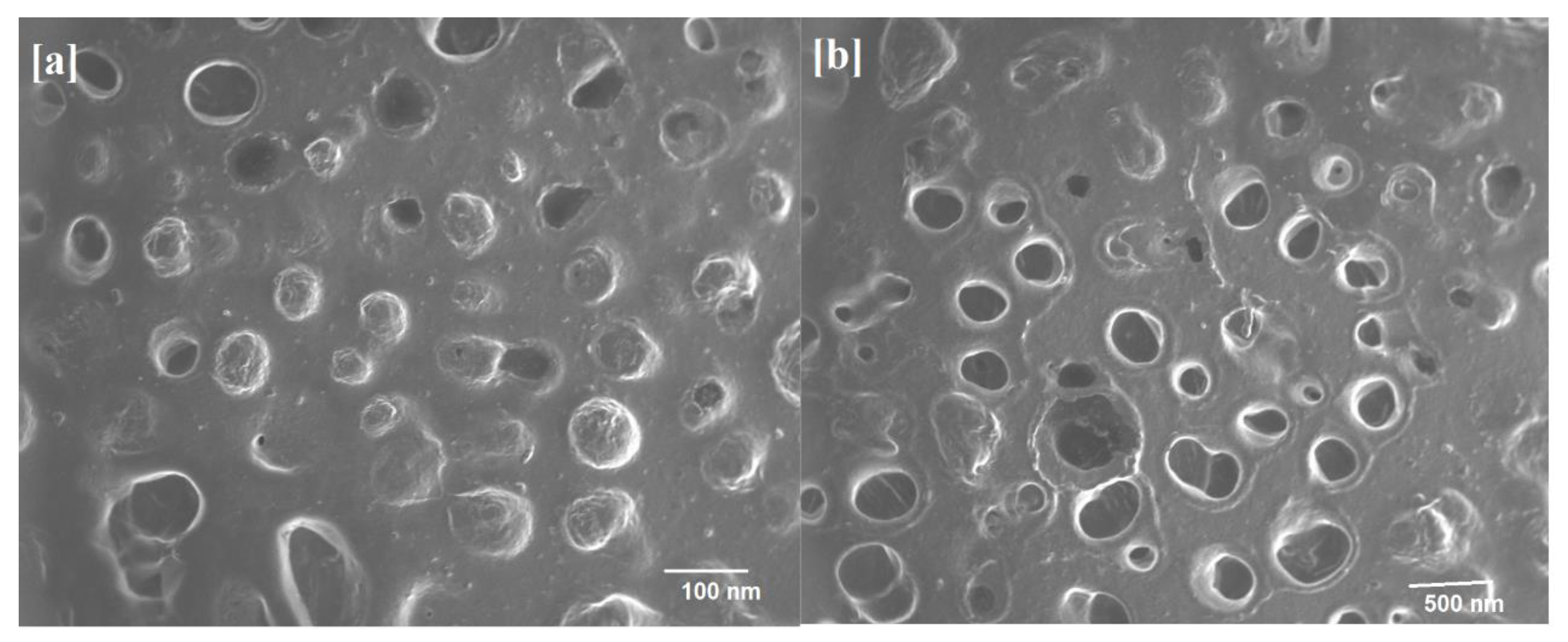

| Batches | Component (% w/v) | Drug Content (mg/5 mL of nPEVs) * | % EE * | Particle Size (nm) * | PDI * | Zeta Potential (mV) * | ||
|---|---|---|---|---|---|---|---|---|
| ITZ | Lipid | Cholesterol | ||||||
| S1 | 1 | 4 | - | 95.36 ± 0.31 | 95.36 ± 0.51 | 196.55 ± 0.025 | 0.092 ± 0.03 | +11.1 ± 0.52 |
| S2 | 1 | 4 | 2 | 96.59 ± 0.44 | 96.59 ± 0.44 | 221.2 ± 0.056 | 0.19 ± 0.045 | +17.2 ± 0.35 |
| S3 | 1 | 7 | 3 | 98.43 ± 0.32 | 97.22 ± 0.46 | 240.33 ± 0.016 | 0.38 ± 0.01 | +19.1 ± 0.87 |
| S4 | 2 | 7 | 3 | 193.89 ± 0.8 | 96.94 ± 0.70 | 252.2 ±0.019 | 0.49 ± 0.011 | +22.5 ± 0.28 |
| ITZ-nPEVs | CMFG-ITZ-nPEVs | ||||||
|---|---|---|---|---|---|---|---|
| Sr. No. | Batches | Deformability Index (mL·s−1) | Viscosity (cP) * | Batches | CMFG (w/v) | Chitosan (w/v) | Viscosity (cP) * |
| 1 | S1 | 1.35 | 0.98 ± 0.02 | G1 | 1% | 1% | 155.7 ± 0.567 |
| 2 | S2 | 0.556 | 1.32 ± 0.015 | G2 | 0.5% | 1% | 116.1 ± 0.85 |
| 3 | S3 | 0.449 | 1.41 ± 0.032 | G3 | 1% | 0.5% | 132 ± 0.737 |
| 4 | S4 | 0.200 | 1.72 ± 0.025 | ||||
| Correlation Coefficient (R2) | ||||||
|---|---|---|---|---|---|---|
| Formulation | Zero-Order | First Order | Higuchi | Hixon Crowell | Korsmeyer- Peppas | Release Exponent (n) |
| Pure ITZ | 0.9612 ± 0.48 | 0.7433 ± 0.67 | 0.9715 ± 0.34 | 0.8920 ± 0.19 | 0.8912 ± 0.17 | 0.417 ± 0.35 |
| S1 | 0.9766 ± 0.12 | 0.7466 ± 0.28 | 0.9726 ± 0.21 | 0.8707 ± 0.14 | 0.8865 ± 0.15 | 0.478 ± 0.38 |
| S2 | 0.9874 ± 0.22 | 0.7762 ± 0.19 | 0.9614 ± 0.23 | 0.9101 ± 0.23 | 0.9244 ± 0.15 | 0.588 ± 0.18 |
| S3 | 0.9895 ± 0.16 | 0.7492 ± 0.15 | 0.9788 ± 0.31 | 0.9189 ± 0.16 | 0.9779 ± 0.33 | 0.613 ± 0.43 |
| S4 | 0.9924 ± 0.31 | 0.7756 ± 0.19 | 0.9732 ± 0.24 | 0.9134 ± 0.23 | 0.9584 ± 0.32 | 0.598 ± 0.31 |
| CMFG-ITZ-PEVs | 0.9912 ± 0.34 | 0.7652 ± 0.23 | 0.982 ± 0.16 | 0.9212 ± 0.31 | 0.9712 ± 0.25 | 0.591 ± 0.43 |
| Sr. No. | Groups | Formulations | Weight of Nails before Hydration (mg) | Weight of Nails after Hydration (mg) | HE24 (h) |
|---|---|---|---|---|---|
| 1 | Control (group I) | Deionized water | 50 | 49.2 | - |
| 2 | Formulation (group II) | S1 | 50 | 62.0 | 1.26 |
| S2 | 50 | 68.2 | 1.38 | ||
| S3 | 50 | 75.6 | 1.53 | ||
| S4 | 50 | 72.7 | 1.47 | ||
| 3 | CMFG-ITZ-nPEVs (group III) | - | 50 | 81.11 | 1.92 |
| 3 | Marketed gel (group IV) | Itrostred gel | 50 | 52.65 | 1.07 |
| Sr. No. | Formulations | Zone of Inhibition * (mm) |
|---|---|---|
| 1 | Control | 5.1 ± 0.12 |
| 2 | Optimized batch (S3) of nPEVs | 27.0 ± 0.25 |
| 3 | CMFG-ITZ-nPEVs gel | 33.2 ± 0.11 |
| 4 | Itrostred Gel | 22.9 ± 0.44 |
| Parameters | 0 Month | 1 Month | 3 Month | 6 Month |
|---|---|---|---|---|
| Drug Content * (mg/ 5 mL of nPEVs) | 98.43 ± 0.32 | 98.39 ± 0.25 | 98.35 ± 0.39 | 97.21 ± 0.12 |
| % Entrapment Efficiency * | 98.43 ± 0.32 | 98.39 ± 0.25 | 98.35 ± 0.39 | 98.21 ± 0.12 |
| Particle size * (nm) | 240.33 ± 0.016 | 241 ± 0.47 | 299.25 ± 0.29 | 349.33 ± 0.92 |
| Zeta potential * (mV) | 19.1 | 19.3 | 19.5 | 19.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, A.; Raut, B.; Khan, S.; Mohamed, J.M.M.; Fatease, A.A.; Alqahtani, T.; Alamri, A.; Ahmad, F.; Krishnaraju, V. The Unique Carboxymethyl Fenugreek Gum Gel Loaded Itraconazole Self-Emulsifying Nanovesicles for Topical Onychomycosis Treatment. Polymers 2022, 14, 325. https://doi.org/10.3390/polym14020325
Alqahtani A, Raut B, Khan S, Mohamed JMM, Fatease AA, Alqahtani T, Alamri A, Ahmad F, Krishnaraju V. The Unique Carboxymethyl Fenugreek Gum Gel Loaded Itraconazole Self-Emulsifying Nanovesicles for Topical Onychomycosis Treatment. Polymers. 2022; 14(2):325. https://doi.org/10.3390/polym14020325
Chicago/Turabian StyleAlqahtani, Ali, Bhavana Raut, Shagufta Khan, Jamal Moideen Muthu Mohamed, Adel Al Fatease, Taha Alqahtani, Ali Alamri, Fazil Ahmad, and Venkatesan Krishnaraju. 2022. "The Unique Carboxymethyl Fenugreek Gum Gel Loaded Itraconazole Self-Emulsifying Nanovesicles for Topical Onychomycosis Treatment" Polymers 14, no. 2: 325. https://doi.org/10.3390/polym14020325
APA StyleAlqahtani, A., Raut, B., Khan, S., Mohamed, J. M. M., Fatease, A. A., Alqahtani, T., Alamri, A., Ahmad, F., & Krishnaraju, V. (2022). The Unique Carboxymethyl Fenugreek Gum Gel Loaded Itraconazole Self-Emulsifying Nanovesicles for Topical Onychomycosis Treatment. Polymers, 14(2), 325. https://doi.org/10.3390/polym14020325








