Synthesis, Hydrophilicity and Micellization of Coil-Brush Polystyrene-b-(polyglycidol-g-polyglycidol) Copolymer—Comparison with Linear Polystyrene-b-polyglycidol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
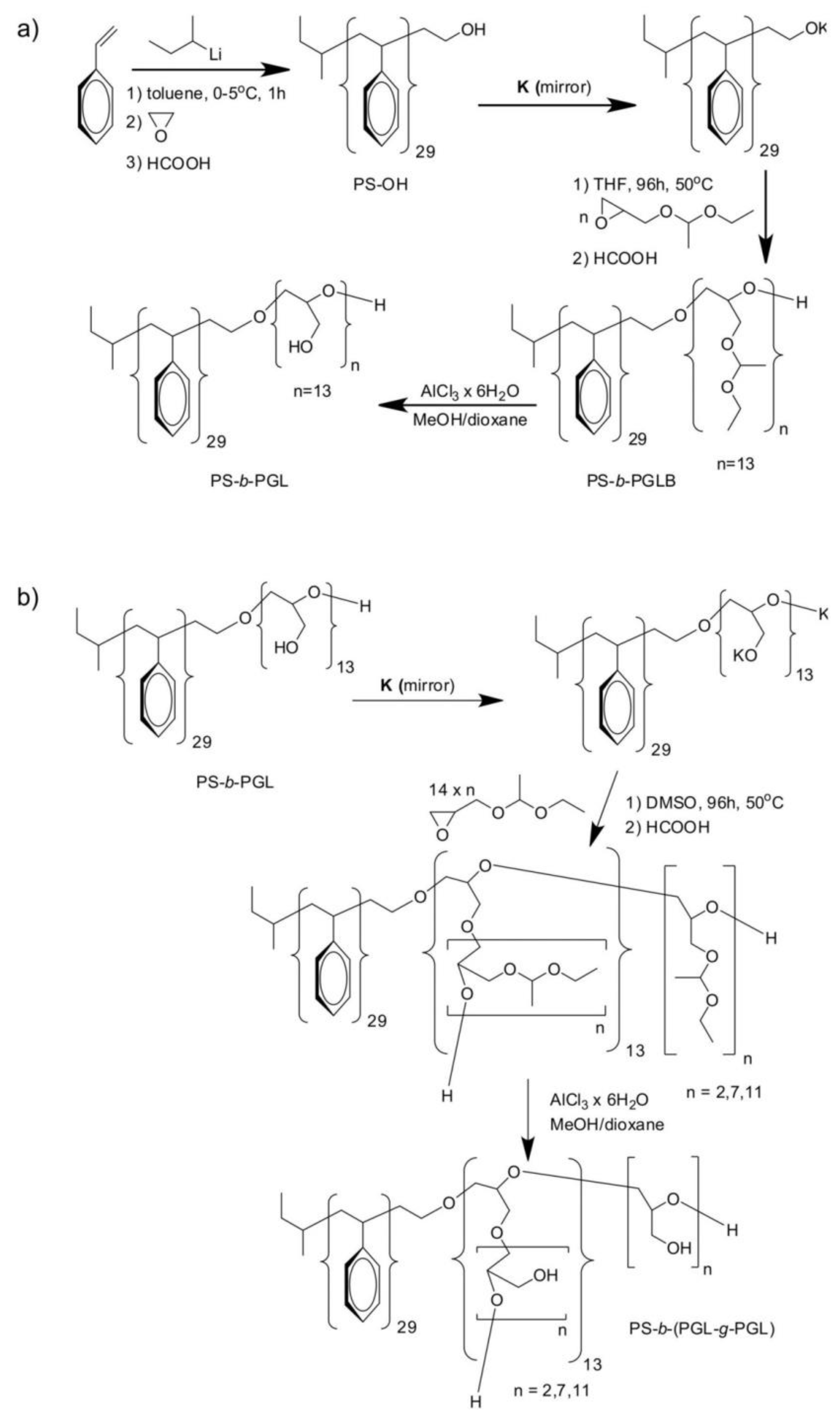
2.2.1. Synthesis of PS-b-(PGL-g-PGL) Copolymers
2.2.2. Micelle Formation
2.3. Characterization
2.3.1. 1H and 13C NMR Spectra
2.3.2. Determination of Mn, Mw/Mn, and dn/dc of PS-b-(PGL-g-PGL) Block Copolymers by GPC-MALLS
2.3.3. FTIR of Coil-Brush Copolymers
2.3.4. Determination of Copolymers CMC Using Spectrophotometric Method
2.3.5. Determination of CMC, Hydrodynamic Diameters of PS-b-(PGL-g-PGL) Particles, and Diameter Dispersity Values by the Dynamic Light Scattering (DLS) Method
2.3.6. Water Contact Angle Measurements of PS-b-PGL and PS-b-(PGL-g-PGL) Films Deposited on Glass Plates
2.3.7. Cryo-TEM of Particles Obtained from PS-b-(PGL-g-PGL) Copolymers
3. Results and Discussion
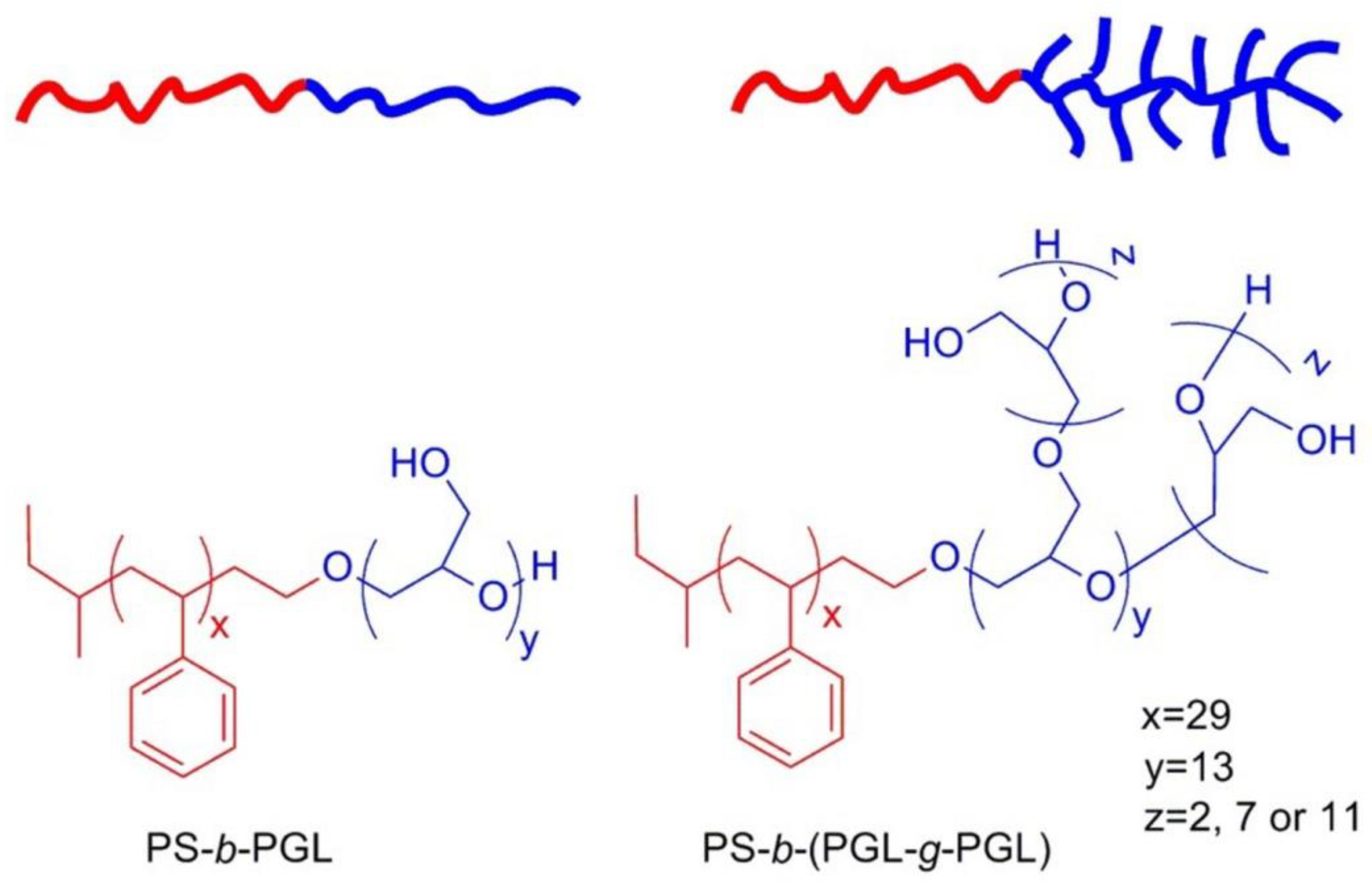
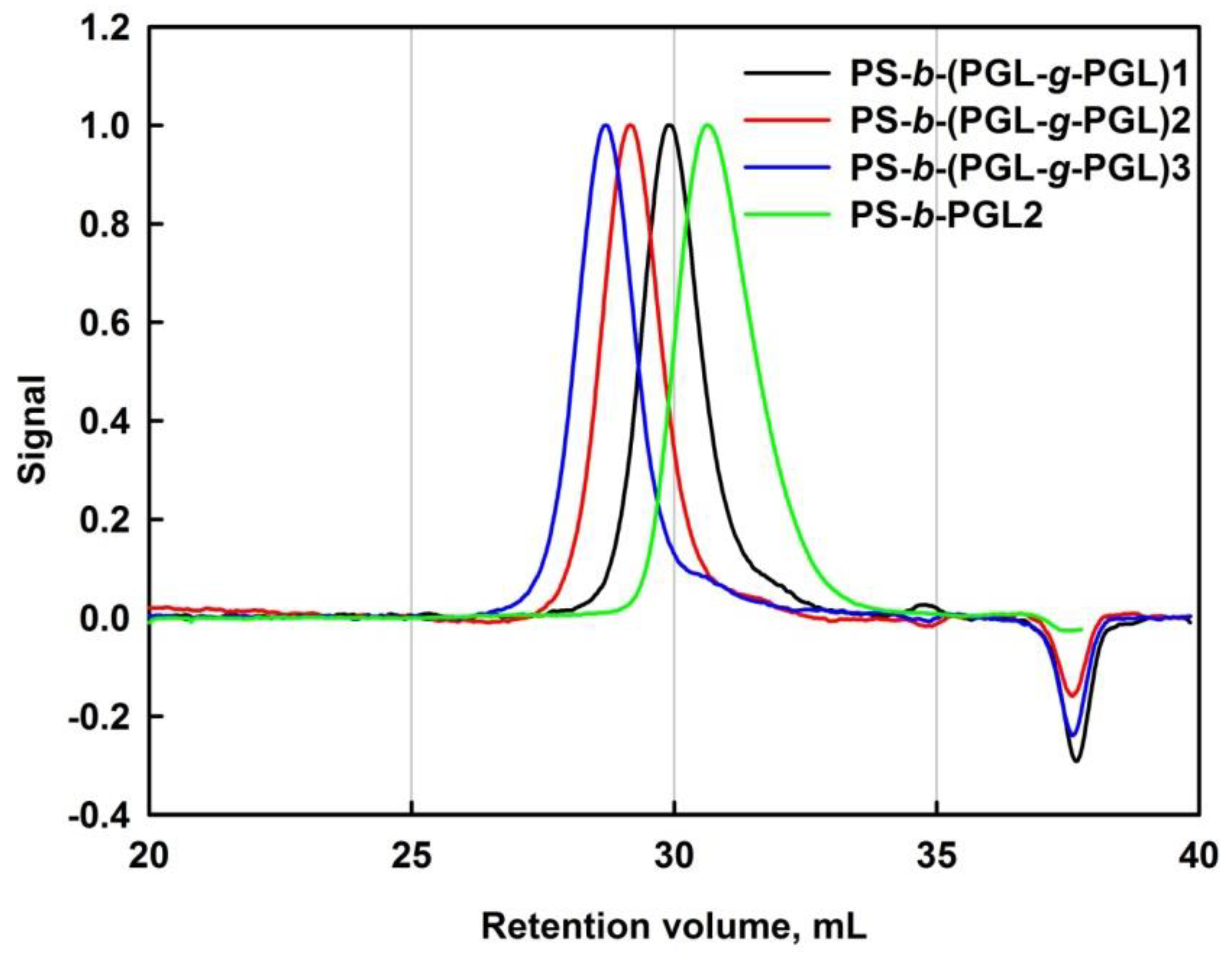
3.1. Synthesis and Characterization of Polystyrene-b-(polyglycidol-g-polyglycidol)
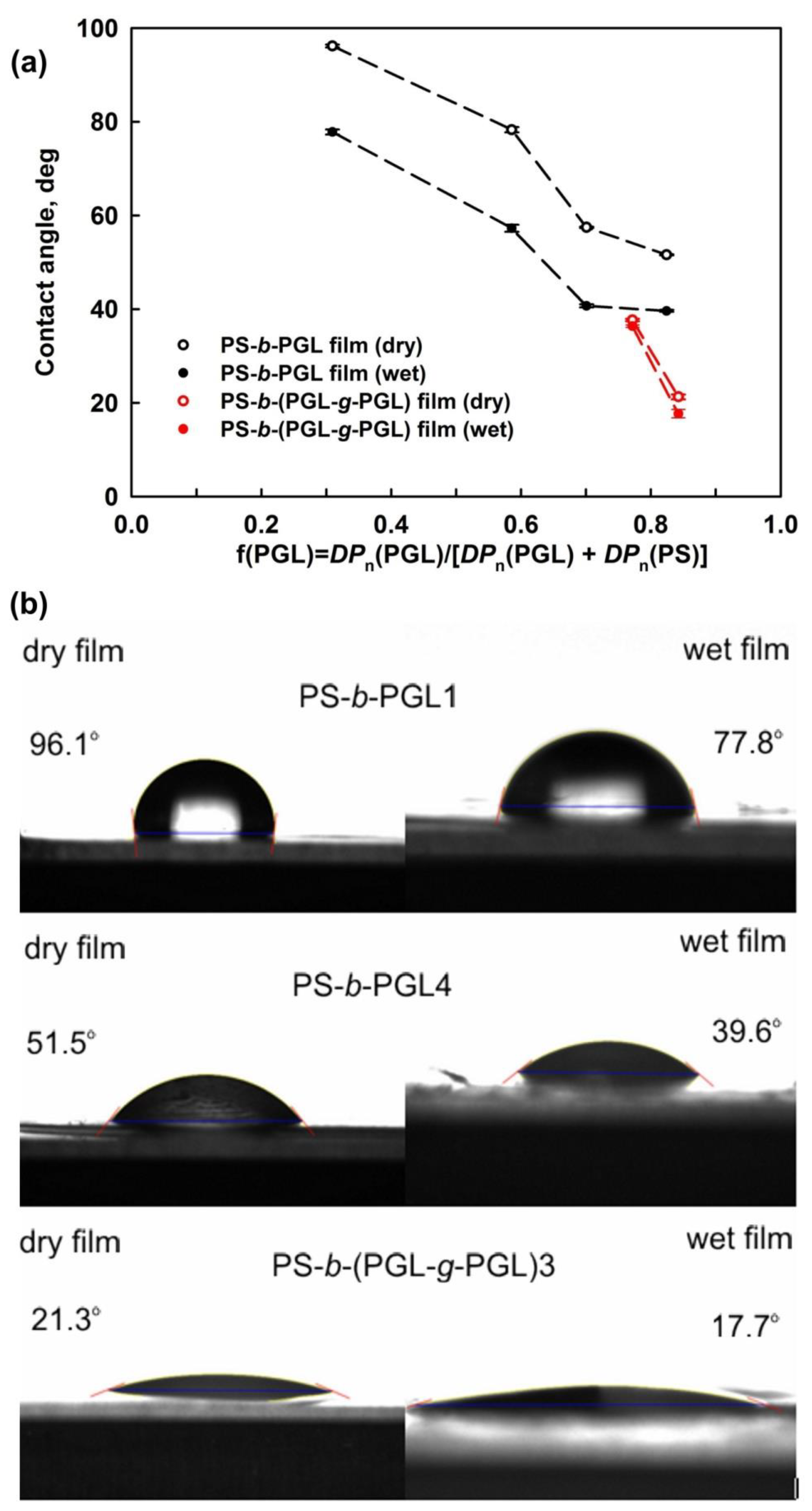
3.2. Properties of PS-b-PGL and PS-b-(PGL-g-PGL) Copolymer Films
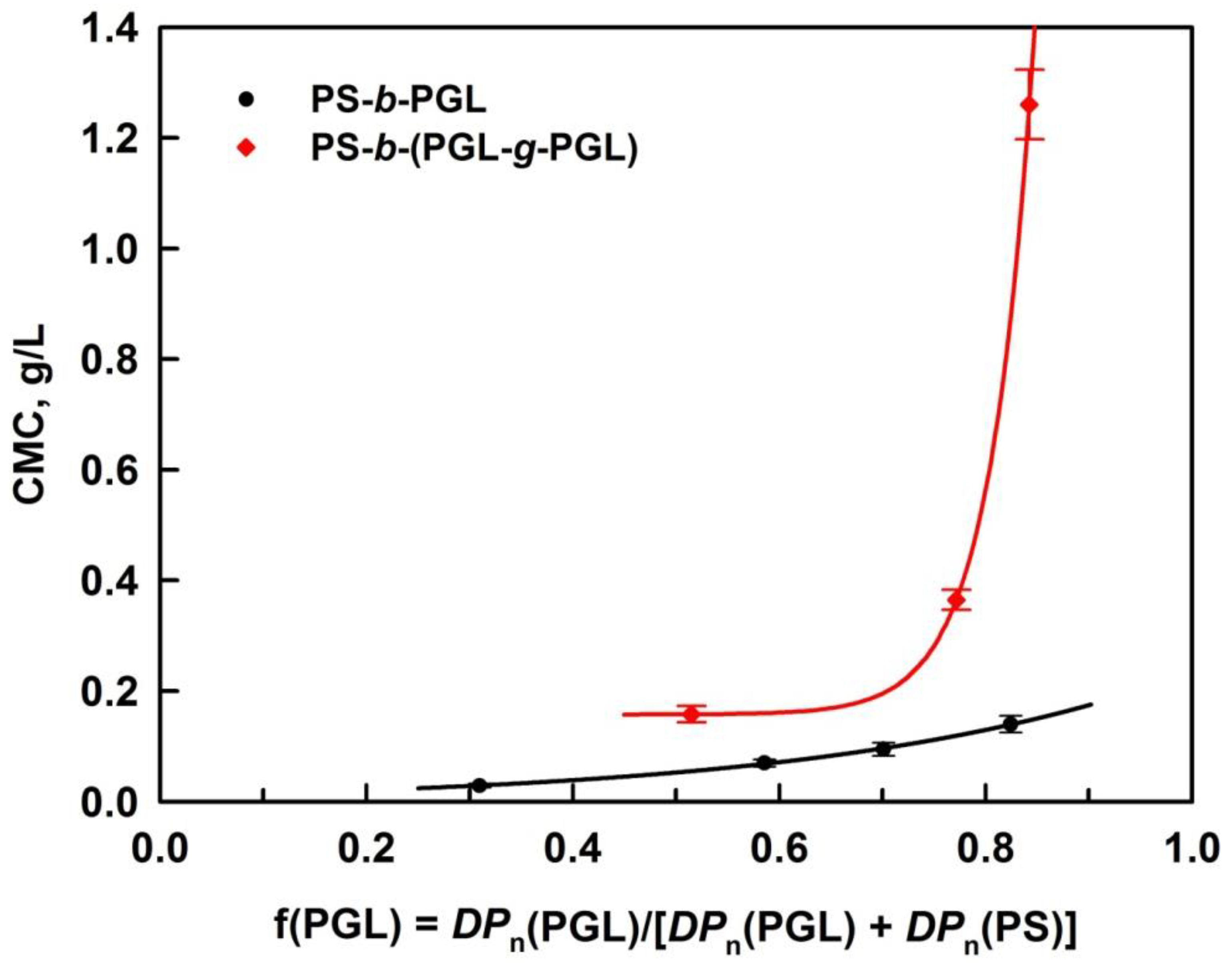
3.3. Determination of Copolymers Critical Micellization Concentration
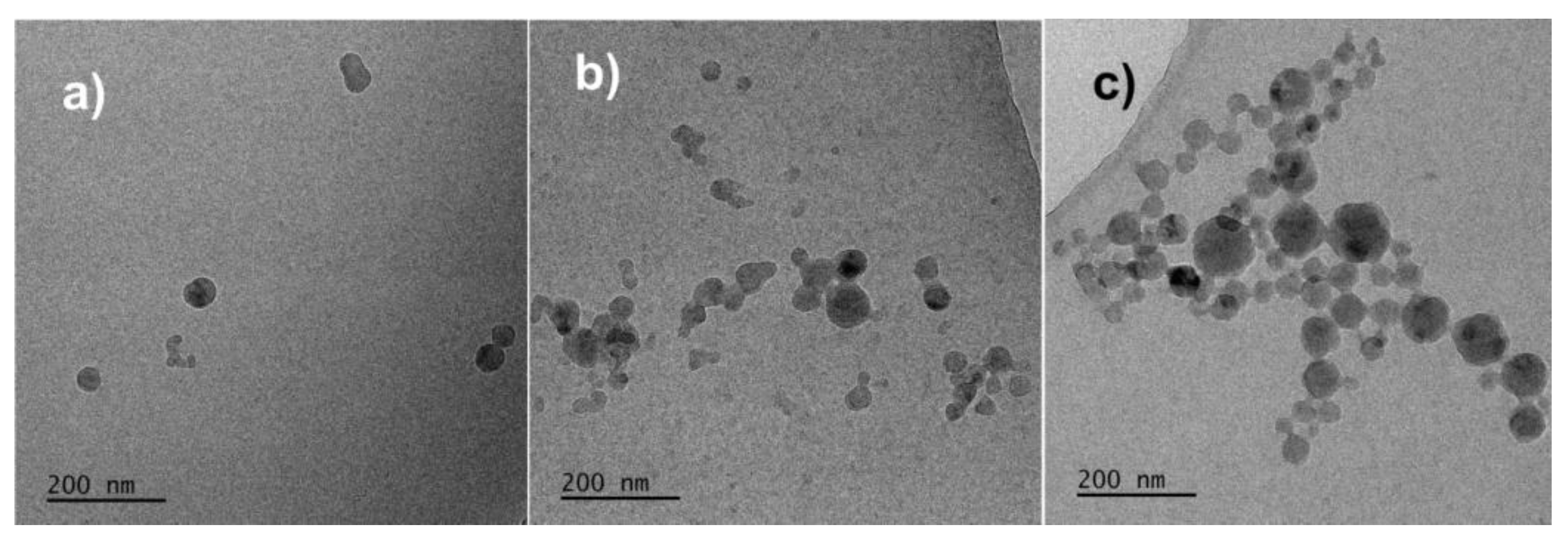
3.4. Aggregation Process of PS-b-PGL and PS-b-(PGL-g-PGL) Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, M.; Hu, H.; Zhao, X.; Huang, C.; Yang, B.; Yin, Z. Construction of Mannose-Modified Polyethyleneimine-Block-Polycaprolactone Cationic Polymer Micelles and Its Application in Acute Lung Injury. Drug Deliv. Transl. Res. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.R.; Mendonça, P.V.; Simões, S.; Serra, A.C.; Coelho, J.F.J. Amphiphilic Well-Defined Degradable Star Block Copolymers by Combination of Ring-Opening Polymerization and Atom Transfer Radical Polymerization: Synthesis and Application as Drug Delivery Carriers. J. Polym. Sci. 2021, 59, 211–229. [Google Scholar] [CrossRef]
- Shahriari, M.; Torchilin, V.P.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. “Smart” Self-Assembled Structures: Toward Intelligent Dual Responsive Drug Delivery Systems. Biomater. Sci. 2020, 8, 5787–5803. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufic, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A Versatile Tri-Block Copolymer for Biomedical Applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Sharma, A.K.; Prasher, P.; Aljabali, A.A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.K.; Tambuwala, M.M.; Dua, K.; et al. Emerging Era of “Somes”: Polymersomes as Versatile Drug Delivery Carrier for Cancer Diagnostics and Therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Blenner, M.; Alexander-Bryant, A.; Larsen, J. Polymersomes for Therapeutic Delivery of Protein and Nucleic Acid Macromolecules: From Design to Therapeutic Applications. Biomacromolecules 2020, 21, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Li, Y.; Lu, J.; Deng, X.; Wu, Y. Amphiphilic Block Copolymers-Guided Strategies for Assembling Nanoparticles: From Basic Construction Methods to Bioactive Agent Delivery Applications. ACS Appl. Bio Mater. 2020, 3, 6546–6555. [Google Scholar] [CrossRef]
- Malik, S.; Sundarrajan, S.; Hussain, T.; Nazir, A.; Ramakrishna, S. Role of Block Copolymers in Tissue Engineering Applications. Cells Tissues Organs 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- LaFreniere, J.M.J.; Roberge, E.J.; Halpern, J.M. Review—Reorientation of Polymers in an Applied Electric Field for Electrochemical Sensors. J. Electrochem. Soc. 2020, 167, 037556. [Google Scholar] [CrossRef] [PubMed]
- Grzetic, D.J.; Delaney, K.T.; Fredrickson, G.H. Electrostatic Manipulation of Phase Behavior in Immiscible Charged Polymer Blends. Macromolecules 2021, 54, 2604–2616. [Google Scholar] [CrossRef]
- Radjabian, M.; Abetz, V. Advanced Porous Polymer Membranes from Self-Assembling block Copolymers. Prog. Polym. Sci. 2020, 102, 101219. [Google Scholar] [CrossRef]
- Spulber, M.; Najer, A.; Winkelbach, K.; Glaied, O.; Waser, M.; Pieles, U.; Meier, W.; Bruns, N. Photoreaction of a Hydroxyalkyphenone with the Membrane of Polymersomes: A Versatile Method to Generate Semipermeable Nanoreactors. J. Am. Chem. Soc. 2013, 135, 9204–9212. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Li, F.; Ai, Y.; Wei, F.; Cui, J.; Fu, J.; Zheng, M.; Liu, S. Diblock Copolymers Directing Construction of Hierarchically Porous Metal-Organic Frameworks for Enhanced-Performance Supercapacitors. Nanotechnology 2021, 32, 165601. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, P.; Peng, W.; Sun, J.; Dong, X.; Ma, Z.; Gan, D.; Liu, P.; Shen, J. Poly(Hexamethylene Biguanide) (PHMB) as High-Efficiency Antibacterial Coating for Titanium Substrates. J. Hazard. Mat. 2021, 411, 125110. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Review of The Research on Anti-Protein Fouling Coatings Materials. Prog. Org. Coat. 2020, 147, 105860. [Google Scholar] [CrossRef]
- Jana, S.; Uchman, M. Poly(2-Oxazoline)-Based Stimulus-Responsive (Co)Polymers: An Overview of Their Design, Solution Properties, Surface-Chemistries and Applications. Prog. Polym. Sci. 2020, 106, 101252. [Google Scholar] [CrossRef]
- Zia, A.; Pentzer, E.; Thickett, S.; Kempe, K. Advances and Opportunities of Oil-in-Oil Emulsions. ACS Appl. Mater. Interfaces 2020, 12, 38845–38861. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, Y.; Tao, Y.; Huang, W. Conjugated Random Terpolymer Donors towards High-Efficiency Polymer Solar Cells. Chin. J. Chem. 2020, 38, 601–624. [Google Scholar] [CrossRef]
- Raisin, S.; Morille, M.; Bony, C.; Noël, D.; Devoisselle, J.-M.; Belamie, E. Tripartite Polyionic Complex (PIC) Micelles as Non-Viral Vectors for Mesenchymal Stem Cell siRNA Transfection. Biomater. Sci. 2017, 5, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yao, X.; Yuan, J.; Ding, T.; Gao, Q. Preparation and Drug Controlled-Release of Polyion Complex Micelles as Drug Delivery Systems. Colloids Surf. B Biointerfaces 2009, 68, 218–224. [Google Scholar] [CrossRef]
- Ruzette, A.-V.; Leibler, L. Block Copolymers in Tomorrow’s Plastics. Nat. Mater. 2005, 4, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hall, A.; Jayapurna, I.; Algharbi, S.; Ginzburg, V.; Xu, T. Nanocomposites Based on Coil-Comb Diblock Copolymers. Macromolecules 2021, 54, 1006–1016. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, T.; Darvish, O.A.; Qian, S.; Tsengam, I.K.M.; John, V.; Zhang, D. Crystallization-Driven Self-Assembly of Coil−Comb-Shaped Polypeptoid Block Copolymers: Solution Morphology and Self-Assembly Pathways. Macromolecules 2019, 52, 8867–8877. [Google Scholar] [CrossRef]
- Jiang, Z.; Qian, Z.; Yang, H.; Wang, R. Disorder to Order Transition and Ordered Morphology of Coil-Comb Block Copolymer by Self-Consistent Field Theory. Nanoscale Res. Lett. 2015, 10, 328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, S.; Moon, S.; Seo, M.; Kim, S.Y. Synthesis of Coil-Comb Block Copolymers Containing Polystyrene Coil and Poly(Methyl Methacrylate) Side Chains via Atom Transfer Radical Polymerization. J. Polym. Sci. A Polym. Chem. 2016, 54, 2971–2983. [Google Scholar] [CrossRef]
- Jonikaite-Svegzdiene, J.; Kudresova, A.; Paukstis, S.; Skapas, M.; Makuska, R. Synthesis and Self-Assembly of Polystyrene-Based Diblock and Triblock Coil-Brush Copolymers. Polym. Chem. 2017, 8, 5621–5632. [Google Scholar] [CrossRef]
- Otulakowski, L.; Dworak, A.; Forys, A.; Gadzinowski, M.; Slomkowski, S.; Basinska, T.; Trzebicka, B. Micellization of Polystyrene-b-Polyglycidol in Dioxane and Water/Dioxane Solutions. Polymers 2020, 12, 200. [Google Scholar] [CrossRef]
- Richards, D.H.; Szwarc, M. Block Polymers of Ethylene Oxide and Its Analogues with Styrene. Trans. Faraday Soc. 1959, 55, 1644–1650. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Wang, G. Synthesis and Characterization of Copolymers with the Same Proportions of Polystyrene and Poly(Ethylene Oxide) Compositions but Different Connection Sequence by the Efficient Williamson Reaction. Polym. Int. 2015, 64, 1202–1208. [Google Scholar] [CrossRef]
- Walach, W.; Trzebicka, B.; Justynska, J.; Dworak, A. High Molecular Arborescent Polyoxyethylene with Hydroxyl Containing Shell. Polymer 2004, 45, 1755–1762. [Google Scholar] [CrossRef]
- Walach, W.; Kowalczuk, A.; Trzebicka, B.; Dworak, A. Synthesis of High-Molar Mass Arborescent-Branched Polyglycidol via Sequential Grafting. Macromol. Rapid Commun. 2001, 22, 1272–1277. [Google Scholar] [CrossRef]
- Otulakowski, L.; Gadzinowski, M.; Slomkowski, S.; Basinska, T.; Forys, A.; Dworak, A.; Trzebicka, B. Micellisation of Polystyrene-b-Polyglycidol Copolymers in Water Solution. Eur. Polym. J. 2018, 99, 72–79. [Google Scholar] [CrossRef]
- Atanase, L.I.; Desbrieres, J.; Riess, G. Micellization of Synthetic and Polysaccharides-Based Graft Copolymers in Aqueous Media. Progr. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Utrata-Wesolek, A.; Walach, W.; Bochenek, M.; Trzebicka, B.; Aniol, J.; Sieron, A.L.; Kubacki, J.; Dworak, A. Branched Polyglycidol and Its Derivatives Grafted-from Poly(Ethylene Terephthalate) and Silica as Surfaces that Reduce Protein Fouling. Europ. Polym. J. 2018, 105, 313–322. [Google Scholar] [CrossRef]
- Feng, Y.H.; Zhang, X.P.; Li, J.Y.; Guo, X.D. How Is a Micelle Formed from Amphiphilic Polymers in a Dialysis Process: Insight from Mesoscopic Studies. Chem. Phys. Lett. 2020, 754, 137711. [Google Scholar] [CrossRef]
- Dimitrov, P.; Utrata-Wesolek, A.; Rangelov, S.; Wałach, W.; Trzebicka, B.; Dworak, A. Synthesis and Self-Association in Aqueous Media of Poly(Ethylene Oxide)/Poly(Ethyl Glycidyl Carbamate) Amphiphilic Block Copolymers. Polymer 2006, 47, 4905–4915. [Google Scholar] [CrossRef]
- The Polymer Data Handbook; Oxford University Press, Inc.: New York, NY, USA, 2009; p. 10016.
- Gam-Derouich, S.; Gosecka, M.; Lepinay, S.; Turmine, M.; Carbonnier, B.; Basinska, T.; Slomkowski, S.; Ben Hassen-Chehimi, D.; Othmane, A.; Chehimi, M.M. Highly Hydrophilic Surfaces from Polyglycidol Grafts with Dual Antifouling and Specific Protein Recognition Properties. Langmuir 2011, 27, 9285–9294. [Google Scholar] [CrossRef] [PubMed]

| Sample | DPn of PS/PGL/gPGL Expected from Amount of Comonomer | DPn of PS/PGL/gPGL Blocks in the Copolymer (c) | f(PGL) (g) | Mn (GPC) (d) g/mol | Mw/Mn | dn/dc, mL/g (Measured) | DPn of PGL Side Block (GPC (f)) |
|---|---|---|---|---|---|---|---|
| PS-OH1 (a) | 29/0 | 29/0 | - | 2800 | 1.013 | 0.1620 (e) | - |
| PS-OH2 (a) | 29/0 | 29/0 | - | 2540 | 1.19 | 0.1620 (e) | - |
| PS-b-PGL1 (b) | 29/12.7 | 29/13 | 0.309 | 3900 | 1.010 | 0.1340 | - |
| PS-b-PGL2 (b) | 29/41.5 | 29/41 | 0.586 | 6100 | 1.030 | 0.1020 | - |
| PS-b-PGL3 (b) | 29/68 | 29/68 | 0.701 | 8100 | 1.040 | 0.0870 | - |
| PS-b-PGL4 (b) | 29/136 | 29/136 | 0.824 | 11,700 | 1.070 | 0.0720 | - |
| PS-b-(PGL-g-PGL)1 | 29/12.7/2 | 29/13/2.2 | 0.515 | 5800 | 1.036 | 0.1156 | 1.78 (23.4 (h)) |
| PS-b-(PGL-g-PGL)2 | 29/12.7/7 | 29/13/6.5 | 0.782 | 12,100 | 1.056 | 0.0939 | 7.57 (98.4 (h)) |
| PS-b-(PGL-g-PGL)3 | 29/12.7/11 | 29/13/10.9 | 0.854 | 16,300 | 1.081 | 0.0880 | 11.40 (148.2 (h)) |
| Copolymer Sample | f(PGL) | CMC, g/L (UV) | CMC, g/L (DLS) |
|---|---|---|---|
| PS-b-PGL1 | 0.309 | - | 0.029 |
| PS-b-PGL2 | 0.586 | - | 0.070 |
| PS-b-PGL3 | 0.701 | - | 0.096 |
| PS-b-PGL4 | 0.824 | - | 0.140 |
| PS-b-(PGL-g-PGL)1 | 0.515 | 0.141 | 0.158 |
| PS-b-(PGL-g-PGL)2 | 0.782 | 0.541 | 0.365 |
| PS-b-(PGL-g-PGL)3 | 0.854 | 1.23 | 1.26 |
| Copolymer Sample | Copolymer Conc., g/L | Dh,n, nm | Dh,si, nm | Dispersity SM |
|---|---|---|---|---|
| PS-b-PGL1 | 0.12 | 27.3 | 27.3/127 | 0.38 |
| PS-b-PGL2 | 0.19 | 49.4 | 49.4/165 | 0.41 |
| PS-b-PGL3 | 0.24 | 50.7 | 50.7/187 | 0.55 |
| PS-b-PGL4 | 0.26 | 102 | 102/239 | 0.70 |
| PS-b-(PGL-g-PGL)1 | 0.38 0.75 | 14.5 8.5 | 14.5/123 8.5/172 | 0.265 0.224 |
| PS-b-(PGL-g-PGL)2 | 0.36 0.60 | 8.5 14.5 | 8.5/154 14.5/136 | 0.204 0.264 |
| PS-b-(PGL-g-PGL)3 | 1.86 | 14.0 | 14.0/129 | 0.285 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadzinowski, M.; Kasprów, M.; Basinska, T.; Slomkowski, S.; Otulakowski, Ł.; Trzebicka, B.; Makowski, T. Synthesis, Hydrophilicity and Micellization of Coil-Brush Polystyrene-b-(polyglycidol-g-polyglycidol) Copolymer—Comparison with Linear Polystyrene-b-polyglycidol. Polymers 2022, 14, 253. https://doi.org/10.3390/polym14020253
Gadzinowski M, Kasprów M, Basinska T, Slomkowski S, Otulakowski Ł, Trzebicka B, Makowski T. Synthesis, Hydrophilicity and Micellization of Coil-Brush Polystyrene-b-(polyglycidol-g-polyglycidol) Copolymer—Comparison with Linear Polystyrene-b-polyglycidol. Polymers. 2022; 14(2):253. https://doi.org/10.3390/polym14020253
Chicago/Turabian StyleGadzinowski, Mariusz, Maciej Kasprów, Teresa Basinska, Stanislaw Slomkowski, Łukasz Otulakowski, Barbara Trzebicka, and Tomasz Makowski. 2022. "Synthesis, Hydrophilicity and Micellization of Coil-Brush Polystyrene-b-(polyglycidol-g-polyglycidol) Copolymer—Comparison with Linear Polystyrene-b-polyglycidol" Polymers 14, no. 2: 253. https://doi.org/10.3390/polym14020253
APA StyleGadzinowski, M., Kasprów, M., Basinska, T., Slomkowski, S., Otulakowski, Ł., Trzebicka, B., & Makowski, T. (2022). Synthesis, Hydrophilicity and Micellization of Coil-Brush Polystyrene-b-(polyglycidol-g-polyglycidol) Copolymer—Comparison with Linear Polystyrene-b-polyglycidol. Polymers, 14(2), 253. https://doi.org/10.3390/polym14020253








