Application Prospect and Preliminary Exploration of GelMA in Corneal Stroma Regeneration
Abstract
1. Introduction
2. Current Status of Polymers Used in Corneal Stromal Regeneration
2.1. Chitosan
2.2. Hydrophilic Gels
2.3. Collagen
2.4. Gelatin Methacrylate
2.5. Silk Fibroin
2.6. Glycerol Sebacate
2.7. Decellularized Extracellular Matrix
3. Research into GelMA Material in Corneal Stromal Regeneration
3.1. Introduction of GelMA and Cornea Stroma
3.2. GelMA Biological Properties
4. Application Perspectives of GelMA Materials in Corneal Stroma Regeneration
5. GelMA in the Future
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, W.Y.; Gao, H.; Li, Y.J. Standardizing the clinical diagnosis and treatment of keratoconus in China. Chin. J. Ophthalmol. 2019, 55, 401–404. [Google Scholar]
- Godefrooij, D.A.; de Wit, G.A.; Uiterwaal, C.S.; Imhof, S.M.; Wisse, R.P. Age-specific Incidence and Prevalence of Keratoconus: A Nationwide Registration Study. Am. J. Ophthalmol. 2017, 175, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Mas Tur, V.; MacGregor, C.; Jayaswal, R.; O’Brart, D.; Maycock, N. A review of keratoconus: Diagnosis, pathophysiology, and genetics. Surv. Ophthalmol. 2017, 62, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Le, Q.; Yao, W.; Xu, J. Indications and Techniques of Pediatric Keratoplasty in Eastern China From 2008 to 2017. Cornea 2019, 38, 1370–1376. [Google Scholar] [CrossRef]
- Kandel, H.; Pesudovs, K.; Watson, S.L. Measurement of Quality of Life in Keratoconus. Cornea 2020, 39, 386–393. [Google Scholar] [CrossRef]
- Saad, S.; Saad, R.; Jouve, L.; Kallel, S.; Trinh, L.; Goemaere, I.; Borderie, V.; Bouheraoua, N. Corneal crosslinking in keratoconus management. J. Fr. Ophtalmol. 2020, 43, 1078–1095. [Google Scholar] [CrossRef]
- Lagali, N. Corneal Stromal Regeneration: Current Status and Future Therapeutic Potential. Curr. Eye Res. 2020, 45, 278–290. [Google Scholar] [CrossRef]
- Alio, J.L.; Montesel, A.; El Sayyad, F.; Barraquer, R.I.; Arnalich-Montiel, F.; Alio Del Barrio, J.L. Corneal graft failure: An update. Br. J. Ophthalmol. 2021, 105, 1049–1058. [Google Scholar] [CrossRef]
- Du, Y.; Sundarraj, N.; Funderburgh, M.L.; Harvey, S.A.; Birk, D.E.; Funderburgh, J.L. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5038–5045. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. WHO 2001, 79, 214–221. [Google Scholar] [PubMed]
- Aghamirsalim, M.; Mobaraki, M.; Soltani, M.; Kiani Shahvandi, M.; Jabbarvand, M.; Afzali, E.; Raahemifar, K. 3D Printed Hydrogels for Ocular Wound Healing. Biomedicines 2022, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, F.; Li, M.; Knorz, M.C.; Zhou, X. Treatment of Corneal Ectasia by Implantation of an Allogenic Corneal Lenticule. J. Refract. Surg. 2018, 34, 347–350. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Yang, S.; Lu, T.C. Tuck-in Lamellar keratoplasty with an lenticule obtained by small incision lenticule extraction for treatment of Post- LASIK Ectasia. Sci. Rep. 2017, 7, 17806. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, M.; Li, N.; Chen, T.; Qi, X.; Xie, L.; Shi, W. Femtosecond laser-assisted minimally invasive lamellar keratoplasty for the treatment of advanced keratoconus. Clin. Exp. Ophthalmol. 2022, 50, 294–302. [Google Scholar] [CrossRef]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018, 22, 834–849. [Google Scholar] [CrossRef]
- Edgar, L.; Pu, T.; Porter, B.; Aziz, J.M.; La Pointe, C.; Asthana, A.; Orlando, G. Regenerative medicine, organ bioengineering and transplantation. Br. J. Surg. 2020, 107, 793–800. [Google Scholar] [CrossRef]
- Alió Del Barrio, J.L.; Alió, J.L. Cellular therapy of the corneal stroma: A new type of corneal surgery for keratoconus and corneal dystrophies. Eye Vis. 2018, 5, 28. [Google Scholar] [CrossRef]
- Dos Santos, A.; Balayan, A.; Funderburgh, M.L.; Ngo, J.; Funderburgh, J.L.; Deng, S.X. Differentiation Capacity of Human Mesenchymal Stem Cells into Keratocyte Lineage. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3013–3023. [Google Scholar] [CrossRef]
- El Zarif, M.; Alió, J.L.; Alió Del Barrio, J.L.; Abdul Jawad, K.; Palazón-Bru, A.; Abdul Jawad, Z.; de Miguel, M.P.; Makdissy, N. Corneal Stromal Regeneration Therapy for Advanced Keratoconus: Long-term Outcomes at 3 Years. Cornea 2021, 40, 741–754. [Google Scholar] [CrossRef]
- Brennan, M.; Layrolle, P.; Mooney, D.J. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv. Funct. Mater. 2020, 30, 1909125. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Ashrafi-Dehkordi, K.; Alizadeh, Z.; Sanami, S.; Banitalebi-Dehkordi, M. Biopolymer-based scaffolds for corneal stromal regeneration: A review. Polim. W. Med. 2020, 50, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, T.H.; Lin, C.C.; Hussain, A.K.; Maas, S.; Lai, H.J.; Linnartz, H.; van den Berg, T.J.; Salvatori, D.C.; Luyten, G.P.; Jager, M.J. A fish scale-derived collagen matrix as artificial cornea in rats: Properties and potential. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3224–3233. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Ritch, R.; Lin, S.M.; Ni, M.H.; Chang, Y.C.; Lu, Y.L.; Lai, H.J.; Lin, F.H. A new fish scale-derived scaffold for corneal regeneration. Eur. Cell Mater. 2010, 19, 50–57. [Google Scholar] [CrossRef]
- Chen, S.C.; Telinius, N.; Lin, H.T.; Huang, M.C.; Lin, C.C.; Chou, C.H.; Hjortdal, J. Use of Fish Scale-Derived BioCornea to Seal Full-Thickness Corneal Perforations in Pig Models. PLoS ONE 2015, 10, e0143511. [Google Scholar] [CrossRef]
- Fagerholm, P.; Lagali, N.S.; Ong, J.A.; Merrett, K.; Jackson, W.B.; Polarek, J.W.; Suuronen, E.J.; Liu, Y.; Brunette, I.; Griffith, M. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 2014, 35, 2420–2427. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef]
- Wang, F.; Shi, W.; Li, H.; Wang, H.; Sun, D.; Zhao, L.; Yang, L.; Liu, T.; Zhou, Q.; Xie, L. Decellularized porcine cornea-derived hydrogels for the regeneration of epithelium and stroma in focal corneal defects. Ocul. Surf. 2020, 18, 748–760. [Google Scholar] [CrossRef]
- Janes, K.A.; Calvo, P.; Alonso, M.J. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects--an update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef]
- Ray, S.D. Potential aspects of chitosan as pharmaceutical excipient. Acta Pol. Pharm. 2011, 68, 619–622. [Google Scholar] [PubMed]
- Sall, K.N.; Kreter, J.K.; Keates, R.H. The effect of chitosan on corneal wound healing. Ann. Ophthalmol. 1987, 19, 31–33. [Google Scholar] [PubMed]
- Xu, W.; Wang, Z.; Liu, Y.; Wang, L.; Jiang, Z.; Li, T.; Zhang, W.; Liang, Y. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr. Polym. 2018, 192, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Felt, O.; Carrel, A.; Baehni, P.; Buri, P.; Gurny, R. Chitosan as tear substitute: A wetting agent endowed with antimicrobial efficacy. J. Ocul. Pharmacol. Ther. 2000, 16, 261–270. [Google Scholar] [CrossRef]
- Aiba, S.I. Studies on chitosan: 6. Relationship between N-acetyl group distribution pattern and chitinase digestibility of partially N-acetylated chitosans. Int. J. Biol. Macromol. 1993, 15, 241–245. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Yuan, S.; Wang, J.; Shen, Y.; Deng, S.; Xie, L.; Yang, Q. The Formation Mechanism of Hydrogels. Curr. Stem Cell Res. Ther. 2018, 13, 490–496. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Hušáková, M.; Bay-Jensen, A.C.; Forejtová, Š.; Zegzulková, K.; Tomčík, M.; Gregová, M.; Bubová, K.; Hořínková, J.; Gatterová, J.; Pavelka, K.; et al. Metabolites of type I, II, III, and IV collagen may serve as markers of disease activity in axial spondyloarthritis. Sci. Rep. 2019, 9, 11218. [Google Scholar] [CrossRef] [PubMed]
- Kutlehria, S.; Dinh, T.C.; Bagde, A.; Patel, N.; Gebeyehu, A.; Singh, M. High-throughput 3D bioprinting of corneal stromal equivalents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2981–2994. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, H.; Zhou, X.; Zhang, J.; Zhou, H.; Hao, H.; Ding, L.; Li, H.; Gu, Y.; Ma, J.; et al. An Overview of Extracellular Matrix-Based Bioinks for 3D Bioprinting. Front. Bioeng. Biotechnol. 2022, 10, 905438. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.N.; Kim, J.; Jang, J.; Kim, H.K.; Cho, D.W. Characterization of cornea-specific bioink: High transparency, improved in vivo safety. J. Tissue Eng. 2019, 10, 2041731418823382. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.S.; Guan, J.; Wu, S.J. Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels. Acta Biomater. 2021, 125, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Lin, Q.; Shao, Z.; Chen, X.; Yang, Y. Preparing 3D-printable silk fibroin hydrogels with robustness by a two-step crosslinking method. RSC Adv. 2020, 10, 27225–27234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Matyszczak, G.; Wrzecionek, M.; Gadomska-Gajadhur, A.; Ruśkowski, P. Kinetics of Polycondensation of Sebacic Acid with Glycerol. Org. Process Res. Dev. 2020, 24, 1104–1111. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, K.; Wang, L.; Fan, Y. A Review: Optimization for Poly(glycerol sebacate) and Fabrication Techniques for Its Centered Scaffolds. Macromol. Biosci. 2021, 21, e2100022. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed. Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, M.; Abbasi, R.; Omidian Vandchali, S.; Ghaffari, M.; Moztarzadeh, F.; Mozafari, M. Corneal Repair and Regeneration: Current Concepts and Future Directions. Front. Bioeng. Biotechnol. 2019, 7, 135. [Google Scholar] [CrossRef]
- Kumar, A.; Yun, H.; Funderburgh, M.L.; Du, Y. Regenerative therapy for the Cornea. Prog. Retin. Eye Res. 2022, 87, 101011. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.; Bandeira, F.; Liu, Y.C.; Devarajan, K.; Yusoff, N.; Htoon, H.M.; Mehta, J.S. Effect of corneal stromal lenticule customization on neurite distribution and excitatory property. J. Adv. Res. 2022, 38, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Santra, M.; Liu, Y.C.; Jhanji, V.; Yam, G.H. Human SMILE-Derived Stromal Lenticule Scaffold for Regenerative Therapy: Review and Perspectives. Int. J. Mol. Sci. 2022, 23, 7967. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Mann, M.M.; Yang, E.; Funderburgh, J.L.; Wagner, W.R. Bioengineering organized, multilamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Eng. Part A 2013, 19, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Matthyssen, S.; van den Bogerd, B.; Dhubhghaill, S.N.; Koppen, C.; Zakaria, N. Corneal regeneration: A review of stromal replacements. Acta Biomater. 2018, 69, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Stone, W., Jr.; Herbert, E. Experimental study of plastic material as replacement for the cornea; a preliminary report. Am. J. Ophthalmol. 1953, 36, 168–173. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 2200. [Google Scholar] [CrossRef]
- Nam, S.; Hu, K.H.; Butte, M.J.; Chaudhuri, O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc. Natl. Acad. Sci. USA 2016, 113, 5492–5497. [Google Scholar] [CrossRef]
- Suo, H.; Zhang, J.; Xu, M.; Wang, L. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111963. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Sricholpech, M.; Terajima, M.; Tomer, K.B.; Perdivara, I. Glycosylation of Type I Collagen. Methods Mol. Biol. 2019, 1934, 127–144. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen-gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Auger, F.A.; Grandbois, E.; Guignard, R.; Giasson, M.; Boisjoly, H.; Guérin, S.L. Reconstructed human cornea produced in vitro by tissue engineering. Pathobiology 1999, 67, 140–147. [Google Scholar] [CrossRef]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.; Mehta, J.S.; Fischer, H.; et al. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater Res. A 2019, 107, 1945–1953. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.Y.; Su, J.; Tang, X.N.; Chen, Q.; Ma, L.W.; Zhang, J.J.; Wu, J.M.; Wang, S.X. Three-dimensional bioprinting of artificial ovaries by an extrusion-based method using gelatin-methacryloyl bioink. Climacteric 2022, 25, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Arica, T.A.; Guzelgulgen, M.; Yildiz, A.A.; Demir, M.M. Electrospun GelMA fibers and p(HEMA) matrix composite for corneal tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111720. [Google Scholar] [CrossRef]
- Zhong, Z.; Deng, X.; Wang, P.; Yu, C.; Kiratitanaporn, W.; Wu, X.; Schimelman, J.; Tang, M.; Balayan, A.; Yao, E.; et al. Rapid bioprinting of conjunctival stem cell micro-constructs for subconjunctival ocular injection. Biomaterials 2021, 267, 120462. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2019, 8, 438–449. [Google Scholar] [CrossRef]
- Salehi, S.; Fathi, M.; Javanmard, S.H.; Bahners, T.; Gutmann, J.S.; Ergün, S.; Steuhl, K.P.; Fuchsluger, T.A. Generation of PGS/PCL Blend Nanofibrous Scaffolds Mimicking Corneal Stroma Structure. Macromol. Mater. Eng. 2014, 299, 455–469. [Google Scholar] [CrossRef]
- Salehi, S.; Czugala, M.; Stafiej, P.; Fathi, M.; Bahners, T.; Gutmann, J.S.; Singer, B.B.; Fuchsluger, T.A. Poly (glycerol sebacate)-poly (ε-caprolactone) blend nanofibrous scaffold as intrinsic bio- and immunocompatible system for corneal repair. Acta Biomater. 2017, 50, 370–380. [Google Scholar] [CrossRef]
- Kanani, A.G.; Bahrami, S.H. Effect of changing solvents on poly (ε-caprolactone) nanofibrous webs morphology. J. Nanomater. 2011, 2011, 1–724153. [Google Scholar] [CrossRef]
- Stafiej, P.; Küng, F.; Thieme, D.; Czugala, M.; Kruse, F.E.; Schubert, D.W.; Fuchsluger, T.A. Adhesion and metabolic activity of human corneal cells on PCL based nanofiber matrices. Mater. Sci. Eng. C 2017, 71, 764–770. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Khonakdar, H.A.; Arjmand, M.; Jafari, S.H.; Bagher, Z.; Moghaddam, Z.S.; Chimerad, M.; Sisakht, M.M.; Shojaei, S. Review of Bioprinting in Regenerative Medicine: Naturally Derived Bioinks and Stem Cells. ACS Appl. Bio. Mater. 2021, 4, 4049–4070. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Rohaina, C.M.; Then, K.Y.; Ng, A.M.H.; Wan Abdul Halim, W.H.; Zahidin, A.Z.M.; Saim, A.; Idrus, R.B.H. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Transl. Res. 2014, 163, 200–210. [Google Scholar] [CrossRef]
- O’Callaghan, A.R.; Dziasko, M.A.; Sheth-Shah, R.; Lewis, M.P.; Daniels, J.T. Oral mucosa tissue equivalents for the treatment of limbal stem cell deficiency. Adv. Biosyst. 2020, 4, 1900265. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Rattan, V.; Jha, V.; Bhattacharyya, S. Secretome Cues Modulate the Neurogenic Potential of Bone Marrow and Dental Stem Cells. Mol. Neurobiol. 2017, 54, 4672–4682. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Katori, R.; Sasamoto, Y.; Taniwaki, Y.; Takayanagi, H.; Tsujikawa, M.; Sekiguchi, K.; Quantock, A.J.; Nishida, K. Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nat. Protoc. 2017, 12, 683–696. [Google Scholar] [CrossRef]
- Zeppieri, M.; Salvetat, M.L.; Beltrami, A.; Cesselli, D.; Russo, R.; Alcalde, I.; Merayo-Lloves, J.; Brusini, P.; Parodi, P.C. Adipose derived stem cells for corneal wound healing after laser induced corneal lesions in mice. J. Clin. Med. 2017, 6, 115. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Cai, S.; Sun, M.; Wang, J.; Li, S.; Li, X.; Tighe, S.; Chen, S.; Xie, H.; et al. Human limbal niche cells are a powerful regenerative source for the prevention of limbal stem cell deficiency in a rabbit model. Sci. Rep. 2018, 8, 6566. [Google Scholar] [CrossRef]
- Van den Bulcke, A.I.; Bogdanov, B.; de Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Meek, K.M.; Quantock, A.J. The use of X-ray scattering techniques to determine corneal ultrastructure. Prog. Retin. Eye Res. 2001, 20, 95–137. [Google Scholar] [CrossRef]
- Avetisov, S.E.; Narbut, M.N. Corneal transparency: Anatomical basis and evaluation methods. Vestn. Oftalmol. 2017, 133, 84–91. [Google Scholar] [CrossRef]
- Leu Alexa, R.; Cucuruz, A.; Ghițulică, C.D.; Voicu, G.; Stamat Balahura, L.R.; Dinescu, S.; Vlasceanu, G.M.; Stavarache, C.; Ianchis, R.; Iovu, H.; et al. 3D Printable Composite Biomaterials Based on GelMA and Hydroxyapatite Powders Doped with Cerium Ions for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 1841. [Google Scholar] [CrossRef]
- Yi, S.; Liu, Q.; Luo, Z.; He, J.J.; Ma, H.L.; Li, W.; Wang, D.; Zhou, C.; Garciamendez, C.E.; Hou, L.; et al. Micropore-Forming Gelatin Methacryloyl (GelMA) Bioink Toolbox 2.0: Designable Tunability and Adaptability for 3D Bioprinting Applications. Small 2022, 18, e2106357. [Google Scholar] [CrossRef]
- Benton, J.A.; DeForest, C.A.; Vivekanandan, V.; Anseth, K.S. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. Part A 2009, 15, 3221–3230. [Google Scholar] [CrossRef]
- Knopf-Marques, H.; Barthes, J.; Wolfova, L.; Vidal, B.; Koenig, G.; Bacharouche, J.; Francius, G.; Sadam, H.; Liivas, U.; Lavalle, P.; et al. Auxiliary Biomembranes as a Directional Delivery System To Control Biological Events in Cell-Laden Tissue-Engineering Scaffolds. ACS Omega 2017, 2, 918–929. [Google Scholar] [CrossRef]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Kumar, H.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent. Ann. Biomed. Eng. 2020, 48, 1955–1970. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, L.; Kong, B.; Huang, Y.; Zhong, S.; Connon, C.; Tan, J.; Yang, S.; Sun, W.; Mi, S. Effects of Gelatin Methacrylate Hydrogel on Corneal Repair and Regeneration in Rats. Transl. Vis. Sci. Technol. 2021, 10, 25. [Google Scholar] [CrossRef]
- Farasatkia, A.; Kharaziha, M. Robust and double-layer micro-patterned bioadhesive based on silk nanofibril/GelMA-alginate for stroma tissue engineering. Int. J. Biol. Macromol. 2021, 183, 1013–1025. [Google Scholar] [CrossRef]
- Farasatkia, A.; Kharaziha, M.; Ashrafizadeh, F.; Salehi, S. Transparent silk/gelatin methacrylate (GelMA) fibrillar film for corneal regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111744. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, Q.; Yu, D.; Shao, Y.; Wang, Z.; Liu, X.; Wang, W.; Chang, L.; Ma, T.; Mok, H.; et al. 3D-bioprinted vascular scaffold with tunable mechanical properties for simulating and promoting neo-vascularization. Smart Mater. Med. 2022, 3, 199–208. [Google Scholar] [CrossRef]
- Sakr, M.A.; Sakthivel, K.; Hossain, T.; Shin, S.R.; Siddiqua, S.; Kim, J.; Kim, K. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J. Biomed. Mater. Res. A 2022, 110, 708–724. [Google Scholar] [CrossRef]
- Lee, J.; Manoharan, V.; Cheung, L.; Lee, S.; Cha, B.H.; Newman, P.; Farzad, R.; Mehrotra, S.; Zhang, K.; Khan, F.; et al. Nanoparticle-Based Hybrid Scaffolds for Deciphering the Role of Multimodal Cues in Cardiac Tissue Engineering. ACS Nano 2019, 13, 12525–12539. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef]
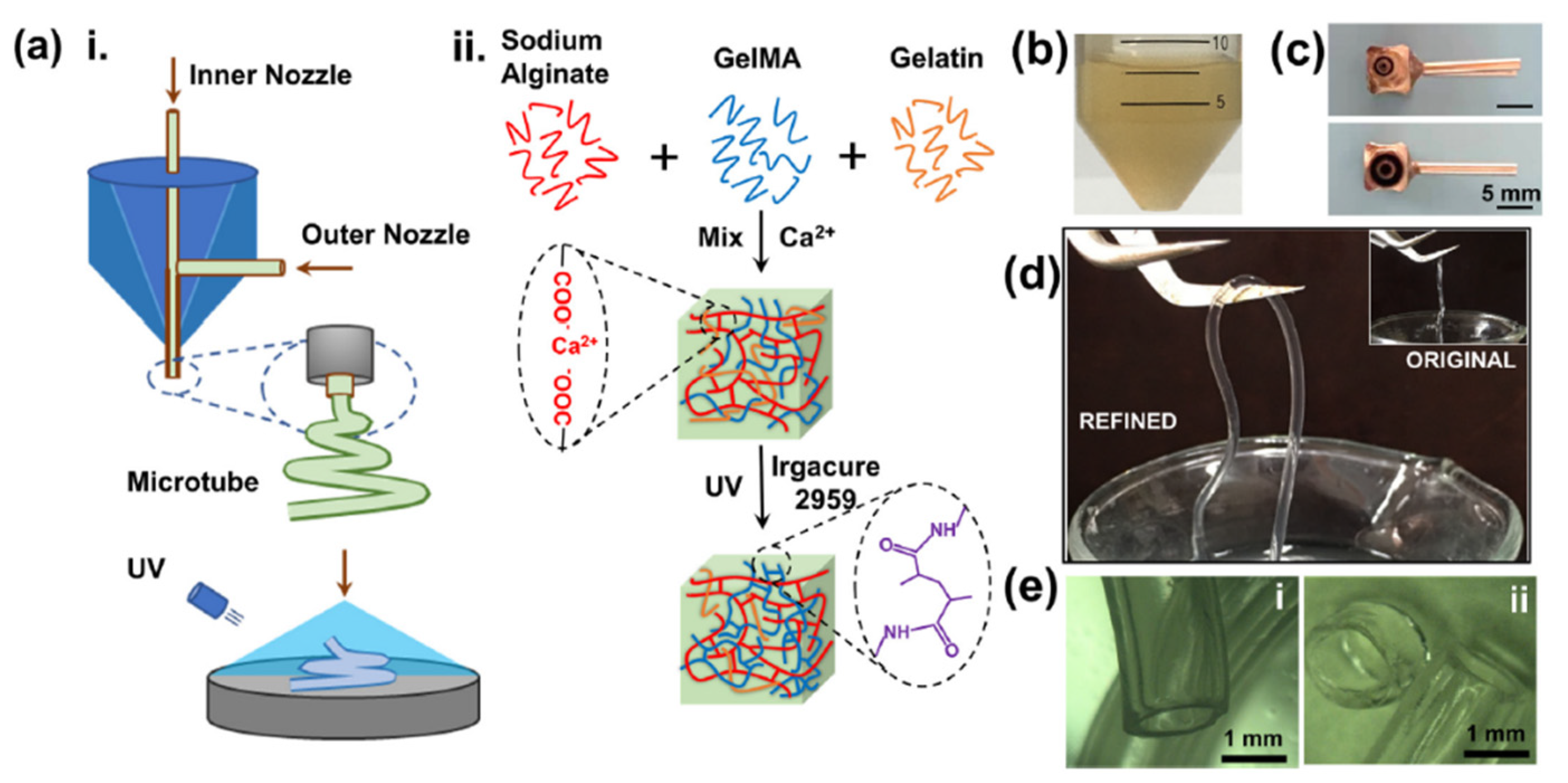
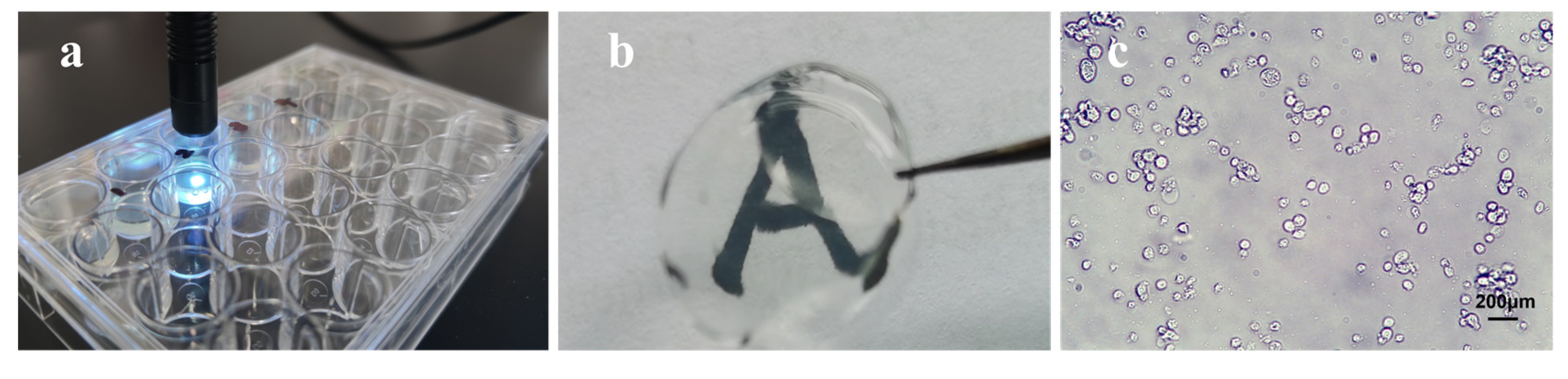
| Name | Natural/Sythetic | Advantage | Disadvantage | Form |
|---|---|---|---|---|
| Chitosan | Natural [37] | Antibacterial and easily biodegraded and removed [33,36,38] | Not revealing enhanced corneal wound-healing [33] | Natural biopolymer [37] |
| Hydrophilic gels | Natural/sythetic [39] | Strong biocompatibility, biodegradability, swelling, and cytocompatibility [40] | Some toxicity reported [39] | Three-dimensional(3D) hydrophilic polymer |
| Collagen | Natural [41] | Close to natural corneal stroma [42] | Low antigenicity and the liquid state at low temperatures [43] | As bioink for 3D printing [42] |
| Gelatin methacrylate (GelMA) | Synthetic [44] | Adhesion, spreading, and proliferation of many cells [44] | There are no studies in the corneal stroma. | As bioink for 3D printing |
| Silk fibroin | Natural [45] | Excellent transparency, biocompatibility, and low cytotoxicity [45]. | Poor mechanical performance [46] | A novel type of bioink [47] |
| Poly(glycerol sebacate) (PGS) | Synthesis [48] | Flexible elasticity with extremely nonlinear stress–strain behavior and biodegradability [48,49]. | Harsh synthesis conditions, rapid degradation rates, and low stiffness [50] | Synthesis of PGS as a robust biodegradable polyester [48] |
| Decellularized Extracellular Matrix (dECM) | Synthesis [45] | Excellent biocompatibility for three-dimensional cell growth [45] | Limited to small tissues or organs [51] | As bioink for 3D printing [45] |
| Extracellular matrix (ECM) | Natural | Good preservation of the natural ECM structure in corneas [52] | The presence of immune rejection [53] | Natural cellular matrix material [53] |
| Decellularized SMILE scaffolds | Natural/synthesis [54] | MSC development into corneal epithelial cells can be aided by decellularized lenticules [54]. | Standard methods are not widely accepted and are only carried out in a few countries [55]. | SMILE-derived stromal lenticules |
| Poly(ester urethane) urea(PEUU) | Synthesis [56,57] | Mimicking the human corneal stromal tissue [56] | - | A highly organized collagen–fibril construct |
| Electrospinning | Synthesis [58] | Simulating the structure of the ECM in the natural corneal stroma [56] | Electrostatic spinning does not show uniform inter-fiber spacing, resulting in optically opaque [58] | Artificial fiber |
| Fish scale derived biocornea | Natural [58] | Good mechanical strength and reasonable optical properties [25] | - | Collagen scaffold |
| Poly(methyl methacrylate) (PMMA) | synthetic | PMMA structures in rabbit eyes were relatively well retained after 24 months [59]. | PMMA is only available in combination with titanium for artificial corneas [59] |
| Cells | Method | In Vivo/Vitro | Result | Publication Time | Author |
|---|---|---|---|---|---|
| Bone marrow mesenchymal stem cells (BMMSC) | Differentiation into corneal epithelial cells can be achieved in 10 days of culture on amniotic membrane | In vitro | CK3 and p63 expression was significantly enhanced | 2014 | Rohaina et al. [81] |
| Oral mucosal epithelial cells | In vitro induction of oral mucosal epithelial cells using human oral mucosal fibroblasts (HOMF) as trophoblast cells for the treatment of (Corneal epithelial stem cell deficiency) CESD | In vivo | Oral mucosal epithelial cells can differentiate into corneal epithelial cells to treat corneal limbal stem cell deficiency | 2020 | O’callaghan et al. [82] |
| Dental pulp stem cells (DPSC) | Reconstruction of corneal surface by DPSC in the form of amniotic cell sheets in a rabbit model of CESD | In vivo | Clearer corneas and less angiogenesis in rabbits with DPSC group intervention | 2017 | Kumar et al. [83] |
| Induced pluripotent stem cells (iPSC) | Induced differentiation using fibroblast-derived induced pluripotent stem cells (iPSC) | In vitro | Can be induced into PAX6(+) and K12(+) corneal epithelial cells after 12 weeks | 2017 | Hayashi et al. [84] |
| Adipose stem cells (ASC) | ASC were also found to induce differentiation into corneal epithelial cells in a laser-induced mouse model for the treatment of CESD in mice | In vivo | Significant healing of corneal epithelial wounds in CESD mice | 2017 | Zeppieri et al. [85] |
| Limbal niche cell (LNC) | Subconjunctival injection of LNC cells in a model of CESD | In vivo | LNC can effectively promote the healing of corneal epithelium | 2020 | Li et al. [86] |
| Polymers | Method | Application Object | Result | Publication Time | Author |
|---|---|---|---|---|---|
| Chitosan | Chitosan 1% was applied to rabbits with central corneal injury, and the eyes were spotted 3 times daily for 1 week. | Rabbits | No difference | 1987 | Sall, K N et al. [33] |
| Carboxymethyl chitosan, hyaluronic acid, and gelatin | Application of mixed preparations of carboxymethyl chitosan, hyaluronic acid, and gelatin to alkali-burned rabbit corneas | Rabbits | Completely healed | 2018 | Xu et al. [34] |
| Chitosan | Solutions containing 0.5% w/v chitosan were assessed for antibacterial efficacy against E. coli and S. aureus in vitro. | E. coli and S. aureus | Effective in inhibiting bacterial growth | 2000 | Felt O et al. [35] |
| GelMA | The subconjunctival injection of this printed hydrogel encapsulates conjunctival stem cells. | Rabbits | Sustain the vitality and proliferative potential of the stem cells | 2020 | Zhong et al. [73] |
| Collagen and gelatin | For the engineering of corneal tissue, type-I collagen–gelatin hydrogel | In vitro | In addition to increasing Young’s modulus and stiffness, COL-I also boosts optical characteristics. | 2019 | Goodarzi, H. et al. [66] |
| Silk fibroin | Co-dECM as a bioink; the bio-ink was injected into mice and rabbits. | Mice and rabbits | Similar to clinical-grade collagen | 2019 | Kim, H. et al. [45] |
| Poly (glycerol sebacate) (PGS) | PGS-PCL nanofibrous scaffolds using a modified electrospinning technique, culture HCEC in PGSPCL nanofibrous scaffolds | In vitro | Cell proliferation and viability compared to pure PCL scaffolds was improved in the presence of PGS within blended scaffolds (4:1 specifically). | 2017 | Salehi et al. [76] |
| Decellularized porcine-cornea-derived hydrogels | An injectable and transparent hydrogel for the regeneration of epithelium and stroma in localized corneal lesions was developed. | Rabbit | The rabbit corneal epithelium regenerated in 3 days, and corneal thickness returned to normal 12 weeks after surgery without significant inflammation or scar formation. | 2020 | Wang et al. [29] |
| Polycaprolactone (PCL) | HCEC and human corneal keratocytes (HCK) cultured in PCL-based matrices | In vitro | HCEC and HCK growth was observed on all aligned PCL-based matrices | 2016 | Stafiej, Piotr et al. [78] |
| Decellularized extracellular matrix (dECM) | Co-dECM as a bioink for corneal regeneration; the Co-dECM gel was heterologously implanted into mice and rabbits for two months and one month. | Mice and rabbits | Only the Co-dECM group showed the ability of hTMSCs to differentiate into a keratocyte lineage. | 2019 | Kim, H. et al. [45] |
| Collagen | Collagen was successfully printed using extrusion bioprinting technology by adding it to a gelatin/alginate system. | In vitro | HCECs can achieve a high cellular viability of 94.6 ± 2.5% after printing. | 2016 | Wu et al. [47] |
| Application | Concentration of the GelMA | Result | Publication Time | Author |
|---|---|---|---|---|
| GelMA as a bioink to 3D bioprint the corneal stroma | - | Only 8% weight loss after 3 weeks with good transparency. | 2019 | Kilic Bektas, C. et al. [74] |
| Structure of 3D printed corneas using different concentrations of GelMA material to compare biological properties | 12.5% and 7.5% | The mechanical and optical transmittance of 12.5% were superior. | 2020 | Mahdavi et al. [96] |
| Taking GelMA as the donor material for rat lamellar corneal transplantation | 5% | GelMA effectively improves repair after corneal injury in rats. | 2021 | Chen et al. [97] |
| SNF/GelMA hybrid films | 0.5 wt% | Significantly improved biocompatibility after mixing with SNF (30/70). | 2020 | Asal Farasatkia [99] |
| Induction of keratocytes regeneration in vitro and in vivo using GelMA | 5%, 10% and 15% | 3D GelMA can induce the regeneration of damaged corneal stroma in vitro and in vivo. | 2020 | Kong, Bin [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, G.; Li, G.; Wang, W.; Xu, L. Application Prospect and Preliminary Exploration of GelMA in Corneal Stroma Regeneration. Polymers 2022, 14, 4227. https://doi.org/10.3390/polym14194227
Su G, Li G, Wang W, Xu L. Application Prospect and Preliminary Exploration of GelMA in Corneal Stroma Regeneration. Polymers. 2022; 14(19):4227. https://doi.org/10.3390/polym14194227
Chicago/Turabian StyleSu, Guanyu, Guigang Li, Wei Wang, and Lingjuan Xu. 2022. "Application Prospect and Preliminary Exploration of GelMA in Corneal Stroma Regeneration" Polymers 14, no. 19: 4227. https://doi.org/10.3390/polym14194227
APA StyleSu, G., Li, G., Wang, W., & Xu, L. (2022). Application Prospect and Preliminary Exploration of GelMA in Corneal Stroma Regeneration. Polymers, 14(19), 4227. https://doi.org/10.3390/polym14194227





