An Ab Initio Investigation on Relevant Oligomerization Reactions of Toluene Diisocyanate (TDI)
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Thermochemistry of the Phenyl Isocyanate Covalent Dimers
3.2. TDI Cyclodimerization
3.3. Validation of the qG3MP2B3 Protocol
3.4. Cyclotrimerization Reactions of 2,4-TDI
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenzi, V.; Crema, A.; Pyrlin, S.; Marques, L. Current State and Perspectives of Simulation and Modeling of Aliphatic Isocyanates and Polyisocyanates. Polymers 2022, 14, 1642. [Google Scholar] [CrossRef] [PubMed]
- Golling, F.E.; Pires, R.; Hecking, A.; Weikard, J.; Richter, F.; Danielmeier, K.; Dijkstra, D. Polyurethanes for Coatings and Adhesives—Chemistry and Applications. Polym. Int. 2019, 68, 848–855. [Google Scholar] [CrossRef]
- Polyurethanes: New Technologies and Applications Drive Global Market Growth 2021–2026. Available online: https://www.researchandmarkets.com/reports/5412105/polyurethanes-new-technologies-and-applications (accessed on 27 June 2022).
- Allport, D.C.; Gilbert, D.S.; Outterside, S.M. MDI and TDI: Safety, Health & Environment, A Source Book and Practical Guide; John Wiley & Sons, Ltd.: West Sussex, England, 1998; ISBN 0471958123. [Google Scholar]
- Parod, R.J. Toluene Diisocyanate, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, ISBN 9780123864543. [Google Scholar]
- Thangaraj, R.; Horváth, T.; Boros, R.Z.; Viskolcz, B.; Szőri, M. A Theoretical Study on the Phosgenation of 2,4-Toluenediamine (2,4-TDA). Polymers 2022, 14, 2254. [Google Scholar] [CrossRef] [PubMed]
- Siebert, M.; Sure, R.; Deglmann, P.; Closs, A.C.; Lucas, F.; Trapp, O. Mechanistic Investigation into the Acetate-Initiated Catalytic Trimerization of Aliphatic Isocyanates: A Bicyclic Ride. J. Org. Chem. 2020, 85, 8553–8562. [Google Scholar] [CrossRef]
- Parodi, F. Isocyanate-Derived Polymers. Compr. Polym. Sci. Suppl. 1989, 5, 387–412. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-Isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Querat, E.; Tighzert, L.; Pascault, J.P.; Dušek, K. Blocked Isocyanate: Reaction and Thermal Behaviour of the Toluene 2,4-Diisocyanate Dimer. Angew. Makromol. Chemie 1996, 242, 1–36. [Google Scholar] [CrossRef]
- Helberg, J.; Oe, Y.; Zipse, H. Mechanistic Analysis and Characterization of Intermediates in the Phosphane-Catalyzed Oligomerization of Isocyanates. Chem. A Eur. J. 2018, 24, 14387–14391. [Google Scholar] [CrossRef]
- Bulian, F.; Graystone, J.A. Raw Materials for Wood Coatings (1)—Film Formers (Binders, Resins and Polymers); Elsevier B.V.: Oxford, UK, 2009; ISBN 9780444528407. [Google Scholar]
- Guo, J.; He, Y.; Xie, D.; Zhang, X. Process Investigating and Modelling for the Self-Polymerization of Toluene Diisocyanate (TDI)-Based Polyurethane Prepolymer. J. Mater. Sci. 2015, 50, 5844–5855. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, H.; Zhou, S.Z.; Garnier, S.; Füldner, S.; Ye, L.; Feng, Z. The Preparation of Hybrid Trimer by Cyclo-Oligomerization of TDI and HDI and Its Curing Process with Polyols to Form Elastic PU Coating. J. Coatings Technol. Res. 2017, 14, 1279–1288. [Google Scholar] [CrossRef]
- Schwarcz, A.; Weehawken, N.J.; Brindell, G.D. Brindell Preparation of Diisocyanate Dimers in Aqueous Medium. U.S. Patent 3,489,744A, 13 January 1970. [Google Scholar]
- Davis, A. Dimerisation and Trimerisation of 2,4 Tolylene Di-Isocyanate. Die Makromol. Chemie 1963, 66, 196–204. [Google Scholar] [CrossRef]
- Okumoto, S.; Yamabe, S. A Computational Study of Base-Catalyzed Reactions between Isocyanates and Epoxides Affording 2-Oxazolidones and Isocyanurates. J. Comput. Chem. 2001, 22, 316–326. [Google Scholar] [CrossRef]
- Gibb, J.N.; Goodman, J.M. The Formation of High-Purity Isocyanurate through Proazaphosphatrane- Catalysed Isocyanate Cyclo-Trimerisation: Computational Insights. Org. Biomol. Chem. 2013, 11, 90–97. [Google Scholar] [CrossRef]
- Guo, Y.; Muuronen, M.; Deglmann, P.; Lucas, F.; Sijbesma, R.P.; Tomović, Ž. Role of Acetate Anions in the Catalytic Formation of Isocyanurates from Aromatic Isocyanates. J. Org. Chem. 2021, 86, 5651–5659. [Google Scholar] [CrossRef]
- Uchimaru, T.; Yamane, S.; Mizukado, J.; Tsuzuki, S. Thermal Stabilities and Conformational Behaviors of Isocyanurates and Cyclotrimerization Energies of Isocyanates: A Computational Study. RSC Adv. 2020, 10, 15955–15965. [Google Scholar] [CrossRef]
- Baboul, A.G.; Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-3 Theory Using Density Functional Geometries and Zero-Point Energies. J. Chem. Phys. 1999, 110, 7650–7657. [Google Scholar] [CrossRef]
- Waleed, H.Q.; Csécsi, M.; Hadjadj, R.; Thangaraj, R.; Pecsmány, D.; Owen, M.; Szőri, M.; Fejes, Z.; Viskolcz, B.; Fiser, B. Computational Study of Catalytic Urethane Formation. Polymers 2022, 14, 8. [Google Scholar] [CrossRef]
- Boros, R.Z.; Farkas, L.; Nehéz, K.; Viskolcz, B.; Szori, M. An Ab Initio Investigation of the 4,4’-Methlylene Diphenyl Diamine (4,4’-MDA) Formation from the Reaction of Aniline with Formaldehyde. Polymers 2019, 11, 398. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 720–723. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, K.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Cheikh, W.; Rózsa, Z.B.; López, C.O.C.; Mizsey, P.; Viskolcz, B.; Szori, M.; Fejes, Z. Urethane Formation with an Excess of Isocyanate or Alcohol: Experimental and Ab Initio Study. Polymers 2019, 11, 1543. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Gonzalez, C.; Schlegel, H.B. An Improved Algorithm for Reaction Path Following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- NIST. Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database 101. Available online: https://cccbdb.nist.gov/ (accessed on 4 May 2022).
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on the Generalized Born Approximation with Asymmetric Descreening. J. Chem. Theory Comput. 2009, 5, 2447–2464. [Google Scholar] [CrossRef]
- Boros, R.Z.; Rágyanszki, A.; Csizmadia, I.G.; Fiser, B.; Guljas, A.; Farkas, L.; Viskolcz, B. Industrial Application of Molecular Computations on the Dimerization of Methylene Diphenyl Diisocyanate. React. Kinet. Mech. Catal. 2018, 124, 1–14. [Google Scholar] [CrossRef]






| ΔE0,G3MP2B3 | ΔH0G3MP2B3 | ΔG0G3MP2B3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | - | ODCB | - | ODCB | - | ODCB | - | ODCB | - | ODCB | |
| T in K | 0 | 298.15 | 423.15 | 298.15 | 423.15 | ||||||
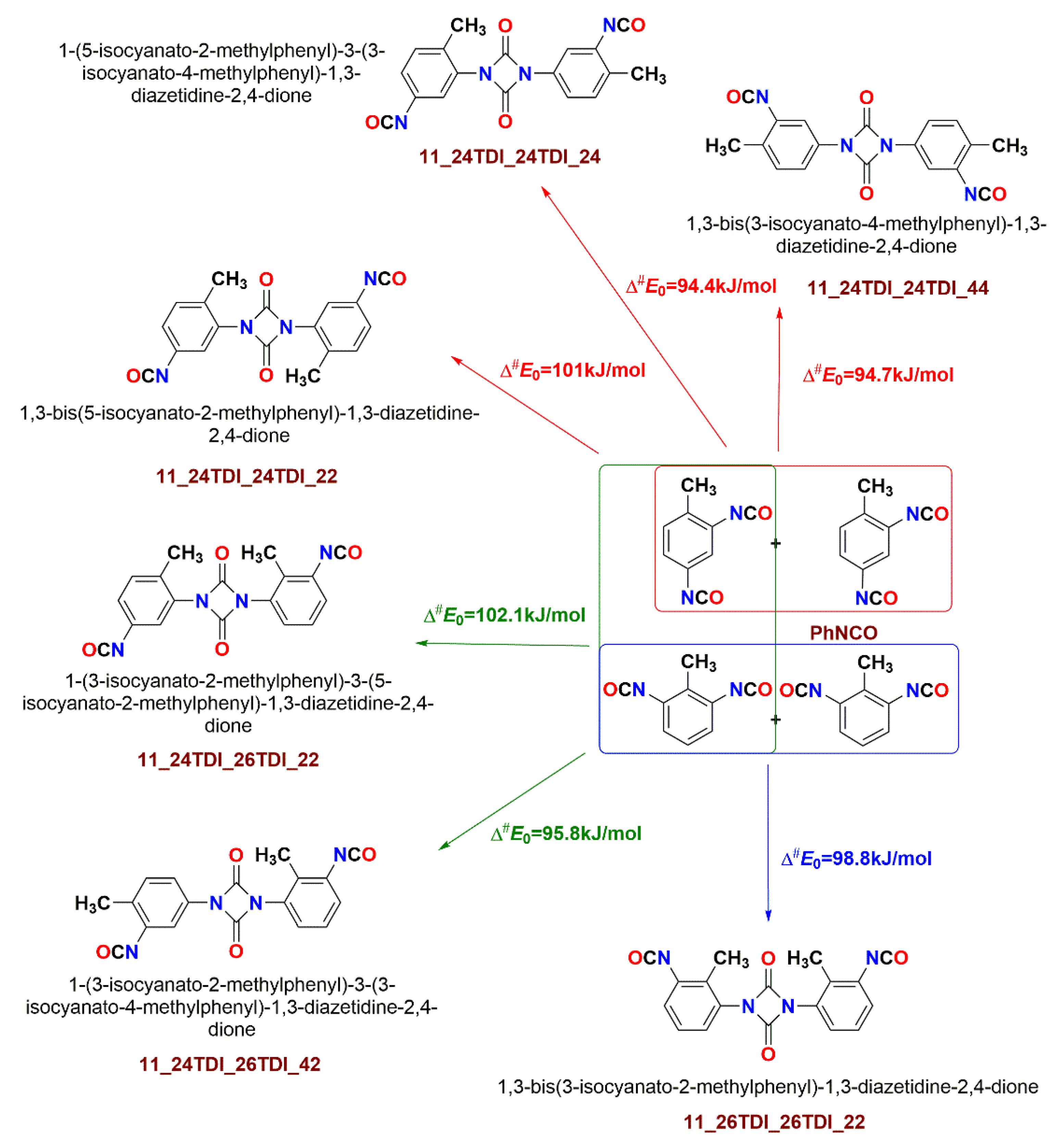
| 11_24TDI_24TDI_22 | TS | 101.0 | 94.0 | 99.8 | 92.4 | 100.4 | 93.0 | 155.4 | 149.9 | 178.7 | 173.6 |
| 11_24TDI_24TDI_24 | TS | 94.4 | 87.0 | 93.3 | 85.9 | 93.8 | 86.4 | 147.7 | 140.3 | 170.5 | 162.5 |
| 11_24TDI_24TDI_44 | TS | 94.7 | 87.1 | 94.2 | 86.1 | 94.8 | 86.6 | 144.5 | 141.3 | 165.5 | 164.4 |
| 11_24TDI_26TDI_22 | TS | 102.0 | 94.5 | 100.7 | 93.0 | 101.3 | 93.5 | 156.9 | 150.6 | 180.4 | 174.6 |
| 11_24TDI_26TDI_42 | TS | 95.8 | 86.6 | 94.7 | 85.4 | 95.3 | 85.9 | 150.5 | 141.9 | 173.8 | 165.1 |
| 11_26TDI_26TDI_22 | TS | 98.8 | 90.1 | 97.0 | 88.0 | 97.6 | 88.3 | 156.1 | 147.6 | 180.8 | 172.1 |
| 1-2_24TDI_24TDI_22 | TS | 121.9 | 102.7 | 120.4 | 101.1 | 120.9 | 101.4 | 178.1 | 158.1 | 202.2 | 182.3 |
| 1-2_24TDI_24TDI_24 | TS | 120.3 | 102.8 | 118.8 | 100.8 | 119.3 | 101.1 | 176.0 | 158.7 | 199.8 | 182.9 |
| 1-2_24TDI_24TDI_44 | TS | 121.3 | 103.2 | 120.0 | 101.3 | 120.5 | 101.6 | 176.0 | 160.0 | 199.5 | 184.5 |
| 1-2_24TDI_26TDI_22 | TS | 124.1 | 104.4 | 122.6 | 102.9 | 123.0 | 103.2 | 179.7 | 160.0 | 203.6 | 183.8 |
| 1-2_24TDI_26TDI_42 | TS | 122.5 | 105.8 | 120.6 | 103.3 | 121.1 | 103.0 | 179.5 | 164.6 | 204.1 | 187.5 |
| 1-2_26TDI_26TDI_22 | TS | 126.7 | 105.2 | 124.7 | 102.4 | 125.3 | 102.7 | 183.7 | 165.7 | 208.3 | 192.1 |
| 11_24TDI_24TDI_22 | Product | −33.6 | −45.4 | −36.3 | −47.6 | −36.0 | −47.3 | 25.6 | 12.0 | 51.5 | 36.9 |
| 11_24TDI_24TDI_24 | Product | −42.0 | −52.7 | −44.4 | −55.0 | −44.2 | −54.7 | 16.1 | 5.8 | 41.5 | 31.3 |
| 11_24TDI_24TDI_44 | Product | −48.5 | −58.1 | −50.2 | −59.8 | −49.9 | −59.5 | 6.3 | -2.9 | 29.9 | 20.9 |
| 11_24TDI_26TDI_22 | Product | −33.6 | −46.1 | −36.3 | −48.4 | −36.1 | −48.1 | 26.5 | 10.7 | 52.9 | 35.4 |
| 11_24TDI_26TDI_42 | Product | −42.0 | −53.5 | −44.6 | −55.7 | −44.3 | −55.4 | 17.2 | 2.9 | 43.1 | 27.4 |
| 11_26TDI_26TDI_22 | Product | −32.7 | −46.6 | −35.7 | −49.3 | −35.4 | −49.0 | 28.4 | 11.5 | 55.3 | 37.0 |
| 1-2_24TDI_24TDI_22 | Product | 27.2 | 22.7 | 24.2 | 19.9 | 24.4 | 20.2 | 85.5 | 80.9 | 111.2 | 106.4 |
| 1-2_24TDI_24TDI_24 | Product | 19.0 | 16.7 | 16.0 | 13.8 | 16.3 | 14.1 | 76.7 | 74.8 | 102.1 | 100.3 |
| 1-2_24TDI_24TDI_44 | Product | 18.3 | 16.5 | 15.5 | 13.6 | 15.7 | 14.0 | 75.2 | 75.1 | 100.3 | 100.9 |
| 1-2_24TDI_26TDI_22 | Product | 28.6 | 23.3 | 25.6 | 20.5 | 25.8 | 20.8 | 86.6 | 81.3 | 112.2 | 106.8 |
| 1-2_24TDI_26TDI_42 | Product | 19.9 | 16.3 | 16.6 | 13.0 | 16.9 | 13.4 | 78.6 | 76.2 | 104.6 | 102.7 |
| 1-2_26TDI_26TDI_22 | Product | 31.0 | 24.6 | 27.4 | 21.1 | 27.7 | 21.5 | 90.9 | 84.4 | 117.5 | 110.9 |
| Species | T = 298.15 K | T = 423.15 K | ||
|---|---|---|---|---|
| ΔrG0 (kJ/mol) | pK | ΔrG0 (kJ/mol) | pK | |
| 11_24TDI_24TDI_22 | 12.0 | 2.1 | 36.9 | 4.6 |
| 11_24TDI_24TDI_24 | 5.8 | 1.0 | 31.3 | 3.9 |
| 11_24TDI_24TDI_44 | −2.9 | −0.5 | 20.9 | 2.6 |
| 11_24TDI_26TDI_22 | 10.7 | 1.9 | 35.4 | 4.4 |
| 11_24TDI_26TDI_42 | 2.9 | 0.5 | 27.4 | 3.4 |
| 11_26TDI_26TDI_22 | 11.5 | 2.0 | 37.0 | 4.6 |
| ΔE0,qG3MP2B3 (kJ/mol) | ΔH0qG3MP2B3 (kJ/mol) | ΔG0qG3MP2B3 (kJ/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | - | ODCB | - | ODCB | - | ODCB | - | ODCB | - | ODCB |
| T (K) | 0 | 298.15 | 423.15 | 298.15 | 423.15 | |||||
| Reactant | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| oTS1 | 247.4 | 260.5 | 246.3 | 259.7 | 248.3 | 261.9 | 350.4 | 364.8 | 393.8 | 408.4 |
| oTS2 | 141.7 | 124.3 | 141.8 | 124.0 | 143.9 | 126.1 | 246.3 | 235.1 | 289.7 | 281.3 |
| oTS3 | 134.9 | 122.4 | 136.0 | 122.4 | 138.3 | 124.4 | 236.2 | 230.1 | 277.8 | 275.0 |
| IM | −48.5 | −58.1 | −50.2 | −59.8 | −49.9 | −59.5 | 6.3 | −2.9 | 29.9 | 20.9 |
| tTS2 | 60.5 | 54.0 | 56.2 | 49.4 | 57.0 | 50.3 | 178.4 | 171.9 | 229.6 | 223.1 |
| substituted 1,3,5-trioxane | 116.1 | 125.3 | 112.8 | 121.6 | 113.8 | 122.6 | 220.8 | 234.2 | 265.9 | 281.2 |
| substituted iminooxadiazinedione | −90.0 | −105.4 | −94.2 | −109.8 | −93.4 | −109.0 | 23.2 | 11.2 | 72.3 | 61.8 |
| substituted isocyanurate | −196.6 | −227.9 | −200.8 | −232.0 | −200.2 | −231.5 | −84.3 | −114.4 | −35.5 | −65.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangaraj, R.; Fiser, B.; Qiu, X.; Li, C.; Viskolcz, B.; Szőri, M. An Ab Initio Investigation on Relevant Oligomerization Reactions of Toluene Diisocyanate (TDI). Polymers 2022, 14, 4183. https://doi.org/10.3390/polym14194183
Thangaraj R, Fiser B, Qiu X, Li C, Viskolcz B, Szőri M. An Ab Initio Investigation on Relevant Oligomerization Reactions of Toluene Diisocyanate (TDI). Polymers. 2022; 14(19):4183. https://doi.org/10.3390/polym14194183
Chicago/Turabian StyleThangaraj, Ravikumar, Béla Fiser, Xuanbing Qiu, Chuanliang Li, Béla Viskolcz, and Milán Szőri. 2022. "An Ab Initio Investigation on Relevant Oligomerization Reactions of Toluene Diisocyanate (TDI)" Polymers 14, no. 19: 4183. https://doi.org/10.3390/polym14194183
APA StyleThangaraj, R., Fiser, B., Qiu, X., Li, C., Viskolcz, B., & Szőri, M. (2022). An Ab Initio Investigation on Relevant Oligomerization Reactions of Toluene Diisocyanate (TDI). Polymers, 14(19), 4183. https://doi.org/10.3390/polym14194183








