Ultrasensitive Functionalized Polymeric-Nanometal Oxide Sensors for Potentiometric Determination of Ranitidine Hydrochloride
Abstract
1. Introduction
2. Materials and Methods
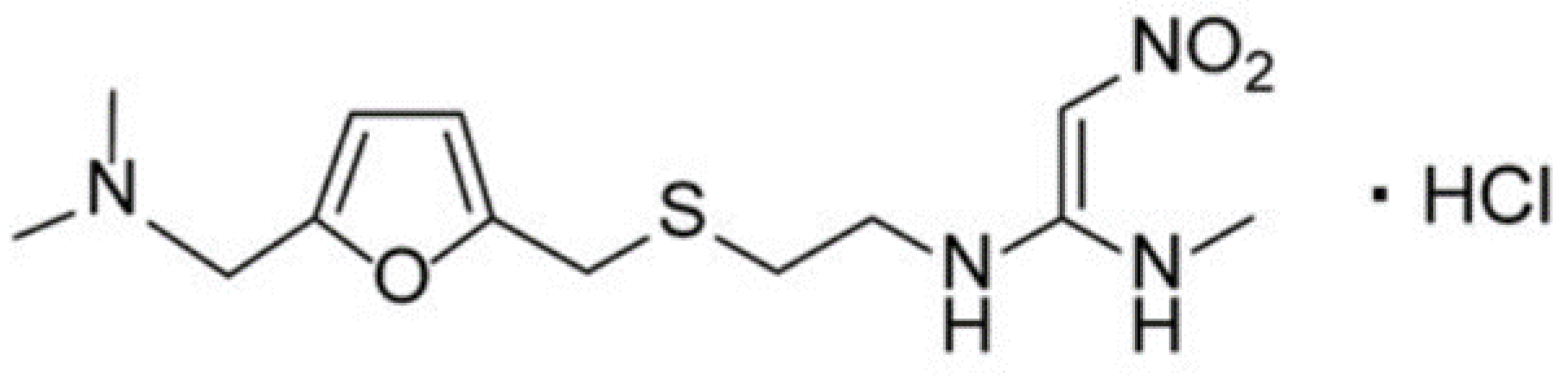
2.1. Chemicals
2.2. Instrumentation
2.3. Green Preparation of Nanoparticles
2.4. Spectroscopic and Microscopic Characterization of Nanoparticles
2.5. Preparation of Standard Solution
2.6. Formation of Ion-Pair Complex
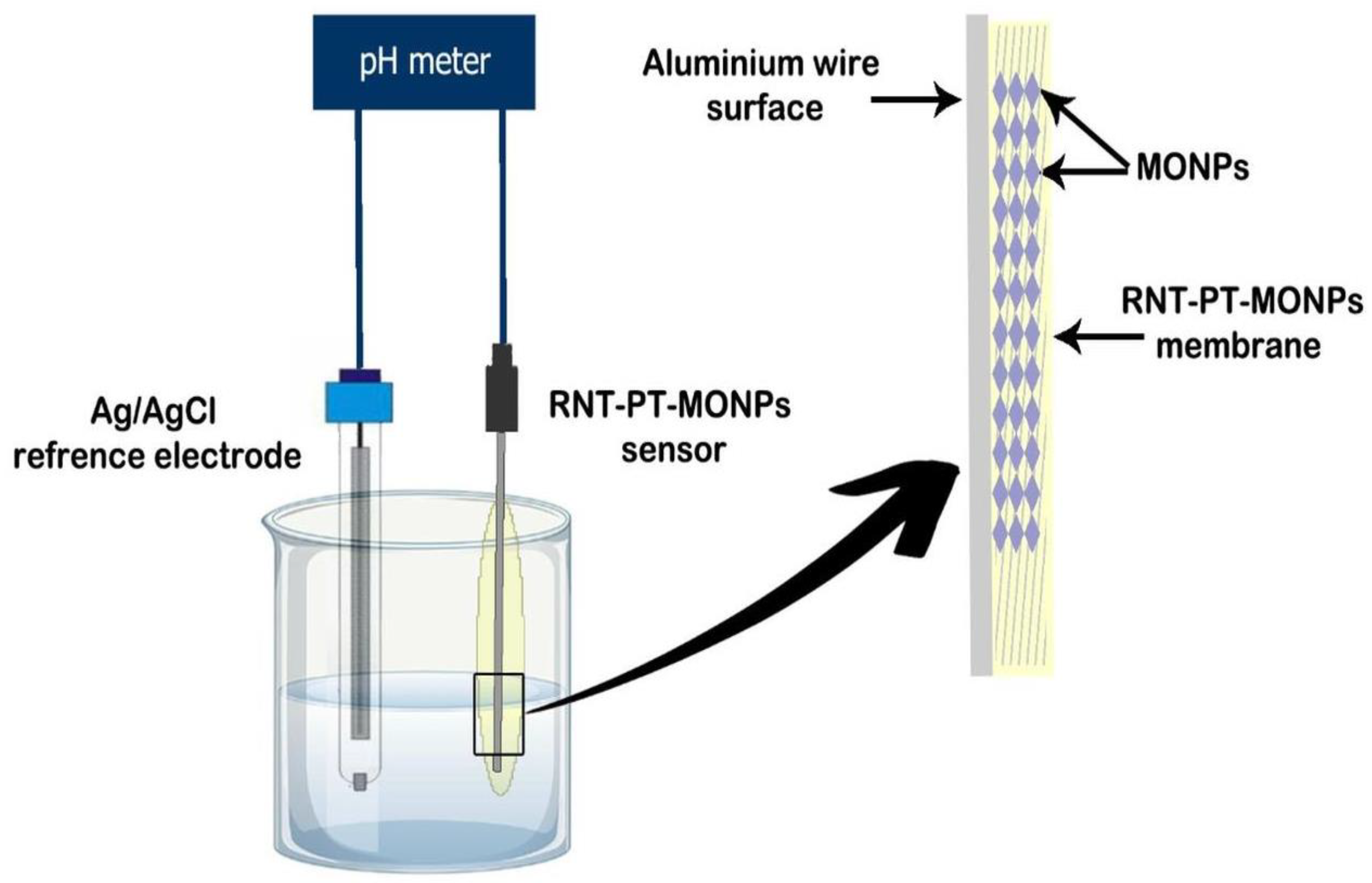
2.7. Sensor Design and Membrane Composition
2.8. Calibration Graphs
2.9. Optimization of Potential Readings’ Conditions
2.10. Analytical Applications
3. Results and Discussion
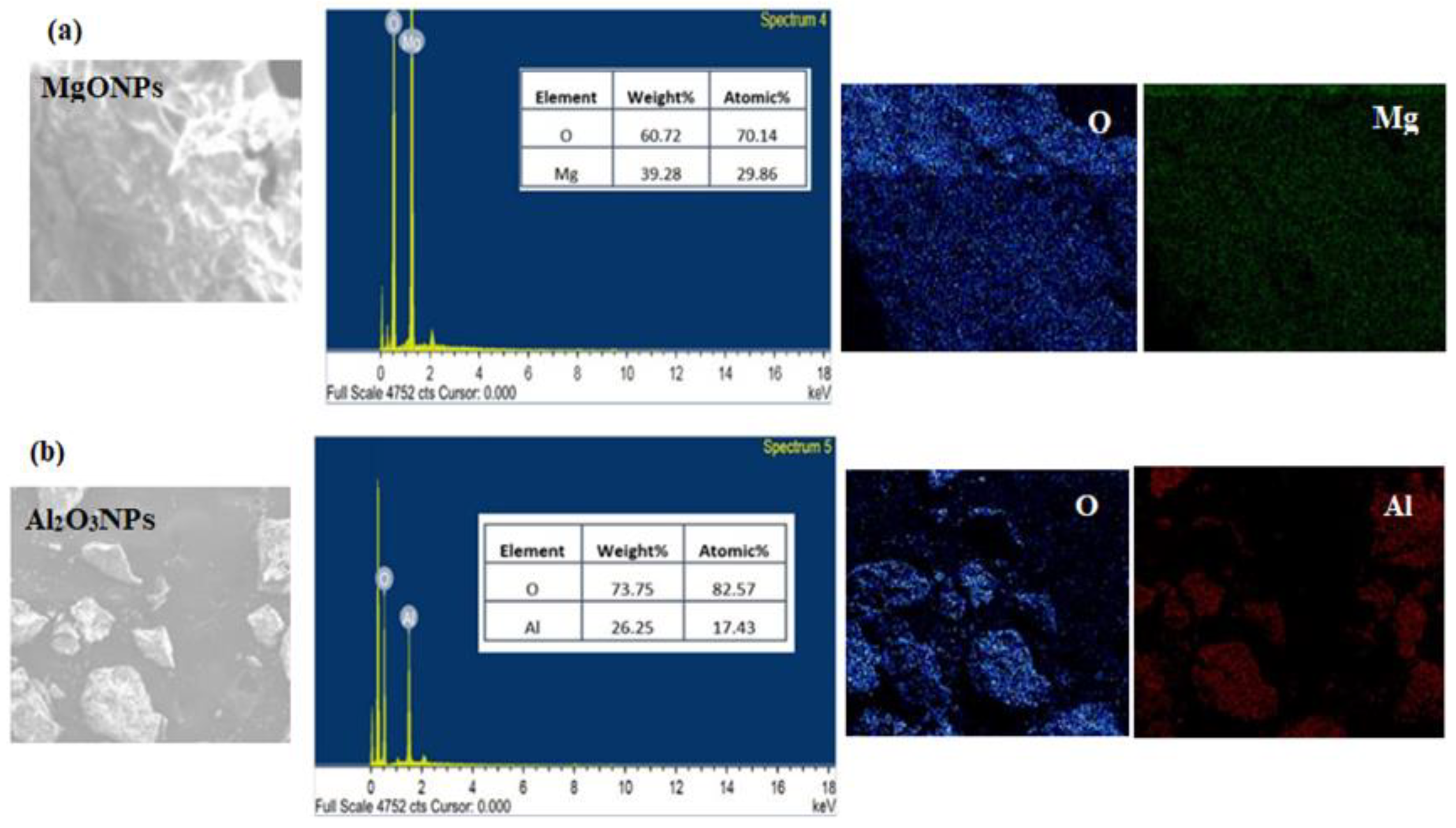
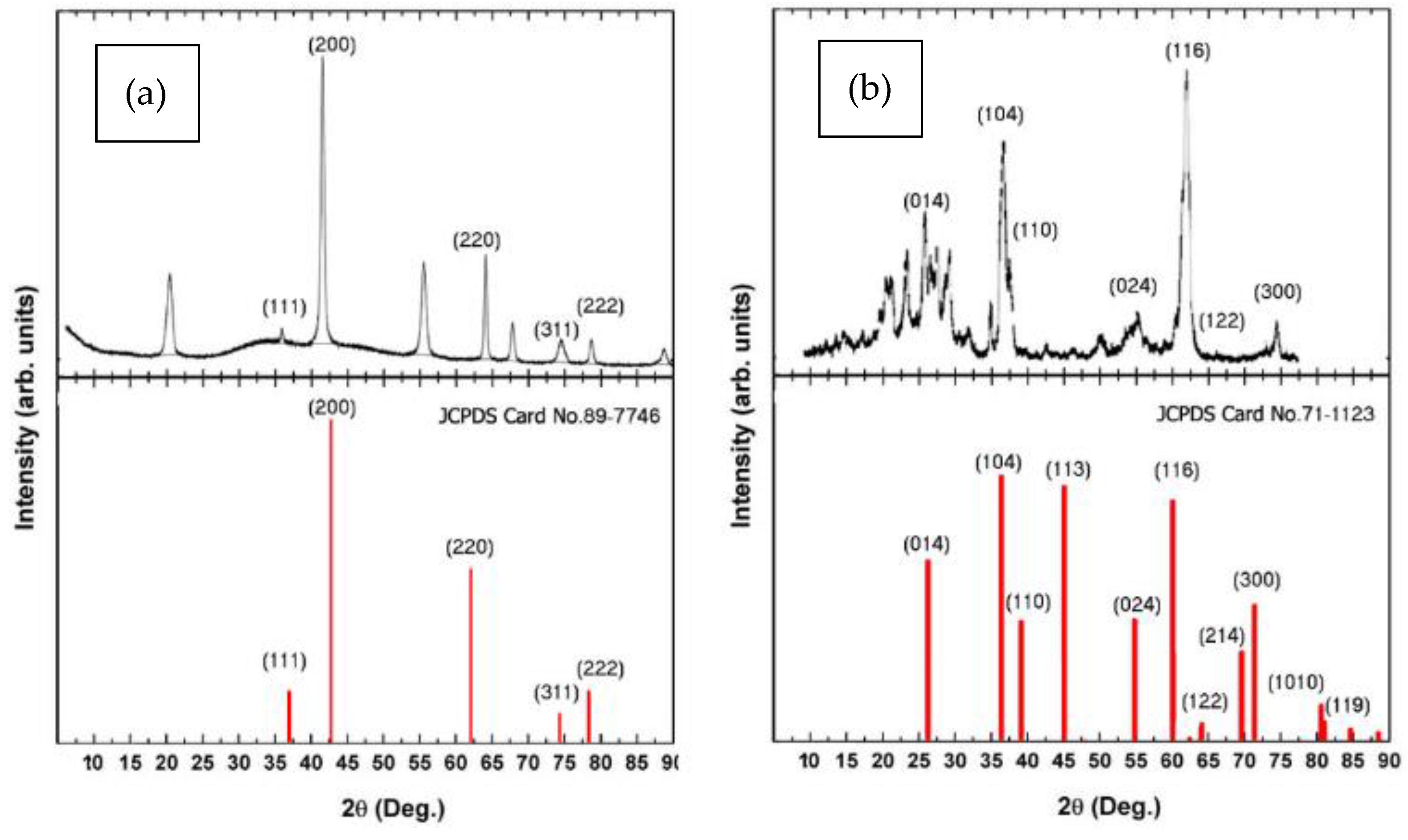
3.1. Characterization of the Synthesized Nanoparticles
3.2. The Fabricated Sensors Behavior
3.3. Quantification of Ranitidine Hydrochloride
3.4. Method Validation
3.5. Quantification of the Drug in Its Tablets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitesides, G.M. Nanoscience, nanotechnology, and chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef]
- Zhu, W.; Bartos, P.J.; Porro, A. Application of nanotechnology in construction. Mater. Struct. 2004, 37, 649–658. [Google Scholar] [CrossRef]
- Mamat, M.H.; Khusaimi, Z.; Suriani, A.B.; Asiah, M.N.; Soga, T.; Mahmood, M.R. (Eds.) Nanoscience, Nanotechnology and Nanoengineering; Trans Tech Publications Ltd.: Bäch SZ, Switzerland, 2013. [Google Scholar]
- Toghan, A.; Abo-bakr, A.M.; Rageh, H.M.; Abd-Elsabour, M. A novel electrochemical sensor for determination of salbutamol based on graphene oxide/poly (o-nitrobenzoic acid) modified glassy carbon electrode and its analytical application in pharmaceutical formulation and human urine. J. Biosens. Bioelectron. 2019, 10, 2. [Google Scholar]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Oprea, M.; Panaitescu, D.M. Nanocellulose hybrids with metal oxides nanoparticles for biomedical applications. Molecules 2020, 25, 4045. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.; Eid, A.M.; Hassan, S.E.D.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The catalytic activity of biosynthesized magnesium oxide nanoparticles (MgO-NPs) for inhibiting the growth of pathogenic microbes, tanning effluent treatment, and chromium ion removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Li, X.; Chen, X.; Mei, Y.; Yang, Y.; An, L. Aluminium oxide nanoparticles compromise spatial memory performance and proBDNF-mediated neuronal function in the hippocampus of rats. Part. Fibre Toxicol. 2022, 19, 34. [Google Scholar] [CrossRef]
- Kusuma, K.B.; Manju, M.; Ravikumar, C.R.; Nagaswarupa, H.P.; Amulya, M.S.; Anilkumar, M.R.; Avinash, B.; Gurushantha, K.; Ravikantha, N. Photocatalytic and electrochemical sensor for direct detection of paracetamol comprising γ-aluminium oxide nanoparticles synthesized via sonochemical route. Sens. Int. 2020, 1, 100039. [Google Scholar] [CrossRef]
- Kaya, S.; Kurbanoglu, S.; Yavuz, E.; Demiroglu Mustafov, S.; Sen, F.; Ozkan, S.A. Carbon-based ruthenium nanomaterial-based electroanalytical sensors for the detection of anticancer drug Idarubicin. Sci. Rep. 2020, 10, 11057. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Ge, L.; Lisak, G. Highly reproducible solid contact ion selective electrodes: Emerging opportunities for potentiometry–A review. Anal. Chim. Acta 2021, 1162, 338304. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, Y.; Zhang, R.; Li, J.; Li, X.; Zang, Z. Conductometric room temperature ammonia sensors based on titanium dioxide nanoparticles decorated thin black phosphorus nanosheets. Sens. Actuators B Chem. 2021, 349, 130770. [Google Scholar] [CrossRef]
- Ali, T.A.; Mohamed, G.G.; Othman, A.R. Design and construction of new potentiometric sensors for determination of copper (II) ion based on copper oxide nanoparticles. Int. J. Electrochem. Sci. 2015, 10, 8041–8057. [Google Scholar]
- Chen, H.; Rim, Y.S.; Wang, I.C.; Li, C.; Zhu, B.; Sun, M.; Goorsky, M.S.; He, X.; Yang, Y. Quasi-two-dimensional metal oxide semiconductors based ultrasensitive potentiometric biosensors. ACS Nano 2017, 11, 4710–4718. [Google Scholar] [CrossRef]
- Willander, M.; Tahira, A.; Ibupoto, Z. Potentiometric Biosensors Based on Metal Oxide Nanostructures. In Encyclopedia of Interfacial Chemistry, Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. TrAC Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F. New functionalized polymeric sensor based NiO/MgO nanocomposite for potentiometric determination of doxorubicin hydrochloride in commercial injections and human plasma. Polymers 2020, 12, 3066. [Google Scholar] [CrossRef]
- Alterary, S.S.; El-Tohamy, M.F. Advanced functionalized CeO2/Al2O3 Nanocomposite sensor for determination of opioid medication tramadol hydrochloride in pharmaceutical formulations. Nanomaterials 2022, 12, 1373. [Google Scholar] [CrossRef]
- Evangelista, S. Overview on gastrointestinal pharmacology. In Pharmacology-Volume I; Eolss Publications: Oxford, UK, 2009; p. 241. [Google Scholar]
- Medicines and Healthcare products Regulatory Agency. British Pharmacopoeia; Medicines and Healthcare products Regulatory Agency: London, UK, 2016; Volume 1–2, pp. 5068–5073.
- Khan, A.; Shabir, D.; Ahmad, P.; Khandaker, M.U.; Faruque, M.R.I.; Din, I.U. Biosynthesis and antibacterial activity of MgO-NPs produced from Camellia-sinensis leaves extract. Mater. Res. Exp. 2020, 8, 015402. [Google Scholar] [CrossRef]
- Ghotekar, S. Plant extract mediated biosynthesis of Al2O3 nanoparticles-a review on plant parts involved, characterization and applications. Nanochem. Res. 2019, 4, 163–169. [Google Scholar]
- Alarfaj, N.A.; El-Tohamy, M.F. New electrochemically-modified carbon paste inclusion β-cyclodextrin and carbon nanotubes sensors for quantification of dorzolamide hydrochloride. Int. J. Mol. Sci. 2016, 17, 2027. [Google Scholar] [CrossRef]
- Egorov, V.V.; Zdrachek, E.A.; Nazarov, V.A. Improved separate solution method for determination of low selectivity coefficients. Anal. Chem. 2014, 86, 3693–3696. [Google Scholar] [CrossRef]
- Pietrzak, K.; Krstulovic, N.; Blazeka, D.; Car, J.; Malinowski, S.; Wardak, C. Metal oxide nanoparticles as solid contact in ion-selective electrodes sensitive to potassium ions. Talanta 2022, 243, 123335. [Google Scholar] [CrossRef] [PubMed]
- FDA. ICH-Q2 (R1) Validation and Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonization Guidelines, Geneva, Switzerland, 17 November 2005. [Google Scholar]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.G. Curriculum Vitae for Prof. Dr. Gehad Genidy Mohamed Professor of Inorganic Chemistry. Ph.D. Thesis, Cairo University, Giza, Egypt, 1993. [Google Scholar]
- Baker, H.M.; Alsaoud, H.A.; Abdel-Halim, H.M. Spectrophotometric method for determination of ranitidine hydrochloride in bulk and in pharmaceutical preparation using ninhydrin. Eur. J. Chem. 2020, 11, 291–297. [Google Scholar] [CrossRef]
- Chen, J.; Shu, J.; Chen, J.; Cao, Z.; Xiao, A.; Yan, Z. Highly luminescent S, N co-doped carbon quantum dots-sensitized chemiluminescence on luminol–H2O2 system for the determination of ranitidine. J. Lumin. 2017, 32, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.H.; Mote, U.S.; Tele, S.S.; Anbhule, P.V.; Rath, M.C.; Patil, S.R.; Kolekar, G.B. A novel method for ranitidine hydrochloride determination in aqueous solution based on fluorescence quenching of functionalised CdS QDs through photoinduced charge transfer process: Spectroscopic approach. Analyst 2011, 136, 2606–2612. [Google Scholar] [CrossRef]
- Kantariya, B.; Agola, A.; Roshani, H.; Gheita, U.; Sai Shivam, S. Development and validation of a RP-HPLC method for the simultaneous estimation of ranitidine hydrochloride and dicyclomine hydrochloride in tablet dosage forms. Int. J. Pharm. Res. 2013, 2, 258–267. [Google Scholar]
- Pınar, P.T.; Yardım, Y.; Şentürk, Z. Electrochemical oxidation of ranitidine at poly (dopamine) modified carbon paste electrode: Its voltammetric determination in pharmaceutical and biological samples based on the enhancement effect of anionic surfactant. Sens. Actuators B Chem. 2018, 273, 1463–1473. [Google Scholar] [CrossRef]
- Merghani, S.M.; Elbashir, A.A. Development of chemically modified electrode using cucurbit (6) uril to detect ranitidine hydrochloride in pharmaceutical formulation by voltammetry. J. Anal. Pharm. Res. 2018, 7, 634–639. [Google Scholar] [CrossRef]
- Li, X.; Xu, G. Simultaneous determination of ranitidine and metronidazole in pharmaceutical formulations at poly (chromotrope 2B) modified activated glassy carbon electrodes. J. Food Drug Anal. 2014, 22, 345–349. [Google Scholar] [CrossRef]
- Rahman, M.M.; Li, X.B.; Jeon, Y.D.; Lee, H.J.; Lee, S.J.; Lee, J.J. Simultaneous determination of ranitidine and metronidazole at poly (thionine) modified anodized glassy carbon electrode. J. Electrochem. Sci. Technol. 2012, 3, 90–94. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Teixeira, M.F.; Fatibello-Filho, O.; Dockal, E.R.; Bonifacio, V.G.; Marcolino-Junior, L.H. Electrochemical sensor for ranitidine determination based on carbon paste electrode modified with oxovanadium (IV) salen complex. Mater. Sci. Eng. C 2013, 33, 4081–4085. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.G.; Khalilb, M.M.; Frag, E.Y. Potentiometric determination of ranitidine hydrochloride utilizing modified carbon paste electrodes. Int. J. Curr. Pharm. Res. 2013, 5, 72–79. [Google Scholar]





| Parameters | Conventional RNT-PT Sensor | Modified RNT-PT-MgONPs Sensor | Modified RNT-PT-Al2O3NPs Sensor |
|---|---|---|---|
| Slope (mV. Decade−1) | 52.2 ± 0.7 | 54.1 ± 0.5 | 58.6 ± 0.2 |
| Intercept | 756.76 | 762.33 | 696.48 |
| Correlation coefficient, r | 0.9992 | 0.9997 | 0.9995 |
| Linear range (mol L−1) | 1.0 × 10−6–1.0 × 10−2 | 1.0 × 10−9–1.0 × 10−2 | 1.0 × 10−10–1.0 × 10−2 |
| LOD | 5.0 × 10−7 | 4.9 × 10−10 | 5.0 × 10−11 |
| Response time/s | 50 | 30 | 25 |
| Working pH range | 3–9 | 3–9 | 3–9 |
| Lifetime/day | 20 | 40 | 50 |
| Temperature, °C | 25 | 25 | 25 |
| Accuracy (%) | 99.04 ± 0.73 | 99.49 ± 0.40 | 99.54 ± 0.53 |
| Interfering Species | Conventional RNT-PT Sensor | Modified RNT-PT-MgONPs Sensor | Modified RNT-PT-Al2O3NPs Sensor |
|---|---|---|---|
| Fe3+ | 3.6 × 10−3 | 1.4 × 10−4 | 4.9 × 10−4 |
| Ca2+ | 7.5 × 10−3 | 1.6 × 10−5 | 7.7 × 10−5 |
| Cr3+ | 4.1 × 10−3 | 5.2 × 10−5 | 5.4 × 10−4 |
| K+ | 5.5 × 10−3 | 5.8 × 10−4 | 4.6 × 10−4 |
| Na+ | 6.9 × 10−3 | 4.2 × 10−4 | 3.5 × 10−5 |
| Ag+ | 2.1 × 10−3 | 6.8 × 10−5 | 1.6 × 10−4 |
| Mg2+ | 6.2 × 10−3 | 8.4 × 10−5 | 8.1 × 10−4 |
| Lactose | 5.2 × 10−3 | 6.3 × 10−4 | 2.4 × 10−5 |
| Glycine | 8.5 × 10−3 | 4.5 × 10−5 | 1.5 × 10−4 |
| Histidine | 6.3 × 10−3 | 3.6 × 10−4 | 2.3 × 10−5 |
| Leucine | 7.9 × 10−3 | 9.8 × 10−4 | 3.9 × 10−4 |
| Niperotidine | 9.5 × 10−3 | 3.5 × 10−4 | 4.8 × 10−4 |
| Conventional RNT-PT Coated Wire Sensor | Modified RNT-PT MgONPs Sensor | Modified RNT-PT-Al2O3NPs Sensor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Taken −log (RNT) mol L−1 | Found mol L−1 | % Recovery | Taken −log (RNT) mol L−1 | Found mol L−1 | % Recovery | Taken −log (RNT) mol L−1 | Found mol L−1 | % Recovery | |
| Statistical Analysis | 6.00 | 5.96 | 99.33 | 9.00 | 9.00 | 100.00 | 9.00 | 8.98 | 99.78 |
| 5.30 | 5.23 | 98.68 | 9.50 | 9.45 | 99.47 | 8.50 | 8.50 | 100.00 | |
| 5.00 | 5.00 | 100.00 | 8.00 | 7.97 | 99.63 | 8.00 | 7.95 | 99.38 | |
| 4.30 | 4.24 | 98.60 | 7.50 | 7.45 | 99.33 | 7.00 | 7.00 | 100.00 | |
| 4.00 | 3.95 | 98.75 | 7.00 | 6.98 | 99.71 | 6.50 | 6.45 | 99.23 | |
| 3.30 | 3.28 | 99.39 | 5.50 | 5.50 | 100.00 | 6.00 | 5.99 | 99.83 | |
| 3.00 | 3.00 | 100.00 | 4.00 | 3.91 | 98.75 | 4.00 | 4.00 | 100.00 | |
| 2.30 | 2.28 | 99.13 | 2.50 | 2.49 | 99.60 | 3.50 | 3.44 | 98.29 | |
| 2.00 | 1.95 | 97.50 | 2.00 | 1.98 | 99.00 | 3.00 | 2.98 | 99.33 | |
| Mean ± SD | 99.04 ± 0.73 | 99.49 ± 0.40 | 99.54 ± 0.53 | ||||||
| n | 9 | 9 | 9 | ||||||
| Variance | 0.54 | 0.16 | 0.28 | ||||||
| * %SE | 0.24 | 0.13 | 0.18 | ||||||
| %RSD | 0.74 | 0.40 | 0.53 | ||||||
| Precision Test | Taken −log (RNT) mol L−1 | % Recovery a | % RSD b | % Error c | |
|---|---|---|---|---|---|
| RNT-PT-MgONPs | Intra-day precision | 10.00 | 99.50 ± 0.5 | 0.5 | 0.32 |
| 5.00 | 98.83 ± 1.2 | 1.2 | 0.70 | ||
| 2.00 | 99.83 ± 0.2 | 0.2 | 0.12 | ||
| Inter-day precision | 10.00 | 98.53 ± 1.2 | 1.2 | 0.72 | |
| 5.00 | 98.83 ± 0.8 | 0.8 | 0.50 | ||
| 2.00 | 97.67 ± 0.6 | 0.6 | 0.36 | ||
| RNT-PT-Al2O3NPs | Intra-day precision | 9.00 | 99.63 ± 0.3 | 0.3 | 0.17 |
| 6.00 | 99.23 ± 0.7 | 0.7 | 0.40 | ||
| 3.00 | 98.37 ± 0.8 | 0.8 | 0.45 | ||
| Inter-day precision | 9.00 | 99.11 ± 0.4 | 0.4 | 0.24 | |
| 6.00 | 98.33 ± 1.3 | 1.3 | 0.73 | ||
| 3.00 | 98.79 ± 0.6 | 0.6 | 0.33 | ||
| Conventional RNT-PT Coated Wire Sensor | Modified RNT-PT MgONPs Sensor | Modified RNT-PT-Al2O3NPs Sensor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Taken −log (RNT) mol L−1 | Found mol L−1 | % Recovery | Taken −log (RNT) mol L−1 | Found mol L−1 | % Recovery | Taken −log (RNT) mol L−1 | Found mol L−1 | % Recovery | |
| Statistical Analysis | 6.00 | 5.94 | 99.0 | 9.0 | 8.98 | 99.8 | 7.0 | 6.98 | 99.7 |
| 5.00 | 4.97 | 99.4 | 8.0 | 7.97 | 99.6 | 6.0 | 5.99 | 99.8 | |
| 4.30 | 4.26 | 99.1 | 7.0 | 6.96 | 99.3 | 5.0 | 4.95 | 99.0 | |
| 4.00 | 3.96 | 99.0 | 6.0 | 5.97 | 99.5 | 4.0 | 4.00 | 100.0 | |
| 3.00 | 2.97 | 99.0 | 3.0 | 2.99 | 98.7 | 3.0 | 2.96 | 98.7 | |
| 2.00 | 1.97 | 98.5 | 2.0 | 1.99 | 99.5 | 2.0 | 1.99 | 99.5 | |
| Mean ± SD | 99.00 ± 0.26 | 99.40 ± 0.37 | 99.45 ± 0.50 | ||||||
| n | 6 | 6 | 9 | ||||||
| Variance | 0.07 | 0.14 | 0.25 | ||||||
| %SE * | 0.11 | 0.15 | 0.20 | ||||||
| %RSD | 0.26 | 0.33 | 0.50 | ||||||
| t-test | 0.569 (2.228) ** | 1.08 (2.228) ** | 1.13 (2.228) ** | ||||||
| F-test | 2.85 (5.05) ** | 1.43 (5.05) ** | 1.25 (5.05) ** | ||||||
| Reported method [27] | 99.13 ± 0.45 | ||||||||
| 6 | |||||||||
| 0.25 | |||||||||
| 0.07 | |||||||||
| Analytical Method | Reagent | Linearity | LOD | Reference |
|---|---|---|---|---|
| Spectrophotometry | RNT, ninhydrin | 8.98 × 103–9.90 × 104 µg L−1 | 0.0997 µg mL−1 | [30] |
| Chemiluminescence | RNT, S, N co-doped carbon quantum dots | 0.5–50 μg mL−1 | 0.12 µg mL−1 | [31] |
| Fluorescence | RNT, CdS quantum dots | 0.50–15.0 μg mL−1 | 0.38 µg mL−1 | [32] |
| Chromatography | RNT, RP-HPLC method, ortho-phosphoric acid 0.1% and acetonitrile pH 3.5 (25:75, %v/v) | 5–25 μg mL−1 | 1.35 µg mL−1 | [33] |
| Electrochemical | RNT, poly(dopamine) modified carbon paste electrode | 1.0 × 10−7–7.5 × 10−6 mol L−1 | 1.9 × 10−8 mol L−1 | [34] |
| RNT, modified pencil graphite electrode (PGE) modified with p-amino benzene sulfonic acid/cucurbit(6) uril | 2 × 10−4–1.7 × 10−2 mol L−1 | 1.57 × 10−4 mol L−1 | [35] | |
| RNT, poly (chromotrope 2B) modified activated glassy carbon electrode (PCHAGCE) | 1.0 ×10−5–4.0 ×10−4 mol L−1 | 5.4 ×10−7 mol L−1 | [36] | |
| RNT, poly(thionine)-modified anodized glassy carbon electrode (PTH/GCE) | 35–500 µmol L−1 | 1.5 µ mol L−1 | [37] | |
| RNT, carbon paste electrode modified with the N,N-ethylene-bis(salicyllideneiminato)oxovanadium (IV) complex ((VO(salen))) | 9.9 × 10−5–1.0 × 10−3 mol L−1 | 6.6 ×10−5 mol L−1 | [38] | |
| Modified carbon paste electrode, tetraphenylborate | 1.0 ×10−6–1.0 ×10−2 mol L−1 | 1.0 ×10−6 mol L−1 | [39] | |
| Proposed method | Potentiometric measurement modified RNT-PT-MgONPs and RNT-PT-Al2O3NPs sensors | 1.0 × 10−9–1.0 × 10−2 mol L−1 | 4.9 × 10−10 mol L−1 | RNT-PT-MgONPs sensor |
| 1.0 × 10−10–1.0 × 10−2 mol L−1 | 5.0 × 10−11 mol L−1 | RNT-PT-Al2O3NPs sensor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, E.M.; Alarfaj, N.A.; Al-Tamimi, S.A.; El-Tohamy, M.F. Ultrasensitive Functionalized Polymeric-Nanometal Oxide Sensors for Potentiometric Determination of Ranitidine Hydrochloride. Polymers 2022, 14, 4150. https://doi.org/10.3390/polym14194150
Alshehri EM, Alarfaj NA, Al-Tamimi SA, El-Tohamy MF. Ultrasensitive Functionalized Polymeric-Nanometal Oxide Sensors for Potentiometric Determination of Ranitidine Hydrochloride. Polymers. 2022; 14(19):4150. https://doi.org/10.3390/polym14194150
Chicago/Turabian StyleAlshehri, Eman M., Nawal A. Alarfaj, Salma A. Al-Tamimi, and Maha F. El-Tohamy. 2022. "Ultrasensitive Functionalized Polymeric-Nanometal Oxide Sensors for Potentiometric Determination of Ranitidine Hydrochloride" Polymers 14, no. 19: 4150. https://doi.org/10.3390/polym14194150
APA StyleAlshehri, E. M., Alarfaj, N. A., Al-Tamimi, S. A., & El-Tohamy, M. F. (2022). Ultrasensitive Functionalized Polymeric-Nanometal Oxide Sensors for Potentiometric Determination of Ranitidine Hydrochloride. Polymers, 14(19), 4150. https://doi.org/10.3390/polym14194150








