Novel Transparent Films Composed of Bisphenol-A Polycarbonate and Copolyester
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Measurements
3. Results and Discussion
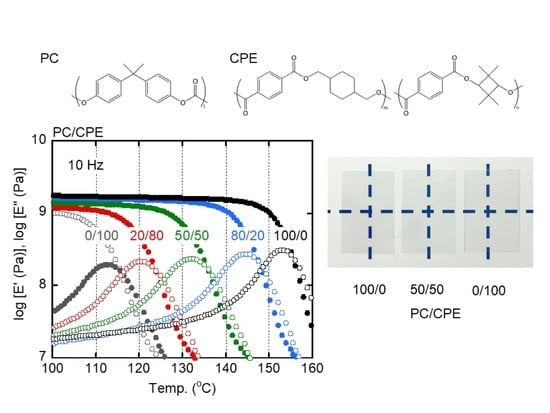
3.1. Transparency of the Films
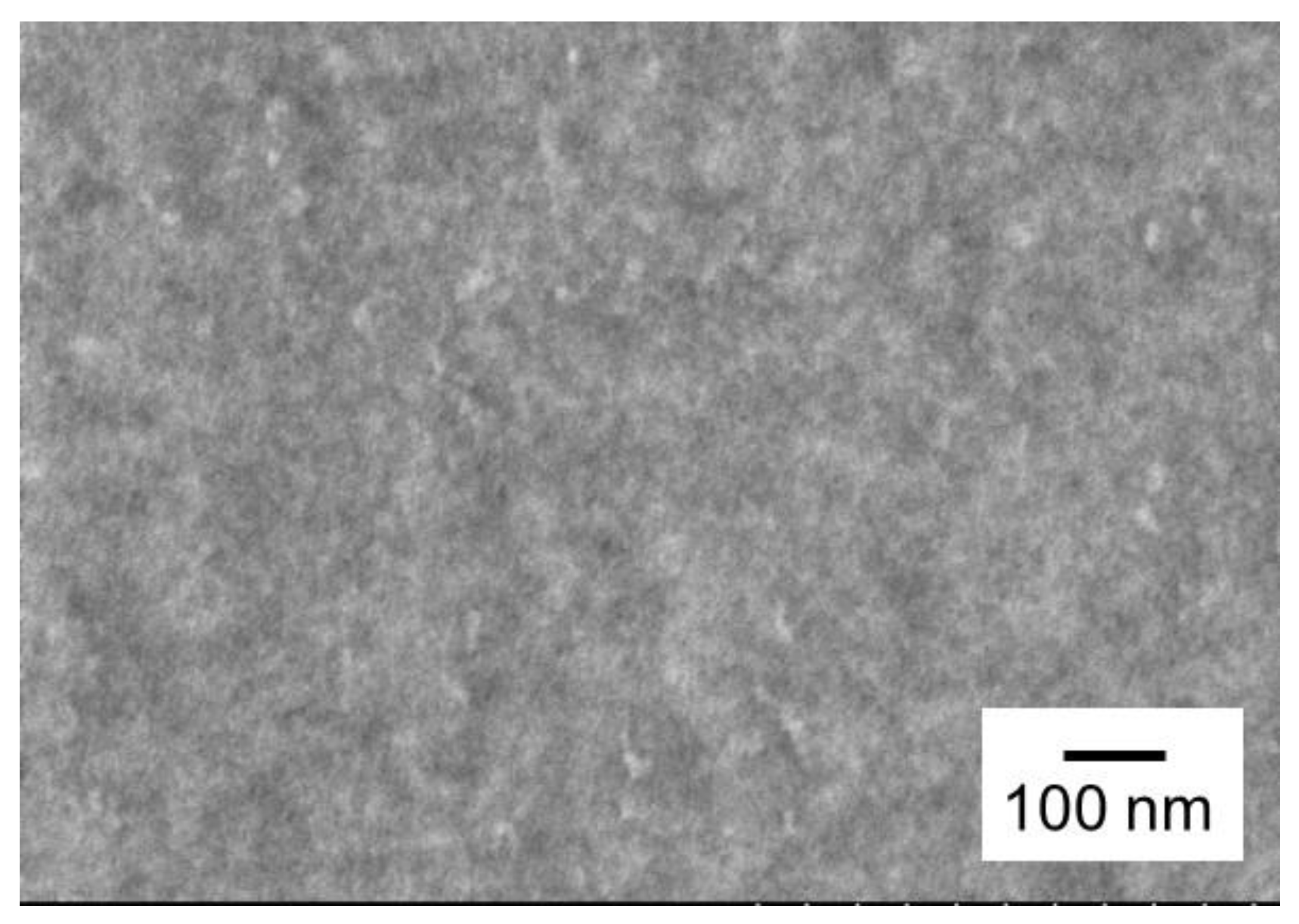
3.2. Morphology Observation
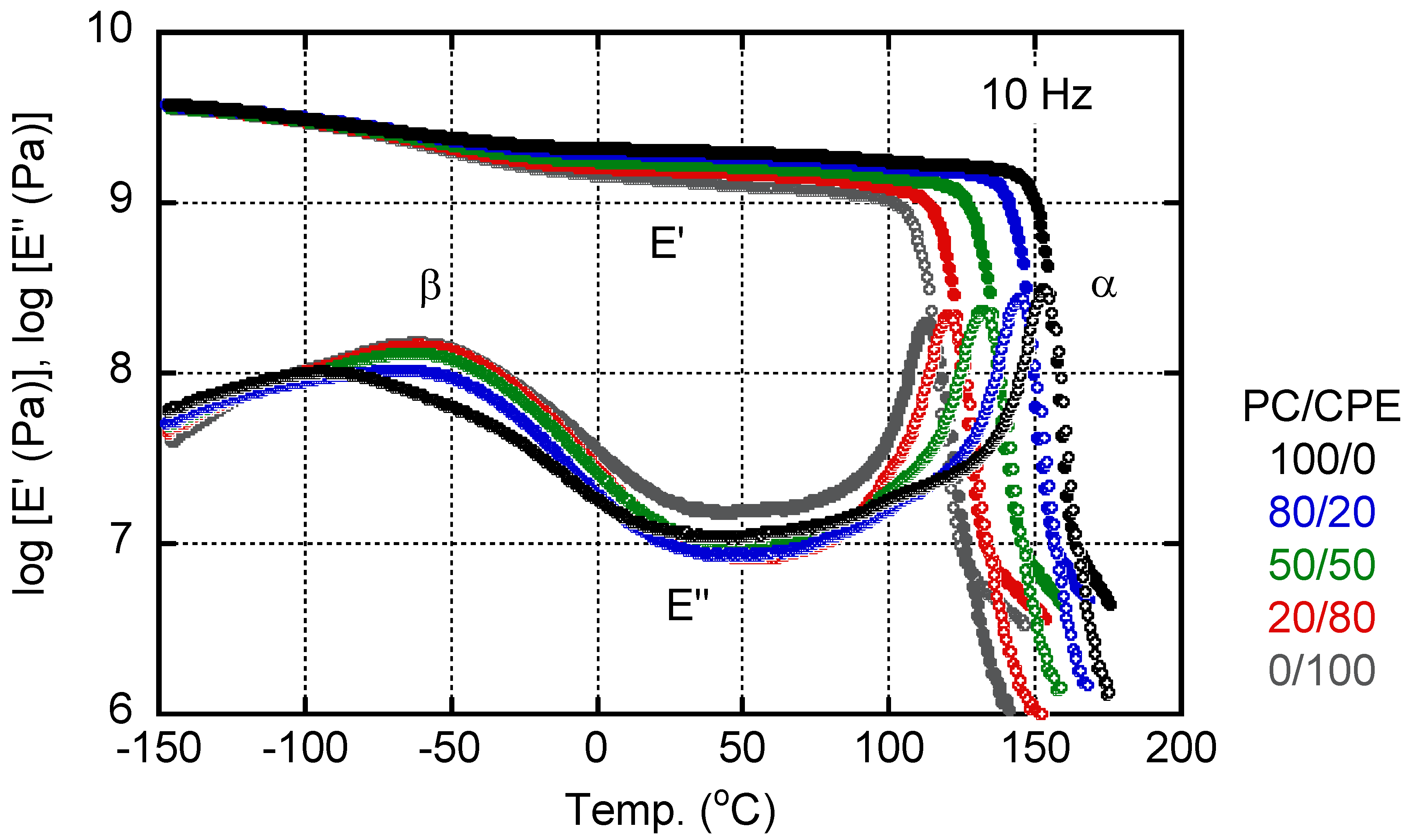
3.3. Viscoelasticity in the Solid State
3.4. Viscoelasticity in the Molten State
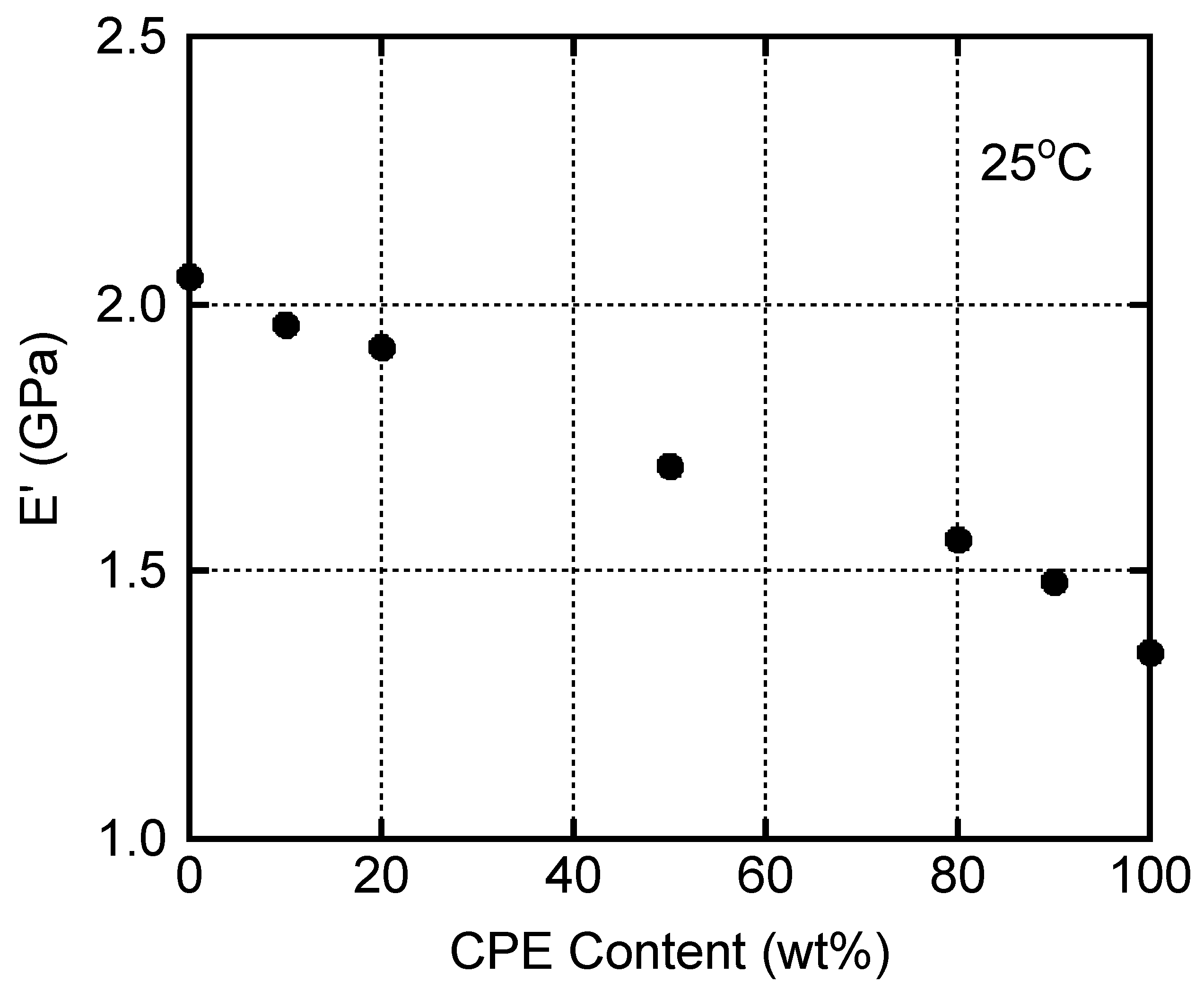
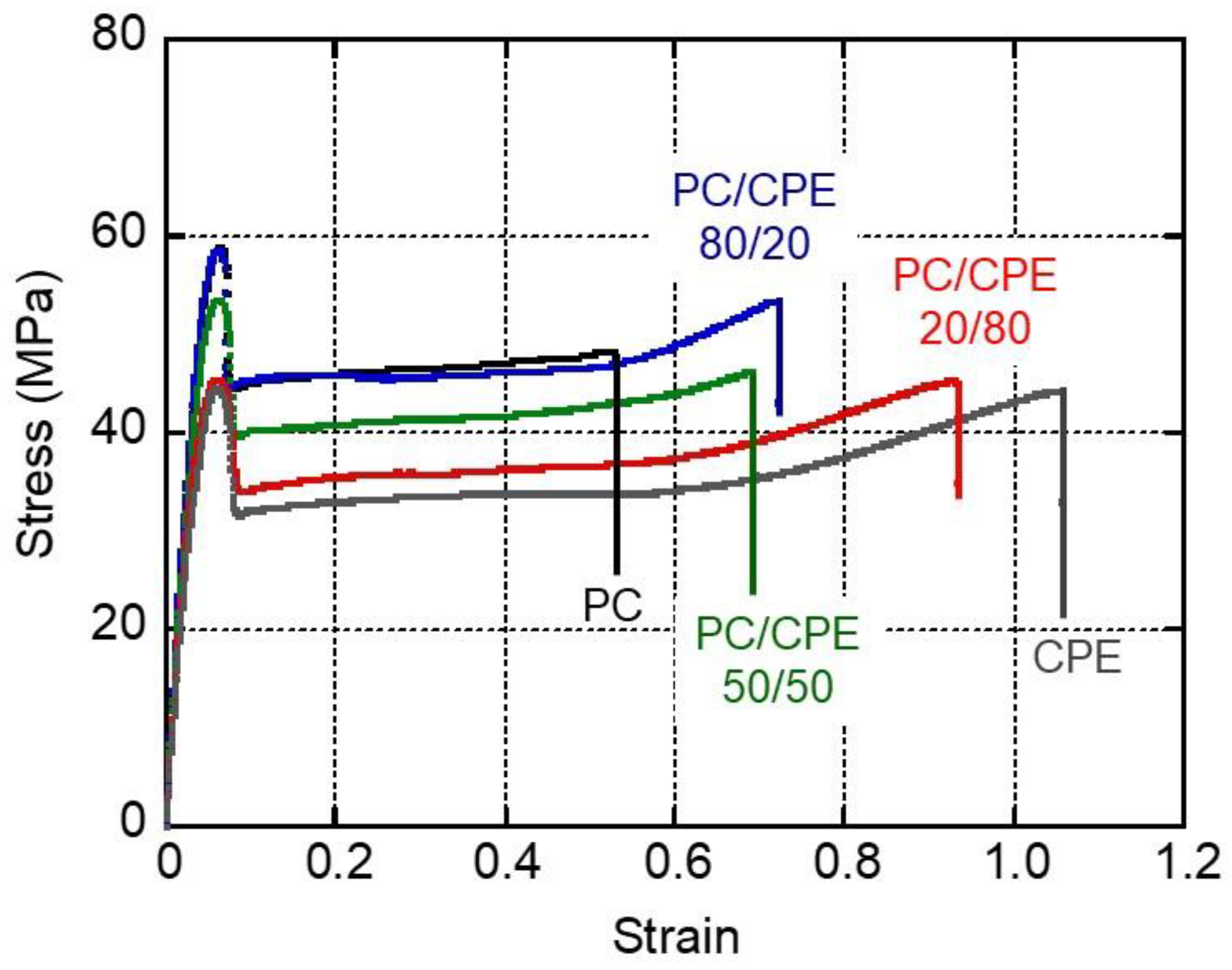
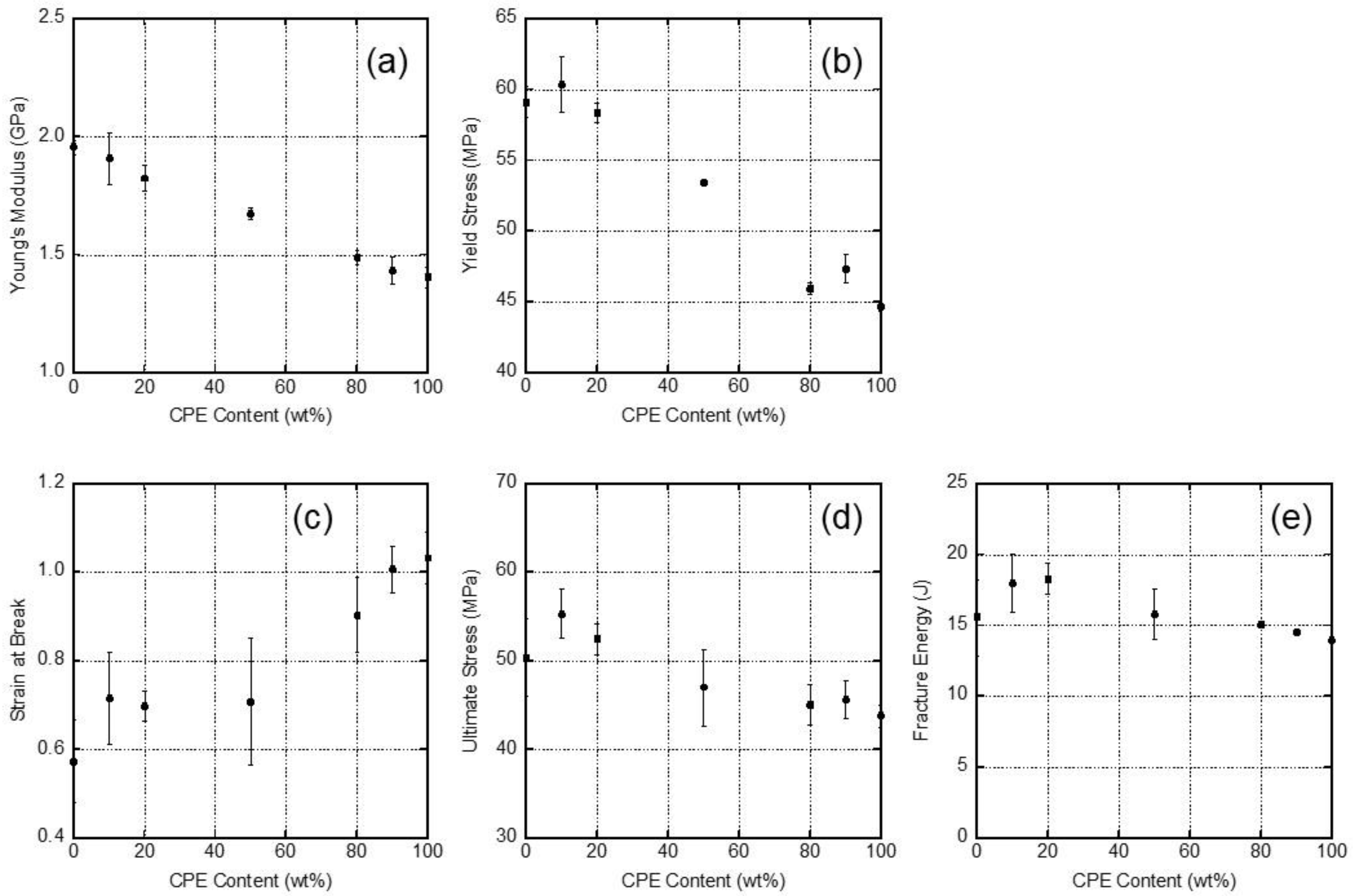
3.5. Mechanical Properties of the Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grigo, U.; Kircher, K.; Muller, P.R. Polycarbonate. In Engineering Thermoplastics; Bottenbruch, L., Ed.; Hanser: Munich, Germany, 1996; Chapter 3. [Google Scholar]
- Legrand, D.G.; Bendler, J.T. Handbook of Polycarbonate: Science and Technology; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Kyriacos, D. Polycarbonates. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 17. [Google Scholar]
- Utracki, L.A. Commercial Polymer Blends; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Greco, R.; Sorrentino, A. Polycarbonate/ABS blends: A literature review. Adv. Polym. Technol. 1994, 13, 249–258. [Google Scholar] [CrossRef]
- Hashima, K.; Nishitsuji, S.; Inoue, T. Structure-properties of super-tough PLA alloy with excellent heat resistance. Polymer 2010, 51, 3934–3939. [Google Scholar] [CrossRef]
- Hentari, F.; Barhoumi, N.; Khlifi, K. Characterization of the surface properties and adhesion behavior of electroplated PC/ABS using nano-indentation and scratch tests. J. Adhes. Sci. Technol. 2022; in press. [Google Scholar] [CrossRef]
- Kanzawa, T.; Tokumitsu, K. Mechanical properties and morphological changes of poly(lactic acid)/polycarbonate/poly(butylene adipate-co-terephthalate) blend through reactive processing. J. Appl. Polym. Sci. 2011, 121, 2908–2918. [Google Scholar] [CrossRef]
- Phuong, V.T.; Coltelli, M.-B.; Cinelli, P.; Cifelli, M.; Verstichel, S.; Lazzeri, A. Compatibilization and property enhancement of poly(lactic acid)/polycarbonate blends through triacetin-mediated interchange reactions in the melt. Polymer 2014, 55, 4498–4513. [Google Scholar] [CrossRef]
- Tejada-Oliveros, R.; Gomez-Caturla, J.; Sanchez-Nacher, L.; Montanes, N.; Quiles-Carrillo, L. Improved toughness of polylactide by binary blends with polycarbonate with glycidyl and maleic anhydride-based compatibilizers. Macromol. Mater. Eng. 2021, 306, 2100480. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nagai, M.; Hamakawa, K.; Nishitsuji, S.; Inoue, T. Design of a super-ductile polypropylene/polycarbonate blend with high heat resistance by using reactive plasticizer. J. Appl. Polym. Sci. 2013, 129, 443–448. [Google Scholar] [CrossRef]
- Jasinska-Walc, L.; Bouyahyi, M.; Kruszynski, J.; Tercjak, A.; Rozanski, A.; Troisi, E.M.; Liu, Y.; Yang, L.; Ivashkiv, O.; Sienkiewicz, M.; et al. Preparation of well-compatibilized PP/PC blends and foams thereof. ACS Appl. Polym. Mater. 2021, 3, 5509–5516. [Google Scholar] [CrossRef]
- Kim, W.N.; Burns, C.M. Blends of polycarbonate and poly(methyl methacrylate) and the determination of the polymer-polymer interaction parameter of the two polymers. Macromolecules 1987, 20, 1876–1882. [Google Scholar] [CrossRef]
- Butzbach, G.D.; Wendorff, J.H. Polycarbonate-poly(methyl methacrylate) blends: The role of molecular interactions on miscibility and antiplasticization. Polymer 1991, 32, 1155–1159. [Google Scholar] [CrossRef]
- Sako, T.; Nobukawa, S.; Yamaguchi, M. Surface localization of poly(methyl methacrylate) in a miscible blend with polycarbonate. Polym. J. 2015, 47, 576–579. [Google Scholar] [CrossRef]
- Moonprasith, N.; Date, J.; Sako, T.; Kida, T.; Hiraoka, T.; Yamaguchi, M. Segregation behavior of miscible PC/PMMA blends during injection molding. Materials 2022, 15, 2994. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Nakamura, K.; Kimura, T.; Moonprasith, N.; Kida, T.; Tsubouchi, K.; Narita, T.; Hiraoka, T. Complicated structure change during capillary extrusion of binary blends of polycarbonate and poly (methyl methacrylate). Materials 2022, 15, 2783. [Google Scholar] [CrossRef] [PubMed]
- Wiwattananukul, R.; Hachiya, Y.; Nobukawa, S.; Yamaguchi, M. Selective localization of carbon nanotubes in PC/PET blends. Polym. Comp. 2017, 38, 1103–1111. [Google Scholar] [CrossRef]
- Nassar, T.R.; Paul, D.R.; Barlow, J.W. Polyester-polycarbonate blends. II. Poly(ethylene terephthalate). J. Appl. Polym. Sci. 1979, 23, 85–99. [Google Scholar] [CrossRef]
- Xue, M.L.; Yu, Y.L.; Sheng, J.; Chuah, H.H.; Geng, C.H. Compatibilization of poly(trimethylene terephthalate)/polycarbonate blends by epoxy. Part 1. Miscibility and morphology. J. Macromol. Sci. Part B Phys. 2005, 44, 317–329. [Google Scholar] [CrossRef]
- Yuryev, Y.; Mohanty, A.K.; Misra, M. Novel super-toughened bio-based blend from polycarbonate and poly(lactic acid) for durable applications. RSC Adv. 2016, 6, 105094–105104. [Google Scholar] [CrossRef]
- Hoeks, T.; Goossens, J.; Vermeulen, H.; Shaikh, A.A.G. Improved chemical resistance of transparent bisphenol A polycarbonate materials. Polym. Eng. Sci. 2022, 62, 1377–1385. [Google Scholar] [CrossRef]
- Wilkinson, A.N.; Cole, D.; Tattum, S.B. The effects of transesterification on structure development in PC-PBT blends. Polym. Bull. 1995, 35, 751–757. [Google Scholar] [CrossRef]
- Marchese, P.; Celli, A.; Fiorini, M. Influence of the activity of transesterification catalysts on the phase behavior of PC-PET blends. Macromol. Chem. Phys. 2002, 203, 695–704. [Google Scholar] [CrossRef]
- Ganguly, A.; Channe, P.; Jha, R.; Mitra, S.; Saha, S. Review on transesterification in polycarbonate–poly(butylene terephthalate) blend. Polym. Eng. Sci. 2021, 61, 650–661. [Google Scholar] [CrossRef]
- Nassar, T.R.; Paul, D.R.; Barlow, J.W. Polyester-polycarbonate blends. III. Polyesters based on 1,4-cyclohexanedimethanol/terephthalic acid/isophthalic acid. J. Appl. Polym. Sci. 1979, 23, 575–587. [Google Scholar]
- Kim, L.U.; Jeon, M.Y.; Kim, C.K.; Kum, C.G. Polycarbonate/Copolyester Blends Having Optical-Grade Clarity and Their Upper Critical Solution Temperature Type Phase Behavior. Ind. Eng. Chem. Res. 2006, 45, 8921–8925. [Google Scholar] [CrossRef]
- Lim, H.C.A. Thermoplastic polyesters. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 20. [Google Scholar]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Sustainable nanocomposites based on halloysite nanotubes and pectin/polyethylene glycol blend. Polym. Degrad. Stab. 2013, 98, 2529–2536. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Tan, X.M.; Rodrigue, D. A review on porous polymeric membrane preparation. Part I: Production techniques with polysulfone and poly (vinylidene fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef]
- Tan, D.Q. Review of polymer-based nanodielectric exploration and film scale-up for advanced capacitors. Adv. Func. Mater. 2020, 30, 1808567. [Google Scholar] [CrossRef]
- Nonkrathok, W.; Trongsatitkul, T.; Suppakarn, N. Role of maleic anhydride-grafted poly(lactic acid) in improving shape memory properties of thermoresponsive poly(ethylene glycol) and poly(lactic acid) blends. Polymers 2022, 14, 3923. [Google Scholar] [CrossRef]
- Seferis, J.C. Refractive indices of polymers. In Polymer Handbook, VI/571; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar]
- Takahashi, S.; Okada, H.; Nobukawa, S.; Yamaguchi, M. Optical properties of polymer blends composed of poly(methyl methacrylate) and ethylene-vinyl acetate copolymer. Eur. Polym. J. 2012, 48, 974–980. [Google Scholar] [CrossRef]
- Kuhn, W.; Grün, F. Beziehungen zwishen elastischen konstanten und dehnungsdoppelbrechung hochelastischer stoffe. Kolloid-Zeitschrift 1942, 101, 248–271. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Okada, K.; Mohd Edeerozey, A.M.; Shiroyama, Y.; Iwasaki, T.; Okamoto, K. Extraordinary wavelength dispersion of orientation birefringence for cellulose esters. Macromolecules 2009, 42, 9034–9040. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. Principles of Optics, 7th ed.; Cambridge University Press: Cambridge, UK, 1999; p. 22. [Google Scholar]
- Wimberger-Friedl, R.; Hut, M.G.T.; Schoo, H.F.M. Chain stiffness of copolycarbonates containing a spiro linkage. Macromolecules 1996, 29, 5453–5458. [Google Scholar] [CrossRef]
- Chen, L.P.; Yee, A.F.; Goets, J.M.; Scharfer, J. Macomolecules, molecular structure effects on the secondary relaxation and impact strength of a series of polyester copolymer glasses. Macromolecules 1998, 31, 5371–5382. [Google Scholar] [CrossRef]
- Alegria, A.; Mitxelena, O.; Colmenero, J. On the molecular motions originating from the dielectric γ-relaxation of bisphenol-A polycarbonate. Macromolecules 2006, 39, 2691–2699. [Google Scholar] [CrossRef]
- Weldeghiorghis, T.; Singh, M.; Schaefer, J. Molecular basis of secondary relaxation in stiff-chain glassy polymers. J. Chem. Phys. 2022, 157, 044901. [Google Scholar] [CrossRef]
- Miyagawa, A.; Ayerdurai, V.; Nobukawa, S.; Yamaguchi, M. Viscoelastic properties of poly(methyl methacrylate) with high glass transition temperature by lithium salt addition. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 2388–2394. [Google Scholar] [CrossRef]
- Wu, S. Predicting chain conformation and entanglement of polymers from chemical structure. Polym. Eng. Sci. 1992, 32, 823–830. [Google Scholar] [CrossRef]
- Fetters, L.J.; Lohse, D.J.; Richter, D.; Witten, T.A.; Zirkel, A. Connection between polymer molecular weight, density, chain dimensions, and melt viscoelastic properties. Macromolecules 1994, 27, 4639–4647. [Google Scholar] [CrossRef]
- Asai, S.; Okabe, N.; Sumita, M.; Miyasaka, K. Molecular examination of fracture toughness of amorphous polyesters as a function of copolymerization component. Polymer 1991, 32, 2400–2405. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1956, 1, 123–127. [Google Scholar]
- Graebling, D.; Muller, R. Rheological behavior of polydimethylsiloxane/polyoxyethylene blends in the melt. Emulsion model of two viscoelastic liquids. J. Rheol. 1990, 34, 193–206. [Google Scholar] [CrossRef]
- Palierne, J.F. Linear rheology of viscoelastic emulsions with interfacial tension. Rheol. Acta 1990, 29, 204–214. [Google Scholar] [CrossRef]
- Mills, N.J. Elasticity of polydimethylsiloxane melts. Nature 1968, 219, 1249–1250. [Google Scholar] [CrossRef]
- Agarwal, P.K. A relationship between steady state shear compliance and molecular weight distribution. Macromolecules 1979, 12, 342–344. [Google Scholar] [CrossRef]
- Auhl, D.; Ramirez, J.; Likhtman, A.E. Linear and nonlinear shear flow behavior of monodisperse polyisoprene melts with a large range of molecular weights. J. Rheol. 2008, 52, 801–835. [Google Scholar] [CrossRef]
- Wu, S. Chain structure, phase morphology, and toughness relationships in polymers and blends. Polym. Eng. Sci. 1990, 30, 753–761. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sako, T.; Hiraoka, T.; Yamaguchi, M.; Yamaguchi, M. Effect of morphology on shear viscosity for binary blends of polycarbonate and polystyrene. J. Appl. Polym. Sci. 2020, 137, 49516. [Google Scholar] [CrossRef]
- Sako, T.; Date, J.; Hagi, M.; Hiraoka, T.; Matsuoka, S.; Yamaguchi, M. Anomalous viscosity decrease of polycarbonate by addition of polystyrene. Polymer 2019, 170, 135–141. [Google Scholar] [CrossRef]
- Yoon, H.; Okamoto, K.; Umishita, K.; Yamaguchi, M. Development of conductive network of multi-walled carbon nanotubes in polycarbonate melt. Polym. Comp. 2011, 32, 97–102. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, H.; Kida, T.; Yamaguchi, M. Novel Transparent Films Composed of Bisphenol-A Polycarbonate and Copolyester. Polymers 2022, 14, 4146. https://doi.org/10.3390/polym14194146
Hasegawa H, Kida T, Yamaguchi M. Novel Transparent Films Composed of Bisphenol-A Polycarbonate and Copolyester. Polymers. 2022; 14(19):4146. https://doi.org/10.3390/polym14194146
Chicago/Turabian StyleHasegawa, Hiroyuki, Takumitsu Kida, and Masayuki Yamaguchi. 2022. "Novel Transparent Films Composed of Bisphenol-A Polycarbonate and Copolyester" Polymers 14, no. 19: 4146. https://doi.org/10.3390/polym14194146
APA StyleHasegawa, H., Kida, T., & Yamaguchi, M. (2022). Novel Transparent Films Composed of Bisphenol-A Polycarbonate and Copolyester. Polymers, 14(19), 4146. https://doi.org/10.3390/polym14194146