Abstract
This review surveys and summarizes the materials and methods used to make liquid filtration membranes. Examples of each method including phase inversion, electrospinning, interfacial polymerization, thin film composites, stretching, lithography and templating techniques, are given and the pros and cons of each method are discussed. Trends of recent literature are also discussed and their potential direction is deliberated. Furthermore, the polymeric materials used in the fabrication process of liquid filtration membranes are also reviewed and trends and similarities are shown and discussed. Thin film composites and selective filtration applications appear to be a growing area of research for membrane technology. Other than the required mechanical properties (tensile strength, toughness and chemical and thermal stability), it becomes apparent that polymer solubility and hydropathy are key factors in determining their applicability for use as a membrane material.
1. Introduction
Filtration is a processing aid that is utilized across a wide range of industries, with its value growing rapidly and expected to reach USD 2.9 billion by 2024 [1]. It is a key technology within the wine, dairy and water purification industries, with many more esoteric applications, such as membranes developed for research purposes, selective removal or capture of targeted species [2,3,4,5,6,7,8]. This can be attributed to the many benefits filtration techniques have when compared to other purification techniques, which include the relatively low energy cost, the simplicity of setup, its non-destructive nature (keeping important components in the liquid intact), little to no waste and it does not require the addition of any external additives. Liquid filtration is a very well-established technology, with early water filtration systems dating back to around 500 BC with the Hippocratic Sleeve. Water filtration first became industrialized in the 1800s, beginning with the use of sand filters to control the spread of cholera [9]. However, it wasn’t until 1937, with the invention of nylon, that saw the first synthetic polymers being used a membrane material [10] and even then, bio-based polymers such as cellulose acetate were still the dominant material [10]. Research in membrane technology is currently focused on mechanically stable, more efficient filters with better rejection, allowing membranes to last longer with lower energy costs. Today, water purification and beverage clarification are among the most common users of liquid filtration, each with their own unique filtration requirements.
The objective of a liquid filtration membrane is to separate particulates from a liquid source, producing a purified output stream and leaving behind a concentrated source. Liquid filtration is categorized based on the size of particles rejected or passed (Figure 1). Membrane performance is evaluated based on transmembrane flux rates, retention/rejection efficiency and long-term stability. One of the main limitations of filters is their rapid fouling [11,12,13,14,15,16], thus a large focus of recent research is aimed at fabricating filters with anti-fouling properties, improving their long-term stability. A related focus is on fabricating filter membranes with specific rejection/retention, which will allow the removal or concentration of undesirables/desirables.
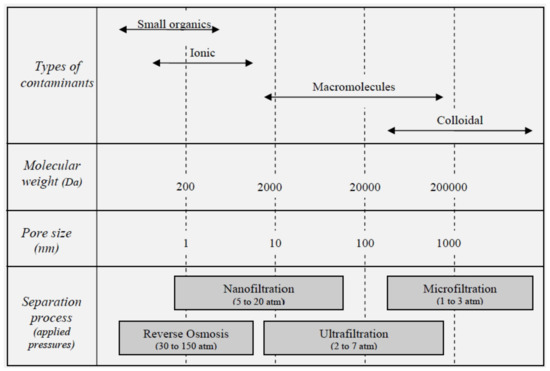
Figure 1.
Filtration levels with equivalent particle rejection ranges and molecular weight cut-offs.
In this review we summarize and discuss polymeric materials and the fabrication methods used to produce polymer membranes for liquid filtration, as well as methods used to modify these filters to enhance their performance. While reverse osmosis (RO) membranes will be mentioned, they will not be discussed in detail as they work on a fundamentally different mechanism to other filtration membranes. However, a detailed discussion on RO membranes can be found in the works by Rana et al. [17] and another detailing specifically thin film composite RO membranes by Ng et al. [18]. Additionally, absorbent materials such as metal organic frameworks (MOF)s [19], ceramics [20] and activated carbon [21], will not be discussed as this review will focus on filters fabricated from polymeric materials.
2. Microfabrication Techniques
Microfabrication is the process by which a device is made with at least one dimension a micrometer or smaller. By this definition almost all modern, and some traditional, membrane fabrication techniques are microfabrication. A successful fabrication process will create a membrane with the highest porosity (for the targeted filtration level), of sufficient mechanical strength and is scalable to fabricate membranes of adequate size. Herein we will discuss the microfabrication methods used by modern processes and researchers, their advantages, disadvantages and their future directions. These will include: phase inversion, electrospinning, interfacial polymerization, stretching, templating, lithography and self-assembly. It is worthy to note that the majority of commercially available filter membranes are prepared by the phase inversion technique (see Synder XT membranes as an example), with some older products still being fabricated using the stretching method (some Whatman and Millipore products as examples).
2.1. Phase Inversion
Phase inversion has been utilized to fabricate porous substrates for several decades [22] and is still today one of the more common methods for the fabrication of filters. There are four different types of phase inversion techniques: precipitation from the vapor phase, precipitation by controlled evaporation, thermally induced phase separation and immersion precipitation. A detailed description of these techniques can be found in a review by Holda et al. [22] but are briefly outlined here. Precipitation from the vapor phase involves exposing a polymer solution to a non-solvent gas saturated with the solvent the polymer is dissolved in. The good solvent (which the polymer is dissolved in) in the gas prevents this solvent from evaporating and instead the non-solvent diffuses into the polymer solution, resulting in precipitation and formation of porous features. Precipitation by controlled evaporation is performed using the polymer dissolved in a solvent and non-solvent mixture, where the solvent is considerably more volatile, this results in the solvent evaporating at a greater rate, thus allowing the polymer to precipitate out, forming a porous film. Thermally induced phase separation involves reducing the temperature of a polymer solution, triggering phase separation, a membrane is formed upon solvent evaporation. Lastly, immersion precipitation involves loading a polymer solution onto an appropriate support that is then pulled through a coagulation bath which contains a non-solvent, causing a porous film to form on contact.
The main advantages of this technique are outlined below. It is a scalable process, as there are no scale limiting steps in this process and thus it can be tailored to produce membranes of any size. This method can naturally form something similar to a thin film composites, with a thinner smaller dimensioned pore layer and wider, more porous support layer (Figure 2). This is called the Loeb-Sourirajan structure and has been shown to have several advantages [17,23,24]. Phase inversion also has a wide selection of polymer materials available to it which is both the cause and a result of it prolific use.
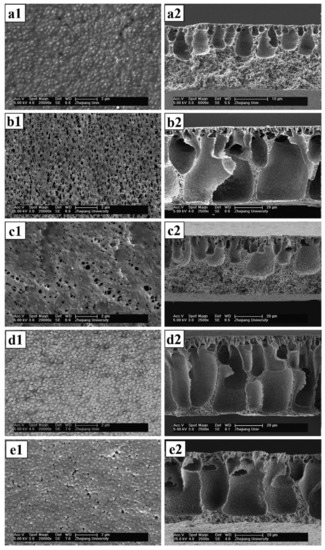
Figure 2.
SEM images of top surface (left) and cross-section (right) of the membranes. (a1,a2) Pure PVDF, (b1,b2) PVDF/EPTBP, (c1,c2) PVDF/ACPS, (d1,d2) PVDF/HPE-g-MPEG and (e1,e2) PVDF/PEG. Provided with permission from Elsevier [25].
The main disadvantages of this technique include the relatively complex apparatus required to control the vapor phase environment and temperature. Furthermore, the required solvents are often hazardous (such as dimethylformamide or chloroform). Lastly, with the expectation of immersion precipitation, it is difficult to produce a continuous manufacturing process.
2.2. Electrospinning
Of the filter fabrication techniques being researched in recent years, electrospinning is one of the more common techniques employed. This is likely due to its simplicity and capability of large-scale production [26]. Electrospinning is not a new invention [27], but has gained momentum due to the recent drive in nanotechnology. As a result, the technique has seen a growing number of industrial applications as well as a growing number of polymer materials available to it.
The technique involves preparing a viscoelastic polymer solution which is delivered through a spinneret tip. A large electric potential difference is applied across the spinneret and a collector (which is usually earthed). As the electric field overcomes the solution surface tension, a Taylor cone [27] forms at the needle tip and a solution jet will escape the droplet and fly towards the collector. During flight, a whipping instability causes the jet to whip erratically, stretching the jet, causing it to become thinner, and allowing solvent evaporation to occur. In this manner, mats of randomly-oriented fibers with widths in the nano-to mico- range are deposited on the collector (Figure 3).
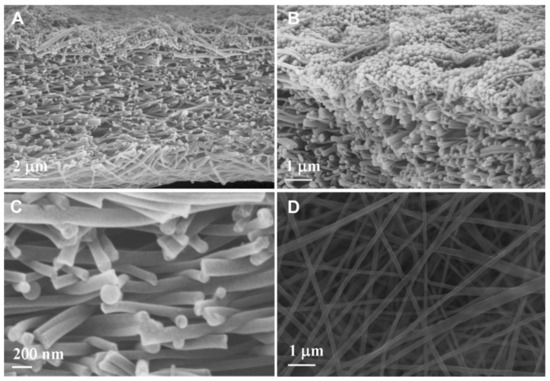
Figure 3.
SEM images of the M2 membrane after particle rejection test in different views: (A) cross-section; (B) top layers of cross-section; (C) middle part of cross-section; (D) bottom surface. Provided with permission from Elsevier [28].
There are several advantages of this technique compared to other microfabrication methods for filtration membranes, these include the following. The large surface to volume ratio of an electrospun membrane and its high porosity [26,29,30] can lead to enhanced flux rates and improved efficiency compared to conventional fabrication techniques [31]. Its simple apparatus, ease of setup and production, making it relatively straightforward for researchers to build or purchase a lab scale system and allowing for industrial scalability. There exists a wide range of polymer materials capable of electrospinning and this is still growing. Additionally, other materials can be included in the electrospinning solution, with a polymer in solution as a carrier [32,33]. This can be important for fabricating anti-fouling or selective filter membranes. Another advantage is its continuous nature. Electrospinning produces a continuous fiber and can be adapted to a continuous sheet production, making it perfect for a continuous industrial fabrication process. There are many easily adjustable parameters that govern the production of an electrospun fiber membrane. This makes electrospinning a very tunable process; porosity [26,29,30], fiber diameter [27,28,29,34], fiber shape [34,35,36] and fiber orientation [37,38,39] can all be controlled during electrospinning by adjusting the some of these parameters.
However, like all methods, there are disadvantages. Like phase inversion, electrospining often requires hazardous solvents. While there are some relatively non-hazardous solvents available (such as water, formic acid or methyl ethyl ketone [40,41]), the majority of solvents used are often flammable or toxic (such as dimethylformamide, chloroform or tetrahydrofuran [15,42,43]). Another disadvantage is that there is usually some material loss which occurs with most electrospinning setups. Due to the uncontrolled nature of the whipping instability, some material will fall outside the region of the collector. This can be taken into account when setting up the apparatus however, reducing this drawback. Additionally, due to the inherent random fiber deposition, a wide range of pore sizes are produced. This is only a drawback for situations where a particular size rejection is desired and extensive research has been conducted in this area to diminish this drawback [38,44,45,46]. Another drawback to electrospinning is that it can be sensitive to ambient relative humidity. The electrospinning process utilizes an electric field between to a highly charged needle and a grounded plate. Sparking occurring in an often-flammable environment is an obvious fire hazard. Therefore, the air resistance between the two is a factor that has to be considered. Often, if the humidity is too high, an electric field strong enough, without sparking between the collector and needle, cannot be achieved to commence electrospinning. This can be avoided with adequate humidity control but not all electrospinning apparatus has such control available.
2.3. Interfacial Polymerization and Thin Film Composites
Interfacial polymerization is a secondary membrane fabrication technique. Here, a pre-existing porous membrane is swollen with a solvent and polymer precursor, the swollen membrane is then exposed to a solution that is non-miscible with the first solvent and contains the remaining polymer precursor, such that a polymer forms at the solution-membrane interface [47]. This technique then allows for the creation of ultrafiltration or reverse osmosis membranes form micro- or nano- filtration membranes [18,48,49]. An added benefit of this method is that it can alter the hydrophilicity of the membrane surface, allowing for tailored compatibility with the desired filtrant solution [18,47,48,50]. As this is a secondary fabrication technique, its main drawback are the additional steps required over and above the steps to fabricate the supporting membrane.
This method aligns well with a recent trend within filtration membrane research, the development of thin film composites (TFCs) [18,47,49]. Typically, a TFC is made from a highly porous membrane with an extra barrier layer added, that provides nano, ultra or reverse osmosis (RO) filtration [18,47,48,49,50]. This makes interfacial polymerization receive additional attention as it is a primary method for forming TFCs. The benefit of a TFC is the added support from the underlying porous membrane, providing a mechanical strength that the thin barrier layer cannot. Furthermore, higher fluxes are possible, due to the porosity of the support layer and thinness of the flux-limiting barrier layer [18,47,50]. Such a membrane is fabricated with a technique such as (but not limited to) interfacial polymerization.
Notice how the TFCs structure in Figure 4 has similarities to the intrinsic structures formed in Figure 2 second column (e.g., d2), with a thick, highly porous layer and a thin, much denser, smaller pored layer, the Loeb-Sourirajan structure. This explains why phase inversion is such a popular choice for membrane fabrication as it can form this favorable structure without additional steps. However, the two layered system (as in Figure 4) is often superior to the coincidental one, as more control is possible over the membrane fabrication process as it is possible to specify the properties of both layers in a TFC [47].

Figure 4.
Cross-section SEM images thin film composite membranes of cellulose (A), chitin (B), and cellulose-chitin blend (C) barrier layers prepared by ionic liquid regeneration. Provided with permission from Elsevier [47].
2.4. Stretching
Like electrospinning and phase inversion, the stretching method is also a well-established technique. This process involves stretching a polymer film at low temperature to induce nucleation points followed by stretching at elevated temperatures to then cause a microporous structure to evolve (Figure 5) [51,52]. This technique requires highly crystalline polymers, with polypropylene being the most common and has an advantage of being solvent free [51,52]. However, relatively little research has been conducted on this technique over the last decade. This is likely due the small number of available polymer materials suitable and then their comparatively inferior properties (solvent resistance, temperature resistance, hydropathy, ability to functionalize etc.) compared to other polymers used in membranes. However, this method is still used industrially (see 3M™ Membrana™ Oxygenation Membrane Series).
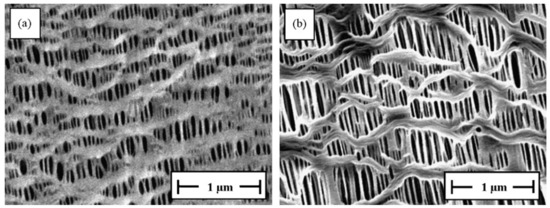
Figure 5.
SEM micrographs of the surface of microporous membranes (20 m thick): (a) PP and (b) HDPE; DR = 90, H-AFR, cold stretching of 55%, followed by hot stretching of75%. Provided with permission by Elsevier. [53].
2.5. Templating, Ablation, Photolithography, Etching and Self-Assembly
These techniques are all labor-intensive methods that are difficult to utilize on an industrial scale. Furthermore, when fabricating membranes using these techniques, often a combination of two or more of these techniques are used to form the resulting membrane. These techniques can be described as follows.
Photo-lithography: Photo-lithography is where a laser or light source is used with a mask to create a micro/nano patterned material. Etching and stripping is followed, resulting in a material with a precisely controlled pattern [54].
Ablation: Ablation is the process whereby part of a material is obliterated, by some means, in a controlled manner [55].
Templating: Templating involves using a, typically, nanoscale “mold” to fabricate porous polymer membranes [56,57]. This technique is often coupled with photolithography in the creation of the mold [56].
Self-assembly: Self-assembly takes advantage of a polymer system’s intrinsic nature to self-assemble into patterned phases. This is usually done with block polymers whose blocks comprise polymers that do not blend [58]. If the casting conditions are controlled, such polymers phase separate into distinct patterns. When using self-assembly to form filter membranes, usually one phase is water permeably or one phase can later be removed by other means, forming filtration membranes with very defined nanoscaled patterns [59]. A drawback to such a method is that it can only be used to make very thin membranes and is hard to upscale, however, like interfacial polymerization, this technique can be combined with others to form composite membranes.
Techniques such as templating, photolithography, etching and self-assembly techniques allow for much more control over intricate details and morphology [60,61,62] of the resulting membrane compared to the aforementioned fabrication techniques and are sometimes used sequentially [62]. These techniques are usually labor intensive, difficult to produce on the large scale and cannot be performed in a continuous process like electrospinning or immersion precipitation. As such, they have not found their way into the industrial membrane fabrication market, however, due to the morphological control they provide, these methods are ideal for more fundamental membrane research, linking morphology to performance [54]. The main advantage of these techniques is the precise control of the nano- and micro- morphology of the resulting membrane, making it an ideal method for selective filtration as the exact pore structure to reject specific particles of specific shape and size can be achieved [55,56,60,61,62].
A good example of these techniques (laser ablation) being used to study the effects of precise membrane morphology is provided by Alderson et al. [55]. In their study, they used laser ablation to generate an auxetic (a material with a negative Poisson ratio) polymer film. The pores in this material opened when stretched (Figure 6), allowing for an easy, mechanical antifouling process.
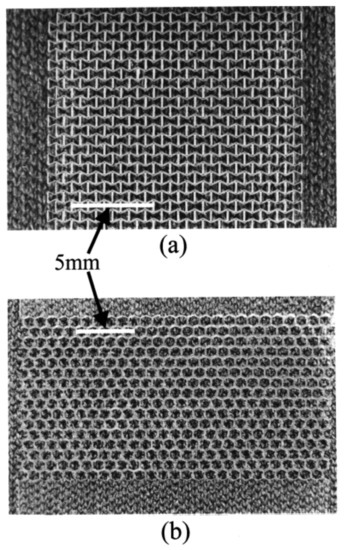
Figure 6.
(a) Polymeric re-entrant honeycomb membrane; (b) polymeric conventional honeycomb membrane. Pores are approximately 1 mm in width (along the x direction). The membranes were fabricated by direct femtosecond laser ablation in air, with pulses at 790 nm (170 fs). Provided with permission from American Chemical Society [55].
One of the benefits of utilizing a mold is that they do not always require a polymer solution. By removing the need of a solvent, the process becomes far less hazardous. As an example Fan et al. [56] created an alumina mold through lithography that then allowed polyethylene films to be hot pressed and imprinted by the mold (Figure 7), producing very defined, porous structure, with wine-bottle shaped pores. Their study revealed that this morphology had superior filtering properties compared to commercial filters with randomized pore structures.

Figure 7.
SEM images of (a,b) the obtained membrane with a well-distributed ordered cylindrical straight through-pore structure (pore size: 2 μm, distance between adjacent pores: 2 μm) in a large area from the imprint process, and (c) the bending of the membrane edge for clearly observing the through-pores. Provided with permission from IOPScience [56].
3. Polymer Materials
To make an effective liquid filter, the material used must possess properties favorable for membrane fabrication such as ease of processing, amenability to clean-in-place procedures (CIP), mechanical robustness, appropriate hydropathy depending on the application, temperature-resistance, chemical inertness, cost-competitivity, and may need to be food-safe [24,51]. Materials which inherently have (or can be modified to provide) added functionality such as anti-fouling, or targeted filtration present increased attractiveness for use [24,51]. These are challenging criteria for any one material to fulfil, and most materials (including the polymer membranes commonly used by industry) are only able to fulfil some of these criteria.
A defining feature of polymeric materials is their ease of processing. Thus, there are many methods for creating highly porous membranes with appropriate pore sizes for the different levels of filtration. Furthermore, many polymers are easily chemically modified to allow for the creating of anti-fouling membranes and other more advanced filtration applications [25,63,64]. However, a critical factor in choosing a polymer material is the fabrication technique chosen to generate the membrane porosity, this is largely due to most techniques depending on the polymer solubility [22,51,65,66]. The dependence of the fabrication technique and polymer choice on solubility presents a unique challenge, as a desired characteristic for filter membranes is solvent resistance, however the fabrication techniques favors polymers that are readily soluble. This requirement of both requiring solubility for fabrication and possessing solvent resistance greatly narrows the range of applicable polymers.
Hydropathy is worth special mention. It has been shown that for aqueous systems, increasing membrane hydrophilicity will improve its anti-fouling properties [34,67,68]. With the theory that many fouling contaminants are hydrophobic and thus are repelled by a hydrophilic surface [34,67,69]. A polymer’s hydropathy or at least its ability to modify the surface functional groups then becomes important for membrane performance and thus for the decision of material choice.
A table expressing the physical properties of some of the common polymers used to make filters is shown in Table 1.

Table 1.
Physical properties of common polymers used in liquid filtration membranes.
3.1. Polyethersulfone and Polysulfone
Of the common polymers used for aqueous filtration applications, polyethersulfone (PES) and polysulfone (PSf) are two of the more common, with many commercially available membranes [86]. This is largely due to their high strength and creep, temperature and chemical resistance [48,87,88]. However, one of the drawbacks with PES and PSf is their hydrophobicity; this likely is why recent research on PES/PSf filters focus on polymer blends or modifications to impart a more hydrophilic nature, [34,48,87] with some focusing on forming TFCs for ultrafiltration membranes, where the PES/PSf is the support layer [48]. These polymers are both suitable for the phase inversion [64,89] and electrospinning techniques [34], while we were unable to identify examples of the other techniques mentioned in this review.
An example of some of the methods that researchers employ to improve the hydropathy is provided by Yoon et al. [34]. Here, the authors demonstrated the use of simple methods to acquire what most studies achieve through more complicated composites [69,90,91,92], that is producing a PES membrane with enhanced mechanical strength and improved hydrophilicity. In their study electrospun PES fibers’ mechanical strength was improved by causing fibers to fuse at the joints as a result of different solvent (% N-Methyl-2-pyrrolidone, NMP) blends during the electrospinning process (Figure 8). The fibers were also oxidized to enhance their hydrophilicity. Figure 8 and Figure 9 reveals the effectiveness of this approach.
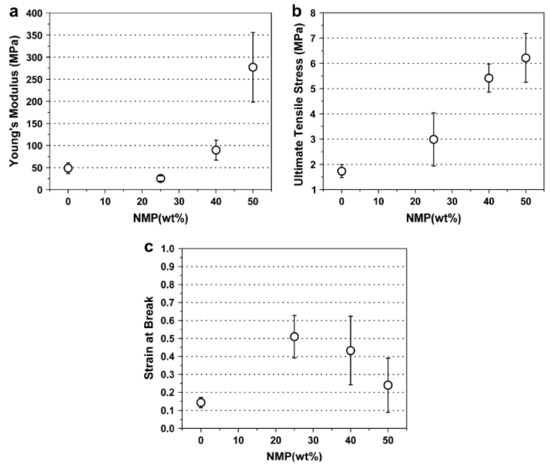
Figure 8.
Mechanical properties of electrospun PES membranes as a function of the mixed solvent: (a) Young’s modulus, (b) ultimate tensile strength, and (c) strain at break. Provided with permission from Elsevier [34].
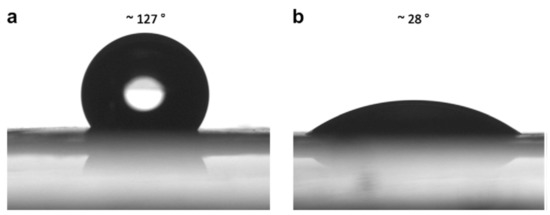
Figure 9.
Water contact angles for oxidized (a) and untreated (b) electrospun PES membranes. Provided with permission from Elsevier [34].
PES can also be readily used with the phase inversion technique [87,90,91]. Another example where researcher attempt to enhance the hydrophicity of PES is provided by Li et al. [90]. Here, the authors used phase inversion and TiO2 nanoparticles to improve both the flux (by 30%) and the strength of the resulting membrane (Figure 10). These results demonstrate that fabrication methods can be modified to improve the performance of membranes and simultaneously provide a method to add further functionality.
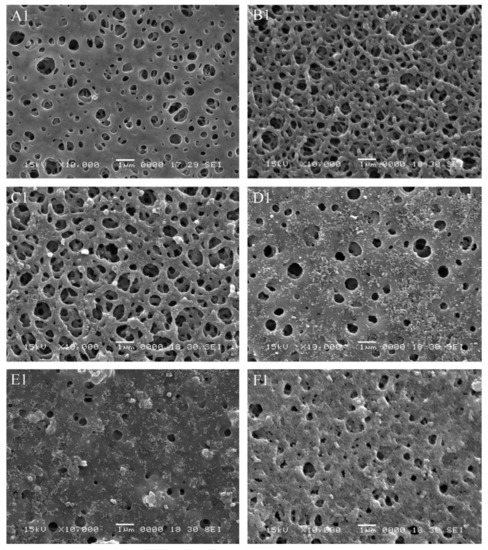
Figure 10.
SEM images of the top surface morphology of the membranes with different TiO2 content: (A1) 0 wt.%, (B1) 1 wt.%, (C1) 2 wt.%, (D1) 3 wt.%, (E1) 4 wt.% and (F1) 5 wt.%. Provided with permission from Elsevier [90].
3.2. Polyacrylonitrile
Polyacrylonitrile (PAN) is another very popular polymer for filtration applications [74]. Like PES, this is due to its desirable physical and chemical properties, particularly its solvent resistance [30,74]. As with PES, PAN is hydrophobic and thus is often blended with more hydrophilic polymers (often chitin or cellulose based polymers) [30,93] to improve its compatibility with aqueous systems. However, PAN is more hydrophilic than PES or PSf and furthermore is more naturally anti-fouling [89], making it a common choice for water filtration membranes [30,50,93,94,95]. A drawback of PAN however is its poor solubility, with the polymer being only soluble in strong polar solvents such as NMP, DMA or DMF. PAN is also suitable for electrospinning or phase inversion techniques.
An example demonstrating a typical use of a PAN based filter membrane is provided by Yeh et al. [96] Here, the authors electrospun PAN as the porous support, in a thin film composite (TFC) filter membrane. Upon this nanofiber support, a thin layer of cellulose nanofiber was cast and lastly up top of that, a graphene oxide layer also deposited. This resulted in a membrane that maintained a high permeate flux (2.2 kg m2 h−1) while showing excellent ethanol dehydration properties. This study can also be considered an example for the trend of using polymers like PAN and PES in composites with more hydrophilic polymers.
3.3. Cellulose and Chitin Derivatives
Biologically derived polymers (such as cellulose or chitin) are obvious choices for aqueous filtration applications, largely due to their innate hydrophilicity and abundance [47,87,97]. However, unlike synthetic polymers, these bio-based polymers often lack the solubility to be easily processed, and for this reason exist as regenerated [98] or derived forms [87,99,100], or are processed without dissolving them, using more esoteric techniques to fabricate composite membranes such as casting methods or spray coating [47,49,97,98]. Thus, these polymers are often blended with other polymers (such as PES or PAN) or used as a barrier layer on more easily processed supports [63,97]. Alternatively, chemically modified derivates, that are more soluble such as cellulose and chitin derivatives (cellulose acetate or chitosan) can be electrospun [95,100,101] or cast using phase inversion [87,99]. Due to is abundance and cost effectiveness, cellulose and its derivatives can be found in many commercially available filters with many filter paper products consisting of cellulose or its variants.
An example of cellulose being processed without dissolving it provided by the work of Wang et al. [97]. Here the authors electrospun PAN to form a fiber mat with a mean fiber diameter of 150 ± 10 nm; after this, a barrier layer of cellulose nanofibers was added using a spray coating method, forming an ultrafiltration thin film composite (TFC) membrane. The spray coating method uses a cellulose nanofiber suspension, thus avoiding the need to dissolve the cellulose. The authors also used PET as a backing for the PAN electrospun fibers, which is a very common practice in preparing such membranes [48,50,102].
3.4. Polyvinylalcohol
Polyvinylalcohol (PVA) is a hydrophilic, water soluble polymer, and thus must be covalently crosslinked if it is to be used as the sole component of a filter membrane [28,41,103]. This gives PVA membranes an added optimization parameter, the crosslinking densities. The hydrophilicity makes it an attractive material for membranes, however, due to its poor mechanical properties [74], especially when swollen [104], PVA is often used in composite membranes with another polymer such as PAN [50].
An example of the use of PVA as a barrier layer is provided by Tang et al. [103]. In their study a barrier PVA layer was added by UV crosslinking to electrospun PVA fibers. To prevent deep penetration of the aqueous based barrier layer solution, the crosslinked (via glutaraldehyde) electrospun fibers were soaked in a borax solution which filled the pores. Thus, the barrier layer remained at the fiber mats surface and was crosslinked through UV light. The barrier layer consisted of an aqueous solution of chemically modified PVA with UV crosslinking capability.
3.5. Polyvinylidene Fluoride
Polyvinylidene fluoride (PVDF) is a fluorinated hydrophobic polymer with good chemical and thermal stability and unique electric properties [74,89,105,106]. Owing to these properties PVDF is used commercially and industrially in many membranes’ applications, such as water treatment, biomedical filtration (western blotting) and electronic components (such as batteries [105,107]).
Atypically, in this case PVDF’s high hydrophobicity is responsible for its use in the biomedical filtration field as it non-specifically binds amino acids, allowing for protein removal [108] and it is often utilized in water desalination as opposed to simple filtration [109,110].
Membranes prepared from PVDF are often prepared by phase inversion. [14,77] PVDF is unique compared to other membrane polymers in that it has piezoelectric properties and possesses high electrochemical stability [106,111,112] and thus these membranes are often used an electrode separators in batteries [105,106,107] and other electronic applications [105,111,113].
However, simple filtration applications have also been explored [12,25,77,114]. For these simple filtration applications, the focus is mostly on fabricating hydrophilic PVDF membranes, as exampled by Zhao et al. [25]. In this work, amphiphilic triblock polymers were blended with PVDF to produce membranes with hydrophilic surfaces that showed superior anti-fouling properties and significantly better flux rates.
A table detailing the polymer used, the fabrication method and some critical membrane properties is shown in Table 2. Notice the wide range of fluxes and pressures used to test these fluxes, illustrating a lack of convention for the systematic study of membranes. This is attributed to the broad conditions that membranes are used in and the multitude of applications that they are required for.

Table 2.
Summary of polymers used and the resulting membrane characteristics.
4. Membrane Modification for Antifouling and Specificity
Modification of membrane properties to provide specific functions is a growing trend in filtration membrane research. This is a logical progression of membrane technology, where more custom applications find the need for specialized membranes. This section details some of the targeted areas associated with the development of specialized liquid filtration membranes, in particular, antifouling functionalities and targeted filtration of specific compounds or organisms. These areas dominate the research in modified filtration membranes due to the growing demand of such materials for pollution and disease control.
Membrane modification techniques, such as implementing TFCs, ref. [47,63,96] play a large role in this field, as well as techniques such as atom transfer radical polymerization (ATRP) [7,123,124] or reversible addition−fragmentation chain-transfer (RAFT) [123]. Additionally, incorporation of smart materials such as conducting polymers can allow for control over recognition events [125] or control of the antifouling nature or hydropathy of the resulting membrane [126,127,128,129].
4.1. Addition of Antifouling Properties
Methods for the addition of antifouling properties can be broken down into four main categories: surface chemistry modification, barrier layer modification, electrical disruption and physical (mechanical) methods. Surface chemistry modification largely involves the addition of antifouling moieties to the liquid-solid interface of the entire filter [12,15,130]. Barrier layer modification mostly applies to ultrafiltration or RO membranes with the use of TFCs and modifying the barrier layer to incorporate antifouling properties [63,64]. Physical methods may involve the use of a physical barrier, physical movement or abrasion of the membrane surface to reduce fouling [11,54,131,132]. Electrical disruption is a method that uses electrokinetic behavior of materials or causes convection disruption close to the surface of the membrane, preventing fouling [11,133].
For surface chemical modification methods and barrier layer modification, PEG based polymers are the most utilized owing to their well-known anti-fouling and protein rejecting properties [13,127]. Another popular surface modification is the use of Zwitter ionic grafts [14,15]. Both of these polymers have excellent antifouling properties and are easily attached to surfaces. Modification of the surface chemistry for membranes prepared through phase inversion usually involves addition of a compound that aggregates at the surface during the phase inversion process, often a surfactant such as Pluronic F127 [92] but these additives are not limited to surfactants [25,67,134].
A good example of surface chemistry modification is provided by Kolewe et al. [130] where electrospun cellulose acetate mats were functionalized with a Zwitter ion-based polymer, poly(2-methacryloyloxyethylphosphorylcholine) (polyMPC), which was bound to the surface of the fibers by physisorption to a polydopamine layer polymerized to the surface (Figure 11). These fibers demonstrated excellent resistance to fouling by protein and bacteria.
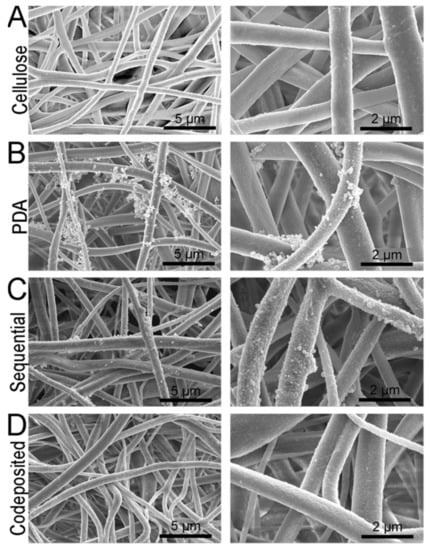
Figure 11.
(A) SEM micrographs of the cellulose nanofiber mats used as the base materials for this study. The morphologies of (B) PDA and (C,D) polyMPC/PDA (sequential and co-deposited) functionalized nanofiber mats are also displayed. Provided with permission from American Chemical Society [130].
An interesting method for developing antifouling membranes utilizes alternating electric fields [131,132,135]. The principle behind the electrokinetic anti-fouling method is that the alternating electric field causes a turbulent Debye’s double layer that prevents the accumulation of foulants. Furthermore, there is an electro-osmotic force that drives solution flux which further improves transmembrane flux rate [11,131,133]. Li et al. [131] described such a method, with a graphene/polyaniline (PANI) coated polyester filter cloth (Figure 12). In their work Li et al. ascribe the improved flux and antifouling properties to the conductance with a correlation between higher conductance and higher flux and superior anti-fouling properties.
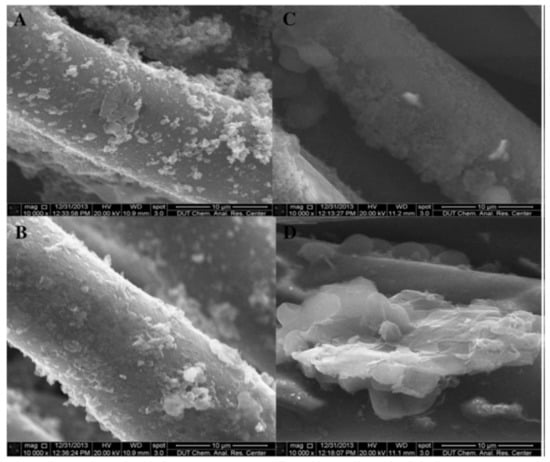
Figure 12.
SEM of the modified membranes (A,B) PANi-PA membrane; (C,D) Gr/PANi-PA membrane) after use in EMBR. (A,C) ×10,000, (B,D) ×10,000. Provided with permission from Elsevier [131].
4.2. Addition of Selective Filtration Properties
Selective filtration refers to the ability to selectively remove or concentrate a contaminant or desirable component from a source by a filtration process. As an example, the western blotting technique can be considered a protein selective filtration process [136,137], and ion exchange membranes can be considered an ion selective filtration process [138].
Designing a selective filter requires significantly more thought than fabricating a simple filtration membrane, owing to the need for a specific recognition/exclusion element in the filter material. The most common examples of this are proton exchange membranes [139], however these are fairly distinctive when compared to other selective filters. The applications of selective filters are often different than those of the typical liquid filtration applications. From ion exchange/exclusion membranes [4,87,140] in such applications as batteries [107] and other electronics [141], to specific pollutant removal [2,142,143,144,145], to medical applications with biomolecule [143,146] extraction/separation, selective filters are generally used for higher-value applications.
However, water purification still plays a large role in this field of research with membrane modification focusing on the specific removal of bacteria, viruses and pollutants [2,3,6,7,8,118,143,146]. As an example, Ma et al. [118] functionalized the surface of PAN electrospun fibers with imidazole or ethylene glycol containing polymers. The presence of the positively charged imidazole resulted in a greatly enhanced retention of viruses and bacteria, whereas the ethylene glycol based polymer had the opposite effect, with lower bacteria retention, when compared to commercial membranes.
Proton exchange membranes have gained interest due to their part in hydrogen fuel cells and thus due to their part in renewable energy, with Nafion membranes being the industry leader [147]. However, there are a number of other candidates for proton exchange membranes [72,139,148]. PES derivatives are one such polymer that have potential in this area. In a study published by Wang et al. [139], the phase inversion technique was used with sulfonated PES based polymers to produce excellent proton exchange membranes, with high water uptake and ion exchange capacity.
While proton exchange membranes have received more interest due to their part in renewable energy, these are not the only ion exchange/exclusion membranes that are being extensively researched. For instance, Qu et al. [140] developed amine and sulfonic acid functionalized PAN/PEO based membranes using the phase inversion technique. These membranes showed selective rejection between Na2SO4 and MgCl2 depending on the functionalization (Figure 13).
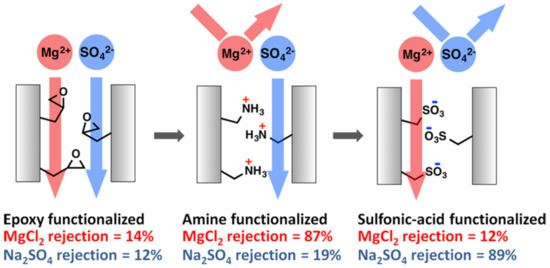
Figure 13.
Membrane rejection properties based on surface functionalization. Provided with permission from American Chemical Society [140].
Additionally, membranes for the specific removal of pollutants, nitrates [2,144] and sulphates [3,149] are receiving greater interest due to environmental concerns.
Selective filtration may require or rely on surface functionalization. A method that is potentially underutilized for surface functionalization is the diazonium salt grafting method [150,151,152]. This method involves reducing a diazonium salt to form radical ions that covalently attach to organic surfaces. This allows for a range of functional groups to be added and work has been undertaken to control the process, allowing monolayers comprised of specific groups to be added [153]. Such a technique is an excellent choice when making membranes for selective filtration, such as enhancing cation exchange membranes [138,154]. For instance, in research by Liu et al. [138], grafted polyethyleneimine layers using the diazonium salt grafting method to a commercially available CEM membrane. This added layer benefitted the membrane by forming a more homogenous surface alleviating concentration polarization effects. Furthermore, salt diffusion was suppressed enhancing the current efficiency.
5. Conclusions
This review takes a close look at the current research on liquid filtration technology. The polymer materials used, the fabrication techniques and the methods for modifying the membranes for more advanced filtration applications are discussed. The summation of methods in recent literature reveals that there are two methods for fabricating liquid filters that are more commonly used: electrospinning and phase inversion. Additionally, the formation of TFCs is a common method being utilized by researchers. The prevalence of TFCs in recent literature is due to that they provide an effective method to drastically improve existing membranes and allows for better rejections at higher fluxes not otherwise achievable. Looking at the materials chosen for the fabrication of filtration membranes, recent literature demonstrates that the polymer materials chosen are often selected due to their chemical and temperature resistance and then their physical properties but most critically they are chosen based on the fabrication method under investigation or intended to use. It is predicted that providing additional functionality to membranes is likely to be a key trend in future research, with provision of antifouling, and/or selective filtration membranes being important targets.
Author Contributions
Conceptualization, T.K.-P.; writing—original draft preparation, T.K.-P.; writing—review and editing, T.K.-P., B.S. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Thomas Kerr-Phillips thanks The Science for Technological Innovation National Science Challenge Clean Water Technology for providing his salary.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liquid Filtration Market, Markets and Markets: 2019; Report Number: CH 6693.
- Samatya, S.; Kabay, N.; Yüksel, Ü.; Arda, M.; Yüksel, M. Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React. Funct. Polym. 2006, 66, 1206. [Google Scholar] [CrossRef]
- Qin, L.; Vervuurt, S.J.N.; Elmes, R.B.P.; Berry, S.N.; Proschogo, N.; Jolliffe, K.A. Extraction and transport of sulfate using macrocyclic squaramide receptors. Chem. Sci. 2020, 11, 201. [Google Scholar] [CrossRef]
- Kim, S.; Nham, J.; Jeong, Y.S.; Lee, C.S.; Ha, S.H.; Park, H.B.; Lee, Y. Biomimetic Selective Ion Transport through Graphene Oxide Membranes Functionalized with Ion Recognizing Peptides. J. Chem. Mater. 2015, 27, 1255. [Google Scholar] [CrossRef]
- Coskun, H.; Aljabour, A.; De Luna, P.; Farka, D.; Greunz, T.; Stifter, D.; Kus, M.; Zheng, X.; Liu, M.; Hassel, A.W.; et al. Biofunctionalized conductive polymers enable efficient CO2 electroreduction. Sci. Adv. 2017, 3, e1700686. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, B.; Tong, X.; Chen, Y. Monovalent-anion selective and antifouling polyelectrolytes multilayer anion exchange membrane for reverse electrodialysis. J. Membr. Sci. 2018, 567, 68. [Google Scholar] [CrossRef]
- Kerr-Phillips, T.E.; Aydemir, N.; Chan, E.W.C.; Barker, D.; Malmström, J.; Plesse, C.; Travas-Sejdic, J. Conducting electrospun fibres with polyanionic grafts as highly selective, label-free, electrochemical biosensor with a low detection limit for non-Hodgkin lymphoma gene. Biosens. Bioelectron. 2018, 100, 549. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, W.; Jiao, Y.; Zhou, C.J. A surface molecularly imprinted electrospun polyethersulfone (PES) fiber mat for selective removal of bilirubin. Mater. Chem. B 2017, 5, 5763. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, A.; Mays, L. Evolution of Water Supply Through the Millennia; IWA Publishing: London, UK, 2014. [Google Scholar]
- Glater, J. The early history of reverse osmosis membrane development. Desalination 1998, 117, 297. [Google Scholar] [CrossRef]
- Yu, B.; Sun, J.; Zhao, K.; Ma, F.; Sun, L.; Shao, J.; Tian, J.; Hu, C. Mitigating membrane fouling by coupling coagulation and the electrokinetic effect in a novel electrocoagulation membrane cathode reactor. Water Res. 2022, 217, 118378. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, W.N. Design of poly(vinylidene fluoride)-g-p(hydroxyethyl methacrylate-co-N-isopropylacrylamide) membrane via surface modification for enhanced fouling resistance and release property. Appl. Surf. Sci. 2017, 398, 103. [Google Scholar] [CrossRef]
- Wagner, V.E.; Koberstein, J.T.; Bryers, J.D. Protein and bacterial fouling characteristics of peptide and antibody decorated surfaces of PEG-poly(acrylic acid) co-polymers. Biomaterials 2004, 25, 2247. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, Y.; Sadam, H.; Ma, J.; Bai, Y.; Shen, X.; Kim, J.; Shao, L. Novel mussel-inspired zwitterionic hydrophilic polymer to boost membrane water-treatment performance. J. Membr. Sci. 2019, 582, 1. [Google Scholar] [CrossRef]
- Yang, W.; Sundaram, H.S.; Ella, J.; He, N.; Jiang, S. Low-fouling electrospun PLLA films modified with zwitterionic poly(sulfobetaine methacrylate)-catechol conjugates. Acta Biomater. 2016, 40, 92. [Google Scholar] [CrossRef] [PubMed]
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. Chapter 1-Introduction to Membranes. Interface Sci. Technol. 2018, 25, 1. [Google Scholar]
- Rana, D.; Matsuura, T.; Mohd, A.K.; Ismail, A.F. Reverse Osmosis Membrane. In Handbook of Membrane Separations; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ng, Z.C.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Thin film nanocomposite RO membranes: Review on fabrication techniques and impacts of nanofiller characteristics on membrane properties. Chem. Eng. Res. Des. 2021, 165, 81. [Google Scholar] [CrossRef]
- Van de Voorde, B.; Bueken, B.; Denayer, J.; De Vos, D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766. [Google Scholar] [CrossRef]
- Voigt, I.; Adler, J.Ö.; Weyd, M.; Kriegel, R. Ceramic Filters and Membranes. In Ceramics Science and Technology; Wiley Online Library: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hassan, M.F.; Sabri, M.A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent trends in activated carbon fibers production from various precursors and applications—A comparative review. J. Anal. Appl. Pyrolysis 2020, 145, 104715. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F.J. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132, 42130. [Google Scholar] [CrossRef]
- Buonomenna, M.G. Design Next Generation Membranes or Rethink the “Old” Asymmetric Membranes? Symmetry 2020, 12, 270. [Google Scholar] [CrossRef]
- Abdullah, N.; Rahman, M.A.; Dzarfan Othman, M.H.; Jaafar, J.; Ismail, A.F. Chapter 2-Membranes and Membrane Processes: Fundamentals. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zhao, Y.; Qian, Y.; Zhu, B.; Xu, Y. Modification of porous poly(vinylidene fluoride) membrane using amphiphilic polymers with different structures in phase inversion process. J. Membr. Sci. 2008, 310, 567. [Google Scholar] [CrossRef]
- Kannan, B.; Cha, H.; Hosie, I.C. Electrospinning—Commercial Applications, Challenges and Opportunities. In Nano-Size Polymers: Preparation, Properties, Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Doshi, J.; Reneker, D.H.J. Electrospinning process and applications of electrospun fibers. Electrostatics 1995, 35, 151. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. High-flux microfiltration filters based on electrospun polyvinylalcohol nanofibrous membranes. Polymer 2013, 54, 548. [Google Scholar] [CrossRef]
- Wang, Z.; Crandall, C.; Sahadevan, R.; Menkhaus, T.J.; Fong, H. Microfiltration performance of electrospun nanofiber membranes with varied fiber diameters and different membrane porosities and thicknesses. Polymer 2017, 114, 64. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, K.; Wang, X.; Fang, D.; Hsiao, B.S.; Chu, B. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer 2006, 47, 2434. [Google Scholar] [CrossRef]
- Qin, X.; Wang, S. Filtration properties of electrospinning nanofibers. J. Appl. Polym. Sci. 2006, 102, 1285. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Baek, P.; Barker, D.; Travas-Sejdic, J. Highly functionalisable polythiophene phenylenes. Polym. Chem. 2015, 6, 7618. [Google Scholar] [CrossRef]
- El-Shazly, A.; Rezk, M.Y.; Gameel, K.M.; Allam, N.K. Electrospun Lead-Free All-Inorganic Double Perovskite Nanofibers for Photovoltaic and Optoelectronic Applications. ACS Appl. Nano Mater. 2019, 2, 7085. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. Formation of functional polyethersulfone electrospun membrane for water purification by mixed solvent and oxidation processes. Polymer 2009, 50, 2893. [Google Scholar] [CrossRef]
- Yue, B.; Zhang, B.; You, J.; Li, Y.; Li, L.; Li, J. “Lotus-effect” tape: Imparting superhydrophobicity to solid materials with an electrospun Janus composite mat. RSC Adv. 2016, 6, 17215. [Google Scholar] [CrossRef]
- Stanishevsky, A.V.; Wetuski, J.D.; Yockell-Lelièvre, H. Crystallization and stability of electrospun ribbon- and cylinder-shaped tungsten oxide nanofibers. Ceram. Int. 2016, 42, 388. [Google Scholar] [CrossRef]
- Fallahi, A.; Mandla, S.; Kerr-Phillip, T.; Seo, J.; Rodrigues, R.O.; Jodat, Y.A.; Samanipour, R.; Hussain, M.A.; Lee, C.K.; Bae, H.; et al. Flexible and Stretchable PEDOT-Embedded Hybrid Substrates for Bioengineering and Sensory Applications. ChemNanoMat 2019, 5, 729. [Google Scholar] [CrossRef]
- Duan, G.; Liu, S.; Jiang, S.; Hou, H. High-performance polyamide-imide films and electrospun aligned nanofibers from an amide-containing diamine. J. Mater. Sci. 2019, 54, 6719. [Google Scholar] [CrossRef]
- Gluais, M.; Clouet, J.; Fusellier, M.; Decante, C.; Moraru, C.; Dutilleul, M.; Veziers, J.; Lesoeur, J.; Dumas, D.; Abadie, J.; et al. In vitro and in vivo evaluation of an electrospun-aligned microfibrous implant for Annulus fibrosus repair. Biomaterials 2019, 205, 81. [Google Scholar] [CrossRef] [PubMed]
- Aussawasathien, D.; Teerawattananon, C.; Vongachariya, A. Separation of micron to sub-micron particles from water: Electrospun nylon-6 nanofibrous membranes as pre-filters. J. Membr. Sci. 2008, 315, 11. [Google Scholar] [CrossRef]
- Wang, X.; Fang, D.; Yoon, K.; Hsiao, B.S.; Chu, B. High performance ultrafiltration composite membranes based on poly(vinyl alcohol) hydrogel coating on crosslinked nanofibrous poly(vinyl alcohol) scaffold. J. Membr. Sci. 2006, 278, 261. [Google Scholar] [CrossRef]
- Kerr-Phillips, T.; Woehling, V.; Agniel, R.; Nguyen, G.T.M.; Vidal, F.; Kilmartin, P.; Plesse, C.; Travas-Sejdic, J. Electrospun rubber fibre mats with electrochemically controllable pore sizes. J. Mater. Chem. B 2015, 3, 4249. [Google Scholar] [CrossRef]
- Homaeigohar, S.S.; Buhr, K.; Ebert, K. Polyethersulfone electrospun nanofibrous composite membrane for liquid filtration. J. Membr. Sci. 2010, 365, 68. [Google Scholar] [CrossRef]
- Grey, C.P.; Newton, S.T.; Bowlin, G.L.; Haas, T.W.; Simpson, D.G. Gradient fiber electrospinning of layered scaffolds using controlled transitions in fiber diameter. Biomaterials 2013, 34, 4993. [Google Scholar] [CrossRef] [PubMed]
- Im, J.S.; Park, S.; Kim, T.J.; Kim, Y.H.; Lee, Y. The study of controlling pore size on electrospun carbon nanofibers for hydrogen adsorption. J. Colloid Interface Sci. 2008, 318, 42. [Google Scholar] [CrossRef]
- Pavliňák, D.; Hnilica, J.; Quade, A.; Schäfer, J.; Alberti, M.; Kudrle, V. Functionalisation and pore size control of electrospun PA6 nanofibres using a microwave jet plasma. Polym. Degrad. Stab. 2014, 108, 48. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Thin-film nanofibrous composite membranes containing cellulose or chitin barrier layers fabricated by ionic liquids. Polymer 2011, 52, 2594. [Google Scholar] [CrossRef]
- Yung, L.; Ma, H.; Wang, X.; Yoon, K.; Wang, R.; Hsiao, B.S.; Chu, B. Fabrication of thin-film nanofibrous composite membranes by interfacial polymerization using ionic liquids as additives. J. Membr. Sci. 2010, 365, 52. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Fabrication and characterization of cellulose nanofiber based thin-film nanofibrous composite membranes. J. Membr. Sci. 2014, 454, 272. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. High flux ultrafiltration nanofibrous membranes based on polyacrylonitrile electrospun scaffolds and crosslinked polyvinyl alcohol coating. J. Membr. Sci. 2009, 338, 145. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77. [Google Scholar] [CrossRef]
- Sarada, T.; Sawyer, L.C.; Ostler, M.I. Three dimensional structure of celgard® microporous membranes. J. Membr. Sci. 1983, 15, 97. [Google Scholar] [CrossRef]
- Tabatabaei, S.H.; Carreau, P.J.; Ajji, A. Microporous membranes obtained from PP/HDPE multilayer films by stretching. J. Membr. Sci. 2009, 345, 148. [Google Scholar] [CrossRef]
- Holdich, R.; Kosvintsev, S.; Cumming, I.; Zhdanov, S. Pore design and engineering for filters and membranes. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 161. [Google Scholar] [CrossRef]
- Alderson, A.; Rasburn, J.; Ameer-Beg, S.; Mullarkey, P.G.; Perrie, W.; Evans, K.E. An Auxetic Filter: A Tuneable Filter Displaying Enhanced Size Selectivity or Defouling Properties. Ind. Eng. Chem. Res. 2000, 39, 654. [Google Scholar] [CrossRef]
- Fan, F.; Wang, L.; Jiang, W.; Chen, B.; Liu, H. A novel polyethylene microfiltration membrane with highly permeable ordered ‘wine bottle’ shaped through-pore structure fabricated via imprint and thermal field induction. J. Phys. D 2016, 49, 125501. [Google Scholar] [CrossRef]
- Liang, H.; Cao, X.; Zhang, W.; Lin, H.; Zhou, F.; Chen, L.; Yu, S. Robust and Highly Efficient Free-Standing Carbonaceous Nanofiber Membranes for Water Purification. Adv. Funct. Mater. 2011, 21, 3851. [Google Scholar] [CrossRef]
- Ross, C.A.; Berggren, K.K.; Cheng, J.Y.; Jung, Y.S.; Chang, J. Three-Dimensional Nanofabrication by Block Copolymer Self-Assembly. Adv. Mater. 2014, 26, 4386. [Google Scholar] [CrossRef] [PubMed]
- Phillip, W.A.; O’Neill, B.; Rodwogin, M.; Hillmyer, M.A.; Cussler, E.L. Self-Assembled Block Copolymer Thin Films as Water Filtration Membranes. ACS Appl. Mater. Interfaces 2010, 2, 847. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lim, J.; Gu, M.; Yu, C.; Hong, M.; Char, K.; Choi, T. Dimensionally controlled water-dispersible amplifying fluorescent polymer nanoparticles for selective detection of charge-neutral analytes. Polym. Chem. 2017, 8, 7507. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Liu, Y.; Chen, T.; Lin, J. Layer-by-layer self-assembly of aramid nanofibers on nonwoven fabric for liquid filtration. Polym. Compos. 2018, 39, 2411. [Google Scholar] [CrossRef]
- Del Barrio, J.; Sánchez-Somolinos, C. Light to Shape the Future: From Photolithography to 4D Printing. Adv. Opt. Mater. 2019, 7, 1900598. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofibrous ultrafiltration membranes containing cross-linked poly(ethylene glycol) and cellulose nanofiber composite barrier layer. Polymer 2014, 55, 366. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Liu, Y.; Xu, Y.; Li, R.; Hong, H.; Shen, L.; Lin, H.; Liao, B. Enhanced permeability and antifouling performance of polyether sulfone (PES) membrane via elevating magnetic Ni@MXene nanoparticles to upper layer in phase inversion process. J. Membr. Sci. 2021, 623, 119080. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298. [Google Scholar] [CrossRef] [PubMed]
- Vandezande, P.; Li, X.; Gevers, L.E.M.; Vankelecom, I.F.J. High throughput study of phase inversion parameters for polyimide-based SRNF membranes. J. Membr. Sci. 2009, 330, 307. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284. [Google Scholar] [CrossRef]
- Li, Z.; Zabihi, O.; Wang, J.; Li, Q.; Wang, J.; Lei, W.; Naebe, M. Hydrophilic PAN based carbon nanofibres with improved graphitic structure and enhanced mechanical performance using ethylenediamine functionalized graphene. RSC Adv. 2017, 7, 2621. [Google Scholar] [CrossRef]
- Xiang, T.; Yue, W.; Wang, R.; Liang, S.; Sun, S.; Zhao, C. Surface hydrophilic modification of polyethersulfone membranes by surface-initiated ATRP with enhanced blood compatibility. Colloids Surf. B Biointerfaces 2013, 110, 15. [Google Scholar] [CrossRef]
- Polyethersulphone (PES)-Supplier Data by Goodfellow. Available online: https://www.azom.com/article.aspx?ArticleID=1953 (accessed on 20 September 2022).
- Overview of Materials for Polyethersulfone (PES). Available online: https://www.matweb.com/search/datasheet.aspx?matguid=6be926d8eb0842abbfb80b5658ade95a (accessed on 20 September 2022).
- Casciola, M.; Donnadio, A.; Pica, M. 3.7 Basic Aspects in Proton-Conducting Membranes for Fuel Cells. In Comprehensive Membrane Science and Engineering, 2nd ed.; Elsevier: Oxford, UK, 2017. [Google Scholar]
- Huang, Y.; Young, R.J. Effect of fibre microstructure upon the modulus of PAN- and pitch-based carbon fibres. Carbon 1995, 33, 97. [Google Scholar] [CrossRef]
- Carraher, C.E. Carraher’s Polymer Chemistry; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Krentsel, L.B.; Kudryavtsev, Y.V.; Rebrov, A.I.; Litmanovich, A.D.; Platé, N.A. Acidic Hydrolysis of Polyacrylonitrile: Effect of Neighboring Groups. Macromolecules 2001, 34, 5607. [Google Scholar] [CrossRef]
- Litmanovich, A.D.; Platé, N.A. Alkaline hydrolysis of polyacrylonitrile. On the reaction mechanism. Macromol. Chem. Phys. 2000, 201, 2176. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.J. Molecular elucidation of morphology and mechanical properties of PVDF hollow fiber membranes from aspects of phase inversion, crystallization and rheology. Membr. Sci. 2009, 340, 192. [Google Scholar] [CrossRef]
- Overview of Materials for Polyvinylidinefluoride (PVDF), Molded/Extruded. Available online: https://www.matweb.com/search/DataSheet.aspx?MatGUID=a011f8ccf4b448a19246773a32085094 (accessed on 15 September 2022).
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2017, 26, 213. [Google Scholar] [CrossRef]
- Freire, T.F.; Quinaz, T.; Fertuzinhos, A.; Quyền, N.T.; de Moura, M.F.S.M.; Martins, M.; Zille, A.; Dourado, N. Thermal, Mechanical and Chemical Analysis of Poly(vinyl alcohol) Multifilament and Braided Yarns. Polymers 2021, 13, 3644. [Google Scholar] [CrossRef]
- Iwamoto, S.; Kai, W.; Isogai, A.; Iwata, T. Elastic Modulus of Single Cellulose Microfibrils from Tunicate Measured by Atomic Force Microscopy. Biomacromolecules 2009, 10, 2571. [Google Scholar] [CrossRef]
- Kubát, J.; Pattyranie, C. Transition in Cellulose in the Vicinity of −30 °C. Nature 1967, 215, 390. [Google Scholar] [CrossRef]
- Greene, J.P. 3-Microstructures of Polymers. In Automotive Plastics and Composites; William Andrew Publishing: Oxford, UK, 2021. [Google Scholar]
- McIntee, O.M.; Welch, B.C.; Greenberg, A.R.; George, S.M.; Bright, V.M. Elastic modulus of polyamide thin films formed by molecular layer deposition. Polymer 2022, 255, 125167. [Google Scholar] [CrossRef]
- Silva, L.; Tognana, S.; Salgueiro, W. Study of the water absorption and its influence on the Young’s modulus in a commercial polyamide. Polym. Test. 2013, 32, 158. [Google Scholar] [CrossRef]
- Urkiaga, A.; De Las Fuentes, L.; Acilu, M.; Uriarte, J. Membrane comparison for wine clarification by microfiltration. Desalination 2002, 148, 115. [Google Scholar] [CrossRef]
- Sivakumar, M.; Mohan, D.R.; Rangarajan, R. Studies on cellulose acetate-polysulfone ultrafiltration membranes: II. Effect of additive concentration. J. Membr. Sci. 2006, 268, 208. [Google Scholar] [CrossRef]
- Gopal, R.; Kaur, S.; Feng, C.Y.; Chan, C.; Ramakrishna, S.; Tabe, S.; Matsuura, T. Electrospun nanofibrous polysulfone membranes as pre-filters: Particulate removal. J. Membr. Sci. 2007, 289, 210. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Membr. Ind. Eng. Chem. Res. 2011, 50, 3798. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Yang, H.; Yu, L.; Liu, M. Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl. Surf. Sci. 2009, 255, 4725. [Google Scholar] [CrossRef]
- Zha, S.; Yu, J.; Zhang, G.; Liu, N.; Lee, R. Polyethersulfone (PES)/cellulose acetate butyrate (CAB) composite hollow fiber membranes for BTEX separation from produced water. RSC Adv. 2015, 5, 105692. [Google Scholar] [CrossRef]
- Zhao, W.; Su, Y.; Li, C.; Shi, Q.; Ning, X.; Jiang, Z. Fabrication of antifouling polyethersulfone ultrafiltration membranes using Pluronic F127 as both surface modifier and pore-forming agent. J. Membr. Sci. 2008, 318, 405. [Google Scholar] [CrossRef]
- Musale, D.A.; Kumar, A.; Pleizier, G. Formation and characterization of poly(acrylonitrile)/Chitosan composite ultrafiltration membranes. J. Membr. Sci. 1999, 154, 163. [Google Scholar] [CrossRef]
- Patel, S.; Hota, G. Synthesis of novel surface functionalized electrospun PAN nanofibers matrix for efficient adsorption of anionic CR dye from water. J. Environ. Chem. Eng. 2018, 6, 5301. [Google Scholar] [CrossRef]
- Makaremi, M.; Lim, C.X.; Pasbakhsh, P.; Lee, S.M.; Goh, K.L.; Chang, H.; Chan, E.S. Electrospun functionalized polyacrylonitrile–chitosan Bi-layer membranes for water filtration applications. RSC Adv. 2016, 6, 53882. [Google Scholar] [CrossRef]
- Yeh, T.; Wang, Z.; Mahajan, D.; Hsiao, B.S.; Chu, B. High flux ethanol dehydration using nanofibrous membranes containing graphene oxide barrier layers. J. Mater. Chem. A 2013, 1, 12998. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Chu, B.; Hsiao, B.S. Fabrication of cellulose nanofiber-based ultrafiltration membranes by spray coating approach. J. Appl. Polym. Sci. 2017, 134, 44583. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169. [Google Scholar] [CrossRef]
- Kutowy, O.; Sourirajan, S. Cellulose acetate ultrafiltration membranes. J. Appl. Polym. Sci. 1975, 19, 1449. [Google Scholar] [CrossRef]
- Demirci, S.; Celebioglu, A.; Uyar, T. Surface modification of electrospun cellulose acetate nanofibers via RAFT polymerization for DNA adsorption. Carbohydr. Polym. 2014, 113, 200. [Google Scholar] [CrossRef]
- Lan, T.; Shao, Z.; Gu, M.; Zhou, Z.; Wang, Y.; Wang, W.; Wang, F.; Wang, J. Electrospun nanofibrous cellulose diacetate nitrate membrane for protein separation. J. Membr. Sci. 2015, 489, 204. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultrafine Polysaccharide Nanofibrous Membranes for Water Purification. Biomacromolecules 2011, 12, 970. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, J.; Yung, L.; Ji, B.; Ma, H.; Qiu, C.; Yoon, K.; Wan, F.; Fang, D.; Hsiao, B.S.; et al. UV-cured poly(vinyl alcohol) ultrafiltration nanofibrous membrane based on electrospun nanofiber scaffolds. J. Membr. Sci. 2009, 328, 1. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Gregersen, Ø.W. Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes. J. Ind. Eng. Chem. 2018, 57, 113. [Google Scholar] [CrossRef]
- Cheng, C.L.; Wan, C.C.; Wang, Y.Y. Microporous PVdF-HFP based gel polymer electrolytes reinforced by PEGDMA network. Electrochem. Commun. 2004, 6, 531. [Google Scholar] [CrossRef]
- Hsu, C.; Liu, R.; Hsu, C.; Kuo, P. High thermal and electrochemical stability of PVDF-graft-PAN copolymer hybrid PEO membrane for safety reinforced lithium-ion battery. RSC Adv. 2016, 6, 18082. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, B.C. Electrospun PVdF-based fibrous polymer electrolytes for lithium ion polymer batteries. Electrochim. Acta 2004, 50, 69. [Google Scholar] [CrossRef]
- Xiang, Y.; Zheng, Y.; Liu, S.; Liu, G.; Li, Z.; Dong, W. Comparison of the sensitivity of Western blotting between PVDF and NC membranes. Sci. Rep. 2021, 11, 12022. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Shih, J.; Huang, M.; Wang, C.; Shih, W.; Liu, Y. A study of hydrophobic electrospun membrane applied in seawater desalination by membrane distillation. Fibers Polym. 2012, 13, 698. [Google Scholar] [CrossRef]
- Wang, K.Y.; Foo, S.W.; Chung, T. Mixed Matrix PVDF Hollow Fiber Membranes with Nanoscale Pores for Desalination through Direct Contact Membrane Distillation. Ind. Eng. Chem. Res. 2009, 48, 4474. [Google Scholar] [CrossRef]
- Cauda, V.; Stassi, S.; Bejtka, K.; Canavese, G. Nanoconfinement: An Effective Way to Enhance PVDF Piezoelectric Properties. ACS Appl. Mater. Interfaces 2013, 5, 6430. [Google Scholar] [CrossRef]
- Sohn, J.; Im, J.S.; Gwon, S.; Choi, J.; Shin, J.; Nho, Y. Preparation and characterization of a PVDF-HFP/PEGDMA-coated PE separator for lithium-ion polymer battery by electron beam irradiation. Radiat. Phys. Chem. 2009, 78, 505. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Wang, L. Poly (3, 4-ethylenedioxythiophene) modified polyvinylidene fluoride membrane for visible photoelectrocatalysis and filtration. J. Colloid Interface Sci. 2019, 553, 220. [Google Scholar] [CrossRef]
- Li, Z.; Kang, W.; Zhao, H.; Hu, M.; Wei, N.; Qiu, J.; Cheng, B. A Novel Polyvinylidene Fluoride Tree-Like Nanofiber Membrane for Microfiltration. Nanomaterials 2016, 6, 152. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, M.; Chen, Y.; He, G.; Wu, M.; Wang, J.J. Preparation and performance of cellulose acetate/polyethyleneimine blend microfiltration membranes and their applications. Membr. Sci. 2004, 235, 73. [Google Scholar] [CrossRef]
- Wang, X.; Yeh, T.; Wang, Z.; Yang, R.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofiltration membranes prepared by interfacial polymerization on thin-film nanofibrous composite scaffold. Polymer 2014, 55, 1358. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Li, B.; Hsiao, B.S.; Chu, B. Electrospun nanofibrous membranes for high flux microfiltration. J. Membr. Sci. 2012, 392–393, 167. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Functionalized electrospun nanofibrous microfiltration membranes for removal of bacteria and viruses. J. Membr. Sci. 2014, 452, 446. [Google Scholar] [CrossRef]
- Tang, N.; Jia, Q.; Zhang, H.; Li, J.; Cao, S. Preparation and morphological characterization of narrow pore size distributed polypropylene hydrophobic membranes for vacuum membrane distillation via thermally induced phase separation. Desalination 2010, 256, 27. [Google Scholar] [CrossRef]
- Dai, J.; Liu, X.; Yang, J.; Zhang, N.; Huang, T.; Wang, Y.; Zhou, Z. Stretching induces pore formation in the β-nucleated polypropylene/graphene oxide composite. Compos. Sci. Technol. 2014, 99, 59. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Olah, A.; Baer, E. Composite nanofibrous microfiltration water filter. J. Appl. Polym. Sci. 2017, 134, 45557. [Google Scholar] [CrossRef]
- Lianchao, L.; Baoguo, W.; Huimin, T.; Tianlu, C.; Jiping, X. A novel nanofiltration membrane prepared with PAMAM and TMC by in situ interfacial polymerization on PEK-C ultrafiltration membrane. J. Membr. Sci. 2006, 269, 84. [Google Scholar] [CrossRef]
- Ameringer, T.; Ercole, F.; Tsang, K.M.; Coad, B.R.; Hou, X.; Rodda, A.; Nisbet, D.R.; Thissen, H.; Evans, R.A.; Meagher, L.; et al. Surface grafting of electrospun fibers using ATRP and RAFT for the control of biointerfacial interactions. Biointerphases 2013, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, C.; Vo, C.D.; Focarete, M.L.; Scandola, M.; Pollicino, A.; Di Silvestro, G.; Tirelli, N. Advantages of Surface-Initiated ATRP (SI-ATRP) for the Functionalization of Electrospun Materials. Macromol. Rapid Commun. 2013, 34, 51. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.; Akbarinejad, A.; Hisey, C.L.; Bryant, D.T.; Wang, J.; Zhu, B.; Evans, C.W.; Williams, D.E.; Chamley, L.W.; Barker, D.; et al. Conducting Polymer-Coated Carbon Cloth Captures and Releases Extracellular Vesicles by a Rapid and Controlled Redox Process. ACS Appl. Mater. Interfaces 2022, 14, 32880. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Schon, B.S.; Travas-Sejdic, J. A Conductive Microfiltration Membrane for In Situ Fouling Detection: Proof-of-Concept Using Model Wine Solutions. Macromol. Rapid Commun. 2020, 41, 2000303. [Google Scholar] [CrossRef]
- Hackett, A.J.; Malmström, J.; Molino, P.J.; Gautrot, J.E.; Zhang, H.; Higgins, M.J.; Wallace, G.G.; Williams, D.E.; Travas-Sejdic, J. Conductive surfaces with dynamic switching in response to temperature and salt. J. Mater. Chem. B 2015, 3, 9285. [Google Scholar] [CrossRef]
- Malmström, J.; Nieuwoudt, M.K.; Strover, L.T.; Hackett, A.; Laita, O.; Brimble, M.A.; Williams, D.E.; Travas-Sejdic, J. Grafting from Poly(3,4-ethylenedioxythiophene): A Simple Route to Versatile Electrically Addressable Surfaces. Macromolecules 2013, 46, 4955. [Google Scholar] [CrossRef]
- Pei, Y.; Travas-Sejdic, J.; Williams, D.E. Reversible Electrochemical Switching of Polymer Brushes Grafted onto Conducting Polymer Films. Langmuir 2012, 28, 8072. [Google Scholar] [CrossRef]
- Kolewe, K.W.; Dobosz, K.M.; Rieger, K.A.; Chang, C.; Emrick, T.; Schiffman, J.D. Antifouling Electrospun Nanofiber Mats Functionalized with Polymer Zwitterions. ACS Appl. Mater. Interfaces 2016, 8, 27585. [Google Scholar] [CrossRef]
- Li, N.; Liu, L.; Yang, F. Highly conductive graphene/PANi-phytic acid modified cathodic filter membrane and its antifouling property in EMBR in neutral conditions. Desalination 2014, 338, 10. [Google Scholar] [CrossRef]
- Brunner, G.; Okoro, E. Reduction of Membrane Fouling by Means of an Electric Field During Ultrafiltration of Protein Solutions. Ber. Bunsenges. Phys. Chem. 1989, 93, 1026. [Google Scholar] [CrossRef]
- Ren, Q.; Liang, C. Insulator-based dielectrophoretic antifouling of nanoporous membrane for high conductive water desalination. Desalination 2020, 482, 114410. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B. Preparation of poly(vinylidene fluoride)(pvdf) ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer 2005, 46, 7701. [Google Scholar] [CrossRef]
- Zumbusch, P.V.; Kulcke, W.; Brunner, G. Use of alternating electrical fields as anti-fouling strategy in ultrafiltration of biological suspensions–Introduction of a new experimental procedure for crossflow filtration. J. Membr. Sci. 1998, 142, 75. [Google Scholar] [CrossRef]
- Moritz, C.P. 40 years Western blotting: A scientific birthday toast. J. Proteom. 2020, 212, 103575. [Google Scholar] [CrossRef]
- Burnette, W.N. “Western Blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Jia, Y. Surface-functionalized cation exchange membrane by covalent immobilization of polyelectrolyte multilayer for effective separation of mono- and multivalent cations. Sep. Sci. Technol. 2016, 51, 2823. [Google Scholar] [CrossRef]
- Wang, F.; Hickner, M.; Kim, Y.S.; Zawodzinski, T.A.; McGrath, J.E. Direct polymerization of sulfonated poly(arylene ether sulfone) random (statistical) copolymers: Candidates for new proton exchange membranes. J. Membr. Sci. 2002, 197, 231. [Google Scholar] [CrossRef]
- Qu, S.; Dilenschneider, T.; Phillip, W.A. Preparation of Chemically-Tailored Copolymer Membranes with Tunable Ion Transport Properties. ACS Appl. Mater. Interfaces 2015, 7, 19746. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Huang, Z.; Yu, X.; Kang, F. Graphene oxide-embedded porous carbon nanofiber webs by electrospinning for capacitive deionization. Colloids Surf. Physicochem. Eng. Asp. 2014, 444, 153. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Electrospun Nanofibrous Membrane for Heavy Metal Ion Adsorption. Curr. Org. Chem. 2013, 17, 1361. [Google Scholar] [CrossRef]
- Wang, R.; Guan, S.; Sato, A.; Wang, X.; Wang, Z.; Yang, R.; Hsiao, B.S.; Chu, B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J. Membr. Sci. 2013, 446, 376. [Google Scholar] [CrossRef]
- Tyagi, S.; Rawtani, D.; Khatri, N.; Tharmavaram, M. Strategies for Nitrate removal from aqueous environment using Nanotechnology: A Review. J. Water Process Eng. 2018, 21, 84. [Google Scholar] [CrossRef]
- Manesiotis, P.; Riley, A.; Bollen, B.J. Polymerisable squaramide receptors for anion binding and sensing. Mater. Chem. C 2014, 2, 8990. [Google Scholar] [CrossRef]
- Papaphilippou, P.C.; Vyrides, I.; Mpekris, F.; Stylianopoulos, T.; Papatryfonos, C.A.; Theocharis, C.R.; Krasia-Christoforou, T. Evaluation of novel, cationic electrospun microfibrous membranes as adsorbents in bacteria removal. RSC Adv. 2015, 5, 67617. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cheng, T.; Zhang, X.; Zhang, W.; Liu, X. Novel composite proton exchange membrane with long-range proton transfer channels constructed by synergistic effect between acid and base functionalized graphene oxide. Polymer 2018, 149, 305. [Google Scholar] [CrossRef]
- Qin, L.; Hartley, A.; Turner, P.; Elmes, R.B.P.; Jolliffe, K.A. Macrocyclic squaramides: Anion receptors with high sulfate binding affinity and selectivity in aqueous media. Chem. Sci. 2016, 7, 4563. [Google Scholar] [CrossRef]
- Hetemi, D.; Noël, V.; Pinson, J. Grafting of Diazonium Salts on Surfaces: Application to Biosensors. Biosensors 2020, 10, 4. [Google Scholar] [CrossRef]
- Mévellec, V.; Roussel, S.; Tessier, L.; Chancolon, J.; Mayne-L’Hermite, M.; Deniau, G.; Viel, P.; Palacin, S. Grafting Polymers on Surfaces: A New Powerful and Versatile Diazonium Salt-Based One-Step Process in Aqueous Media. Chem. Mater. 2007, 19, 6323. [Google Scholar] [CrossRef]
- Allongue, P.; Delamar, M.; Desbat, B.; Fagebaume, O.; Hitmi, R.; Pinson, J.; Savéant, J. Covalent Modification of Carbon Surfaces by Aryl Radicals Generated from the Electrochemical Reduction of Diazonium Salts. J. Am. Chem. Soc. 1997, 119, 201. [Google Scholar] [CrossRef]
- Lee, L.; Ma, H.; Brooksby, P.A.; Brown, S.A.; Leroux, Y.R.; Hapiot, P.; Downard, A.J. Covalently Anchored Carboxyphenyl Monolayer via Aryldiazonium Ion Grafting: A Well-Defined Reactive Tether Layer for On-Surface Chemistry. Langmuir 2014, 30, 7104. [Google Scholar] [CrossRef] [PubMed]
- Le, X.T.; Viel, P.; Jégou, P.; Garcia, A.; Berthelot, T.; Bui, T.H.; Palacin, S. Diazonium-induced anchoring process: An application to improve the monovalent selectivity of cation exchange membranes. J. Mater. Chem. 2010, 20, 3750. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).