Emerging Food Packaging Applications of Cellulose Nanocomposites: A Review
Abstract
1. Introduction
2. Source and Structure of Cellulose and Its Derived Nanocellulose
2.1. Cellulose Source and Structure
2.1.1. Wood
2.1.2. Plant
2.1.3. Tunicate
2.1.4. Algae
2.1.5. Bacteria
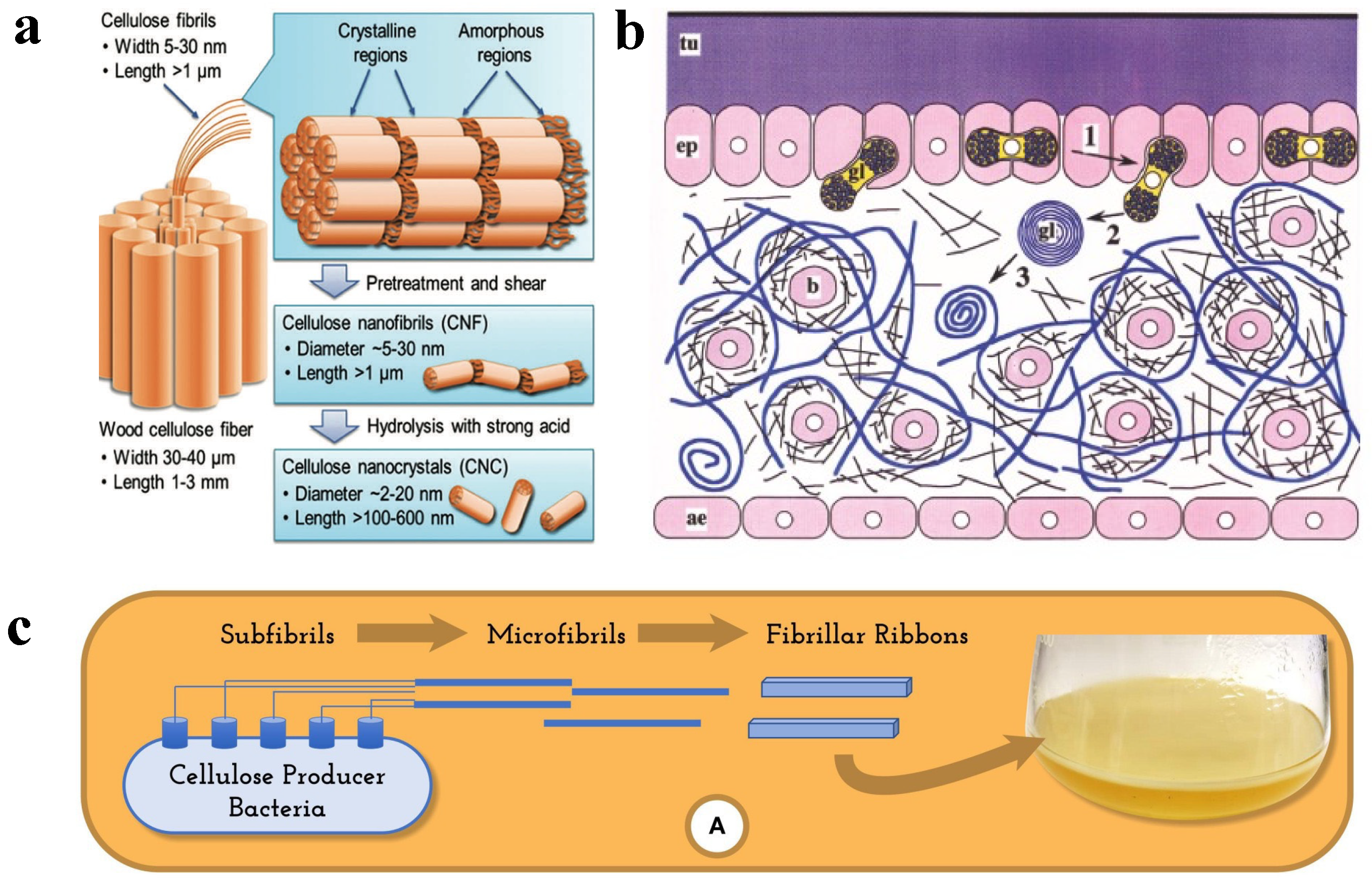
2.2. Nanocellulose Obtained from Different Sources
3. Preparation and Characteristics of Nanocellulose
3.1. Pretreatment of Cellulose Fibers for Nanocellulose Production from Biomass
| Extraction Method | Cellulose | Diameter (nm) | Length (nm) | Crystallinity Index (%) | Degradation Onset Temperature (°C) | Tensile Strength (MPa) | References |
|---|---|---|---|---|---|---|---|
| H2SO4 hydrolysis | Microcrystalline cellulose | 10.8 ± 2.4 | 111.2 ± 25.6 | / | 270 | 114 | [55] |
| HCl hydrolysis | Bleached kraft pulp | 28.5 | 481 | 88.2 | 310 | / | [56] |
| H2SO4 hydrolysis and sonicated | Industrial pepper waste (Piper nigrum L.) | 33.4 ± 11.7 | / | 69.9 | 300.6 | / | [57] |
| HCl hydrolysis and sonication | 50.7 ± 9.6 | / | 73.7 | 291.5 | |||
| H3PO4 hydrolysis, sonicated | 67.8 ± 3.1 | / | 75.8 | 298.3 | |||
| Oxalic acid hydrolysis and sonicated | 21.7 ± 4.9 | / | 77.8 | 311.2 | |||
| Citric acid hydrolysis and sonicated | 23.2 ± 0.6 | 258.8 ± 58.4 | 76.4 | 310.1 | |||
| Acetic acid hydrolysis and sonicated | 48.7 ± 9.4 | 343.7 ± 2.3 | 78.3 | 308.2 | |||
| Alkaline treatment and blending | Oil palm empty fruit bunch | 89 | / | / | / | 33.0 | [58] |
| Disc grinder | Raw wood | 5 ± 3 | / | 67 | 235 | 233 | [59] |
| Ball mill | Raw sisal | 12.35 | / | 53.6% | / | 92.73 | [60] |
| Ultrafine grinder | Unbleached Eucalyptus kraft pulp | 38 ± 16 | 3000 | / | / | / | [61] |
| High pressure homogenization | Cellulose powder (cotton linters) | 46.4 ± 7.5 | 417.7 ± 37.6 | / | 270 | 114 | [55] |
| Homogenizer and sonication | BC (K. oboediens R37-9) | 6.06 ± 0.96 | 815 ± 0.95 | 75.64 | / | 142 | [62] |
| High pressure homogenization | Bleached softwood kraft pulp board | 18.85 ± 4.51 | / | 67.3 | / | 177.99 | [63] |
| Grinder | Hardwood bleached kraft pulp | 5.5 ± 1.6 | / | / | / | / | [64] |
3.1.1. TEMPO Oxidation

3.1.2. Carboxymethylation
3.1.3. Enzymatic Hydrolysis
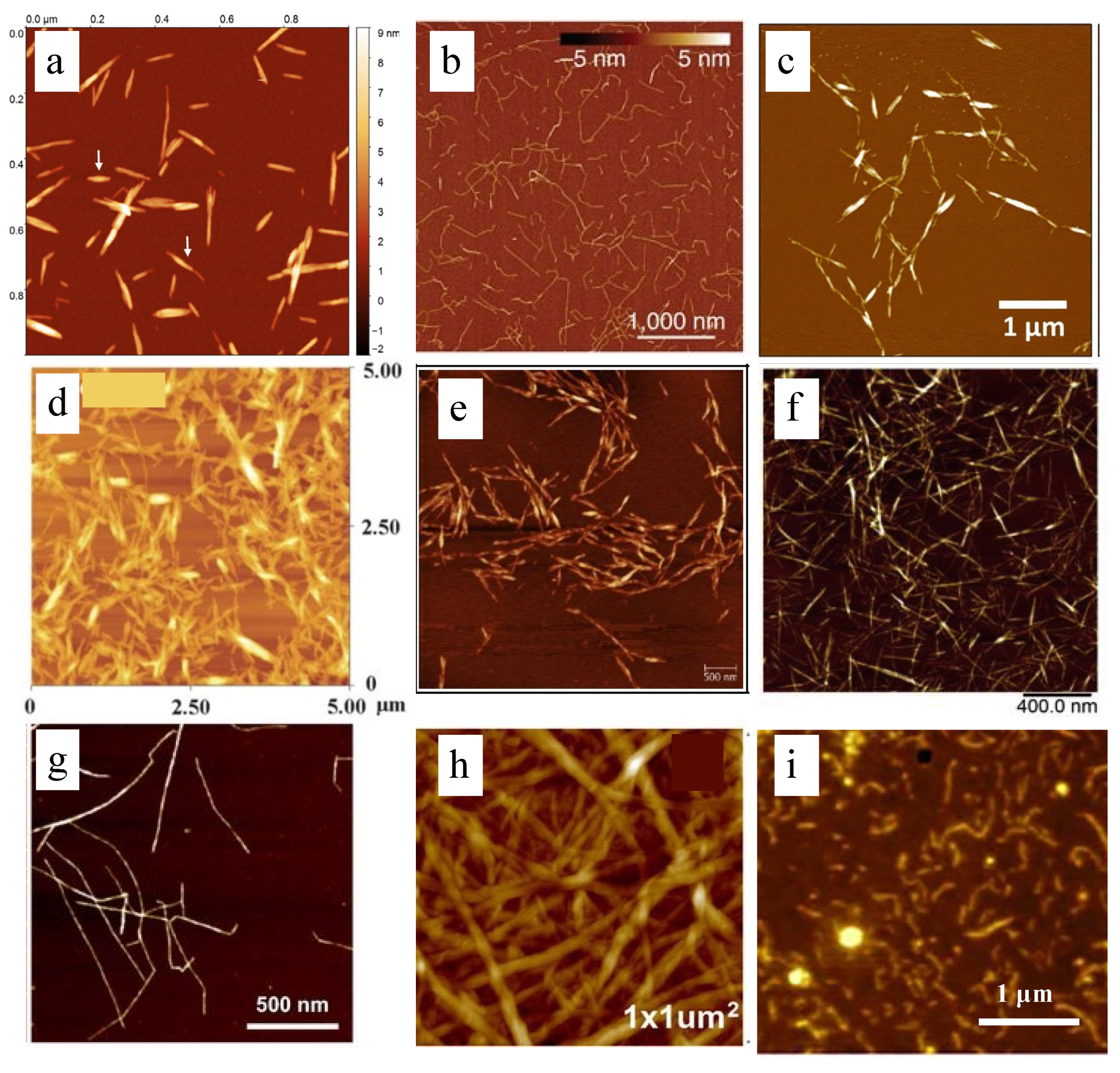
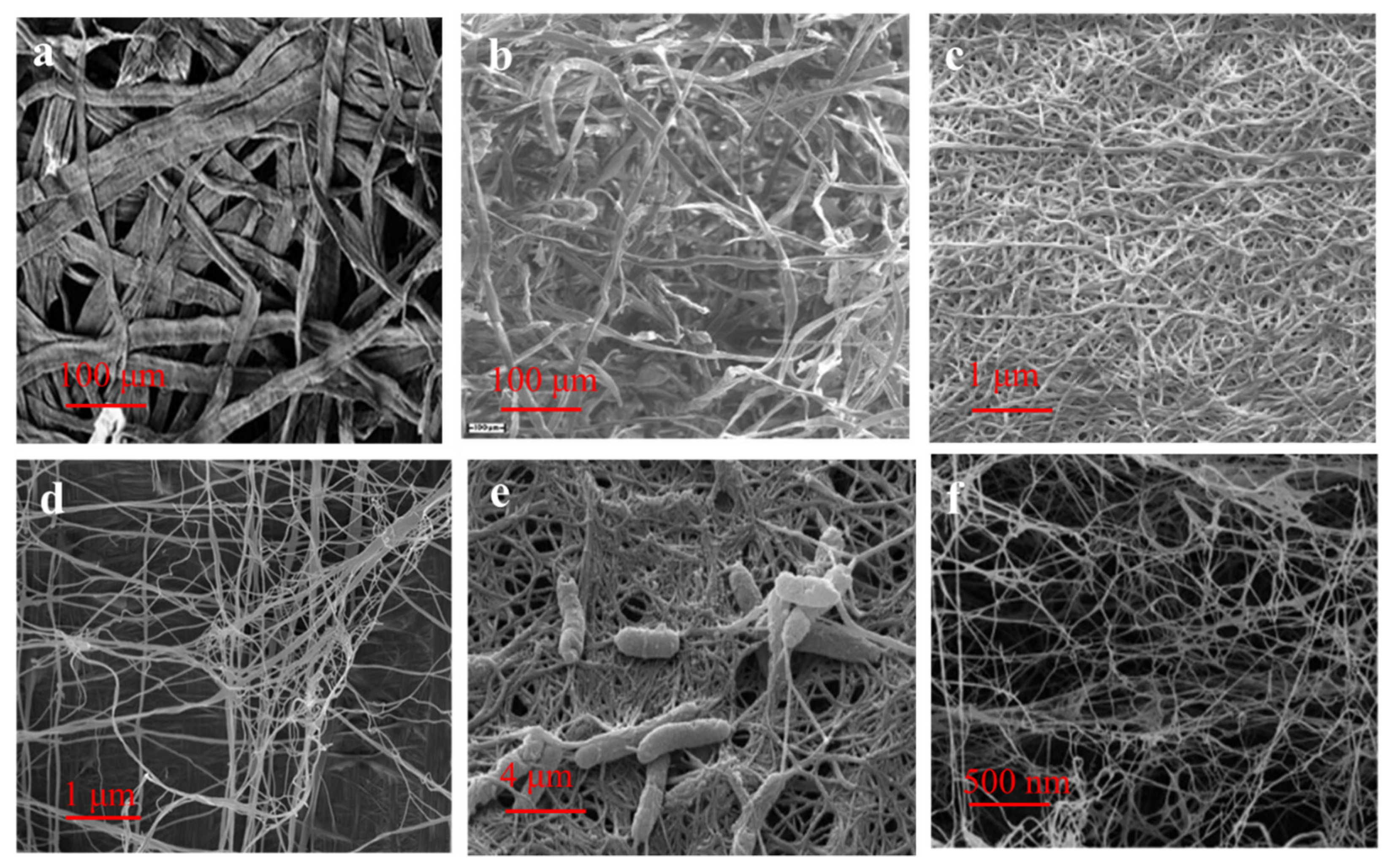
3.2. Extraction Method of Nanocellulose from Biomass
3.2.1. Acid Hydrolysis
Inorganic Acid Hydrolysis
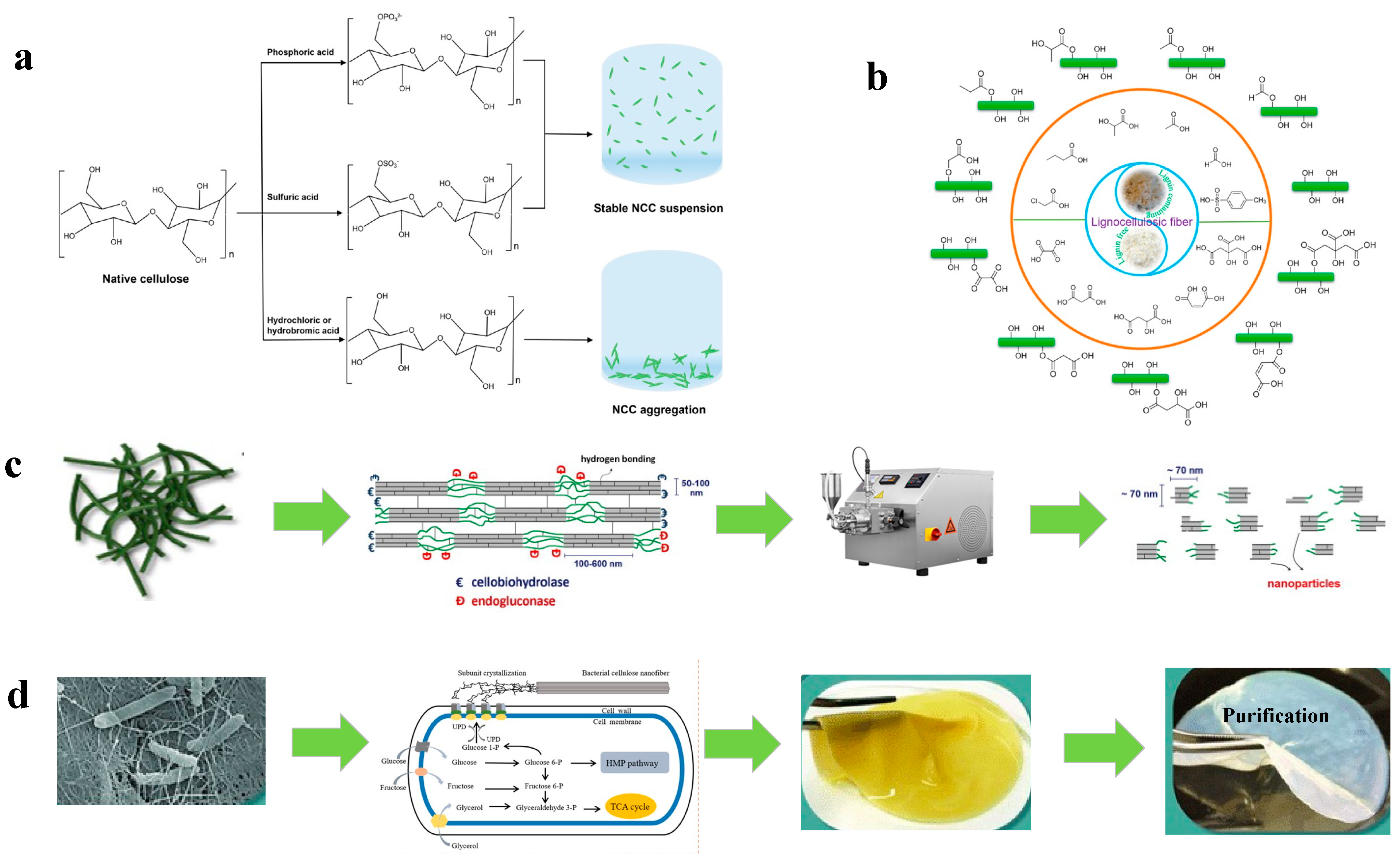
Organic Acid Hydrolysis
3.2.2. Mechanical Process
3.3. Preparation of BC
4. Fabrication Strategies of Cellulose Nanocomposites for Food Packaging
4.1. Solution Casting
4.2. Layer-by-Layer Assembly
4.3. Extrusion
4.4. Direct Coating Method
4.5. Hydrogels
4.6. Spray Drying Method
4.7. Electrostatic Spinning Technology
4.8. Micronanotechnology and Nanoemulsions
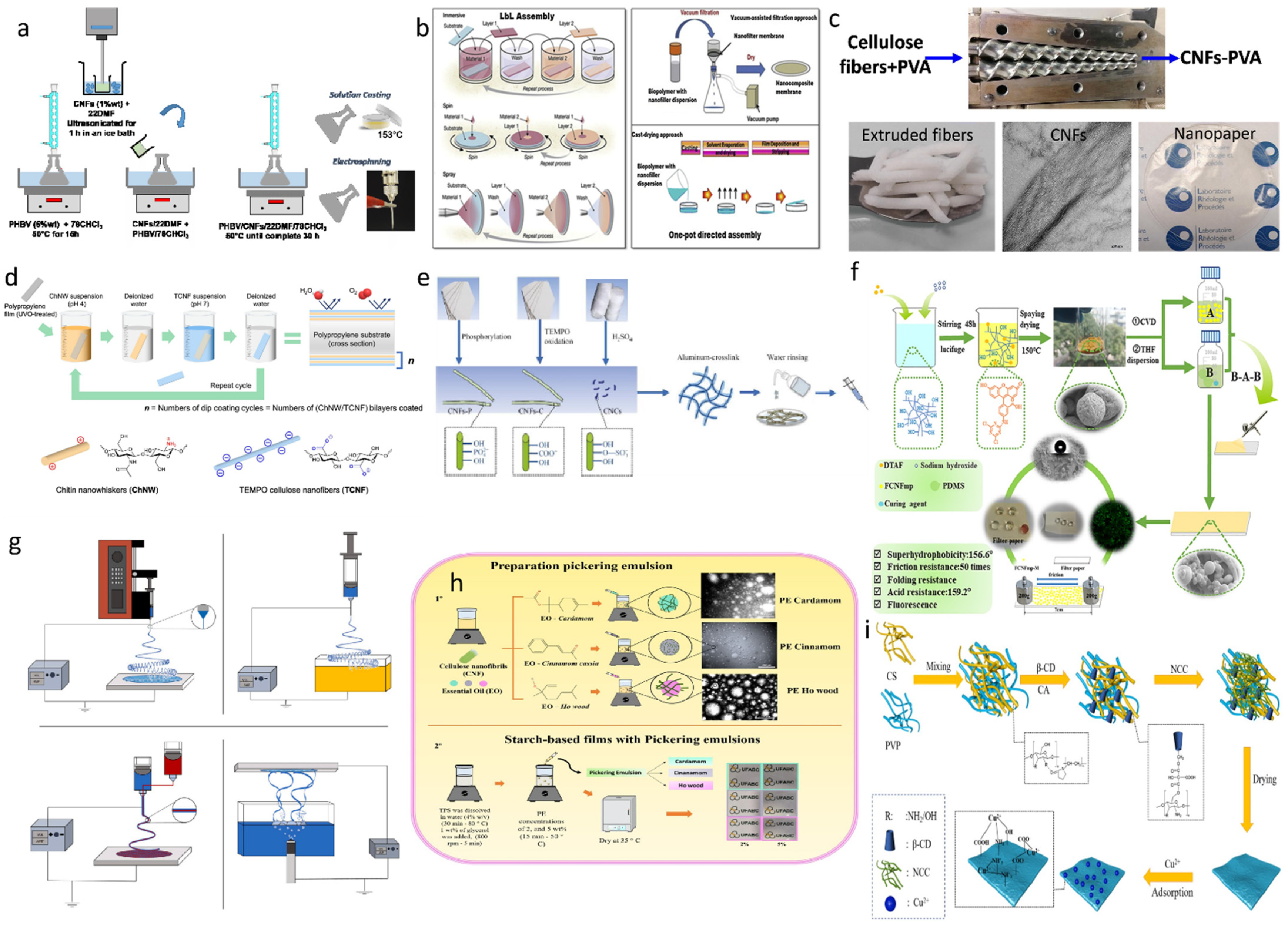
4.9. Adsorption
5. Performance of Nanocellulose-Based Composites as Food Packaging Materials
5.1. Barrier and Mechanical Properties
5.2. Antibacterial Property
5.3. Intelligent Packaging

5.4. Preservation
6. Industrialization of Nanocellulose Production Worldwide
7. Conclusions and Outlook
- Expansion of the cellulose sources to other biomass besides the traditional raw materials, such as tunicate and BC, for higher quality nanocellulose to develop many advanced applications. Until now, the nanocellulose on the market is dominantly produced from wood and other plant-based sources. Though several works of research focused on the nanocellulose preparation from tunicate and BC and demonstrated their better performance than the woody nanocellulose, further investigation on the preparation–characterization–performance correlation of this specific nanocellulose is necessary.
- Development of facial, cost-effective, efficient, and environment-friendly nanocellulose extraction method. Though several novel extraction methods have been developed, sulfuric acid hydrolysis and mechanical refining are still the most widely used ones. However, the harsh acid hydrolysis, high water consumption, huge amount of polluted wastewater, intense energy consumption, and low yield greatly prohibited industrially feasible nanocellulose production. Therefore, more efforts should be put into developing new nanocellulose preparation methods, such as organic acid-based methods, which already showed potential to be a green approach to preparing functionalized nanocellulose.
- Development of new cellulose nanocomposite fabrication approaches. In the lab, the solution casting method is still widely used for research purposes, which is unsuitable for industrial production. In the pilot scale, extrusion is used, though it is not a perfect method since nanocellulose is always dispersed in water, which negatively affects the extrusion performance. Therefore, developing a scalable strategy to prepare nanocellulose-based composites for food packaging materials is vital.
- Improvement of the performance of nanocellulose-based composites as packaging materials. As discussed above, the ideal food packaging materials require UV-proof, gas and vapor barrier properties, excellent mechanical force, and good hydrophobicity. Especially for the last one, new strategies need to be developed to alter the hygroscopic nature of nanocellulose and enhance the wet strength, thus making its applications more practical in daily life. For example, esterification as a pretreatment or coating with natural wax seems suitable to fulfil this purpose.
- Development of nanocellulose-based intelligent packaging materials. Currently, achieving the cellulose nanocomposites’ responsive properties is mainly realized by incorporating various organic and inorganic fillers. However, the release and migration of functional fillers and their potential health risks have not been comprehensively evaluated. Future studies should not only focus on the safety issue of the nanocellulose itself but also on the functional fillers used.
- Design of the food-specific, nanocellulose-based packaging materials. Though many research works generally focused on the preparation and properties of packaging materials, they paid little attention to the interaction between the materials and the food, and even ignored various aspects which would influence the application of the materials. For example, the influence of the environmental conditions on the quality change of both food products and the packaging materials should be investigated to prove the feasibility and suitability of the packaging materials for the specific food.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Alojaly, H.; Benyounis, K.Y. Packaging With Plastics and Polymeric Materials. In Encyclopedia of Materials: Plastics and Polymers; Hashmi, M.S.J., Ed.; Elsevier: Oxford, UK, 2022; pp. 485–501. [Google Scholar]
- Rovera, C.; Türe, H.; Hedenqvist, M.S.; Farris, S. Water vapor barrier properties of wheat gluten/silica hybrid coatings on paperboard for food packaging applications. Food Packag. Shelf Life 2020, 26, 100561. [Google Scholar] [CrossRef]
- Foraboschi, P. Analytical modeling to predict thermal shock failure and maximum temperature gradients of a glass panel. Mater. Des. 2017, 134, 301–319. [Google Scholar] [CrossRef]
- Videira-Quintela, D.; Guillén, F.; Martin, O.; Montalvo, G. Antibacterial LDPE films for food packaging application filled with metal-fumed silica dual-side fillers. Food Packag. Shelf Life 2021, 31, 100772. [Google Scholar] [CrossRef]
- Suleman, R.; Amjad, A.; Ismail, A.; Javed, S.; Ghafoor, U.; Fahad, S. Impact of plastic bags usage in food commodities: An irreversible loss to environment. Environ. Sci. Pollut. Res. 2022, 29, 49483–49489. [Google Scholar] [CrossRef] [PubMed]
- Tedjani, C.F.; Ben Mya, O.; Rebiai, A. Isolation and characterization of cellulose from date palm tree spathe sheath. Sustain. Chem. Pharm. 2020, 17, 100307. [Google Scholar] [CrossRef]
- Gabriel, T.; Belete, A.; Syrowatka, F.; Neubert, R.H.; Gebre-Mariam, T. Extraction and characterization of celluloses from various plant byproducts. Int. J. Biol. Macromol. 2020, 158, 1248–1258. [Google Scholar] [CrossRef]
- Chanthathamrongsiri, N.; Petchsomrit, A.; Leelakanok, N.; Siranonthana, N.; Sirirak, T. The comparison of the properties of nanocellulose isolated from colonial and solitary marine tunicates. Heliyon 2021, 7, e07819. [Google Scholar] [CrossRef]
- Han, J.S.; Kim, S.Y.; Seo, Y.B. Disk-shaped cellulose fibers from red algae, Eucheuma cottonii and its use for high oxygen barrier. Int. J. Biol. Macromol. 2022, 210, 752–758. [Google Scholar] [CrossRef]
- Rastogi, A.; Sahoo, S.; Bandyopadhyay, T.K.; Mukherjee, R.; Banerjee, R. Detailed morphological and kinetic studies of cellulose biosynthesis from Leifsonia soli. Polymer 2022, 242, 124568. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Spiliopoulos, P.; Spirk, S.; Pääkkönen, T.; Viljanen, M.; Svedström, K.; Pitkänen, L.; Awais, M.; Kontturi, E. Visualizing Degradation of Cellulose Nanofibers by Acid Hydrolysis. Biomacromolecules 2021, 22, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Serial, M.; Velichko, E.; Nikolaeva, T.; Adel, R.D.; Terenzi, C.; Bouwman, W.; van Duynhoven, J. High-pressure homogenized citrus fiber cellulose dispersions: Structural characterization and flow behavior. Food Struct. 2021, 30, 100237. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Acharya, S.; Rumi, S.S.; Liyanage, S.; Parajuli, P.; Abidi, N. Cryogenic grinding of cotton fiber cellulose: The effect on physicochemical properties. Carbohydr. Polym. 2022, 289, 119408. [Google Scholar] [CrossRef]
- Qian, M.; Lei, H.; Villota, E.; Zhao, Y.; Wang, C.; Huo, E.; Zhang, Q.; Mateo, W.; Lin, X. High yield production of nanocrystalline cellulose by microwave-assisted dilute-acid pretreatment combined with enzymatic hydrolysis. Chem. Eng. Process. Process Intensif. 2020, 160, 108292. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Nanocellulose for industrial use: Cellulose nanofibers (CNF), cellulose nanocrystals (CNC), and bacterial cellulose (BC). In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 74–126. [Google Scholar]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2020, 6, 124–144. [Google Scholar] [CrossRef]
- Ceccherini, S.; Ståhl, M.; Sawada, D.; Hummel, M.; Maloney, T.C. Effect of Enzymatic Depolymerization of Cellulose and Hemicelluloses on the Direct Dissolution of Prehydrolysis Kraft Dissolving Pulp. Biomacromolecules 2021, 22, 4805–4813. [Google Scholar] [CrossRef]
- Casaburi, A.; Rojo, Ú.M.; Cerrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocoll. 2018, 75, 147–156. [Google Scholar] [CrossRef]
- Baron, R.I.; Coseri, S. Preparation of water-soluble cellulose derivatives using TEMPO radical-mediated oxidation at extended reaction time. React. Funct. Polym. 2020, 157, 104768. [Google Scholar] [CrossRef]
- Boufi, S.; González, I.; Delgado-Aguilar, M.; Tarrès, Q.; Pèlach, M.; Mutjé, P. Nanofibrillated cellulose as an additive in papermaking process: A review. Carbohydr. Polym. 2016, 154, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Parte, F.G.B.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T. Cellulose: Structure and properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Cham, Switzerland, 2015; pp. 1–52. [Google Scholar]
- Mazeau, K.; Heux, L. Molecular Dynamics Simulations of Bulk Native Crystalline and Amorphous Structures of Cellulose. J. Phys. Chem. B 2003, 107, 2394–2403. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Jarvis, M. Cellulose stacks up. Nature 2003, 426, 611–612. [Google Scholar] [CrossRef]
- Islam, M.S.; Chen, L.; Sisler, J.; Tam, K.C. Cellulose nanocrystal (CNC)-inorganic hybrid systems: Synthesis, properties and applications. J. Mater. Chem. B 2018, 6, 864–883. [Google Scholar] [CrossRef]
- Unbehaun, H.; Dittler, B.; Kühne, G.; Wagenführ, A. Investigation into the biotechnological modification of wood and its application in the wood-based material industry. Acta Biotechnol. 2000, 20, 305–312. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites-A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Rajala, S.; Siponkoski, T.; Sarlin, E.; Mettänen, M.; Vuoriluoto, M.; Pammo, A.; Juuti, J.; Rojas, O.J.; Franssila, S.; Tuukkanen, S. Cellulose Nanofibril Film as a Piezoelectric Sensor Material. ACS Appl. Mater. Interfaces 2016, 8, 15607–15614. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.M.; Saxena, I.M. Cellulose: Molecular and Structural Biology; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 106530. [Google Scholar] [CrossRef]
- Tsekos, I. The sites of cellulose synthesis in algae: Diversity and evolution of cellulose-synthesizing enzyme complexes. J. Phycol. 1999, 35, 635–655. [Google Scholar] [CrossRef]
- Hu, S.-Q.; Gao, Y.-G.; Tajima, K.; Sunagawa, N.; Zhou, Y.; Kawano, S.; Fujiwara, T.; Yoda, T.; Shimura, D.; Satoh, Y.; et al. Structure of bacterial cellulose synthase subunit D octamer with four inner passageways. Proc. Natl. Acad. Sci. USA 2010, 107, 17957–17961. [Google Scholar] [CrossRef]
- Sani, A.; Dahman, Y. Improvements in the production of bacterial synthesized biocellulose nanofibres using different culture methods. J. Chem. Technol. Biotechnol. 2009, 85, 151–164. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour. Technol. 2017, 234, 8–14. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Lu, C.; Deng, Y. Aerogels from crosslinked cellulose nano/micro-fibrils and their fast shape recovery property in water. J. Mater. Chem. 2012, 22, 11642–11650. [Google Scholar] [CrossRef]
- Morais, J.P.S.; Rosa, M.F.; Filho, M.M.S.; Nascimento, L.D.; Nascimento, D.M.; Cassales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Lindström, M.E.; Li, J. Tunicate cellulose nanocrystals: Preparation, neat films and nanocomposite films with glucomannans. Carbohydr. Polym. 2015, 117, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Gao, W.; Chen, L.; Lan, W.; Zhu, J.Y.; Runge, T. A comparison of cellulose nanofibrils produced from Cladophora glomerata algae and bleached eucalyptus pulp. Cellulose 2016, 23, 493–503. [Google Scholar] [CrossRef]
- Das, A.A.K.; Bovill, J.; Ayesh, M.; Stoyanov, S.D.; Paunov, V.N. Fabrication of living soft matter by symbiotic growth of unicellular microorganisms. J. Mater. Chem. B 2016, 4, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Ajdary, R.; Abidnejad, R.; Lehtonen, J.; Kuula, J.; Raussi-Lehto, E.; Kankuri, E.; Tardy, B.; Rojas, O.J. Bacterial nanocellulose enables auxetic supporting implants. Carbohydr. Polym. 2022, 284, 119198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J. Unique and outstanding quantum dots (QD)/tunicate cellulose nanofibrils (TCNF) nanohybrid platform material for use as 1D ink and 2D film. Carbohydr. Polym. 2020, 242, 116396. [Google Scholar] [CrossRef]
- Kassab, Z.; Ben Youcef, H.; Hannache, H.; El Achaby, M. Isolation of Cellulose Nanocrystals from Various Lignocellulosic Materials: Physico-chemical characterization and Application in Polymer Composites Development. Mater. Today Proc. 2019, 13, 964–973. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Zhao, Y.; Ma, M.; Song, Y.; Zheng, B.; Zhou, R.; Ostrikov, K. Nisin electroadsorption-enabled multifunctional bacterial cellulose membranes for highly efficient removal of organic and microbial pollutants in water. Chem. Eng. J. 2022, 440, 135922. [Google Scholar] [CrossRef]
- Almeida, T.; Silvestre, A.; Vilela, C.; Freire, C. Bacterial Nanocellulose toward Green Cosmetics: Recent Progresses and Challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef]
- Noremylia, M.; Hassan, M.Z.; Ismail, Z. Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: A review. Int. J. Biol. Macromol. 2022, 206, 954–976. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Kwon, G.; Lee, K.; Kim, D.; Jeon, Y.; Kim, U.-J.; You, J. Cellulose nanocrystal-coated TEMPO-oxidized cellulose nanofiber films for high performance all-cellulose nanocomposites. J. Hazard. Mater. 2020, 398, 123100. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; An, X.; Seta, F.T.; Ma, M.; Shen, M.; Dai, L.; Liu, H.; Ni, Y. Improving dispersion stability of hydrochloric acid hydrolyzed cellulose nano-crystals. Carbohydr. Polym. 2019, 222, 115037. [Google Scholar] [CrossRef] [PubMed]
- Holilah, H.; Bahruji, H.; Ediati, R.; Asranudin, A.; Jalil, A.A.; Piluharto, B.; Nugraha, R.E.; Prasetyoko, D. Uniform rod and spherical nanocrystalline celluloses from hydrolysis of industrial pepper waste (Piper nigrum L.) using organic acid and inorganic acid. Int. J. Biol. Macromol. 2022, 204, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Sukmawan, R.; Kusmono; Rahmanta, A.P.; Saputri, L.H. The effect of repeated alkali pretreatments on the morphological characteristics of cellulose from oil palm empty fruit bunch fiber-reinforced epoxy adhesive composite. Int. J. Adhes. Adhes. 2022, 114, 103095. [Google Scholar] [CrossRef]
- Kaffashsaie, E.; Yousefi, H.; Nishino, T.; Matsumoto, T.; Mashkour, M.; Madhoushi, M.; Kawaguchi, H. Direct conversion of raw wood to TEMPO-oxidized cellulose nanofibers. Carbohydr. Polym. 2021, 262, 117938. [Google Scholar] [CrossRef]
- Yu, W.; Yi, Y.; Wang, H.; Yang, Y.; Zeng, L.; Tan, Z. Light-colored cellulose nanofibrils produced from raw sisal fibers without costly bleaching. Ind. Crop. Prod. 2021, 172, 114009. [Google Scholar] [CrossRef]
- Dias, M.C.; Belgacem, M.N.; de Resende, J.V.; Martins, M.A.; Damásio, R.A.P.; Tonoli, G.H.D.; Ferreira, S.R. Eco-friendly laccase and cellulase enzymes pretreatment for optimized production of high content lignin-cellulose nanofibrils. Int. J. Biol. Macromol. 2022, 209, 413–425. [Google Scholar] [CrossRef]
- Chitbanyong, K.; Pisutpiched, S.; Khantayanuwong, S.; Theeragool, G.; Puangsin, B. TEMPO-oxidized cellulose nanofibril film from nano-structured bacterial cellulose derived from the recently developed thermotolerant Komagataeibacter xylinus C30 and Komagataeibacter oboediens R37–9 strains. Int. J. Biol. Macromol. 2020, 163, 1908–1914. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, S.; Zhao, P.; Wu, M.; Song, X.; Ragauskas, A.J. Effect of endoglucanase and high-pressure homogenization post-treatments on mechanically grinded cellulose nanofibrils and their film performance. Carbohydr. Polym. 2020, 253, 117253. [Google Scholar] [CrossRef]
- Song, H.Y.; Park, S.Y.; Kim, S.; Youn, H.J.; Hyun, K. Linear and nonlinear oscillatory rheology of chemically pretreated and non-pretreated cellulose nanofiber suspensions. Carbohydr. Polym. 2021, 275, 118765. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, Y.; Guo, Y.; Yue, J. Isolation and Characterization of Nanocellulose with a Novel Shape from Walnut (Juglans regia L.) Shell Agricultural Waste. Polymers 2019, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-J.; Huang, P.-L.; Chen, J.-H.; Wu, Y.-Y.; Liu, Q.-Y.; Sun, R.-C. Comparison of acid-hydrolyzed and TEMPO-oxidized nanocellulose for reinforcing alginate fibers. BioResources 2017, 12, 8180–8198. [Google Scholar] [CrossRef]
- Xu, R.; Du, H.; Wang, H.; Zhang, M.; Wu, M.; Liu, C.; Yu, G.; Zhang, X.; Si, C.; Choi, S.-E.; et al. Valorization of Enzymatic Hydrolysis Residues from Corncob into Lignin-Containing Cellulose Nanofibrils and Lignin Nanoparticles. Front. Bioeng. Biotechnol. 2021, 9, 677963. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Chen, J.; Chen, D.; Chai, X.; He, L.; Peng, L.; Zhang, J.; Li, J. A new sight on the catalytic oxidation kinetic behaviors of bamboo cellulose fibers under TEMPO-oxidized system: The fate of carboxyl groups in treated pulps. J. Catal. 2019, 370, 304–309. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Zhou, Z.; Xia, K.; Liu, T.; Guo, H.; Liu, X.; Zhang, X. Preparation of carboxymethyl cellulose nanofibers and their application in warp size of textile. Int. J. Biol. Macromol. 2022, 207, 40–47. [Google Scholar] [CrossRef]
- Phirom-On, K.; Apiraksakorn, J. Development of cellulose-based prebiotic fiber from banana peel by enzymatic hydrolysis. Food Biosci. 2021, 41, 101083. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Wang, Z.; Xie, J. Structure, function and food applications of carboxymethylated polysaccharides: A comprehensive review. Trends Food Sci. Technol. 2021, 118, 539–557. [Google Scholar] [CrossRef]
- Pinto, E.; Aggrey, W.N.; Boakye, P.; Amenuvor, G.; Sokama-Neuyam, Y.A.; Fokuo, M.K.; Karimaie, H.; Sarkodie, K.; Adenutsi, C.D.; Erzuah, S.; et al. Cellulose processing from biomass and its derivatization into carboxymethylcellulose: A review. Sci. Afr. 2021, 15, e01078. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Etherification of Cellulose. In Cellulose Derivatives; Springer: Berlin/Heidelberg, Germany, 2018; pp. 429–477. [Google Scholar] [CrossRef]
- Toğrul, H.; Arslan, N. Production of carboxymethyl cellulose from sugar beet pulp cellulose and rheological behaviour of carboxymethyl cellulose. Carbohydr. Polym. 2003, 54, 73–82. [Google Scholar] [CrossRef]
- Yeasmin, M.S.; Mondal, M.I.H. Synthesis of highly substituted carboxymethyl cellulose depending on cellulose particle size. Int. J. Biol. Macromol. 2015, 80, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Q.; Pan, Y.; Wei, S.; Xia, Q.; Liu, S.; Ji, H.; Deng, C.; Hao, J. Investigation on the correlation between changes in water and texture properties during the processing of surimi from golden pompano (Trachinotus ovatus). J. Food Sci. 2021, 86, 376–384. [Google Scholar] [CrossRef]
- Dinesh, G.; Kandasubramanian, B. Fabrication of transparent paper devices from nanocellulose fiber. Mater. Chem. Phys. 2022, 281, 125707. [Google Scholar] [CrossRef]
- Arantes, V.; Dias, I.K.R.; Berto, G.L.; Pereira, B.; Marotti, B.S.; Nogueira, C.F.O. The current status of the enzyme-mediated isolation and functionalization of nanocelluloses: Production, properties, techno-economics, and opportunities. Cellulose 2020, 27, 10571–10630. [Google Scholar] [CrossRef]
- Chen, M.; Parot, J.; Hackley, V.A.; Zou, S.; Johnston, L.J. AFM characterization of cellulose nanocrystal height and width using internal calibration standards. Cellulose 2021, 28, 1933–1946. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure–properties relationship at the single fibril level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Reid, M.S.; Bras, J.; Heux, L.; Godoy-Vargas, J.; Panga, M.K.R.; Cranston, E.D. Insight into thermal stability of cellulose nanocrystals from new hydrolysis methods with acid blends. Cellulose 2018, 26, 507–528. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Wang, R.; Chen, L.; Zhu, J.; Yang, R. Tailored and Integrated Production of Carboxylated Cellulose Nanocrystals (CNC) with Nanofibrils (CNF) through Maleic Acid Hydrolysis. ChemNanoMat 2017, 3, 328–335. [Google Scholar] [CrossRef]
- Henschen, J.; Li, D.; Ek, M. Preparation of cellulose nanomaterials via cellulose oxalates. Carbohydr. Polym. 2019, 213, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Nakamura, Y.; Zhou, Y.; Horikawa, Y.; Isogai, A. Linear and branched structures present in high-molar-mass fractions in holocelluloses prepared from chara, haircap moss, adiantum, ginkgo, Japanese cedar, and eucalyptus. Cellulose 2021, 28, 3935–3949. [Google Scholar] [CrossRef]
- Song, J.; Yang, F.; Zhang, Y.; Hu, F.; Wu, S.; Jin, Y.; Guo, J.; Rojas, O.J. Interactions between fungal cellulases and films of nanofibrillar cellulose determined by a quartz crystal microbalance with dissipation monitoring (QCM-D). Cellulose 2017, 24, 1947–1956. [Google Scholar] [CrossRef]
- Marakana, P.G.; Dey, A.; Saini, B. Isolation of nanocellulose from lignocellulosic biomass: Synthesis, characterization, modification, and potential applications. J. Environ. Chem. Eng. 2021, 9, 106606. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Zheng, D.; Li, M.; Yue, J. Isolation and characterization of nanocellulose crystals via acid hydrolysis from agricultural waste-tea stalk. Int. J. Biol. Macromol. 2020, 163, 927–933. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Xu, M.; Chen, J.; Shi, Y.; Huang, C.; Wang, S.; An, S.; Li, C. Preparation and mechanical properties of modified nanocellulose/PLA composites from cassava residue. AIP Adv. 2018, 8, 025116. [Google Scholar] [CrossRef]
- Lin, K.-H.; Enomae, T.; Chang, F.-C. Cellulose Nanocrystal Isolation from Hardwood Pulp using Various Hydrolysis Conditions. Molecules 2019, 24, 3724. [Google Scholar] [CrossRef]
- Hastuti, N.; Kanomata, K.; Kitaoka, T. Hydrochloric Acid Hydrolysis of Pulps from Oil Palm Empty Fruit Bunches to Produce Cellulose Nanocrystals. J. Polym. Environ. 2018, 26, 3698–3709. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Le Gars, M.; Douard, L.; Belgacem, N.; Bras, J. Cellulose Nanocrystals: From Classical Hydrolysis to the Use of Deep Eutectic Solvents. In Smart Nanosystems for Biomedicine, Optoelectronics and Catalysis; IntechOpen: London, UK, 2020. [Google Scholar]
- Septevani, A.A.; Rifathin, A.; Sari, A.A.; Sampora, Y.; Ariani, G.N.; Sudiyarmanto; Sondari, D. Oil palm empty fruit bunch-based nanocellulose as a super-adsorbent for water remediation. Carbohydr. Polym. 2019, 229, 115433. [Google Scholar] [CrossRef] [PubMed]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.-Y.; Lee, C.Y.; Goh, C.F. Nanocrystalline cellulose isolation via acid hydrolysis from non-woody biomass: Importance of hydrolysis parameters. Carbohydr. Polym. 2022, 286, 119285. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef] [PubMed]
- Chiulan, I.; Panaitescu, D.M.; Radu, E.-R.; Vizireanu, S.; Sătulu, V.; Biţă, B.; Gabor, R.A.; Nicolae, C.A.; Raduly, M.; Rădiţoiu, V. Influence of TEMPO oxidation on the properties of ethylene glycol methyl ether acrylate grafted cellulose sponges. Carbohydr. Polym. 2021, 272, 118458. [Google Scholar] [CrossRef]
- Chen, C.; Ding, W.; Zhang, H.; Zhang, L.; Huang, Y.; Fan, M.; Yang, J.; Sun, D. Bacterial cellulose-based biomaterials: From fabrication to application. Carbohydr. Polym. 2021, 278, 118995. [Google Scholar] [CrossRef]
- Wang, H.; Du, H.; Liu, K.; Liu, H.; Xu, T.; Zhang, S.; Chen, X.; Zhang, R.; Li, H.; Xie, H.; et al. Sustainable preparation of bifunctional cellulose nanocrystals via mixed H2SO4/formic acid hydrolysis. Carbohydr. Polym. 2021, 266, 118107. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef]
- Teo, H.L.; Wahab, R.A. Towards an eco-friendly deconstruction of agro-industrial biomass and preparation of renewable cellulose nanomaterials: A review. Int. J. Biol. Macromol. 2020, 161, 1414–1430. [Google Scholar] [CrossRef]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. Nanocellulose Preparation methods and applications. In Cellulose-Reinforced Nanofibre Composites; WP Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Naderi, A.; Lindström, T.; Sundström, J. Carboxymethylated nanofibrillated cellulose: Rheological studies. Cellulose 2014, 21, 1561–1571. [Google Scholar] [CrossRef]
- Kondo, T.; Kose, R.; Naito, H.; Kasai, W. Aqueous counter collision using paired water jets as a novel means of preparing bio-nanofibers. Carbohydr. Polym. 2014, 112, 284–290. [Google Scholar] [CrossRef]
- Yokota, S.; Tagawa, S.; Kondo, T. Facile surface modification of amphiphilic cellulose nanofibrils prepared by aqueous counter collision. Carbohydr. Polym. 2020, 255, 117342. [Google Scholar] [CrossRef]
- Ishida, K.; Yokota, S.; Kondo, T. Localized surface acetylation of aqueous counter collision cellulose nanofibrils using a Pickering emulsion as an interfacial reaction platform. Carbohydr. Polym. 2021, 261, 117845. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-M.; Chen, J.-H.; Nagarajan, D.; Lee, C.-K.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Immobilization of Chlorella sorokiniana AK-1 in bacterial cellulose by co-culture and its application in wastewater treatment. J. Taiwan Inst. Chem. Eng. 2022; in press. [Google Scholar] [CrossRef]
- Nascimento, E.S.; Barros, M.O.; Cerqueira, M.A.; Lima, H.L.; Borges, M.D.F.; Pastrana, L.M.; Gama, F.M.; Rosa, M.F.; Azeredo, H.M.; Gonçalves, C. All-cellulose nanocomposite films based on bacterial cellulose nanofibrils and nanocrystals. Food Packag. Shelf Life 2021, 29, 100715. [Google Scholar] [CrossRef]
- Nam, J.; Hyun, Y.; Oh, S.; Park, J.; Jin, H.-J.; Kwak, H.W. Effect of cross-linkable bacterial cellulose nanocrystals on the physicochemical properties of silk sericin films. Polym. Test. 2021, 97, 107161. [Google Scholar] [CrossRef]
- Vedove, T.M.; Maniglia, B.C.; Tadini, C.C. Production of sustainable smart packaging based on cassava starch and anthocyanin by an extrusion process. J. Food Eng. 2020, 289, 110274. [Google Scholar] [CrossRef]
- Lin, S.-P.; Huang, S.-H.; Ting, Y.; Hsu, H.-Y.; Cheng, K.-C. Evaluation of detoxified sugarcane bagasse hydrolysate by atmospheric cold plasma for bacterial cellulose production. Int. J. Biol. Macromol. 2022, 204, 136–143. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2020, 113, 106530. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, K.; Chang, Y.-H.; Chiu, F.-C. Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers 2020, 12, 202. [Google Scholar] [CrossRef]
- Shen, S.; Wang, N.; Jia, J.; Song, D.; Zuo, T.; Liu, K.; Che, Q. Constructing the basal nanofibers suit of layer-by-layer self-assembly membranes as anion exchange membranes. J. Mol. Liq. 2022, 350, 118536. [Google Scholar] [CrossRef]
- Dhar, P.; Gaur, S.S.; Soundararajan, N.; Gupta, A.; Bhasney, S.M.; Milli, M.; Kumar, A.; Katiyar, V. Reactive Extrusion of Polylactic Acid/Cellulose Nanocrystal Films for Food Packaging Applications: Influence of Filler Type on Thermomechanical, Rheological, and Barrier Properties. Ind. Eng. Chem. Res. 2017, 56, 4718–4735. [Google Scholar] [CrossRef]
- Wang, W.; Du, G.; Li, C.; Zhang, H.; Long, Y.; Ni, Y. Preparation of cellulose nanocrystals from asparagus (Asparagus officinalis L.) and their applications to palm oil/water Pickering emulsion. Carbohydr. Polym. 2016, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Wang, Y.; Luo, X.; Li, Y.; Li, B.; Wang, J.; Liu, S. Surface modification of cellulose nanofibrils with protein nanoparticles for enhancing the stabilization of O/W pickering emulsions. Food Hydrocoll. 2019, 97, 105180. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid/chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W. Fabrication of antibacterial chitosan-PVA blended film using electrospray technique for food packaging applications. Int. J. Biol. Macromol. 2018, 107, 848–854. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Fabrication of silver nanoparticles embedded into polyvinyl alcohol (Ag/PVA) composite nanofibrous films through electrospinning for antibacterial and surface-enhanced Raman scattering (SERS) activities. Mater. Sci. Eng. C 2016, 69, 462–469. [Google Scholar] [CrossRef]
- Padrão, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Dourado, F. Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocoll. 2016, 58, 126–140. [Google Scholar] [CrossRef]
- Patel, A.S.; Lakshmibalasubramaniam, S.; Nayak, B.; Tripp, C.; Kar, A.; Sappati, P.K. Improved stability of phycobiliprotein within liposome stabilized by polyethylene glycol adsorbed cellulose nanocrystals. Int. J. Biol. Macromol. 2020, 163, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Krstić, M.; Radojević, M.; Stojanović, D.; Radojević, V.; Uskoković, P.; Ibrić, S. Formulation and characterization of nanofibers and films with carvedilol prepared by electrospinning and solution casting method. Eur. J. Pharm. Sci. 2017, 101, 160–166. [Google Scholar] [CrossRef]
- Oldoni, F.C.; Bernardo, M.P.; Filho, J.G.O.; de Aguiar, A.C.; Moreira, F.K.; Mattoso, L.H.; Colnago, L.A.; Ferreira, M.D. Valorization of mangoes with internal breakdown through the production of edible films by continuous solution casting. LWT 2021, 145, 111339. [Google Scholar] [CrossRef]
- Rajeswari, A.; Christy, E.J.S.; Swathi, E.; Pius, A. Fabrication of improved cellulose acetate-based biodegradable films for food packaging applications. Environ. Chem. Ecotoxicol. 2020, 2, 107–114. [Google Scholar] [CrossRef]
- Koca, N.; Bayramoğlu, B. Layer-by-layer assembly of lysozyme with iota-carrageenan and gum Arabic for surface modification of food packaging materials with improved barrier properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128391. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef]
- Thomas, G.; Sathish Kumar, K.; Panda, S.K.; Bindu, J. Nanocellulose Incorporated Polylactic Acid Films for Chilled Preservation of Indian Anchovy (Stolephorus indicus) (van Hasselt, 1823); Society of Fisheries Technologists: Kerala, India, 2021. [Google Scholar]
- Karkhanis, S.S.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Potential of extrusion-blown poly(lactic acid)/cellulose nanocrystals nanocomposite films for improving the shelf-life of a dry food product. Food Packag. Shelf Life 2021, 29, 100689. [Google Scholar] [CrossRef]
- Karkhanis, S.S.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Water vapor and oxygen barrier properties of extrusion-blown poly(lactic acid)/cellulose nanocrystals nanocomposite films. Compos. Part A Appl. Sci. Manuf. 2018, 114, 204–211. [Google Scholar] [CrossRef]
- Quintana, S.E.; Llalla, O.; García-Risco, M.R.; Fornari, T. Comparison between essential oils and supercritical extracts into chitosan-based edible coatings on strawberry quality during cold storage. J. Supercrit. Fluids 2021, 171, 105198. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A novel pH-sensing indicator based on bacterial cellulose nanofibers and black carrot anthocyanins for monitoring fish freshness. Carbohydr. Polym. 2019, 222, 115030. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Terol, J.; Fages, E.; Balart, R.; Quiles-Carrillo, L.; Prieto, C.; Torres-Giner, S. Microencapsulation of Copper(II) Sulfate in Ionically Cross-Linked Chitosan by Spray Drying for the Development of Irreversible Moisture Indicators in Paper Packaging. Polymers 2020, 12, 2039. [Google Scholar] [CrossRef]
- Pasaoglu, M.E.; Koyuncu, I. Substitution of petroleum-based polymeric materials used in the electrospinning process with nanocellulose: A review and future outlook. Chemosphere 2020, 269, 128710. [Google Scholar] [CrossRef] [PubMed]
- Leena, M.M.; Yoha, K.; Moses, J.; Anandharamakrishnan, C. Edible coating with resveratrol loaded electrospun zein nanofibers with enhanced bioaccessibility. Food Biosci. 2020, 36, 100669. [Google Scholar] [CrossRef]
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Molet-Rodríguez, A.; Turmo-Ibarz, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Incorporation of antimicrobial nanoemulsions into complex foods: A case study in an apple juice-based beverage. LWT 2021, 141, 110926. [Google Scholar] [CrossRef]
- Benini, K.C.C.D.C.; Cioffi, M.O.H.; Voorwald, H.J.C. PHBV/cellulose nanofibrils composites obtained by solution casting and electrospinning process. In Proceedings of the 3rd Brazilian Conference on Composite Materials, Gramado, Rio Grande do Sul, Brazil, 28–31 August 2016. [Google Scholar] [CrossRef]
- Trigui, K.; Magnin, A.; Putaux, J.-L.; Boufi, S. Twin-screw extrusion for the production of nanocellulose-PVA gels with a high solid content. Carbohydr. Polym. 2022, 286, 119308. [Google Scholar] [CrossRef]
- Nguyen, H.-L.; Tran, T.H.; Hao, L.T.; Jeon, H.; Koo, J.M.; Shin, G.; Hwang, D.S.; Hwang, S.Y.; Park, J.; Oh, D.X. Biorenewable, transparent, and oxygen/moisture barrier nanocellulose/nanochitin-based coating on polypropylene for food packaging applications. Carbohydr. Polym. 2021, 271, 118421. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, R.; Wu, J.; Penkova, A.; Qi, W.; He, Z.; Su, R. Injectable self-healing nanocellulose hydrogels crosslinked by aluminum: Cellulose nanocrystals vs. cellulose nanofibrils. Chin. J. Chem. Eng. 2022 in press. [CrossRef]
- Yi, K.; Fu, S.; Huang, Y. Nanocellulose-based superhydrophobic coating with acid resistance and fluorescence. Prog. Org. Coat. 2022, 168, 106911. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-based films enriched with nanocellulose-stabilized Pickering emulsions containing different essential oils for possible applications in food packaging. Food Packag. Shelf Life 2020, 27, 100615. [Google Scholar] [CrossRef]
- Zeng, H.; Hao, H.; Wang, X.; Shao, Z. Chitosan-based composite film adsorbents reinforced with nanocellulose for removal of Cu(II) ion from wastewater: Preparation, characterization, and adsorption mechanism. Int. J. Biol. Macromol. 2022, 213, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Na, Z.; Xi, C.; Xiaoyun, X.; Siyi, P.; Lueng, W. Development situation and prospects of food lightweight packaging materia. Sci. Technol. Foood Ind. 2014, 35, 363–367. [Google Scholar]
- Bharimalla, A.K.; Deshmukh, S.P.; Vigneshwaran, N.; Patil, P.G.; Prasad, V. Nanocellulose Based Polymer Composites for Applications in Food Packaging:Future Prospects and Challenges. Polym. Plast. Technol. Eng. 2017, 56, 805–823. [Google Scholar] [CrossRef]
- Shi, H.; Wu, L.; Luo, Y.; Yu, F.; Li, H. A facile method to prepare cellulose fiber-based food packaging papers with improved mechanical strength, enhanced barrier, and antibacterial properties. Food Biosci. 2022, 48, 101729. [Google Scholar] [CrossRef]
- Salim, M.H.; Kassab, Z.; Abdellaoui, Y.; García-Cruz, A.; Soumare, A.; Ablouh, E.-H.; El Achaby, M. Exploration of multifunctional properties of garlic skin derived cellulose nanocrystals and extracts incorporated chitosan biocomposite films for active packaging application. Int. J. Biol. Macromol. 2022, 210, 639–653. [Google Scholar] [CrossRef]
- Thongsrikhem, N.; Taokaew, S.; Sriariyanun, M.; Kirdponpattara, S. Antibacterial activity in gelatin-bacterial cellulose composite film by thermally crosslinking with cinnamaldehyde towards food packaging application. Food Packag. Shelf Life 2021, 31, 100766. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohydr. Polym. 2020, 252, 117156. [Google Scholar] [CrossRef]
- Dai, Q.; Huang, X.; Jia, R.; Fang, Y.; Qin, Z. Development of antibacterial film based on alginate fiber, and peanut red skin extract for food packaging. J. Food Eng. 2022, 330, 111106. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of Echium amoenum anthocyanins into bacterial cellulose film: A novel colorimetric pH indicator for freshness/spoilage monitoring of shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Yang, J.-X.; Liu, E.-J.; Hu, R.-Z.; Yao, X.-H.; Chen, T.; Zhao, W.-G.; Liu, L.; Fu, Y.-J. Soft and elastic silver nanoparticle-cellulose sponge as fresh-keeping packaging to protect strawberries from physical damage and microbial invasion. Int. J. Biol. Macromol. 2022, 211, 470–480. [Google Scholar] [CrossRef]
- Thivya, P.; Bhosale, Y.; Anandakumar, S.; Hema, V.; Sinija, V. Development of active packaging film from sodium alginate/carboxymethyl cellulose containing shallot waste extracts for anti-browning of fresh-cut produce. Int. J. Biol. Macromol. 2021, 188, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, F.; Xie, X.; Xie, C.; Li, A.; Xia, N.; Gong, X.; Zhang, H. Development and characterization of chitosan/guar gum active packaging containing walnut green husk extract and its application on fresh-cut apple preservation. Int. J. Biol. Macromol. 2022, 209, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Lema, S.; Nilsson, K.; Trifol, J.; Langton, M.; Gomez-Caturla, J.; Balart, R.; Garcia-Garcia, D.; Moriana, R. Faba bean protein films reinforced with cellulose nanocrystals as edible food packaging material. Food Hydrocoll. 2021, 121, 107019. [Google Scholar] [CrossRef]
- Dissanayake, T.; Chang, B.P.; Mekonnen, T.H.; Ranadheera, C.S.; Narvaez-Bravo, C.; Bandara, N. Reinforcing canola protein matrix with chemically tailored nanocrystalline cellulose improves the functionality of canola protein-based packaging materials. Food Chem. 2022, 383, 132618. [Google Scholar] [CrossRef] [PubMed]
- Trifol, J.; Moriana, R. Barrier packaging solutions from residual biomass: Synergetic properties of CNF and LCNF in films. Ind. Crop. Prod. 2022, 177, 114493. [Google Scholar] [CrossRef]
- Shi, C.; Ji, Z.; Zhang, J.; Jia, Z.; Yang, X. Preparation and characterization of intelligent packaging film for visual inspection of tilapia fillets freshness using cyanidin and bacterial cellulose. Int. J. Biol. Macromol. 2022, 205, 357–365. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Priyadarshi, R.; Han, S. Cellulose nanofiber-based coating film integrated with nitrogen-functionalized carbon dots for active packaging applications of fresh fruit. Postharvest Biol. Technol. 2022, 186, 111845. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, Z.; Xie, F.; Tang, S.; Fang, J.; Wang, X. 3D printed nanocellulose-based label for fruit freshness keeping and visual monitoring. Carbohydr. Polym. 2021, 273, 118545. [Google Scholar] [CrossRef]
- Haishun, D.; Chao, L.; Miaomiao, Z.; Qingshan, K.; Bin, L.; Mo, X. Preparation and industrialization status of nanocellulose. Prog. Chem. 2018, 30, 448. [Google Scholar]
- Nelson, K.; Retsina, T.; Iakovlev, M.; Van Heiningen, A.; Deng, Y.; Shatkin, J.A.; Mulyadi, A. American Process: Production of Low Cost Nanocellulose for Renewable, Advanced Materials Applications. In Artificial Intelligence for Materials Science; Springer: Berlin/Heidelberg, Germany, 2016; pp. 267–302. [Google Scholar]
- Bondancia, T.J.; de Aguiar, J.; Batista, G.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Production of Nanocellulose Using Citric Acid in a Biorefinery Concept: Effect of the Hydrolysis Reaction Time and Techno-Economic Analysis. Ind. Eng. Chem. Res. 2020, 59, 11505–11516. [Google Scholar] [CrossRef]
- Ioelovich, M.J. Microcellulose Vs Nanocellulose–A Review. World J. Adv. Eng. Technol. Sci. 2022, 5, 001–015. [Google Scholar] [CrossRef]
- Amoroso, M.; Apelgren, P.; Säljö, K.; Montelius, M.; Orrhult, L.S.; Engström, M.; Gatenholm, P.; Kölby, L. Functional and morphological studies of in vivo vascularization of 3D-bioprinted human fat grafts. Bioprinting 2021, 23, e00162. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Isogai, A. Emerging Nanocellulose Technologies: Recent Developments. Adv. Mater. 2020, 33, 630. [Google Scholar] [CrossRef]
- Lyne, B. Market prospects for nanocellulose. In The Royal Institute of Technology; Alberta Biomaterials Development Centre: Edmunton, AB, Canada, 2013. [Google Scholar]
- Isogai, A. Cellulose Nanofibers: Recent Progress and Future Prospects. J. Fiber Sci. Technol. 2020, 76, 310–326. [Google Scholar] [CrossRef]
- Tom, L.; Christian, A.; Magnus, G.; Torgny, P. The Emergence of Practical Nanocellulose Applications For A More Sustainable Paper/Board Industry. Q. J. Indian Pulp Pap. Technol. Assoc. 2015, 1, 53–61. [Google Scholar]
- Härkönen, M.; Tammelin, T.; Lille, M.; Qvintus, P.; Laine, J.; Koskinen, T.M. The Nanocellulose Challenge. In Proceedings of the 2009 Wood and Fiber Product Seminar, Espoo, Finland, 22–23 September 2009; p. 59. [Google Scholar]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Hawkins, K.; Lewis, A.; Maffeis, T.; Charbonneau, C.; Gazze, A.; Francis, L.W.; Iakovlev, M.; et al. Characterization of pulp derived nanocellulose hydrogels using AVAP® technology. Carbohydr. Polym. 2018, 198, 270–280. [Google Scholar] [CrossRef]

| Source | Length (nm) | Width (nm) | Crystallinity Index (%) | References |
|---|---|---|---|---|
| Softwood | 483 ± 232 | 4.1 ± 1.2 | / | [49] |
| Cotton linter | 177 | 12 | 90.45 | [44] |
| Algae | 315 ± 30 | 9 ± 3 | 81 | [50] |
| Bacteria | / | 64.6 ± 15.3 | 90.3 | [51] |
| Tunicate | 2100 ± 700 | 8.7 ± 2.4 | / | [49] |
| Culture Medium | Bacteria | Conditions | Characteristics | References |
|---|---|---|---|---|
| Wine pomace | Komagataeibacter rhaeticus K15 | 30 °C, 10 days | Yield 1.95 ± 0.22 g/L, nanocellulose concentration 91.67 ± 2.76%, crystallinity index 90.61%, diameter range 30–130 nm | [113] |
| HS | Taonella mepensis | 30 °C, 7 days | Yield 2.472 g/L, crystallinity index 90.3%, average width 64.6 ± 15.3 nm | [51] |
| ST | Taonella mepensis | 30 °C, 7 days | Yield 1.784 g/L, crystallinity index 82.8%, average width 53.3 ± 20.1 nm | [51] |
| Sugarcane bagasse | Komagateibacter xylinus | 28 °C, 9 days | Fiber diameter 47 ± 10 nm, crystallinity index 79%, water content 99.43 ± 0.03% | [114] |
| Cellulose Component | Other Components | Method | Performance | References |
|---|---|---|---|---|
| CNC | Chitosan | Solution casting | The tensile strength and Young’s modulus of the film have been increased by 39% and 78%, respectively. Water solubility has been reduced by 26.5–35.7%, with good UV resistance and water repellency. | [116] |
| CNF | Polyurethane(PU), quaternized chitosan (QCS) and negatively charged phosphotungstic acid | Layer-by-Layer assembly | The conductivity of hydroxide reached 14.3 mS/cm at 80 °C and lasted for more than one month. | [117] |
| CNC | Polylactic acid (PLA) | Extrusion | Improved processability, melt strength, and rheological properties. Good performance in storing oil-based and dairy products can prolong their shelf life. | [118] |
| CNC | Palm oil/water | Emulsions | Checking whether food is spoiled or not. | [119] |
| BC | Protein nanoparticles | Hydrogel | Good wettability, interfacial adsorption capacity, and higher antioxidant property. | [120] |
| CNF | Polylactic acid (PLA), Chitosan, rosin | Spray drying | Great antibacterial effect and increased elasticity and water vapor permeability. | [121] |
| CNC | Chitosan, polyvinyl alcohol | Electrospinning | Preventing the growth of pathogenic bacteria | [122] |
| CNF | Polyvinyl alcohol (PVA), Silver Nanoparticles | Electrospinning | Good antibacterial activity against Staphylococcus aureus, E. coli, and P. aeruginosa. | [123] |
| BC | Bovine Lactoferrin (BLF) | Adsorption | Strong bactericidal effect on E. coli and Staphylococcus aureus. | [124] |
| CNC | Polyethylene glycol, algal bile protein | Adsorption | Improved protein stability. | [125] |
| Composition | Performance | References |
|---|---|---|
| CS/carboxymethylated nanocellulose | Increased resistance to grease, oil, water, air, and water vapor. Good mechanical characteristics and enhanced antibacterial activity against both E. coli and Staphylococcus aureus. | [154] |
| Corn starch/BC | Improved barrier to water vapor and oxygen. | [155] |
| Gelatin/BC/cinnamaldehyde | Increased tensile strength and lower water vapor permeability. Inhibited against Staphylococcus aureus and E. coli germs. | [156] |
| CMC/CNC/AgNPs | Excellent mechanical strength, water vapor and air barrier characteristics, and antibacterial activities. | [157] |
| SA/CNF/Ca2+/PSE | High strength, good water resistance, excellent ultraviolet barrier performance, and significant antibacterial effects against both gram-negative and gram-positive bacteria. | [158] |
| Anthocyanins/BC | Consumers can judge the freshness of fish by comparing the label color with the standard color. | [136] |
| EAE/BC | The fabricated BC-EAE indicator responded to pH by changing color from red to yellow over the pH range of 2–12. | [159] |
| AgNPs@CS-1:1 | The storage time of strawberries packaged by AgNPs@CS-1:1 was extended to 12 days without microbial invasion. | [160] |
| SA/Carboxymethylated nanocellulose/SOWEs | Great effect on controlling browning index in fresh-cut apple and potato over the storage of 12 days and 5 days. | [161] |
| CS/guar gum/walnut green husk extract | Good performance in reducing firmness, weight loss, total soluble solids, and inhibiting browning and microbial growth of fresh-cut apples. | [162] |
| Company | Country | Method | Products | Production Capacity |
|---|---|---|---|---|
| INNVENTIA | Sweden | Enzymatic and microfluidizer | CNF | 100 kg/d |
| Nippon paper | Japan | TEMPO oxidation and mechanical defibrillation | CNF | 150 kg/d |
| Stora Enso | Sweden | Enzymatic and mechanical defibrillation | MFC | n.a. |
| CellForce | Canada | Sulfuric acid hydrolysis | CNC | 1000 kg/d |
| U.S. Forest Service | U.S. | Sulfuric acid hydrolysis and drinder | CNC and CNF | CNC (10 kg/d) and CNF (1000 kg/d) |
| Cellulose Lab | Canada | n.a. | CNC, CNF and BC | n.a. |
| American Process | U.S. | AVAP | CNF | 1000 kg/d |
| University of Maine | U.S. | Mechanical refining method | CNF | 1000 kg/d |
| VTT | Finland | Enzymatic pretreated with Masuko grinder | CNF | 15 kg/d |
| FineCell | Sweden | Oxalic acid hydrolysis | CNF | n.a. |
| Ocean TuniCell AS | Norway | Enzymatic, TEMPO oxidation, carboxylmethylation, and microfluidizer | Tunicate CNF | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, F.; Zhong, Y.; Zhao, Y.; Gao, P.; Tian, F.; Zhang, X.; Zhou, R.; Cullen, P.J. Emerging Food Packaging Applications of Cellulose Nanocomposites: A Review. Polymers 2022, 14, 4025. https://doi.org/10.3390/polym14194025
Li J, Zhang F, Zhong Y, Zhao Y, Gao P, Tian F, Zhang X, Zhou R, Cullen PJ. Emerging Food Packaging Applications of Cellulose Nanocomposites: A Review. Polymers. 2022; 14(19):4025. https://doi.org/10.3390/polym14194025
Chicago/Turabian StyleLi, Jingwen, Feifan Zhang, Yaqi Zhong, Yadong Zhao, Pingping Gao, Fang Tian, Xianhui Zhang, Rusen Zhou, and Patrick J. Cullen. 2022. "Emerging Food Packaging Applications of Cellulose Nanocomposites: A Review" Polymers 14, no. 19: 4025. https://doi.org/10.3390/polym14194025
APA StyleLi, J., Zhang, F., Zhong, Y., Zhao, Y., Gao, P., Tian, F., Zhang, X., Zhou, R., & Cullen, P. J. (2022). Emerging Food Packaging Applications of Cellulose Nanocomposites: A Review. Polymers, 14(19), 4025. https://doi.org/10.3390/polym14194025