Abstract
The objective is to develop immediate release buccal films of Eletriptan Hydrobromide (EHBR) using hydroxypropyl methylcellulose (HPMC) E5. The buccal films have the ability to disintegrate rapidly and provide both systemic and local effects. The solvent casting method was employed to prepare the films and the central composite rotatable design (CCRD) model was used for film optimization. All the formulated films were characterized for physicochemical evaluation (Fourier transform infrared spectroscopy (FTIR), X-ray Diffraction (XRD), differential scanning calorimetry (DSC), and Scanning electron microscopy (SEM), in in-vitro, ex-vivo, and in-vivo drug release. The fabricated films were transparent, colorless, and evenly distributed. The FTIR spectra showed no chemical interaction between the drug and excipients. In in-vitro analysis, the film has the highest% drug release (102.61 ± 1.13), while a maximum of 92.87 ± 0.87% drug was diffused across the cellulose membrane having a pore size of 0.45 µm. In the ex-vivo study, drug diffusion across the goat mucosa was performed and 80.9% of the drug was released in 30 min. In-vivo results depict a mean half-life (t½) of 4.54 ± 0.18 h and a Cmax of 128 ± 0.87 (ng/mL); Tmax was achieved in 1 h. Furthermore, instability and histopathological studies buccal films were proven to be safe and act as an effective dosage form. In a nutshell, optimized and safe instant release EHBR buccal films were prepared that have the tendency to provide effect effectively.
1. Introduction
The oral drug administration route is the oldest and most suitable route of drug administration [1]. The oral cavity is highly enriched with blood capillaries and is more permeable to low molecular drugs than any other route [2]. This route can be used for both systemic and localized effects. However, this route encounters limitations, such as swallowing distress, especially in conditions, such as vomiting, dysphagia, and Parkinson’s disease. At least 28% of the overall population showed uneasiness consuming the solid dosage form [1]. In the 1970s, a new dosage form was introduced to overcome these disadvantages and was termed fast dissolving films (FDF) [3]. These films are prepared using hydrophilic polymers that become hydrated and stick in the oral cavity upon encountering salivary fluids. After hydration, these polymers disintegrate the drugs in the oral cavity due to a network of capillaries in sublingual mucosa, causing an increase in systemic absorption of the drug [4]. These films are soft and flexible, due to which they remain in the oral cavity. Additionally, they take a longer time to disintegrate, causing an increased residence in the oral cavity, and ultimately reducing the dosing frequency [5].
The main constituents of these films are polymers that may be natural, synthetic, or semi-synthetic. Disintegration time (DT) depends on the polymer’s nature, which affects the drug release rate, and mechanical properties [6]. A promising function of these films is mucoadhesion, a process by which the polymers, despite their origin, adhere to the oral cavity. The mechanism of mucoadhesion provides for the phenomenon of wetting and then entanglement of polymer chains at the mucous side within the oral cavity. The most commonly used polymer is hydroxypropyl methylcellulose (HPMC) [7]. As the polymer becomes hydrated in oral mucosa, it develops gel-like consistency. Other additives, such as plasticizers and surfactants, are also added to formulations to confer desirable properties [8].
Low molecular weight and non-volatile plasticizers are used to improve the flexibility of the films. Plasticizers are chosen based on polymer and film compatibility. The mechanical strength of the films is dependent upon the concentration and type of plasticizer used. Natural polymers are currently used more widely, including glycerol, polyethylene glycol, and propylene glycol [9]. Incorporation of surfactant within formulation improves the DT of films and thus improves drug release. Both surfactant and plasticizer enhance the properties of formulated films [4].
Approximately 1 billion people are affected by migraines worldwide [10]. Women (20.7%) are affected more than men (9%). Migraine is characterized by harsh throbbing pain which lasts from 4 to 72 h [11,12]. Further, it is marked by some symptoms, including gastric distress and photophobia [13,14,15]. The condition worsens when the patient cannot take medication due to a high frequency of pain and nausea [15].
Triptans are the first line of therapy for migraineurs. They can activate 5-HT1B and 5-HT1D receptors and cause vasoconstriction [16]. Eletriptan hydrobromide (EHBR) is considered a safe and effective medication for severe to moderate migraine attacks [17]. The drug achieves 50% bioavailability after oral administration [18,19]. Its peak plasma concentrations (Tmax) are achieved within 1 h [20,21]. EHBR is approximately 85% protein-bound [22]. While, the high first-pass effect demands another route of drug administration [17,23].
The current study aimed to develop and characterize an optimized buccal film by incorporating EHBR to ensure a safe and prompt effect. A total of 13 runs were made by using Design Expert, followed by a central composite rotatable design (CCRD). Two variables were chosen, including HPMC E5 as X1 and glycerol as X2 variables. Different responses, including disintegration time (DT), total dissolving time (TDT), folding endurance (FE), and drug release were studied.
2. Materials and Methods
EHBR was received as a gift by Wilshire labs, Lahore, Pakistan. Highnoon Laboratories, Lahore, Pakistan gifted HPMC E5 (Molar mass: 324.285), Tween 80, and glycerol. Ethanol and all other chemicals of analytical grade were purchased from Fisher Chemical, Loughborough, UK.
2.1. Preparation of Film Formulations
Films were prepared by using the solvent evaporation method [24]. To make the procedure more convenient, solutions of the drug and each of the excipients were prepared separately. Firstly, the drug solution was prepared using ethanol as solvent under continuous stirring on a magnetic stirrer for 5 min at 500 rpm. Afterward, 4% aqueous solution of HPMC E5, 5% of Tween 80, and 3% of glycerol were prepared in separate beakers and used according to the formulation design (Table 1 and Table 2). Initially, the polymeric solution was subjected to stirring using a magnetic stirrer, followed by the addition solution of Tween 80, glycerol, and the drug, one by one until a homogenous mixture was formed [25,26,27]. The solution was covered by aluminum foil to prevent evaporation. The final mixture was then poured into Petri dishes (Norman, China) and allowed to dry at 40 °C for 24 h in the oven. After that, the prepared films were peeled off and kept in aluminium foil for further use [26].

Table 1.
Factors studied by RSM for preparation of EHBR-loaded films.

Table 2.
Calculated formulation designs for preparation of EHBR films.
2.2. Analytical Method
Regarding better elution properties of EHBR-based oral solution and film formulation through high-performance liquid chromatography (HPLC), the wavelength was adjusted to 220 nm. High-performance liquid chromatographic analysis was conducted to study the pharmacokinetics of the drug, both in film formulation as well as in oral solution (Reference formulation). For this purpose, 1260 Infinity II LC System, Agilent Technologies, Santa Clara, CA, USA, equipped with a C 18 column having dimensions of 250 mm × 4.6 mm and a particle size of 5 μm was used. Different combinations of the solvent systems were tried to find better elution of the drug, however, the mobile phase consisted of phosphate buffer and acetonitrile in a 70:30 ratio, with adjusted pH to 4.6, a column flow of 1 mL/min at ambient temperature, and a detection wavelength of up to 220 nm [28].
2.3. Numerical Optimization
A widely used software to optimize formulations is Design Expert, which was used to optimize the relation between different variables by varying their concentrations [29]. In the current studies, CCRD was used to foresee 13 runs reliant upon the variables. The data was subsequently fitted in the polynomial equation (Equation (1)), and analysis of variance (ANOVA) with a 95% confidence interval was employed [30]:
Y = β0 + b1X1 + b2X2 + b12X1X2 + b1X12 + b2X22
β0 represents the arithmetic mean response of the selected runs, and Y represents the dependent variable. β1 is the estimated coefficient for the factor X1, X2. In this study, we used two variables designated X1 and X2, which were changed from low to high values. A polynomial equation judges the positive or negative result [31].
2.4. Characterization of Films
All the prepared instant release buccal films were characterized for their organoleptic, physical, and chemical evaluation.
2.4.1. Organoleptic Evaluation
Prepared films were evaluated for the presence of any lumps, bubble formation, and any color deposition [32].
2.4.2. Weight Variation
The prepared films were cut into 2 × 2 cm2 dimensions, followed by weighing of individual film strips, using a digital weighing balance. All measurements were performed in triplicate, and the average weight was calculated [32].
2.4.3. Film Thickness
The formulated films were subjected to a thickness test, using a digital micrometer (Mitutoyo, IP65, Waltham, MA, USA). Thickness from three different locations from each strip was determined and their average thickness was calculated [33].
2.4.4. Content Uniformity
Uniformity of content is an important test to ensure homogeneity in the distribution of the drug and polymer within film preparation. Three strips each of 1 cm2 in size were cut and dissolved in 5.7 pH buffer under continuous stirring, using a magnetic stirrer. The solution was filtered using syringe filters (0.22-μm, Sterlitech, Bedford, MA, USA) of nylon material to remove undissolved particles. The prepared mixture was analyzed using a UV-Visible double beam spectrophotometer (Halo DB-20 UV, Progen Scientific, Livingston, UK) at 220 nm wavelength [34]. The content uniformity of each formulation was determined in triplicate and finally calculated by using the following Equation (2).
2.4.5. Surface pH
The pH of the developed films is an important parameter. As films are placed in the buccal cavity, so, to avoid any damage to the cavity, the pH of the formulation must be in accordance with the buccal mucosa. Strips of films were cut into 1 cm2 and the surface was moistened by using a few drops of distilled water. The pH of wetted films was analyzed using a pH meter (Bench Meter, AD1030 Professional, Adwa, Hungary). The bulb of the pH electrode was touched to the films and, after stabilizing, the pH value was determined in triplicate [34].
2.4.6. FTIR Analysis
FTIR analysis was done to determine any interaction between the used active ingredient and the excipients. Bruker Alpha II (Compact series, Fremont, CA, USA) apparatus was used within the range of 500 to 4000 cm−1 wavelength [35].
2.4.7. DSC Analysis
Differential Scanning Calorimeter (Q2000, TA Instruments, New Castle, DE, USA) was used to perform the DSC analysis. This test was performed on formulated film and pure drugs. The sample size of 8 mg for both EHBR and EHBR-based film was carefully weighed and encapsulated in pans of aluminum metal. The instrument was earlier calibrated by using indium and zinc. For the sake of analysis, the Nitrogen gas at a flow rate of 50 mL/minute was used. The heating of the sample was done between 40 to 280 °C and maintained at 20 °C/min [36,37].
2.4.8. X-Ray diffraction (XRD)
XRD of pure drug sample and formulated film of EHBR was determined using X-Rays Diffractometer (JDX-3532 JEOL, Tokyo, Japan) equipped with CuKa (Wavelength = 1.5418 Å). The current was set at 10 mA and the voltage at 40 kV. Scanned at 2°/min and 2 theta ranges from 0 to 160°. The 2 theta values, and intensities of the samples were scanned, and the graph was plotted. XRD ascertained whether the sample was crystalline or amorphous [38].
2.4.9. Optical Microscopy
Prepared films were studied at a micro-level using an optical microscope at 40× magnification [39].
2.4.10. Surface Electron Microscopy (SEM)
The optimized formulation of EHBR was evaluated using FEI Nova 450 Nano SEM (Thermo Fisher, Waltham, MA, USA) under different resolutions ranging from 1000× to 10,000× [40].
2.4.11. Percentage Moisture Content
Karl Fischer titration (KFT) was used to determine the moisture content [41] present in formulated films at 80 °C.
2.4.12. Disintegration Time (DT) and Total Dissolving Time (TDT)
Formulated films were placed in a 10 mL saliva-based buffer having 5.7 pH, which was maintained at 37 °C and agitated slowly. The time in which films start to crack is termed DT, while the time in which the film completely dissolves in buffer was termed TDT [42,43].
2.4.13. Mechanical Strength
The mechanical strength, including tensile strength (TS) and folding endurance (FE), is the most promising factor in determining the integrity of the film preparation. TS and FE ensure that the films endure the applied stress or pressure through manufacturing, packaging, and transportation [44].
2.4.14. Tensile Strength (TS)
The mechanical strength of the developed films was determined using a Tensile strength tester (Lloyd Instruments, LS series, Ametek, British Columbia, Vancouver, Canada). The film with an area of 5.7 × 1 cm2 was placed between the two jaws of the machine. The lower jaw moves downward, while the upper jaw is fixed in position. The point at which the film was ruptured was determined by using Equation (3).
where F represents the breaking load measured in units of Newton’s (N), and the area was designated as A measured in units of centimeter square (cm2) [45].
TS = F/A
2.4.15. Percentage Elongation at break (% EB)
% EB is determined using the film’s original length, thickness, and width until it breaks under the influence of applied force [46]. It was calculated using Equation (4).
where L1 is the film’s original length and L2 is the change in length at the break.
%EB = L2/L1 × 100
2.4.16. Strain
When a force is applied to film, it causes an extension in film, referred to as a strain. The strain is used to directly measure the film’s elastic behavior calculated by using Equation (5) [47].
Strain = ((L2 − L1))/(L1)
The film’s original length was represented as L1, while the change in length was presented as L2.
2.4.17. Folding Endurance (FE)
The FE of formulated films determines the film’s strength and integrity. Strips from formulated films were folded at 180° at the same point until the films were ruptured. The number of times after which the film was ruptured was determined. The test was done in triplicate, and the mean was calculated [48].
2.4.18. In Vitro Drug Release Studies
The dissolution paddle apparatus was used to determine in vitro drug release studies. A simulated saliva-based buffer (used as dissolution media) having 5.7 pH was used for dissolution purposes. Film strips having a dimension of 2 × 2 cm2 (equivalent to a one-time dose of EHBR, which is 20 mg) were cut and fastened on a glass slide. The glass slide was dipped into a dissolution basket. Baskets were already filled with 250 mL of dissolution media. The media temperature was set at 37 ± 0.5 °C and paddles were rotated at 50 rpm. Samples were withdrawn after 2, 4, 6, 8, 10, 15, 20, 25, 30 min. A sample size of 5 mL was withdrawn each time, which was replaced with the same amount of buffer maintained at the same temperature (mentioned above 37 ± 0.5 °C). At the end of the test, the absorbance of the samples was analyzed at 220 nm using a UV-Vis spectrophotometer [25].
2.4.19. In Vitro Diffusion Studies
Franz diffusion cell was used to determine the drug diffusion studies. The cell has a receptor compartment of 5 mL, which was filled with a simulated saliva-based buffer having pH 5.7, and its temperature was maintained at 37 ± 0.5 °C. The formulated films were placed on cellulose acetate membranes, having a pore size of 0.45 µm. The prepared films were placed on the membrane. The placement of films was such that the drug diffusion faces toward the receptor chamber. Magnet speed was set at 50 rpm. The sample size of 1 mL was withdrawn, while the remaining conditions remained the same, as mentioned above [49].
2.4.20. Kinetic Modeling
The following statistical methods were applied to determine drug release patterns from formulations, including ANOVA, Model dependent methods (first order, zero order, Hixson Crowell model, Korsmeyer Peppas model, and Higuchi model), and model-independent models (difference factor, similarity factor).
2.4.21. Numerical Optimization
The formulations were optimized based on DT, TDT, in vitro drug release, and drug diffusion by using the Design Expert software. The results were interpreted by using the software, and estimated results were designed using polymer, plasticizer, and Tween 80 in 3.87 mL, 0.7 mL, and 0.155 mL concentrations.
2.4.22. Ex Vivo Permeation
After numerical optimization, an optimized film was selected and further tests, which included ex vivo and in vivo analysis, were performed. Fresh excised goat (a local breed of Sahiwal) buccal mucosa was used in 2 h, as received from the slaughterhouse. For 2 h, the mucosa was placed in a normal saline solution. Underlying tissues and fat of mucosa were properly cleaned. It was then placed in Franz’s cell; meanwhile, all conditions remained the same, as discussed [50].
2.4.23. In Vivo Analysis
As there appeared no commercial EHBR film in the market, EHBR films were compared with the EHBR dispersion.
2.4.24. Selection of animals
For in vivo analysis, albino rabbits with an average weight of 3 kg were selected. Animals were provided with an ad libitum quantity of water, but they were kept fastened for a period of approximately 24 h before the start of analysis [51]. The study was conducted according to the guidelines provided by the ethical committee of the University of Central Punjab (Ref. No.: UCP/IRC/2019/144) to conduct animal studies. All male rabbits, having good health, an average weight of at least 3 kg, and no previous history were included in the study. However, underweight rabbits those showing any signs of disease, or having a history of medications were excluded.
2.4.25. Dose Administration
Rabbits were given an oral drug dispersion and buccal film (for comparison purposes) according to their body weight which was 1 mg/kg. Meanwhile, during the administration of buccal films, the animals were anesthetized lightly [52] by using diazepam intramuscular (IM) injection at a dose of 1 mg/kg of their body weight in conjunction with 10 mg/kg of ketamine. As soon as the animals were anesthetized, butterfly catheters were instilled in their marginal ear vein [53]. Prepared films were then placed and gently pressed on the buccal mucosa of the animal for adhesion. Then, 1 mL of blood was removed from the marginal ear vein of the rabbit at a time interval of 0, 1, 2, 3, 4, 6, 8, 12, and 24 h and rapidly transferred to heparinized tubes [54,55].
2.4.26. Drugs Extraction from Animal Plasma
The collected samples were centrifuged at 5000 rpm for 10 min. The supernatant was separated and vortex again for 10 min with the addition of 3 mL methanol and centrifuged again at 5000 rpm for 20 min. The supernatant was again transferred in a test tube containing 3 mL of diethyl ether and centrifuged at 5000 rpm for 10 min. The organic solvent was dried at 40 °C by placing the test tubes in the water bath. Obtained samples were then reconstituted with a mobile phase of approximately 200 μL out of which 20 μL was further used for the HPLC study [55]. Samples were analyzed at 220 nm wavelength. Different pharmacokinetic factors were studied, including Tmax, Cmax, t½, area under the curve (AUC) at the first moment (AUC 0-t) (ng/mL × h), and mean residence time (MRT) (h).
2.4.27. Statistical Analysis
One-way ANOVA at a confidence interval of 95% was performed on results obtained from experimental work. Differences were statistically significant if p < 0.05 [56]. Trials were conducted at n = 6, and their mean ± SD was recorded.
2.4.28. Stability Studies
Formulated films were placed in a stability chamber to identify their stability. Films were placed at 40 °C and 75% ± 5% relative humidity (RH) [57,58] and 30 °C and 65% ± 5% RH [30,59] for a period of 3 months [57]. At the end of 3 months, films were investigated for drug content, appearance, in vitro drug release study [60], TDT, DT, and FE.
2.4.29. Histopathological Slides
At the end of the experiment, rabbits were sacrificed, and their major body organs which include buccal mucosa, heart, liver, kidneys, and lungs, were removed. The organs were placed in a 10% formalin solution and further studied for the formation of any lesions or signs of abnormality [61,62,63].
3. Results and Discussions
3.1. Organoleptic Evaluation
It was observed that formulated films were transparent, had no odor, and were flexible with no bubble formation.
3.2. Weight Variation
Weight variation of formulated films ranges from 0.013 ± 0.0004 for F12 to 0.027 ± 0.0004 mg for F4. It is an important parameter to demonstrate the even distribution of polymer, drug, and excipients within the formulation, as uneven distribution of ingredients may cause dose dumping, as seen in a previous study conducted by Kassem et al. in which uniform and even weight films were formulated by using the solvent casting method [64].
3.3. Film Thickness
Film thickness varied from a maximum of 0.191 ± 0.023 mm for F8 and a minimum of 0.091 ± 0.023 mm for F2. Results showed that all formulated films were of uniform thickness and could be easily used for buccal administration [27].
3.4. Content Uniformity
The content uniformity fell between 97.16 ± 2.5% to a maximum of 104.6 ± 1.7% for F1 and F10, respectively. As per United States Pharmacopeia (USP) guidance, drug contents could be varied between 90% to 110%. The results suggested the drug was evenly distributed throughout the film [65].
3.5. Surface pH
The pH of the oral cavity ranges from 5.5 to 7 [66,67]. The pH of all the formulations ranged between 5.85 ± 0.13 for F4 to 6.2 ± 0.13 for F1, respectively. As pH was in accordance with the oral cavity, it is plausible to predict no interaction with the buccal cavity.
3.6. FTIR Analysis
FTIR of Tween 80 showed a band at 1717 cm−1, possibly due to C=O and reinforced the presence of α, β-unsaturated ester. In comparison, a sharp band at 1121 cm−1 confirms C-O extending. FTIR spectrum of pure HPMC E5 demonstrated a broad absorption band at 1050 cm−1 possibly due to the CO-O-CO group. FTIR band of glycerol indicated an extending at 3288, which confirmed the presence of the carboxylic group. In contrast, alkanes were established at the peak of 2878 cm−1.
Meanwhile, a sharp stretch confirms the presence of C-O at 1027 cm−1 [68]. FTIR of EHBR represents a sharp peak at 1140 cm−1, which depicts a strong C-O bond and the presence of aliphatic ether. Finally, the FTIR spectrum of film elaborated that the drug’s peak, previously at 1140 cm−1, remained at 1140 cm−1. In the case of glycerol, an O-H stretch was observed at 3288 cm−1 before the formulation; the same peak was observed at 3279 cm−1 after the formulation, suggesting no significant interaction between the drug and excipients, as shown in Figure 1.
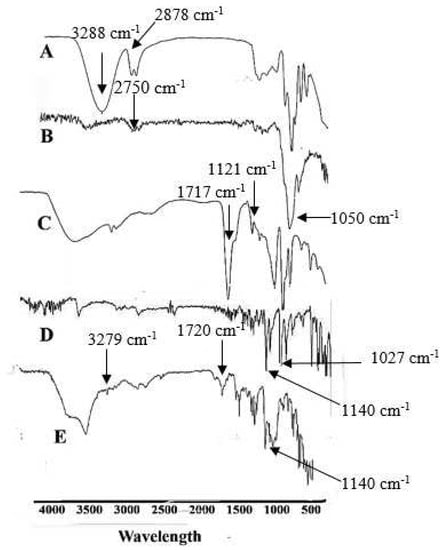
Figure 1.
Represents the FTIR spectrum (A) Glycerol (B) HPMC E5 (C) Tween 80 (D) EHBR (E) Film.
3.7. Differential Scanning Calorimetry (DSC) Analysis
It was used as an analytical method to determine the thermal properties of drugs and polymers. DSC graphs were plotted by taking heat flow values on the y-axis and temperature on the x-axis. It is widely used for the decomposition performance of materials employed [69].
In the case of EHBR, as shown in Figure 2, glass transition temperature (Tg) was observed at ~40 °C, which is supported by an exotherm attributable to crystallization (Tc), having onset at ~169.49 °C and a melting temperature (Tm) at ~173.93 °C. As the concentration of glycerol content increases, a decrease in glass transition, crystallization, and melting temperatures was observed [70]. In the EHBR film, Tg was observed at ~40 °C, supported by an exotherm due to Tc, having onset at ~92 °C and Tm at ~225 °C. DSC of pure drug EHBR showed a sharp temperature peak at 169.49 °C, as represented in Figure 2 [71]. DSC of film formulation changes the endotherms and now appears at 235 °C, suggesting reduced purity of ingredients due to the mixing of drug and excipients that resulted in the broader melting point [72].
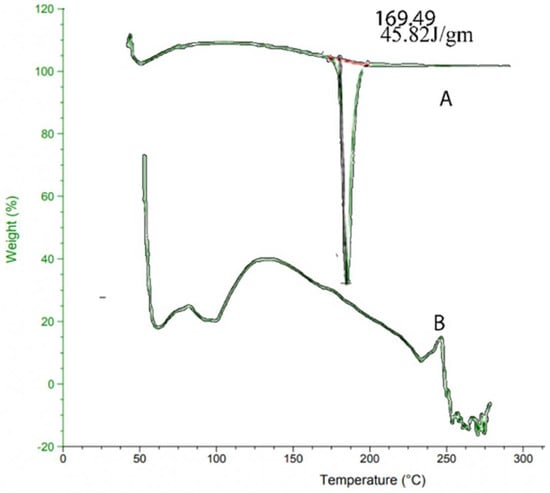
Figure 2.
DSC pattern for (A) Pure drug (B) Formulation.
3.8. X-ray Diffraction (XRD)
The pure drug form appears to be crystalline, and it exhibits visible peaks at 2 θ = 11°, 22°, and 28°, respectively [73]. Following film formulation, XRD of films demonstrated the same peaks at 2 θ = 11°, 22°, suggesting no interactions between the drug and the developed formulation, as shown in Figure 3. However, the peak intensity of pure EHBR drug is reduced in the case of EHBR-based films, which could be possible because the physical mixture decreases the crystalline nature of the drug, and now it shifts toward its amorphous form. After, film formulation XRD of films exhibited a fused peaks pattern, which suggested that the drug is stable in the film formulation as well and merged fully with the used polymer [74].
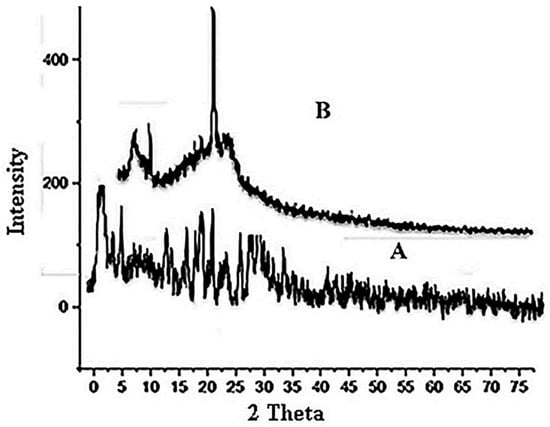
Figure 3.
XRD pattern (A) Pure drug (B) Formulation.
3.9. Optical Microscopy
Optical microscopy was done at 40× magnification. Meanwhile, a continuous texture showed a complete mesh of polymer in which drug and excipients are dispersed completely, as evident from Figure 4.
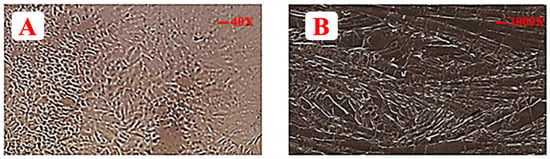
Figure 4.
Illustrating (A) optical microscope image of EHBR film and (B) SEM image of EHBR film.
3.10. SEM
The SEM of formulated films revealed some larger broken pieces that were formed during the process of preparation. It is evident from SEM that drug, polymer, and excipients particles are merged or clustered together, as shown in Figure 4 [75]. Thus, it is justified that the drug and excipients are blended prudently [76].
3.11. Percentage Moisture Content
In the case of ELEHBR film, values of moisture content range from 4.25 ± 0.15% to 9.33 ± 0.25% for formulation F13 and F3, respectively. The presence of plasticizer and surfactant reduces the surface tension and relaxes the polymer chains, so it could be the cause of more entrapment of water molecules inside the meshwork of the polymer [77].
3.12. Disintegration Time (DT) and Total Dissolving Time (TDT)
DT for film ranges from 7 ± 1 s for formulation F12 to a maximum of 13 ± 1 s for formulation F4. DT has a direct relation with polymer concentration. As the polymer concentration increases, the stiffness of films increases and so DT increases [78]. It was evident by using polynomial Equation 6 and the 3 D plot, that both have a cumulative impact on film DT. The ANOVA for DT showed a value of R2 of 0.9082 and an adjusted R2 of 0.8163. The p-value was <0.05 (0.0125).
Polynomial Equation (7) describes that the TDT and polymer have a constructive impact on TDT. A negative sign for plasticizers represents a decrease in TDT [79]. TDT ranges from 40.5 ± 1 for F4 to a minimum of 22 ± 1 for F7. The ANOVA for TDT shows a value of R2 of 0.8566 and an adjusted R2 of 0.7113. The p-value was < 0.05 (0.0366).
DT = β0 + 9.63 + 1.72 X1 − 0.036 X2 – 0.32 X1X2 + 0.16 X12 + 0.66 X22
TDT = β0 + 25 + 4.42 X1 − 3.12 X2 − 0.63 X1X2 + 3.16 X12 − 0.34 X22
3.13. Mechanical Strength
3.13.1. TS
TS is affected by the concentration of plasticizers. Polynomial Equation (8) and 3D graph Figure 5 clearly showed that the higher the concentration of plasticizer, the lower the TS [80,81]. The incorporation of plasticizer reduces the inside chain entanglement of the polymer and ultimately increases the flexibility of the developed film [82]. The higher the concentrations of HPMC E5, the higher the TS [83,84]. HPMC E5 will possibly enhance the TS of the films [80].
TS = β0+ 0.050 + 0.28 X1 − 4.59×10−0.005 X2 + 0.036 X1X2 + 0.19 X12 + 0.017 X22
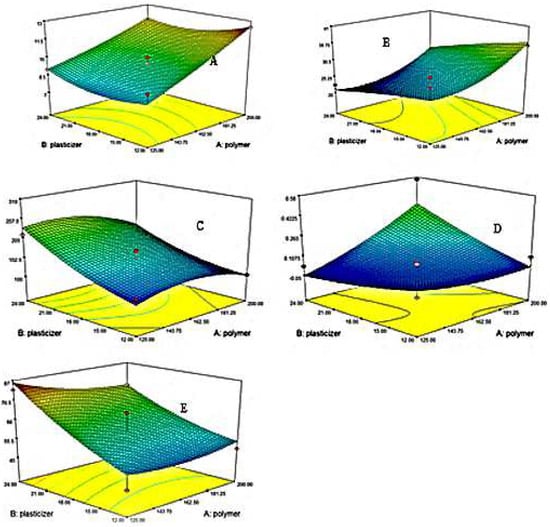
Figure 5.
3D Contour plots represents (A) DT (B) TDT (C) FE (D) TS (E)%EB.
ANOVA for TS showed a value of R2 of 0.9656 and an adjusted R2 of 0.9313. The p was <0.05 (0.0011).
3.13.2. % EB
From polynomial Equation (9) and 3D contour plot Figure 5, it was concluded that Tween 80 and glycerol have a synergistic impact on % EB of formulated films [85].
EB = β0+ 60.93 − 2.50 X1 + 13.28 X2 − 3.25 X1X2 − 2.97 X12 + 2.97 X22
By utilizing ANOVA for%, EB, the value of R2 was 0.8360, and adjusted R2 was 0.6721. The p-value was less than 0.05 (0.0491). As the% EB value increases, the films become more elastic, and a low value indicates tough films. So, an optimized elastic film must be ensured to be safe and effective during production, transportation, and administration.
3.13.3. Strain
When a force is applied to films, it produces an elongation in them under the influence of force. So, strain defines the flexibility of established films [86]. It was evident from the polynomial equation 10 that the amount of plasticizer increases the system’s flexibility and polymer decreases it.
Strain = β0+ 39 − 2.47 X1 + 5.87 X2– 2.25 X1X2 + 2.09 X12 + 0.97 X22
Using ANOVA for strain, the value of R2 was 0.8485, and the adjusted R2 was 0.6970. The analysis was significant as the p-value was less than 0.05 (0.0409).
3.13.4. FE
To confirm the flexible nature of films, they are folded at the same point approximately 200 to 300 times. If the formulated films have an FE value of more than 200, they are considered flexible [46]. In the case of EHBR films folding values ranged from 305 ± 1 time for formulation F8 to 105 ± 1 time for formulation F9. By engaging polynomial Equation (11) and 3 D contour plot Figure 5, it was obvious that the negative values of HPMC (X1) impart a negative effect and reduce the FE. Meanwhile, the positive values of X2 showed that plasticizer increases the flexibility of formulated films.
FE = β0+ 169 − 14.16 X1 + 50.46 X2 − 11.25 X1X2 − 26.69 X12 + 15.81 X22
Using ANOVA for FE, the value of R2 was 0.8990, and the adjusted R2 was 0.7980. The analysis was significant as the value of p was less than 0.05 (0.0157).
3.14. In Vitro Drug Release
Formulated buccal films were developed for immediate drug release studies, so a 30-min study was conducted. A drug release of a maximum of 102.61 ± 1.13% for F7 and the lowest release of 74.5 ± 1.15% for F2 was observed, as shown in Figure 6. Using polynomial Equation (12), the X1 variable has a negative value, indicating that the drug release would decrease if the polymer concentration increased [79]. The possible justification was that the increased polymer concentration increases the polymer meshwork, and the drug’s release decreases [87]. HPMC E5 is a hydrophilic polymer used as a film-forming agent. Its increased concentration makes a thick and more concentrated gel around the drug molecules, which retards the release of drug components. Using ANOVA for drug release, the value of R2 was 0.9013, and the adjusted R2 was 0.8025. The analysis was significant as the value of p was less than 0.05 (0.0149).
Drug release = β0+ 76.97 − 3.15 X1 + 3.93 X2 + 3.00 X1X2 + 9.58 X12 + 9.33 X22
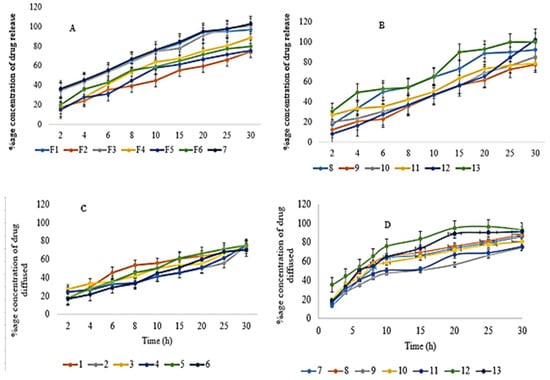
Figure 6.
Represents (A) In vitro drug release for EHBR formulations from 1–7 (B) In vitro drug release for EHBR formulations from 8–13 (C) DD for EHBR formulations from 1–6 (D) DD for EHBR formulations from 7–13.
3.15. Drug Diffusion (DD)
Glycerol was used as a plasticizer in the formulation of films. It provides the films with an elastic and plastic nature. As these plasticizers are hygroscopic, they drag water molecules from their surroundings and enhance the solubility and permeability of the formulated films; more water in the surroundings means more uptake and more drug disintegration [88].
Tween 80, which was used as a surfactant, also improves drug permeability. It encompasses the skin membrane parameters to enhance permeability, for instance, causing conjugation of molecules at the skin site. In addition, they decrease the stratum corneum properties as a skin barrier, and they modify the skin by loosening the lipid layer, increasing permeability [89].
A DD of 92.87 ± 0.87% for F12 and the lowest release of 70.3 ± 1.13% for F6 was observed, as shown in Figure 6. Using ANOVA, the drug diffusion value of R2 was 0.8468, and the adjusted R2 was 0.6936. The analysis was significant as the value of p was less than 0.05 (0.0420). The polynomial Equation (13) clearly shows the negative impact of polymer on drug diffusion.
DD = β0+ 74.65 − 5.88 X1 +0.43 X2– 0.62 X1X2 + 7.38 X12 + 5.57 X22
3.16. Kinetic Modeling
Drug release profiling of all formulated films was analyzed for first order, zero order, Korsmeyer model, Hixson Crowell model, Higuchi model, and the value of n, as shown in Table 3. Formulations F4 and F8 follow first-order kinetics, while the remaining formulations follow the Korsmeyer Peppas model. The n value ranges between 0.378 to 0.807. Suppose the value on n is less than 0.5. In that case, it indicates the Fickian model and drug release followed the diffusion mechanism, which states that the solvent transportation and diffusion rate was far larger than the development of polymeric chain leisure. Moreover, if the value of n is greater than 0.5 but less than 0.89, it follows the non-Fickian or anomalous type of transport mechanism. Both swelling and diffusion mechanism was favored for drug release.

Table 3.
Represents the R2 and value of all formulated films.
3.17. Model-Independent Approach
As per the recommendations of the Food and Drug Administration (FDA), if the similarity factor of f2 falls between 50 to 100, we declare the two formulations as similar [90]. Both f1 and f2 have an important function when we compare dissolution profiling. The f2 value should be nearer to 100 when the formulation is identical to the reference formulation. The value of f2 ranges from 0 to 100 and a formulation is considered similar if the value falls between 50 to 100 [91]. Shah et al. conducted a study and reported that if the calculated value of f2 would be between 50 to 65 of the dissolution profile, the formulations were similar with only a difference of 10 to 5% respectively between reference and test formulation [92]. In the case of drug release, when computed for formulations of EHBR, there appeared a similarity of up to 75% with formulation F5 and a maximum dissimilarity of 50% with formulation F7. In a study conducted by M. C. Gohe et al., they described the similarity of their prepared formulation with the reference up to 75% [92]. While comparing the optimized formulation with test formulations in the case of DD, the results depict that formulation F6 was 68% similar, and F12 was 53% dissimilar.
3.18. Numerical Optimization
The formulation was optimized based on the lowest DT time; the lowest DT time indicated rapid drug release. In addition, a lower TDT time will result in a high drug release of DD. In the optimized formulation, the quantity of polymer plasticizer was so adjusted that DT and TDT would be lowered. Results of the optimized formulation show a DT of 10.3 ± 2.30, while TDT was 24 ± 1.2 s. Cumulative drug release was 101 ± 1.5%, and DD was 79.03 ± 0.65%.
3.19. Ex Vivo Permeation
Ex vivo permeation was conducted on optimized formulations, using fresh goat buccal mucosa. During the permeation studies of 30 min, 80.9% drug was found to be permeated across the buccal mucosa describing the prompt permeation of the drug and satisfying the objectives of the study.
3.20. In Vivo Pharmacokinetics
Pure drug solution of EHBR was evaluated with formulated EHBR film. HPLC method was previously validated for accuracy, precession, sensitivity, and assay for both EHBR in film and solution form. Figure 7 represents the difference between an oral solution and administered film. A high release of the drug was observed in the case of buccal film. A remarkable drug was detected at the end of the 8th hour. Pharmacokinetic parameters, such as t1/2, Tmax, Cmax, AUC 0-t, and MRT of oral solution and film, were studied and compiled in Table 4. The pharmacokinetic parameters suggest a relatively higher drug release profile with buccal film than the oral solution. The aim to formulate a buccal film providing immediate release appeared fulfilled.
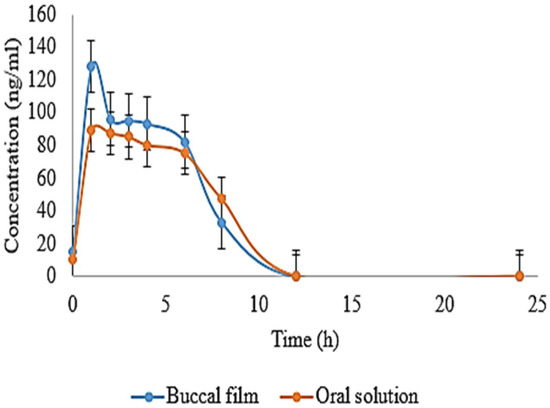
Figure 7.
Represents mean drug concentration for a comparison between oral solution and film.

Table 4.
Pharmacokinetic parameters were evaluated and are presented.
Furthermore, a t-test was applied to the results compiled in Table 5. The t-test involves the null hypothesis, which states that there appeared no difference in the mean of the compared samples. p-value at a value of 0.05 was also calculated, which is linked with the null hypothesis by considering the tails of the test at both ends. When we compared two samples using a t-test, we assumed that there was no difference between the means of both samples or that the mean of one was higher than the other. The possible reason for adopting the two-tailed t-test was that it could reject or accept the null hypothesis [93]. When we compared the parameters, such as t1/2, Cmax, and AUC, it was observed that the result of both parameters was significant as the value of p < 0.05.

Table 5.
Possible outcomes after applying t-test.
3.21. Stability Studies
Optimized formulations were kept at 40 °C and 75% ± 5% RH and 30 °C and 65% ± 5% for 3 months. Parameters that include DT, TDT, drug content, color, in vitro drug release, and DD studies were conducted in the 1st and 3rd months. Results are represented in Table 6. There appeared no significant changes, which suggests that our formulation is suitable for a long period of storage.

Table 6.
Stability profiling of films.
3.22. Histopathological Slides
The histopathological images of the kidney, liver, heart, lung, and buccal mucosa were prepared, as shown in Figure 8. The tissues of the kidneys were normal, and no sign of localized or general inflammation in the tubular or ductal cells was observed. In the case of the liver, well-defined hepatocytes were observed, and no sign of inflammation or cytoplasmic ballooning was investigated. However, fat deposition was observed in the sinusoids, but overall tissue health was good. Likewise, no sign of infection, necrosis, or cardiomegaly was noted in cardiomyocytes. Normal lung tissue with expanded alveoli with appropriate thickness was observed with no fluid deposition, granuloma, or emphysema symptoms. Overall, tissue health was good. The buccal mucosa was normal, with no eruption at the surface. All layers of the cells were in a good state. There was no sign of hyperactivity or accumulation of immune cells indicating inflammation.
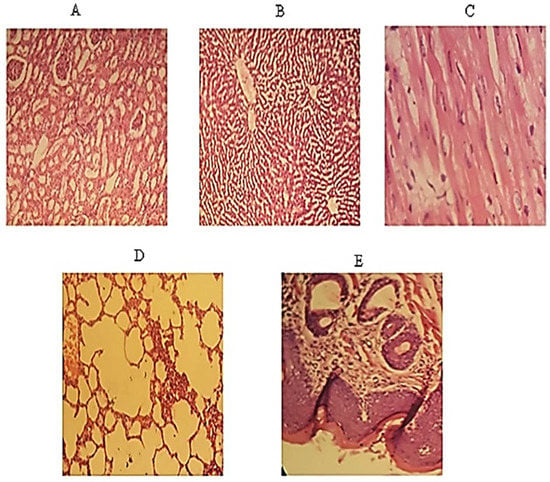
Figure 8.
Represents histopathological images of (A) Kidney (B) Liver (C) Heart (D) Lung (E) Buccal mucosa.
4. Conclusions
All of the results mentioned above declared the suitability for the formulation of EHBR in the form of buccal film. The suggested or optimized formulation has successfully achieved immediate release drug delivery, rapidly improving migraine conditions and enhancing patient compliance.
Author Contributions
Conceptualization, W.S., M.Z. and R.M.S.; data curation, M.H.B. and N.F.; formal analysis, W.S., M.H.B., A.U.R. and R.B.; funding acquisition, R.B., M.Y.A. and M.M.A.-D.; investigation, W.S., M.Z., M.H.B., A.U.R. and G.M.A.; methodology, M.Z., R.M.S., M.H.B., G.M.A., R.B. and M.M.A.-D.; project administration, M.Z. and R.M.S.; resources, W.S., M.Z., R.M.S., G.M.A., R.B. and M.Y.A.; software, M.H.B., A.U.R., and N.F.; supervision, M.Z., R.M.S. and M.M.A.-D.; validation, R.M.S., A.U.R. and M.M.A.-D.; writing—original draft, W.S., M.H.B., N.F., G.M.A. and R.B.; writing—review and editing, M.Z., R.M.S., A.U.R., M.M.A.-D. and M.Y.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R30), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through large Groups (Project under grant number R.G.P. 2/78/43).
Institutional Review Board Statement
The study was approved by ethical committee of the University of Central Punjab (Ref. No.: UCP/IRC/2019/144) to conduct animal studies.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R30), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through large Groups (Project under grant number R.G.P.2/78/43).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V. Orodispersible thin films: A new patient centered innovation. J. Drug Deliv. Sci. Technol. 2020, 59, 1–10. [Google Scholar] [CrossRef]
- Gupta, P. An overview of applications of mucoadhesive buccal film in Oral Medicine. J. Orofac. Res. 2020, 9, 14–19. [Google Scholar]
- Bilal, Q.; Unhale, S.; Shelke, S.; Kale, P.; Sarode, P.; Biyani, D. A review on mouth dissolving films. Eur. J. Pharm. Med. Res. 2020, 7, 232–238. [Google Scholar]
- Tizkam, H.H.; Fadhil, O.Q.; Ghazy, E. Formulation and Evaluation of Metoclopramide Fast Dissolving Film (FDF). Syst. Rev. Pharm. 2020, 11, 1641–1646. [Google Scholar]
- Winarti, L.; Laksono, B.T.; Sari, L.O.R.K. Optimization of Hydroxy Propyl Methyl Cellulose and Carbomer In Diltiazem Hydrochloride Mucoadhesive Buccal Film. Indones. J. Pharm. 2021, 32, 43–51. [Google Scholar] [CrossRef]
- Tawfik, E.; Scarpa, M.; Abdelhakim, H.; Bukhary, H.; Craig, D.; Barker, S.; Orlu, M. A Potential Alternative Orodispersible Formulation to Prednisolone Sodium Phosphate Orally Disintegrating Tablets. Pharmaceutics 2021, 13, 120. [Google Scholar] [CrossRef]
- A Ahmed, T.; O Bawazir, A.; Alharbi, W.S.; Safo, M.K. Enhancement of Simvastatin ex vivo Permeation from Mucoadhesive Buccal Films Loaded with Dual Drug Release Carriers. Int. J. Nanomed. 2020, 15, 4001–4020. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.-M.; Peña, J.; Veiga, M.-D. Development of mucoadhesive vaginal films based on HPMC and zein as novel formulations to prevent sexual transmission of HIV. Int. J. Pharm. 2019, 570, 1–14. [Google Scholar] [CrossRef]
- Müller, K.; Zollfrank, C.; Schmid, M. Natural polymers from biomass resources as feedstocks for thermoplastic materials. Macromol. Mater. Eng. 2019, 304, 1–17. [Google Scholar] [CrossRef]
- Ahmad, A.; Butt, M.H.; Misbah, S.; Saleem, R.T.; Jamshaid, M.; Alvi, M.N. Development and evaluation of orodispersible films by solvent casting method using eletriptan hydrobromide as a model drug. Lat. Am. J. Pharm. 2020, 39, 1951–1956. [Google Scholar]
- Bhatt, P.; Singh, S.; Sharma, S.K.; Rabiu, S. Development and Characterization of Fast Dissolving Buccal Strip of Frovatriptan Succinate Monoydrate for Buccal Delivery. Int. J. Pharm. Investig. 2021, 11, 69–75. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Jacob, S.; Saraiya, V.; Attimarad, M.; SreeHarsha, N.; Akrawi, S.H.; Shehata, T.M. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm. J. 2020, 28, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Hassan, R.; Razzaq, S.; Mahmood, A.; Amjad, M.W.; Raja, M.A.G.; Qaisar, A.A.; Majeed, A.; Hanif, M.; Tahir, R.A. Fabrication of polyvinyl alcohol based fast dissolving oral strips of sumatriptan succinate and metoclopramide HCL. Sci. Prog. 2020, 103, 1–21. [Google Scholar] [CrossRef]
- Doulberis, M.; Saleh, C.; Beyenburg, S. Is there an association between migraine and gastrointestinal disorders? J. Clin. Neurol. 2017, 13, 215. [Google Scholar]
- Aurora, S.K.; Shrewsbury, S.B.; Ray, S.; Hindiyeh, N.; Nguyen, L. A link between gastrointestinal disorders and migraine: Insights into the gut–brain connection. Headache J. Head Face Pain 2021, 61, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.; Schuster, N.M. Lasmiditan for the treatment of acute migraine: A review and potential role in clinical practice. J. Pain Res. 2018, 11, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L.; Keating, G.M. Eletriptan. Drugs 2006, 66, 1129–1149. [Google Scholar] [CrossRef]
- Kothapuvari, P.K.; Rawat, S.; Bhikshapathi, D. Estimation of Eletriptan Hydrobromide in Oral Film Dosage Form by. Int. J. Pharm. Sci. Drug Res. 2015, 7, 484–488. [Google Scholar]
- Spandana, B.; Shashidher, B.; Dinesh, S.; Nagaraj, B. Eletriptan hydrobromide Orodispersible tablets: Design, Development and In vitro characterization. Res. J. Pharm. Technol. 2020, 13, 5339–5344. [Google Scholar]
- Scott, L.J. Repaglinide. Drugs 2012, 72, 249–272. [Google Scholar] [CrossRef]
- Shah, A.K.; Harris, S.C.; Greenhalgh, C.; Morganroth, J. The pharmacokinetics and safety of single escalating oral doses of eletriptan. J. Clin. Pharmacol. 2002, 42, 520–527. [Google Scholar] [CrossRef]
- Capi, M.; Curto, M.; Lionetto, L.; De Andrés, F.; Gentile, G.; Negro, A.; Martelletti, P. Eletriptan in the management of acute migraine: An update on the evidence for efficacy, safety, and consistent response. Ther. Adv. Neurol. Disord. 2016, 9, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Esim, O.; Savaser, A.; Kurbanoglu, S.; Ozkan, C.K.; Ozkan, S.A.; Ozkan, Y. Development of assay for determination of eletriptan hydrobromide in loaded PLGA nanoparticles. J. Pharm. Biomed. Anal. 2017, 142, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Hassan, R.; Amjad, M.W.; Khan, S.M.; Raja, M.A.G.; Shah, S.S.; Siddique, W.; Aman, W.; Abid, Z.; Butt, M.H. Formulation of instant disintegrating buccal films without using disintegrant: An in-vitro study. Pak. J. Pharm. Sci. 2021, 34, 2357–2364. [Google Scholar]
- El-Maghraby, G.M.; Abdelzaher, M.M. Formulation and evaluation of simvastatin buccal film. J. Appl. Pharm. Sci. 2015, 5, 70–77. [Google Scholar] [CrossRef]
- Bîrsan, M.; Apostu, M.; Todoran, N.; Antonoaea, P.; Rusu, A.; Ciurba, A. Development of dermal films containing miconazole nitrate. Molecules 2018, 23, 1640. [Google Scholar] [CrossRef]
- Salehi, S.; Boddohi, S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog. Biomater. 2017, 6, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016, 23, 2154–2162. [Google Scholar] [CrossRef]
- Khan, R.; Zaman, M.; Salawi, A.; Khan, M.A.; Iqbal, M.O.; Riaz, R.; Ahmed, M.M.; Butt, M.H.; Alvi, M.N.; Almoshari, Y. Synthesis of Chemically Cross-Linked PH-Sensitive Hydrogels for the Sustained Delivery of Ezetimibe. Gels 2022, 8, 281. [Google Scholar] [CrossRef]
- Nagaich, U.; Chaudhary, V.; Nagaich, J. Development and Statistical Optimisation of Buspirone Hydrochloride Buccoadhesive Films. Int. Sch. Res. Not. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Ikram; Gilhotra, N.; Gilhotra, R.M. Formulation and optimization of mucoadhesive buccal patches of losartan potassium by using response surface methodology. Adv. Biomed. Res. 2015, 4, 1–13. [Google Scholar]
- Hussain, M.W.; Kushwaha, P.; Rahman, M.A.; Akhtar, J. Development and evaluation of fast dissolving film for oro-buccal drug delivery of chlorpromazine. Indian J. Pharm. Educ. Res. 2017, 51 (Suppl. S4), 539–547. [Google Scholar] [CrossRef]
- Basahih, T.S.; AlAmoudi, A.A.; El-Say, K.M.; Alhakamy, N.A.; Ahmed, O.A.A. Improved Transmucosal Delivery of Glimepiride via Unidirectional Release Buccal Film Loaded with Vitamin E TPGS-Based Nanocarrier. Dose-Response 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Siddique, W.; Sarfraz, R.M.; Zaman, M.; Butt, M.H.; Hayat, Z.; Gul, M.; Gul, M.; Asghar, F. Impact of polymer and plasticizer on mechanical properties of film: A quality by design approach. Lat. Am. J. Pharm. 2021, 40, 3002–3008. [Google Scholar]
- Rashid, R.; Zaman, M.; Ahmad, M.; Khan, M.A.; Butt, M.H.; Salawi, A.; Almoshari, Y.; Alshamrani, M.; Sarfraz, R.M. Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations. Pharmaceuticals 2022, 15, 326. [Google Scholar] [CrossRef]
- Alves, T.F.; Rios, A.C.; da Silva Pontes, K.; Portella, D.L.; Aranha, N.; Severino, P.; Souto, E.B.; Gonsalves, J.K.; de Souza Nunes, R.; Chaud, M.V. Bilayer mucoadhesive buccal film for mucosal ulcers treatment: Development, characterization, and single study case. Pharmaceutics 2020, 12, 657. [Google Scholar] [CrossRef]
- Salawi, A.; Khan, A.; Zaman, M.; Riaz, T.; Ihsan, H.; Butt, M.H.; Aman, W.; Khan, R.; Majeed, I.; Almoshari, Y.; et al. Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir. Polymers 2022, 14, 2369. [Google Scholar] [CrossRef]
- Alshamrani, M.; Mubeen, I.; Iqbal, M.H.; Farooq, M.; Zaman, M.; Chaudhry, M.A.; Qureshi, O.S.; Butt, M.H.; Salawi, A.; Khan, M.R. Fast dissolving oral films: An approach to co-load and deliver the atorvastatin and ezetimibe for better therapeutic response. Pak. J. Pharm. Sci. 2022, 35, 1229–1239. [Google Scholar] [CrossRef]
- Anwar, S.; Zaman, M.; Raja, M.A.G.; Mahmood, A.; Amjad, M.W. Rosuvastatin, Perindopril and Ezetimibe loaded instant release buccal films: Development and in vitro characterization. J. Appl. Biomed. 2020, 18, 115–125. [Google Scholar] [CrossRef]
- Faisal, W.; Farag, F.; Abdellatif, A.A.; Abbas, A. Taste masking approaches for medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef]
- Khalid, G.M.; Selmin, F.; Musazzi, U.M.; Gennari, C.G.; Minghetti, P.; Cilurzo, F. Trends in the characterization methods of orodispersible films. Curr. Drug Deliv. 2021, 18, 935–946. [Google Scholar] [CrossRef]
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive polymeric films to enhance barbaloin penetration into buccal mucosa: A novel approach to chemoprevention. AAPS PharmSciTech. 2019, 20, 18. [Google Scholar] [CrossRef]
- Mubeen, I.; Zaman, M.; Farooq, M.; Mehmood, A.; Azeez, F.K.; Rehman, W.; Akhtar, S.; Chaudhry, M.A.; Butt, M.H.; Shamim, Q.-U.; et al. Formulation of Modified-Release Bilayer Tablets of Atorvastatin and Ezetimibe: An In-Vitro and In-Vivo Analysis. Polymers 2022, 14, 3770. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Lin, S.; Madan, P. Preparation and evaluation of nicotine hydrogen tartrate fast dissolving films for smoking cessation. Asian J. Pharm. Sci. 2012, 7, 181–192. [Google Scholar]
- Abouhussein, D.; El Nabarawi, M.A.; Shalaby, S.H.; El-Bary, A.A. Cetylpyridinium chloride chitosan blended mucoadhesive buccal films for treatment of pediatric oral diseases. J. Drug Deliv. Sci. Technol. 2020, 59, 1–9. [Google Scholar] [CrossRef]
- Dangre, P.V.; PhaD, R.D.; Surana, S.J.; Chalikwar, S.S. Quality by design (QbD) assisted fabrication of fast dissolving buccal film for clonidine hydrochloride: Exploring the quality attributes. Adv. Polym. Technol. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Choi, A.; Ben-Nissan, B.; Bendavid, A.; Latella, B. Mechanical behavior and properties of thin films for biomedical applications. Thin Film. Coat. Biomater. Biomed. Appl. 2016, 117–141. [Google Scholar]
- Fernandes, F.P.; Fortes, A.C.; Fonseca, S.G.D.C.; Breitkreutz, J.; Ferraz, H.G. Manufacture and characterization of mucoadhesive buccal films based on pectin and gellan gum containing triamcinolone acetonide. Int. J. Polym. Sci. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Adhikari, S.N.R.; Panda, S. Development Evaluation and characterization of losartan Potassium buccal patches using hydrophilic polymers. Int. J. Pharm. Sci. Res. 2018, 9, 5474–5484. [Google Scholar]
- Naz, K.; Shahnaz, G.; Ahmed, N.; Qureshi, N.A.; Sarwar, H.S.; Imran, M.; Khan, G.M. Formulation and in vitro characterization of thiolated buccoadhesive film of fluconazole. Aaps Pharmscitech. 2017, 18, 1043–1055. [Google Scholar] [CrossRef]
- Thimmasetty, J.; Pandey, G.S.; Babu, P.R.S. Design and in vivo evaluation of carvedilol buccal mucoadhesive patches. Pak. J. Pharm. Sci. 2008, 21, 241–248. [Google Scholar]
- Koland, M.; Charyulu, R.N.; Vijayanarayana, K.; Prabhu, P. In vitro and in vivo evaluation of chitosan buccal films of ondansetron hydrochloride. Int. J. Pharm. Investig. 2011, 1, 164. [Google Scholar] [CrossRef]
- Rana, P.; Murthy, R. Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: A potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv. 2013, 20, 224–235. [Google Scholar] [CrossRef]
- Singh, A.V.; Nath, L.K.; Pani, N.R. Development and validation of analytical method for the estimation of lamivudine in rabbit plasma. J. Pharm. Anal. 2011, 1, 251–257. [Google Scholar] [CrossRef]
- Kothapuvari, P.K.; Rawat, S.; Bhikshapathi, D. Preparation, optimization and in vivo evaluation of eletriptan hbr fast dissolving oral films. Int. J. Drug Deliv. 2015, 7, 141–154. [Google Scholar]
- Hadi, M.A.; Rao, N.G.R.; Rao, A.S. Pharmacokinetic Parameters Determination and In Vitro–In Vivo Correlation of Ileocolonic-Targeted pH-Responsive Coated Mini-Tablets of Naproxen. Sci. Pharm. 2015, 83, 645–658. [Google Scholar] [CrossRef][Green Version]
- Ammanage, A.; Rodriques, P.; Kempwade, A.; Hiremath, R. Formulation and evaluation of buccal films of piroxicam co-crystals. Future J. Pharm. Sci. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- El Sharawy, A.M.; Shukr, M.H.; Elshafeey, A.H. Formulation and optimization of duloxetine hydrochloride buccal films: In vitro and in vivo evaluation. Drug Deliv. 2017, 24, 1762–1769. [Google Scholar] [CrossRef]
- World Health Organization. Stability testing of active substances and pharmaceutical products. Restricted Work. Doc. QAS/06 2006, 179, 10–32. [Google Scholar]
- Cherian, A.K.; Thomas, N.; Raj, P.; Jacob, L.; George, B.J. Formulation and evaluation of cefpodoxime proxetil buccal film. Int. J. Pharm. Chem. Anal. 2015, 2, 1–13. [Google Scholar]
- Erum, A.; Bashir, S.; Saghir, S.; Tulain, U.R.; Saleem, U.; Nasir, M.; Kanwal, F.; Malik, M.N.H. Acute toxicity studies of a novel excipient arabinoxylan isolated from Ispaghula (Plantago ovata) husk. Drug Chem. Toxicol. 2015, 38, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Bajwa, R.I.; Qureshi, O.S.; Rehman, A.U.; Saeed, S.; Amjad, M.W.; Raja, M.A.G.; Hussain, M.A. Synthesis of Thiol-Modified Hemicellulose, Its Biocompatibility, Studies, and Appraisal as a Sustained Release Carrier of Ticagrelor. Front. Pharmacol. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, V.S.; Menon, R.B.; Raju, K.; Aiswarya, M.; Nair, S.C. Formulation and Evaluation of Lorazepam Encapsulated Collagen/Pectin Buccal Patch. Int. J. Appl. Pharm. 2019, 11, 200–209. [Google Scholar]
- Kassem, A.A.; Issa, D.A.E.; Kotry, G.S.; Farid, R.M. Thiolated alginate-based multiple layer mucoadhesive films of metformin forintra-pocket local delivery: In vitro characterization and clinical assessment. Drug Dev. Ind. Pharm. 2017, 43, 120–131. [Google Scholar] [CrossRef]
- Abbas, I.K.; Rajab, N.A.; Hussein, A.A. Formulation and In-Vitro Evaluation of Darifenacin Hydrobromide as Buccal Films. Iraqi J. Pharm. Sci. 2019, 28, 83–94. [Google Scholar] [CrossRef]
- Patel, R.S.; Poddar, S.S. Development and characterization of mucoadhesive buccal patches of salbutamol sulphate. Curr. Drug Deliv. 2009, 6, 140–144. [Google Scholar] [CrossRef]
- Patel, V.F.; Liu, F.; Brown, M. Modeling the oral cavity: In vitro and in vivo evaluations of buccal drug delivery systems. J. Control. Release 2012, 161, 746–756. [Google Scholar] [CrossRef]
- Ahmed, M.K.; McLeod, M.P.; Nézivar, J.; Giuliani, A.W. Fourier transform infrared and near-infrared spectroscopic methods for the detection of toxic diethylene glycol (DEG) contaminant in glycerin based cough syrup. Spectroscopy 2010, 24, 601–608. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Microbial Poly-3-Hydroxybutyrate and Related Copolymers. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 575–605. [Google Scholar]
- Fung, M.H.; Suryanarayanan, R. Use of a plasticizer for physical stability prediction of amorphous solid dispersions. Cryst. Growth Des. 2017, 17, 4315–4325. [Google Scholar] [CrossRef]
- Kommavarapu, P.; Maruthapillai, A.; Palanisamy, K. Identification and quantitative determination of eletriptan hydrobromide polymorphs: Thermal, diffractometric and spectrometric studies. J. Taibah Univ. Sci. 2015, 9, 586–593. [Google Scholar] [CrossRef][Green Version]
- Shah, S.; Madan, S.; Agrawal, S. Formulation and evaluation of microsphere based oro dispersible tablets of itopride hcl. DARU J. Pharm. Sci. 2012, 20, 24. [Google Scholar] [CrossRef]
- Preetam, A.; Devi, A.S.; Sarbajna, R.M.; Suryanarayana, M.; Radhakrishna, S.; Rao, K.N.; Datta, D.; Lakshmi, P.A. Crystallographic studies of eletriptan hydrobromide: α-form, β-form and its physicochemical characterisation. Mol. Cryst. Liq. Cryst. 2013, 570, 128–147. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.M.; Mahmoud, A.A.; Shahin, H.I. Design and in vitro/in vivo evaluation of ultra-thin mucoadhesive buccal film containing fluticasone propionate. AAPS PharmSciTech. 2017, 18, 93–103. [Google Scholar] [CrossRef]
- Okonogi, S.; Khongkhunthian, S.; Jaturasitha, S. Development of mucoadhesive buccal films from rice for pharmaceutical delivery systems. Drug Discov. Ther. 2014, 8, 262–267. [Google Scholar] [CrossRef]
- Puri, V.; Sharma, A.; Kumar, P.; Singh, I. Thiolation of biopolymers for developing drug delivery systems with enhanced mechanical and mucoadhesive properties: A review. Polymers 2020, 12, 1803. [Google Scholar] [CrossRef]
- Pedada, R.B.; Vanka, E.; Babu, A.S.; Desu, P.K.; Bharathi, P.R.; Rao, P.V. Enhancement of Solubility; an Over View. PharmaTutor 2013, 1, 60–74. [Google Scholar]
- Bin Liew, K.; Tan, Y.T.F.; Peh, K.-K. Effect of polymer, plasticizer and filler on orally disintegrating film. Drug Dev. Ind. Pharm. 2014, 40, 110–119. [Google Scholar] [CrossRef]
- Zaman, M.; Hanif, M. In vitro and ex vivo assessment of hydrophilic polymer-and plasticizer-based thin buccal films designed by using central composite rotatable design for the delivery of meloxicam. Adv. Polym. Technol. 2018, 37, 1823–1836. [Google Scholar] [CrossRef]
- Priya, S.; Rathnanand, M.; Udupa, N.; Ongole, R.; Joshi, U. Preparation and evaluation of buccal mucoadhesive patch of betamethasone sodium phosphate for the treatment of oral submucous fibrosis. J. Chem. Pharm. Res. 2011, 3, 56–65. [Google Scholar]
- Sharma, S.K.; Kumar, A.; Jaimini, M.; Chauhan, B.S. Development and In Vitro Evaluation of Fast Dissolving Tablets of Tizanidine Hydrochloride By Direct Compression Method. J. Drug Deliv. Ther. 2014, 4, 52–58. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Ikeda, N.; Tahara, K.; Takeuchi, H. Mechanical characteristics of orally disintegrating films: Comparison of folding endurance and tensile properties. Int. J. Pharm. 2020, 589, 1–10. [Google Scholar] [CrossRef]
- Skulason, S.; Asgeirsdottir, M.; Magnusson, J.; Kristmundsdottir, T. Evaluation of polymeric films for buccal drug delivery. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 197–201. [Google Scholar]
- Satyanarayana, D.A.; Keshavarao, K.P. Fast disintegrating films containing anastrozole as a dosage form for dysphagia patients. Arch. Pharmacal Res. 2012, 35, 2171–2182. [Google Scholar] [CrossRef]
- Rodríguez, M.; Osés, J.; Ziani, K.; Maté, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res. Int. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Bonsu, M.A.; Ofori-Kwakye, K.; Kipo, S.L.; El Boakye-Gyasi, M.; Fosu, M.-A. Development of oral dissolvable films of diclofenac sodium for osteoarthritis using Albizia and Khaya gums as hydrophilic film formers. J. Drug Deliv. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Prabhu, P.; Malli, R.; Koland, M.; Vijaynarayana, K.; D′souza, U.; Harish, N.; Shastry, C.; Charyulu, R. Formulation and evaluation of fast dissolving films of levocitirizine di hydrochloride. Int. J. Pharm. Investig. 2011, 1, 99. [Google Scholar] [CrossRef]
- Hanif, M.; Zaman, M. Thiolation of arabinoxylan and its application in the fabrication of controlled release mucoadhesive oral films. DARU J. Pharm. Sci. 2017, 25, 1–13. [Google Scholar] [CrossRef]
- Akhtar, N.; Rehman, M.; Khan, H.; Rasool, F.; Saeed, T.; Murtaz, G. Penetration enhancing effect of polysorbate 20 and 80 on the in vitro percutaneous absorption of lascorbic acid. Trop. J. Pharm. Res. 2011, 10, 281–288. [Google Scholar] [CrossRef]
- Boateng, J.; Okeke, O. Evaluation of clay-functionalized wafers and films for nicotine replacement therapy via buccal mucosa. Pharmaceutics 2019, 11, 104. [Google Scholar] [CrossRef]
- Diaz, D.A.; Colgan, S.T.; Langer, C.S.; Bandi, N.T.; Likar, M.D.; Van Alstine, L. Dissolution similarity requirements: How similar or dissimilar are the global regulatory expectations? AAPS J. 2016, 18, 15–22. [Google Scholar] [CrossRef]
- Gohel, M.C.; Ramkishan, A.; Patel, T.M.; Pandya, R.; Suthar, V.; Koradia, H.; Madat, D.V.; Bariya, S.; Mehta, T. Nomogram for computing the value of similarity factor. Indian J. Pharm. Sci. 2014, 76, 245–251. [Google Scholar]
- Ruxton, G.D.; Neuhäuser, M. When should we use one-tailed hypothesis testing? Methods Ecol. Evol. 2010, 1, 114–117. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).