Durability of Implanted Low-Density Polyacrylamide Hydrogel Used as a Scaffold for Microencapsulated Molecular Probes inside Small Fish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
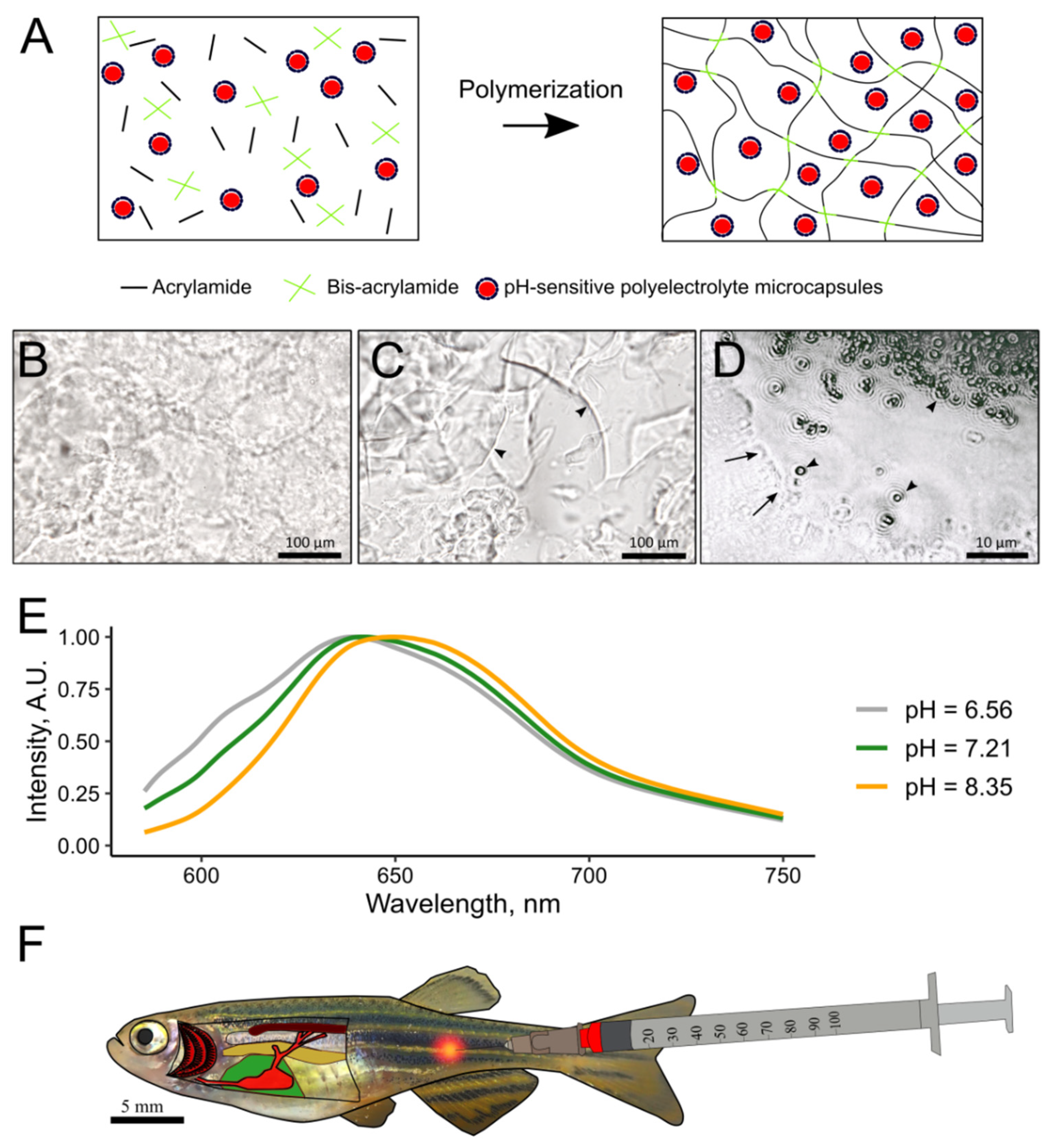
2.2. Preparation of pH-Sensitive Hydrogel
2.3. Animal Maintenance
2.4. Injection of Polyacrylamide Hydrogel
2.5. Histological Examination of Fish with Implanted pH-Sensitive Hydrogel
2.6. pH Measurements Using SNARF-1
2.7. Hypercapnic Exposure
3. Results
3.1. In Vitro Characterization of 2.7% PAAH
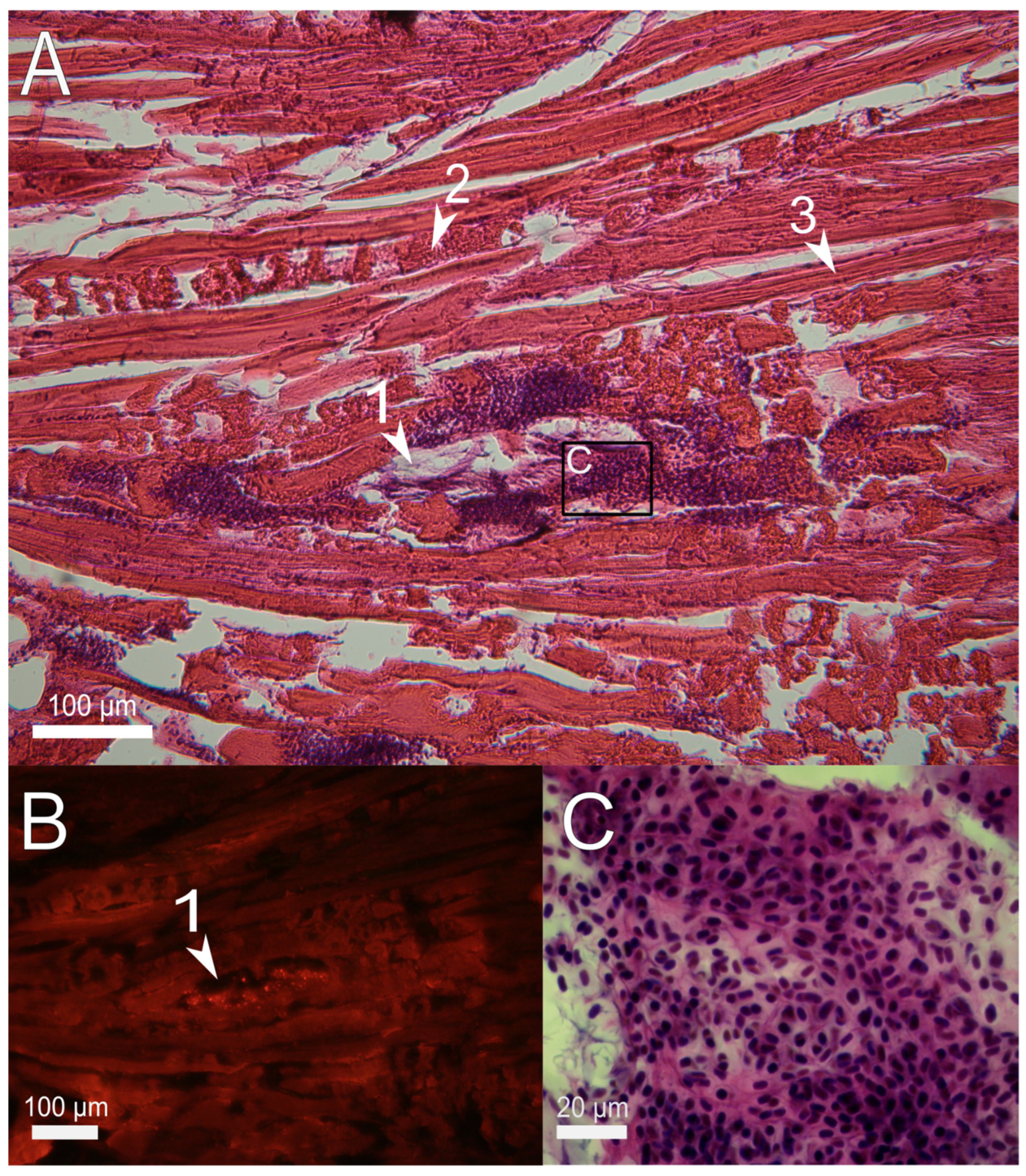
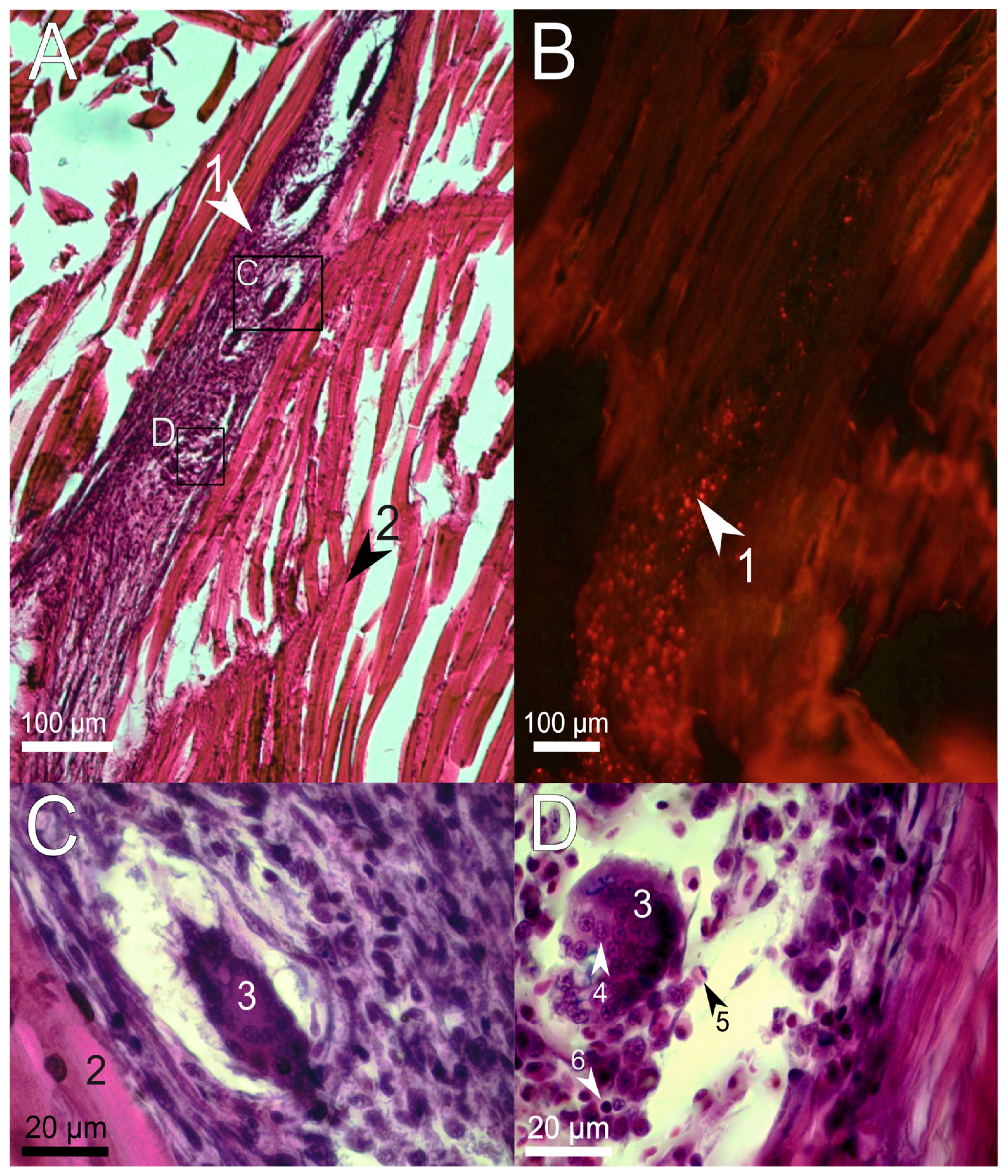
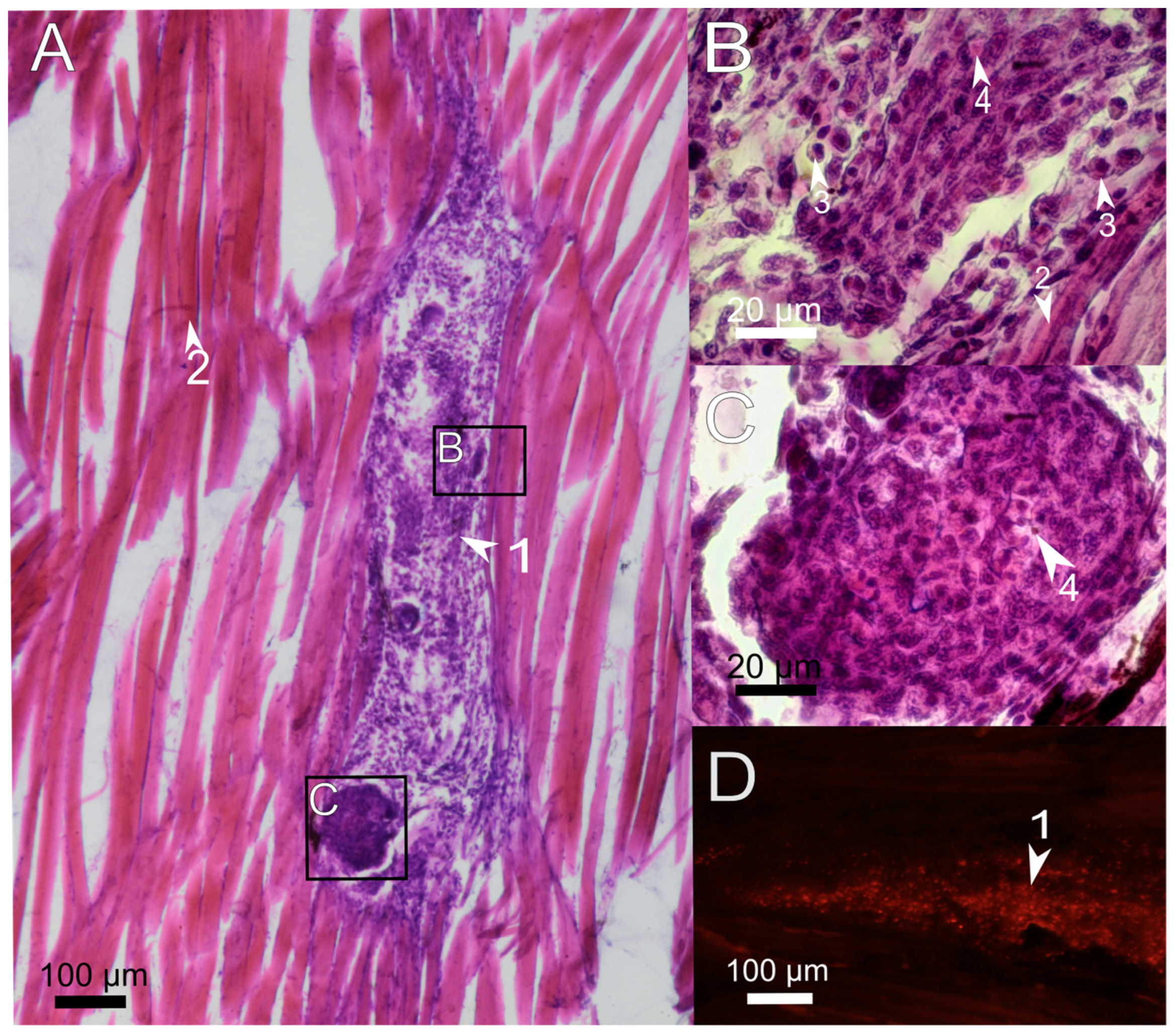
3.2. Histological Examination of the Immune Response to the Implanted Hydrogel in Fish Muscles
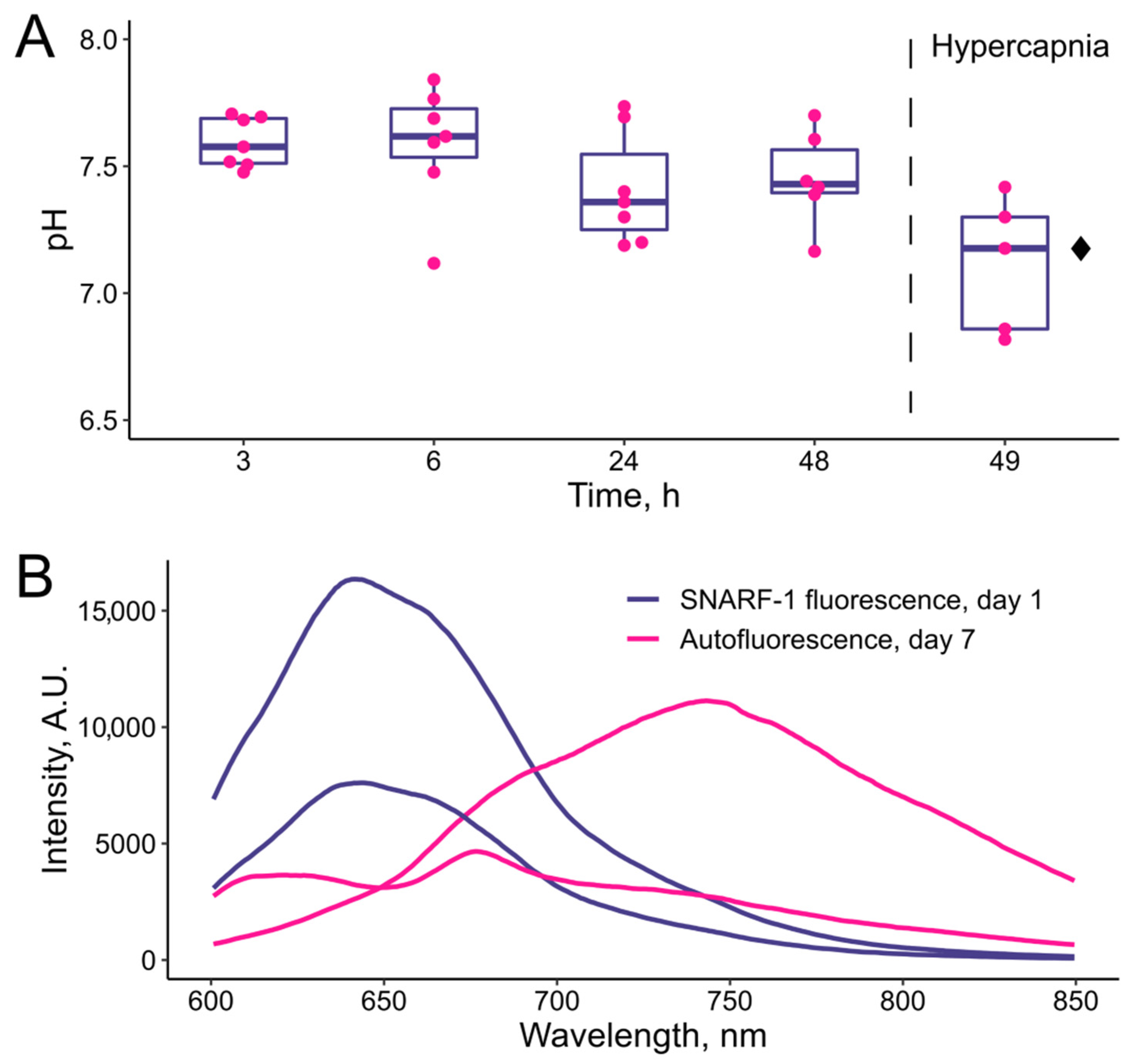
3.3. In Vivo Tests of the pH-Sensitive Hydrogel
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bian, S.; Zhu, B.; Rong, G.; Sawan, M. Towards Wearable and Implantable Continuous Drug Monitoring: A Review. J. Pharm. Anal. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Halachmi, I.; Guarino, M.; Bewley, J.; Pastell, M. Smart Animal Agriculture: Application of Real-Time Sensors to Improve Animal Well-Being and Production. Annu. Rev. Anim. Biosci. 2019, 7, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Clifford, A.; Das, J.; Yousefi, H.; Mahmud, A.; Chen, J.B.; Kelley, S.O. Strategies for Biomolecular Analysis and Continuous Physiological Monitoring. J. Am. Chem. Soc. 2021, 143, 5281–5294. [Google Scholar] [CrossRef]
- Mei, X.; Ye, D.; Zhang, F.; Di, C. Implantable Application of Polymer-Based Biosensors. J. Polym. Sci. 2022, 60, 328–347. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Hernandez, A.L.; Unluturk, B.D.; Quintero, S.A.; de Barros, N.R.; Hoque Apu, E.; Bin Shams, A.; Ostrovidov, S.; Li, J.; Contag, C.; et al. Biodegradable Implantable Sensors: Materials Design, Fabrication, and Applications. Adv. Funct. Mater. 2021, 31, 2104149. [Google Scholar] [CrossRef]
- Kanick, S.C.; Schneider, P.A.; Klitzman, B.; Wisniewski, N.A.; Rebrin, K. Continuous Monitoring of Interstitial Tissue Oxygen Using Subcutaneous Oxygen Microsensors: In Vivo Characterization in Healthy Volunteers. Microvasc. Res. 2019, 124, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Marinchenko, T.E. Aquaculture in The World and Russia: State and Prospects. IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012052. [Google Scholar] [CrossRef]
- Messina, A.; Potrich, D.; Schiona, I.; Sovrano, V.A.; Vallortigara, G. The Sense of Number in Fish, with Particular Reference to Its Neurobiological Bases. Animals 2021, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Li, P.; Zhang, S.-Q.; He, S.-W.; Xing, S.-Y.; Cao, Z.-H.; Lu, R.; Li, Z.-H. Effects of Environmental Norfloxacin Concentrations on the Intestinal Health and Function of Juvenile Common Carp and Potential Risk to Humans. Environ. Pollut. 2021, 287, 117612. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Tuttle, E.E.; Neal Reilly, A.; Clark, H.A. Recent Developments in Nanosensors for Imaging Applications in Biological Systems. Annu. Rev. Anal. Chem. 2019, 12, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Sindeeva, O.A.; Verkhovskii, R.A.; Abdurashitov, A.S.; Voronin, D.V.; Gusliakova, O.I.; Kozlova, A.A.; Mayorova, O.A.; Ermakov, A.V.; Lengert, E.V.; Navolokin, N.A.; et al. Effect of Systemic Polyelectrolyte Microcapsule Administration on the Blood Flow Dynamics of Vital Organs. ACS Biomater. Sci. Eng. 2020, 6, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Baduev, B.; Meglinski, I.; Timofeyev, M. Distribution of PEG-Coated Hollow Polyelectrolyte Microcapsules after Introduction into the Circulatory System and Muscles of Zebrafish. Biol. Open 2018, 7, bio030015. [Google Scholar] [CrossRef]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Baduev, B.; Shatilina, Z.; Sadovoy, A.; Meglinski, I.; Timofeyev, M. Parallel In Vivo Monitoring of PH in Gill Capillaries and Muscles of Fishes Using Microencapsulated Biomarkers. Biol. Open 2017, 6, 673–677. [Google Scholar] [CrossRef]
- Kaefer, K.; Krüger, K.; Schlapp, F.; Uzun, H.; Celiksoy, S.; Flietel, B.; Heimann, A.; Schroeder, T.; Kempski, O.; Sönnichsen, C. Implantable Sensors Based on Gold Nanoparticles for Continuous Long-Term Concentration Monitoring in the Body. Nano Lett. 2021, 21, 3325–3330. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Nguyen, F.T.; Scott, K.; Chan, N.Y.L.; Bakh, N.A.; Jones, K.K.; Pham, C.; Garcia-Salinas, P.; Garcia-Parraga, D.; Fahlman, A.; et al. Implanted Nanosensors in Marine Organisms for Physiological Biologging: Design, Feasibility, and Species Variability. ACS Sens. 2019, 4, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.R.; Sakiyama-Elbert, S.E.; Zhang, G.; Yaszemski, M.J. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Li, L.; Zhang, K.; Wang, T.; Wang, P.; Xue, B.; Cao, Y.; Zhu, L.; Jiang, Q. Biofabrication of a Biomimetic Supramolecular-Polymer Double Network Hydrogel for Cartilage Regeneration. Mater. Des. 2020, 189, 108492. [Google Scholar] [CrossRef]
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701. [Google Scholar] [CrossRef]
- Dahlin, C.; Johansson, A.; Hoffman, M.; Molenberg, A. Early Biocompatibility of Poly(Ethylene Glycol) Hydrogel Barrier Materials for Guided Bone Regeneration. An in Vitro Study Using Human Gingival Fibroblasts (HGF-1). Clin. Oral Implant. Res. 2014, 25, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, N.A.; Nichols, S.P.; Gamsey, S.J.; Pullins, S.; Au-Yeung, K.Y.; Klitzman, B.; Helton, K.L. Tissue-Integrating Oxygen Sensors: Continuous Tracking of Tissue Hypoxia. In Oxygen Transport to Tissue XXXIX; Halpern, H.J., LaManna, J.C., Harrison, D.K., Epel, B., Eds.; Adances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 377–383. [Google Scholar] [CrossRef]
- Lim, S.L.; Ooi, C.-W.; Low, L.E.; Tan, W.S.; Chan, E.-S.; Ho, K.L.; Tey, B.T. Synthesis of Poly(Acrylamide)-Based Hydrogel for Bio-Sensing of Hepatitis B Core Antigen. Mater. Chem. Phys. 2020, 243, 122578. [Google Scholar] [CrossRef]
- Xue, B.; Tang, D.; Wu, X.; Xu, Z.; Gu, J.; Han, Y.; Zhu, Z.; Qin, M.; Zou, X.; Wang, W.; et al. Engineering Hydrogels with Homogeneous Mechanical Properties for Controlling Stem Cell Lineage Specification. Proc. Natl. Acad. Sci. USA 2021, 118, e2110961118. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Z.; Shi, J.; Wang, X.; Han, P.; Qian, W. An Efficient, Recyclable, and Stable Immobilized Biocatalyst Based on Bioinspired Microcapsules-in-Hydrogel Scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 25152–25161. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Sun, M.; Bu, Y.; Luo, F.; Lin, C.; Lin, Z.; Weng, Z.; Yang, F.; Wu, D. Microcapsule-Embedded Hydrogel Patches for Ultrasound Responsive and Enhanced Transdermal Delivery of Diclofenac Sodium. J. Mater. Chem. B 2019, 7, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, L.; Fu, X.; Liu, J.; Liang, Z.; Tan, H.; Li, W.; Zhao, Y. Engineering Microcapsules to Construct Vascularized Human Brain Organoids. Chem. Eng. J. 2021, 424, 130427. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels Based Drug Delivery Synthesis, Characterization and Administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic Acid/Acrylamide Based Hydrogels and Its Properties—A Review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Zamboni, F.; Ryan, E.; Culebras, M.; Collins, M.N. Labile Crosslinked Hyaluronic Acid via Urethane Formation Using Bis(β-Isocyanatoethyl) Disulphide with Tuneable Physicochemical and Immunomodulatory Properties. Carbohydr. Polym. 2020, 245, 116501. [Google Scholar] [CrossRef] [PubMed]
- Gurkov, A.; Sadovoy, A.; Shchapova, E.; Teh, C.; Meglinski, I.; Timofeyev, M. Microencapsulated Fluorescent PH Probe as Implantable Sensor for Monitoring the Physiological State of Fish Embryos. PLoS ONE 2017, 12, e0186548. [Google Scholar] [CrossRef] [PubMed]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Karnaukhov, D.; Sadovoy, A.; Meglinski, I.; Timofeyev, M. Simple and Effective Administration and Visualization of Microparticles in the Circulatory System of Small Fishes Using Kidney Injection. JoVE 2018, 136, e57491. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.N.; Kumar, M. Experimental Determination of the Kinematic Viscosity of Glycerol-Water Mixtures. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1994, 444, 573–581. [Google Scholar] [CrossRef]
- Korzhevskiy, D.E.; Gilyarov, A.V. Osnovy Gistologicheskoy Tekhniki (Basics of Histological Techniques). Saint Petersburg. SpetsLit. 2010, 95. [Google Scholar]
- Gurkov, A.; Shchapova, E.; Bedulina, D.; Baduev, B.; Borvinskaya, E.; Meglinski, I.; Timofeyev, M. Remote in vivo Stress Assessment of Aquatic Animals with Microencapsulated Biomarkers for Environmental Monitoring. Sci. Rep. 2016, 6, 36427. [Google Scholar] [CrossRef]
- Wahli, T.; Verlhac, V.; Girling, P.; Gabaudan, J.; Aebischer, C. Influence of Dietary Vitamin C on the Wound Healing Process in Rainbow Trout (Oncorhynchus Mykiss). Aquaculture 2003, 225, 371–386. [Google Scholar] [CrossRef]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult Zebrafish as a Model System for Cutaneous Wound-Healing Research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef]
- Claver, J.A.; Quaglia, A.I.E. Comparative Morphology, Development, and Function of Blood Cells in Nonmammalian Vertebrates. J. Exot. Pet. Med. 2009, 18, 87–97. [Google Scholar] [CrossRef]
- Wizenty, J.; Ashraf, M.I.; Rohwer, N.; Stockmann, M.; Weiss, S.; Biebl, M.; Pratschke, J.; Aigner, F.; Wuensch, T. Autofluorescence: A Potential Pitfall in Immunofluorescence-Based Inflammation Grading. J. Immunol. Methods 2018, 456, 28–37. [Google Scholar] [CrossRef]
- Montero-Baker, M.F.; Au-Yeung, K.Y.; Wisniewski, N.A.; Gamsey, S.; Morelli-Alvarez, L.; Mills, J.L.; Campos, M.; Helton, K.L. The First-in-Man “Si Se Puede” Study for the Use of Micro-Oxygen Sensors (MOXYs) to Determine Dynamic Relative Oxygen Indices in the Feet of Patients with Limb-Threatening Ischemia during Endovascular Therapy. J. Vasc. Surg. 2015, 61, 1501–1509.e1. [Google Scholar] [CrossRef]
- Hu, X.; Gong, X. A New Route to Fabricate Biocompatible Hydrogels with Controlled Drug Delivery Behavior. J. Colloid Interface Sci. 2016, 470, 62–70. [Google Scholar] [CrossRef]
- Arjmandi, M.; Ramezani, M.; Nand, A.; Neitzert, T. Experimental Study on Friction and Wear Properties of Interpenetrating Polymer Network Alginate-Polyacrylamide Hydrogels for Use in Minimally-Invasive Joint Implants. Wear 2018, 406, 194–204. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Gu, Z.; Cheng, W.; Lei, H.; Qin, M.; Xue, B.; Wang, W.; Cao, Y. Regulating mechanical properties of polymer-supramolecular double-network hydrogel by supramolecular self-assembling structures. Chin. J. Chem. 2021, 39, 2711–2717. [Google Scholar] [CrossRef]
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-Thinning and Self-Healing Hydrogels as Injectable Therapeutics and for 3D-Printing. Nat. Protoc. 2017, 12, 1521–1541. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.; Ju, Y.M.; West, L.; Harmon, J.; Moussy, Y.; Moussy, F. Use of Hydrogel Coating to Improve the Performance of Implanted Glucose Sensors. Biosens. Bioelectron. 2008, 23, 1278–1284. [Google Scholar] [CrossRef]
- Christensen, L.H.; Breiting, V.B.; Aasted, A.; Jørgensen, A.; Kebuladze, I. Long-Term Effects of Polyacrylamide Hydrogel on Human Breast Tissue. Plast. Reconstr. Surg. 2003, 111, 1883–1890. [Google Scholar] [CrossRef]
- Chen, L.; Sha, L.; Huang, S.-P.; Li, S.-R.; Wang, Z.-X. Treatment for Displacement of PAAG Mixture after Injection Augmentation Mammoplasty. Int. J. Clin. Exp. Med. 2015, 8, 3360–3370. [Google Scholar]
- Peng, H.L.; Cheng, Y.H.; Lin, Y.H.; Ko, C.H. Unusual presentation of a late complication in a polyacrylamide gel-injected breast. Formos. J. Surg. 2017, 50, 77. [Google Scholar]
- DeLuca, M.; Shapiro, A.; Banayan, E.; Zielinski, G.; Karanetz, I.; Asarian, A.; Xiao, P. Complications 18 Years after Polyacrylamide Hydrogel Augmentation Mammoplasty: A Case Report and Histopathological Analysis. J. Surg. Case Rep. 2021, 2021, rjab276. [Google Scholar] [CrossRef]
- Zhou, Z.; Ru, K.-H. Observation on biologic characterization of polyacrylamide gel and silicon gel infused into mammary gland of domestic musk swine. J. Pract. Aesth. Plast. Surg. 2001, 12, 69–71. (In Chinese) [Google Scholar]
- Antonio, N.; Bønnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P. The Wound Inflammatory Response Exacerbates Growth of Pre-Neoplastic Cells and Progression to Cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Mutin, A.; Timofeyev, M. Histopathological Analysis of Zebrafish after Introduction of Non-Biodegradable Polyelectrolyte Microcapsules into the Circulatory System. PeerJ 2021, 9, e11337. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Patel, P.S.; Wu, Z.J.; Chien, J.S.; Wisniewski, N.A.; Mohammed, M.M.; Klitzman, B. Detection of Flap Tissue Ischemia in a Rat Model: Real-Time Monitoring of Changes in Oxygenation and Perfusion through Injectable Biosensors. Surgery 2020, 168, 926–934. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Fahimipour, F.; Keith, A.N.; Vashahi, F.; Popryadukhin, P.; Vatankhah-Varnosfaderani, M.; Sheiko, S.S. Injectable Non-Leaching Tissue-Mimetic Bottlebrush Elastomers as an Advanced Platform for Reconstructive Surgery. Nat. Commun. 2021, 12, 3961. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shchapova, E.; Titov, E.; Gurkov, A.; Nazarova, A.; Borvinskaya, E.; Timofeyev, M. Durability of Implanted Low-Density Polyacrylamide Hydrogel Used as a Scaffold for Microencapsulated Molecular Probes inside Small Fish. Polymers 2022, 14, 3956. https://doi.org/10.3390/polym14193956
Shchapova E, Titov E, Gurkov A, Nazarova A, Borvinskaya E, Timofeyev M. Durability of Implanted Low-Density Polyacrylamide Hydrogel Used as a Scaffold for Microencapsulated Molecular Probes inside Small Fish. Polymers. 2022; 14(19):3956. https://doi.org/10.3390/polym14193956
Chicago/Turabian StyleShchapova, Ekaterina, Evgeniy Titov, Anton Gurkov, Anna Nazarova, Ekaterina Borvinskaya, and Maxim Timofeyev. 2022. "Durability of Implanted Low-Density Polyacrylamide Hydrogel Used as a Scaffold for Microencapsulated Molecular Probes inside Small Fish" Polymers 14, no. 19: 3956. https://doi.org/10.3390/polym14193956
APA StyleShchapova, E., Titov, E., Gurkov, A., Nazarova, A., Borvinskaya, E., & Timofeyev, M. (2022). Durability of Implanted Low-Density Polyacrylamide Hydrogel Used as a Scaffold for Microencapsulated Molecular Probes inside Small Fish. Polymers, 14(19), 3956. https://doi.org/10.3390/polym14193956





