Imidazolium Salt for Enhanced Interfacial Shear Strength in Polyphenylene Sulfide/Ex-PAN Carbon Fiber Composites
Abstract
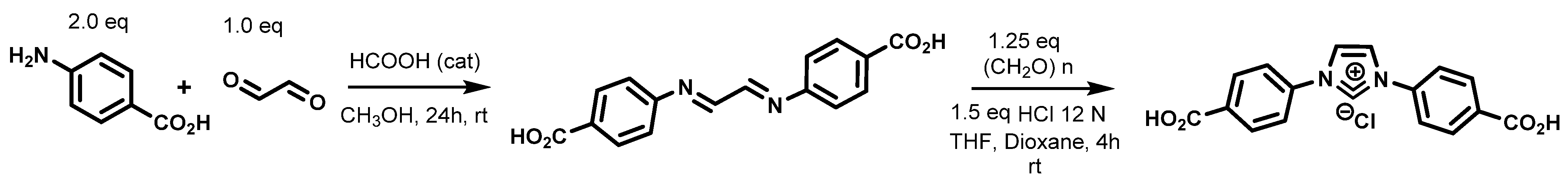
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Processing of PPS and Salt Modified PPS Blends
2.2.1. Polymer Films
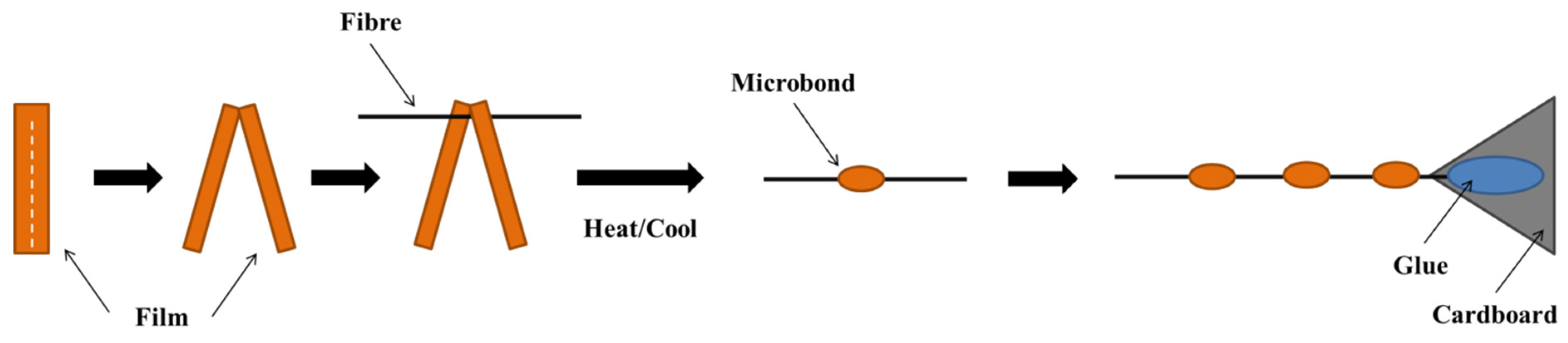
2.2.2. Microcomposite Processing
2.2.3. Thermal Treatments of Microcomposites
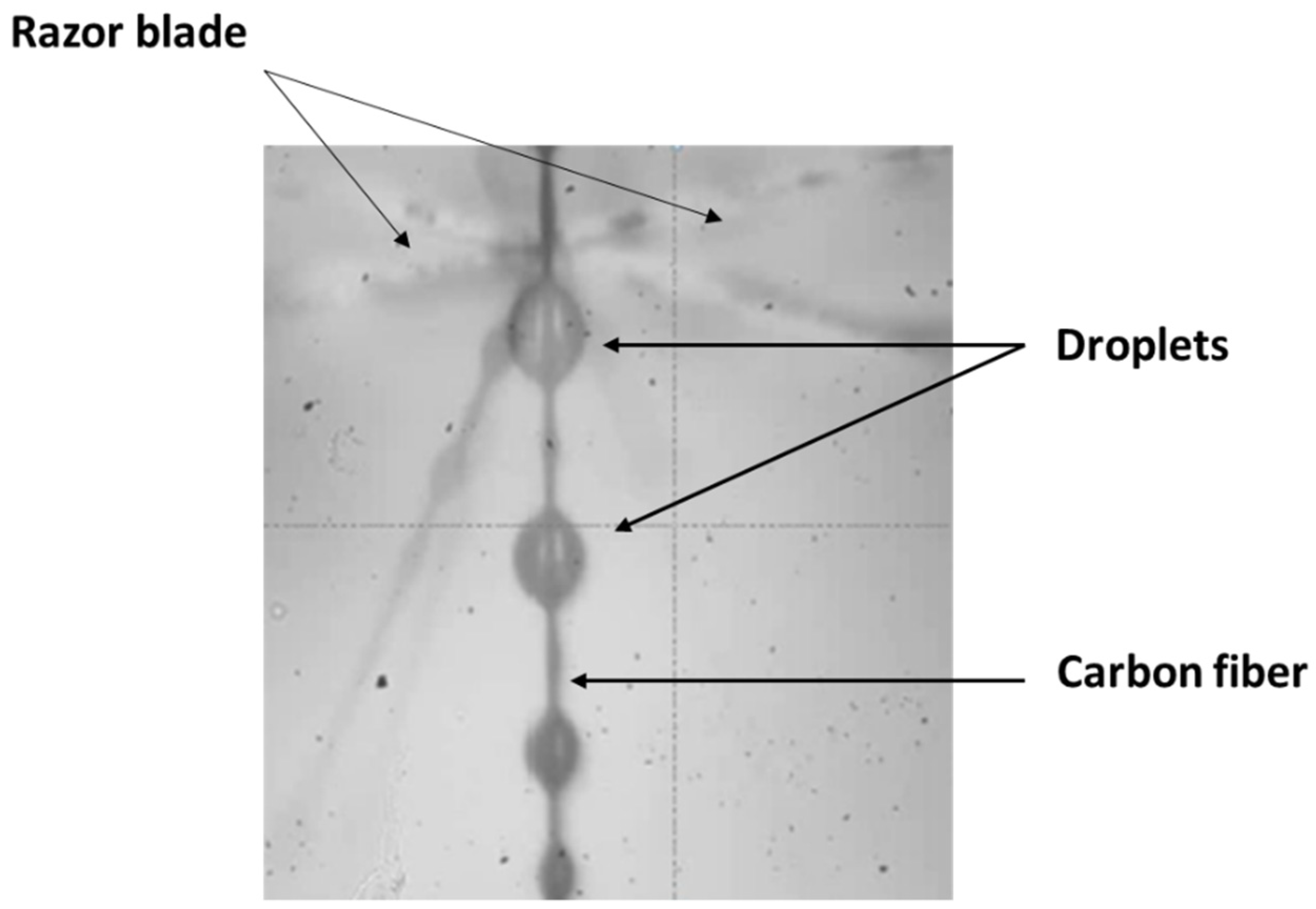
2.3. Characterization Methods
3. Results and Discussion
3.1. Behavior of Pps and Pps_ImCl
3.1.1. Thermal Stability of Pps and Pps_ImCl Matrices
3.1.2. Confirmation of the Presence of ImCl by FTIR Analysis
3.1.3. Surface Energies
3.1.4. Crystallization Behavior of the Matrices
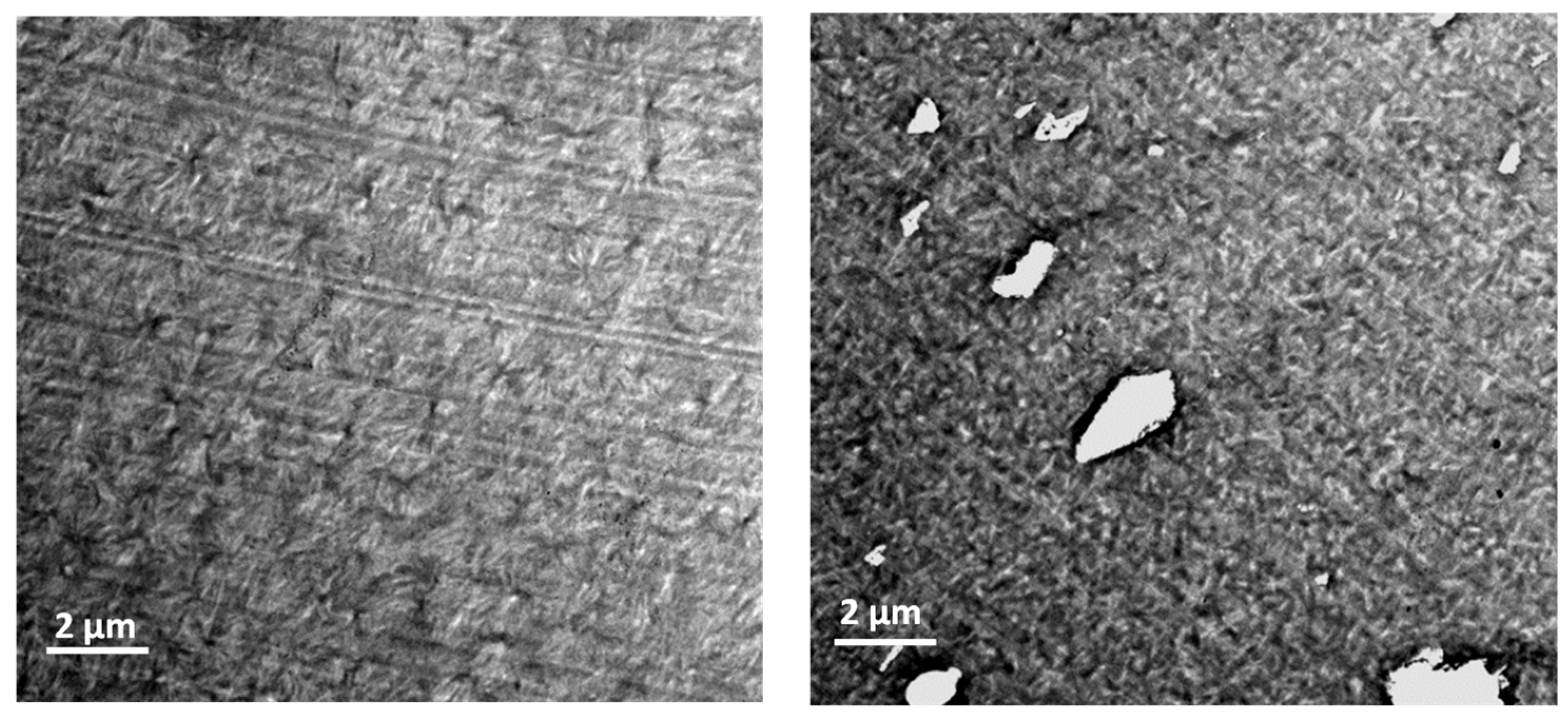
3.1.5. Crystalline Morphology of PPS Containing ImCl Observed by TEM
3.2. Study of Three ex-PAN Carbon Fiber Surfaces
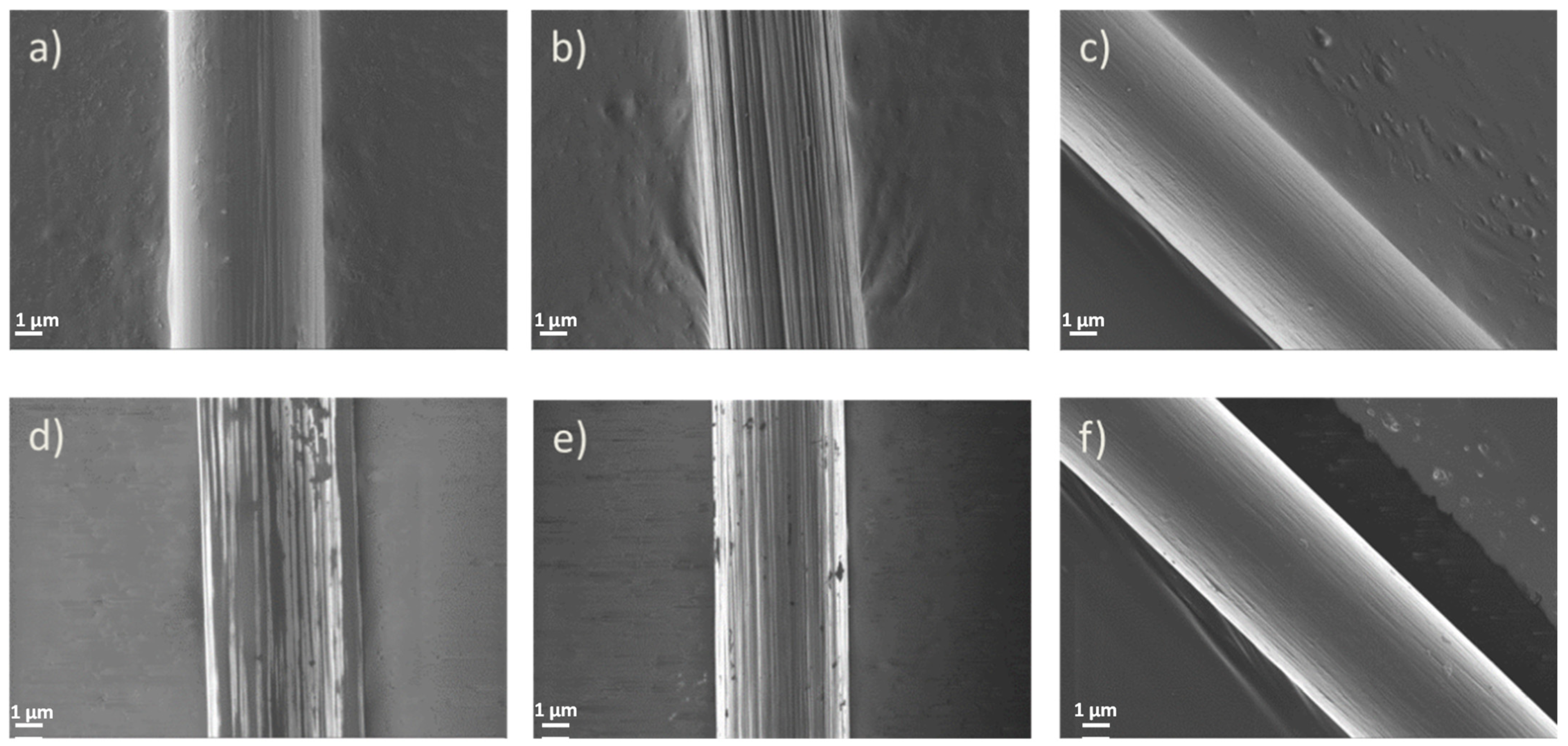
3.2.1. SEM Observations of the Carbon Fibers
3.2.2. Surface Energies of Carbon Fibers
3.2.3. Chemical Analysis of Carbon Fiber’s Surfaces
3.3. Determination of the Matrix-Fiber Adhesion by Micromechanical Tests
3.3.1. Influence of the Chemical Surfaces of Carbon Fibers
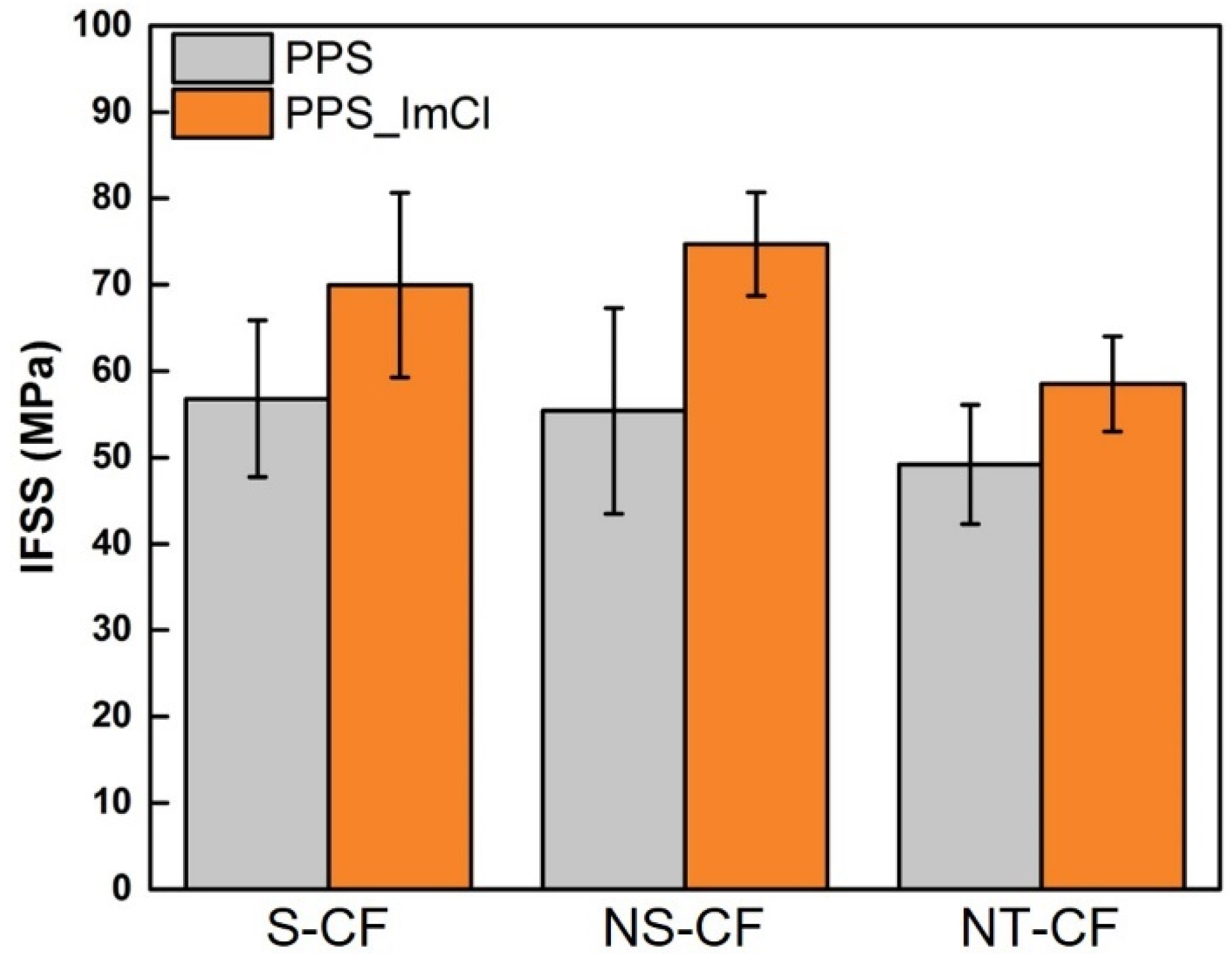
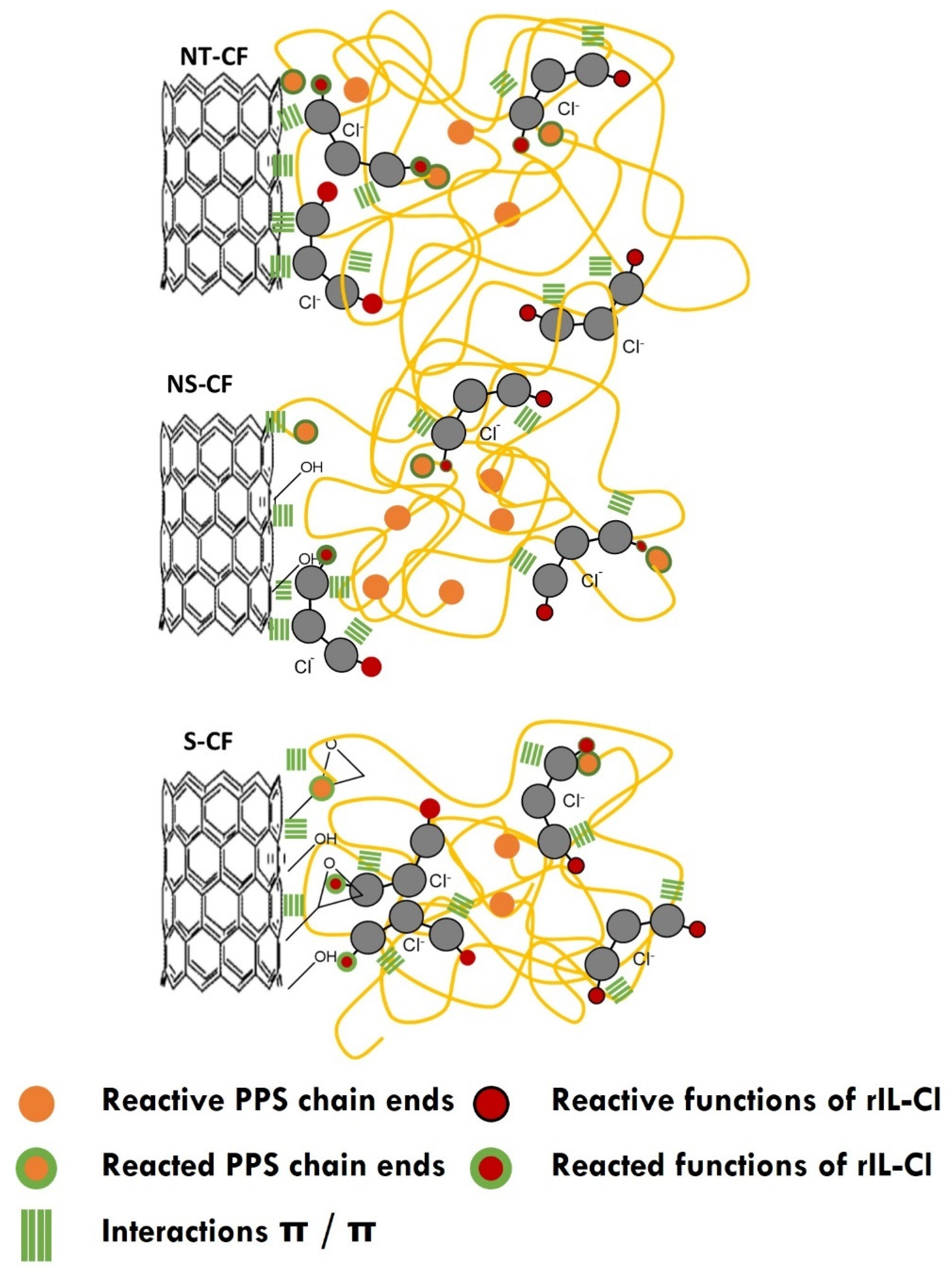
3.3.2. Influence of the Addition of the Imidazolium Salt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beck, H.N. Solubility Characteristics of Poly(Etheretherketone) and Poly(Phenylene Sulfide). J. Appl. Polym. Sci. 1992, 45, 1361–1366. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Aravinthan, T. Bond behavior of composite sandwich panel and epoxy polymer matrix: Taguchi design of experiments and theoretical predictions. Constr. Build. Mater. 2017, 145, 76–87. [Google Scholar] [CrossRef]
- Yuan, L.Y.; Shyu, S.S.; Lai, J.Y. Plasma surface treatments of carbon fibers. Part 2: Interfacial adhesion with poly(phenylene sulfide). Compos. Sci. Technol. 1992, 45, 9–16. [Google Scholar] [CrossRef]
- Xu, D.; Liu, B.; Zhang, G.; Long, S.; Wang, X.; Yang, J. Effect of air plasma treatment on interfacial shear strength of carbon fiber–reinforced polyphenylene sulfide. High Perform. Polym. 2016, 28, 411–424. [Google Scholar] [CrossRef]
- Drzal, L.T.; Rich, M.J.; Lloyd, P.F. Adhesion of Graphite Fibers to Epoxy Matrices: I. The Role of Fiber Surface Treatment. J. Adhes. 1983, 16, 1–30. [Google Scholar] [CrossRef]
- Xu, J.; Xu, D.; Wang, X.; Long, S.; Yang, J. Improved interfacial shear strength of carbon fiber/polyphenylene sulfide composites by graphene. High Perform. Polym. 2017, 29, 913–921. [Google Scholar] [CrossRef]
- Gonon, L.; Chabert, B.; Bernard, A.; Van Hoyweghen, D.; Gerard, J.F. New coupling agents as adhesion promoters at the poly(phenylene sulfide)/glass interface-studies with micro and macro composites. J. Adhes. 1997, 61, 271–292. [Google Scholar] [CrossRef]
- Gao, X.; Wang, R.; Zhang, A. Synthesis of Poly(ether ether ketone)s containing tertiary amine. Mater. Lett. 2007, 61, 3647–3651. [Google Scholar] [CrossRef]
- Ren, H.; Xu, D.-X.; Yan, G.-M.; Zhang, G.; Wang, X.-J.; Long, S.-R.; Yang, J. Effect of carboxylic polyphenylene sulfide on the micromechanical properties of polyphenylene sulfide/carbon fiber composites. Compos. Sci. Technol. 2017, 146, 65–72. [Google Scholar] [CrossRef]
- Savas, L.A.; Tayfun, U.; Dogan, M. The use of polyethylene copolymers as compatibilizers in carbon fiber reinforced high density polyethylene composites. Compos. Part B Eng. 2016, 99, 188–195. [Google Scholar] [CrossRef]
- Livi, S.; Duchet-Rumeau, J.; Gérard, J.F.; Pham, T.N. Polymers and ionic liquids: A successful wedding. Macromol. Chem. Phys. 2015, 216, 359–368. [Google Scholar] [CrossRef]
- Donato, R.K.; Benvegnú, M.A.; Furlan, L.G.; Mauler, R.S.; Schrekker, H.S. Imidazolium salts as liquid coupling agents for the preparation of polypropylene-silica composites. J. Appl. Polym. Sci. 2009, 116, 304–307. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Servinis, L.; Scheffler, C.; Wölfel, E.; Demir, B.; Walsh, T.R.; Henderson, L.C. Synergistic interfacial effects of ionic liquids as sizing agents and surface modified carbon fibers. J. Mater. Chem. A 2018, 6, 4504–4514. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Stojcevski, F.; Hendlmeier, A.; Arnold, C.L.; Randall, J.D.; Perus, M.D.; Servinis, L.; Gengenbach, T.R.; Demir, B.; Walsh, T.R.; et al. An efficient high-throughput grafting procedure for enhancing carbon fiber-to-matrix interactions in composites. Chem. Eng. J. 2018, 353, 373–380. [Google Scholar] [CrossRef]
- Sen, S.; Nair, N.N.; Yamada, T.; Kitagawa, H.; Bharadwaj, P.K. High proton conductivity by a metal-organic framework incorporating Zn8O clusters with aligned imidazolium groups decorating the channels. J. Am. Chem. Soc. 2012, 134, 19432–19437. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, M.; Livi, S.; Duchet-Rumeau, J. Ionic liquids: A new way for the compatibilization of thermoplastic blends. Chem. Eng. J. 2014, 255, 513–524. [Google Scholar] [CrossRef]
- Deporter, J.; Baird, D.G. The effects of thermal history on the structure/property relationship in polyphenylenesulfide/carbon fiber composites. Polym. Compos. 1993, 14, 201–213. [Google Scholar] [CrossRef]
- Batista, N.L.; Olivier, P.; Bernhart, G.; Rezende, M.C.; Botelho, E.C. Correlation between degree of crystallinity, morphology and mechanical properties of PPS/carbon fiber laminates. Mater. Res. 2016, 19, 195–201. [Google Scholar] [CrossRef]
- Trudel-Boucher, D.; Esquirol, A.L.; Bureau, M.N. Influence of cooling rates on microstructure and mechanical performance of continuous fiber reinforced PPS composites. In Proceedings of the Society for the Advancement of Material and Process Engineering International Symposium and Exhibition 2012 (SAMPE 2012), Baltimore, MD, USA, 21–24 May 2012. [Google Scholar]
- Kobayashi, H.; Hayakawa, E.; Kikutani, T.; Takaku, A. Effect of quenching and annealing on fiber pull-out from crystalline polymer matrices. Adv. Compos. Mater. 1991, 1, 155–168. [Google Scholar] [CrossRef]
- Meretz, S.; Auersch, W.; Marotzke, C.; Schulz, E. Investigation of morphology-dependent fracture behaviour with the single-fibre pull-out test. Compos. Sci. Technol. 1993, 48, 285–290. [Google Scholar] [CrossRef]
- Schulz, E.; Kalinka, G.; Auersch, W. Effect of transcrystallization in carbon fiber reinforced poly(p-phenylene sulfide) composites on the interfacial shear strength investigated with the single fiber pull-out test. J. Macromol. Sci. Part B 1996, 35, 527–546. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Long, S.; Yang, J. Interfacial micromechanics of carbon fiber-reinforced polyphenylene sulfide composites. Compos. Interfaces 2014, 21, 359–369. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.-H.; Lee, J.; Lee, D.-M.; Yu, H.; Jeong, H.S.; Kim, S.M.; Lee, K.-H. Accurate measurement of specific tensile strength of carbon nanotube fibers with hierarchical structures by vibroscopic method. RSC Adv. 2017, 7, 8575–8580. [Google Scholar] [CrossRef]
- Awad, W.H. Thermal Degradation Studies of Alkyl-Imidazolium Salts and Their Application in Nanocomposites. ECS Proc. Vol. 2002, 2002-19, 200–212. [Google Scholar] [CrossRef]
- Pucci, A.; Liuzzo, V.; Melai, B.; Pomelli, C.S.; Chiappe, C. Polymerizable ionic liquids for the preparation of polystyrene/clay composites. Polym. Int. 2012, 61, 426–433. [Google Scholar] [CrossRef]
- Livi, S.; Duchet-Rumeau, J.; Pham, T.N.; Gérard, J.F. A comparative study on different ionic liquids used as surfactants: Effect on thermal and mechanical properties of high-density polyethylene nanocomposites. J. Colloid Interface Sci. 2010, 349, 424–433. [Google Scholar] [CrossRef]
- Zimmerman, D.A.; Koenig, J.L.; Ishida, H. Infrared spectroscopic analysis of poly(p-phenylene sulfide). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 2397–2409. [Google Scholar] [CrossRef]
- Ezugwu, C.I.; Asraf, M.A.; Li, X.; Liu, S.; Kao, C.M.; Zhuiykov, S.; Verpoort, F. Selective and adsorptive removal of anionic dyes and CO2 with azolium-based metal-organic frameworks. J. Colloid Interface Sci. 2018, 519, 214–223. [Google Scholar] [CrossRef]
- Piglowski, J.; Coen, M.C.; Schäfer, R.; Kressler, J.; Mülhaupt, R. Surface modification and bonding of poly(p-phenylene sulfide). Angew. Makromol. Chem. 1997, 246, 97–107. [Google Scholar] [CrossRef]
- Wang, Y.; Rak, S. Surface modification of polyphenylene sulfide plastics to improve their adhesion to a dielectric adhesive. Adhes. Asp. Polym. Coat. 2003, 2, 121–136. [Google Scholar]
- Hong, S.M.; Kim, B.C.; Kim, K.U.; Chung, I.J. Crystallization Kinetics of Poly(phenylene sulfide) Containing a Thermotropic Liquid Crystalline Polyesteramide. Polym. J. 2005, 24, 727–736. [Google Scholar] [CrossRef]
- Minkova, L.I.; Magagnini, P.L. Non-isothermal crystallization kinetics of poly(phenylene sulfide)/Vectra-B blends. Polymer 1995, 36, 2059–2063. [Google Scholar] [CrossRef]
- Gopakumar, T.G.; Ghadage, R.S.; Ponrathnam, S.; Rajan, C.R.; Fradet, A. Poly(phenylene sulfide)/liquid crystalline polymer blends: 1. Non-isothermal crystallization kinetics. Polymer 1997, 38, 2209–2214. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Singh, R.K.; Chandra, S. Effect of ionic liquid on the crystallization kinetics behaviour of polymer poly(ethylene oxide). Cryst. Eng. Comm. 2013, 15, 6022–6034. [Google Scholar] [CrossRef]
- Xing, C.; Zhao, L.; You, J.; Dong, W.; Cao, X.; Li, Y. Impact of ionic liquid-modified multiwalled carbon nanotubes on the crystallization behavior of poly(vinylidene fluoride). J. Phys. Chem. B 2012, 116, 8312–8320. [Google Scholar] [CrossRef]
- Kataria, S.; Chaurasia, S.K.; Singh, R.K. Crystallization behaviour of a polymeric membrane based on the polymer PVdF-HFP and the ionic liquid BMIMBF4. RSC Adv. 2014, 4, 50914–50924. [Google Scholar] [CrossRef]
- Lins, L.C.; Livi, S.; Maréchal, M.; Duchet-Rumeau, J.; Gérard, J.-F. Structural dependence of cations and anions to building the polar phase of PVDF. Eur. Polym. J. 2018, 107, 236–248. [Google Scholar] [CrossRef]
- Zhang, W. Modification of Carbon Fiber/Époxy Matrix Interphase in a Composite Material: Design of a Self-Healing Interphase by Introducing Thermally Reversible Diels-Alder Adducts. Ph.D. Thesis, INSA-Lyon, Villeurbanne, France, 2014. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Hoffmann, P. Influence des Propriétés de la Surface des Fibres Sur les Caractéristiques Mécaniques de Composites Unidirectionnels Carbone/Epoxide; INSA de Lyon: Villeurbanne, France, 1985. [Google Scholar]
- Schultz, J.; Martin, C. The Role of the Interface in Carbon Fibre-Epoxy Composites. J. Adhes. 1987, 23, 45–60. [Google Scholar] [CrossRef]
- Zinck, P.; Wagner, H.D.; Salmon, L.; Gerard, J.F. Are microcomposites realistic models of the fibre/matrix interface? I. Micromechanical modelling. Polymer 2001, 42, 5401–5413. [Google Scholar] [CrossRef]
- Gao, S.-L.; Kim, J.-K. Cooling rate influences in carbon fibre/PEEK composites. Part 1. Crystallinity and interface adhesion. Compos. Part A Appl. Sci. Manuf. 2000, 31, 517–530. [Google Scholar] [CrossRef]
- Perchacz, M.; Benes, H.; Matejka, L.; Konefal, R.; Seixas, L.; Livi, S.; Baudoux, J.; Donato, R.K. Solvent-free direct ionic modification of epoxy-based materials using self-catalysed Brønsted-acidic imidazolium salts. ACS Sustain. Chem. Eng. 2019, 7, 19050–19061. [Google Scholar] [CrossRef]




| Material | Notation | Chemical Structure | Tm (°C) |
|---|---|---|---|
| Polyphenylene sulfide | PPS |  | 280 |
| 1,3-Bis(4-carboxyphenyl)imidazolium chloride | ImCl | 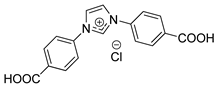 | >300 |
| Material | Td-1st peak (°C) | Td-2nd peak (°C) | Td5% (°C) |
|---|---|---|---|
| ImCl | 349 | 435 | 318 |
| PPS | 531 | 531 | 501 |
| PPS_ImCl | 435 | 536 | 498 |
| Matrix | Water CA (°) | Methylene Diiodide CA (°) |
|---|---|---|
| PPS | 78.8 ± 4.3 | 31.5 ± 3.1 |
| PPS_ImCl | 63.8 ± 6.0 | 36.4 ± 2.5 |
| Matrix | Surface Energy γ (mN/m) | Dispersive Component γd (mN/m) | Non Dispersive Component γnd (mN/m) |
|---|---|---|---|
| PPS | 46.7 ± 0.4 | 43.6 ± 1.4 | 3.1 ± 1.8 |
| PPS_ImCl | 51.2 ± 2.4 | 41.4 ± 1.2 | 9.9 ± 3.6 |
| PPS (0214) [31] | 45.5 | 44.4 | 1.1 |
| PPS (0214) [32] | 49.6 | 47.5 | 2.2 |
| Matrix | Tc (°C) | ΔHc (J/g) | Tm (°C) |
|---|---|---|---|
| PPS | 195 | 42.4 | 281 |
| PPS_ImCl | 232 | 42.2 | 280 |
| Fiber | Water CA (°) | Ethylene Glycol CA (°) | Methylene Diiodide CA (°) |
|---|---|---|---|
| S-CF | 67.2 ± 4.8 | 59.5 ± 3.0 | 35.4 ± 2.6 |
| NS-CF | 45.5 ± 2.2 | 30.8 ± 2.4 | 44.5 ± 2.1 |
| NT-CF | 78.8 ± 1.1 | 54.3 ± 0.7 | 40.0 ± 3.0 |
| Fiber | Surface Energy γ (mN/m) | Dispersive Component γd (mN/m) | Non Dispersive Component γnd (mN/m) |
|---|---|---|---|
| S-CF | 42.2 ± 1.7 | 34.0 ± 1.1 | 8.2 ± 2.8 |
| NS-CF | 53.0 ± 0.9 | 30.9 ± 1.0 | 22.1 ± 1.9 |
| NT-CF | 39.6 ± 0.7 | 35.4 ± 1.5 | 4.2 ± 0.7 |
| Fiber | C | O | N | Si | Other (<0.5%at.) |
|---|---|---|---|---|---|
| S-CF | 76.5 | 21.6 | 1.5 | - | S, Cl, Sn |
| NS-CF | 84.5 | 9.5 | 5.5 | - | Sn |
| NT-CF | 93.9 | 3.3 | 2.4 | 0.5 | - |
| Fiber | PPS | PPS_ImCl | ||
|---|---|---|---|---|
| IFSS (MPa) | N# | IFSS (MPa) | N# | |
| S-CF | 56.8 ± 9.9 | 52 | 69.9 ± 10.7 | 48 |
| NS-CF | 55.4 ± 11.9 | 55 | 74.4 ± 7.3 | 45 |
| NT-CF | 49.8 ± 7.3 | 49 | 58.6 ± 5.5 | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaumond, B.; Livi, S.; Gérard, J.-F.; Duchet-Rumeau, J. Imidazolium Salt for Enhanced Interfacial Shear Strength in Polyphenylene Sulfide/Ex-PAN Carbon Fiber Composites. Polymers 2022, 14, 3692. https://doi.org/10.3390/polym14173692
Gaumond B, Livi S, Gérard J-F, Duchet-Rumeau J. Imidazolium Salt for Enhanced Interfacial Shear Strength in Polyphenylene Sulfide/Ex-PAN Carbon Fiber Composites. Polymers. 2022; 14(17):3692. https://doi.org/10.3390/polym14173692
Chicago/Turabian StyleGaumond, Baptiste, Sébastien Livi, Jean-François Gérard, and Jannick Duchet-Rumeau. 2022. "Imidazolium Salt for Enhanced Interfacial Shear Strength in Polyphenylene Sulfide/Ex-PAN Carbon Fiber Composites" Polymers 14, no. 17: 3692. https://doi.org/10.3390/polym14173692
APA StyleGaumond, B., Livi, S., Gérard, J.-F., & Duchet-Rumeau, J. (2022). Imidazolium Salt for Enhanced Interfacial Shear Strength in Polyphenylene Sulfide/Ex-PAN Carbon Fiber Composites. Polymers, 14(17), 3692. https://doi.org/10.3390/polym14173692








