Efficient Reaction Systems for Lignocellulosic Biomass Conversion to Furan Derivatives: A Minireview
Abstract
:1. Introduction
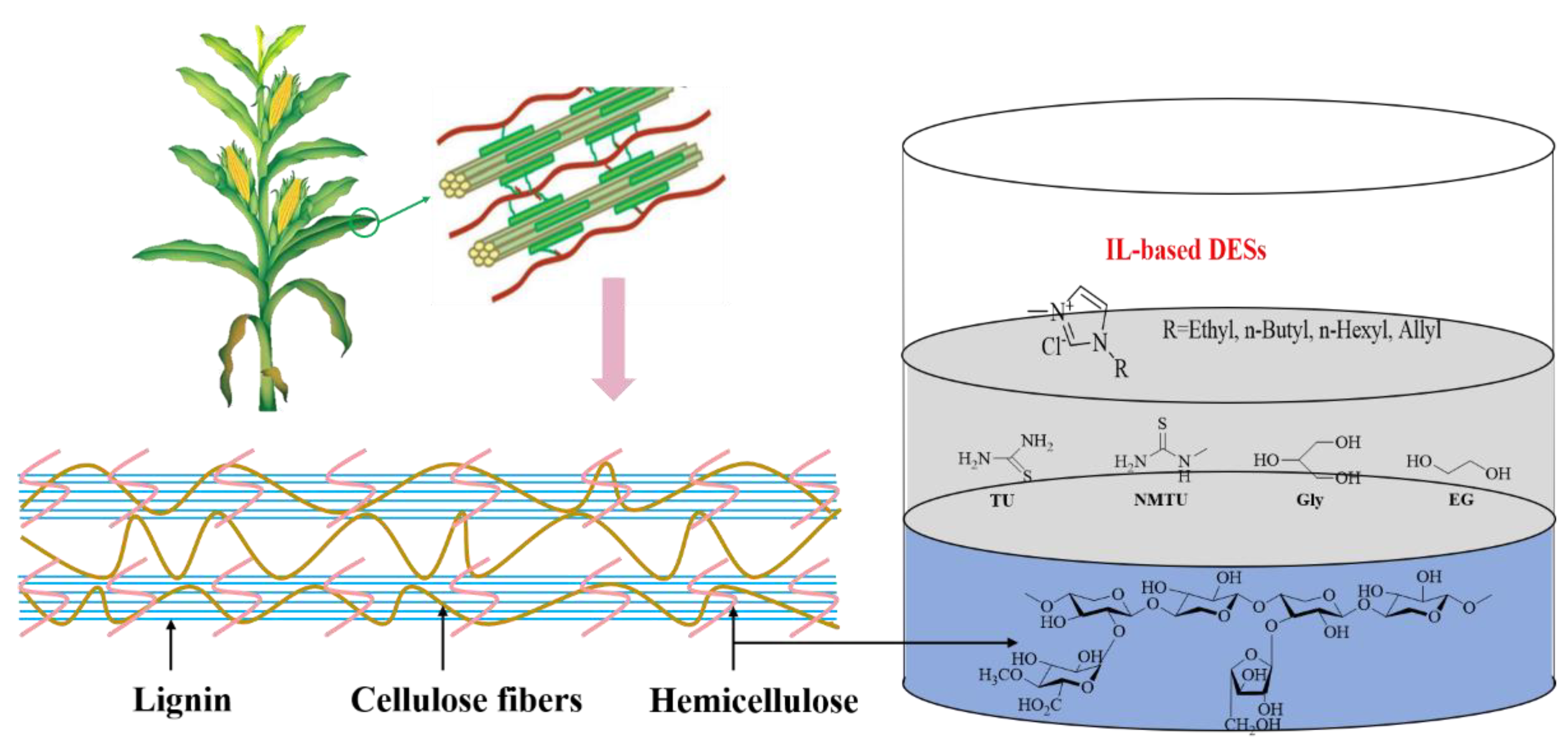
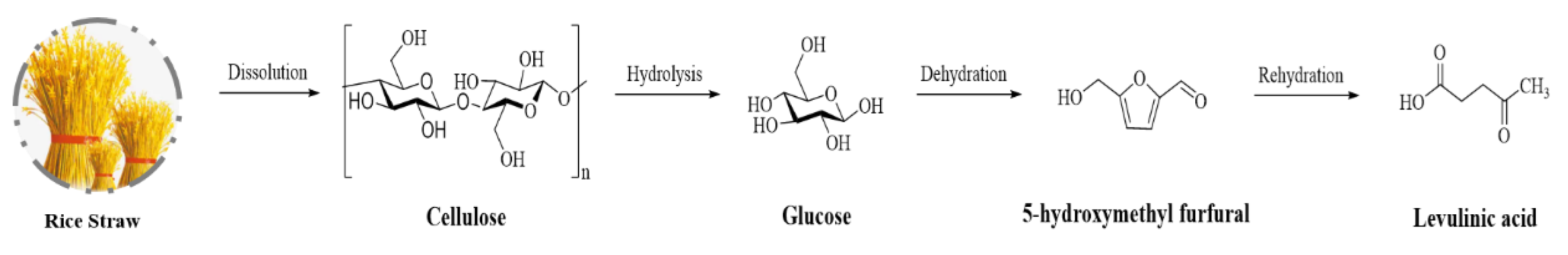
2. Structural Composition of Lignocellulosic Biomass
3. Efficient Solvent Systems for Lignocellulosic Biomass Conversion
3.1. Ionic Liquid System
3.2. Deep Eutectic Solvent System
3.3. Biphasic System
4. Conclusions
- (1)
- To discover an optimized and cost-effective system for lignocellulosic biomass conversion;
- (2)
- The research on DESs recycling and stability needs to be studied in-depth;
- (3)
- The thorough exploration of solvent effect mechanisms;
- (4)
- The commercial synthesis of furan derivatives from biomass on a large scale requires further innovation;
- (5)
- Assistive technologies such as supercritical CO2, microwave, and ultrasound have been introduced into the various reported solvent system; the synergistic effect should be realized effectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richard, G.; Newell, D.R.; Gloria, A. Global Energy Outlook 2019: The Next Generation of Energy; Genaraton of Energy: Dallas, TX, USA, 2019. [Google Scholar]
- Jing, Y.; Guo, Y.; Xia, Q.; Liu, X.; Wang, Y. Catalytic production of value-added chemicals and liquid fuels from lignocellulosic biomass. Chem 2019, 5, 2520–2546. [Google Scholar] [CrossRef]
- Chen, C.C.; Dai, L.; Ma, L.; Guo, R.T. Enzymatic degradation of plant biomass and synthetic polymers. Nat. Rev. Chem. 2020, 4, 114–126. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.U.; Chae, T.U.; Cho, J.S.; Kim, J.W.; Shin, J.H.; Kim, D.I.; Ko, Y.S.; Jang, W.D.; Jang, Y.S. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019, 2, 18–33. [Google Scholar] [CrossRef]
- Luo, N.C.; Montini, T.; Zhang, J.; Fornasiero, P.; Fonda, E.; Hou, T.T.; Nie, W.; Lu, J.M.; Liu, J.X.; Heggen, M.; et al. Visible-light-driven coproduction of diesel precursors and hydrogen from lignocellulose-derived methylfurans. Nat. Energy 2019, 4, 575–584. [Google Scholar] [CrossRef]
- Wagle, A.; Angove, M.J.; Mahara, A.; Wagle, A.; Mainali, B.; Martins, M.; Goldbeck, R.; Paudel, S.R. Multi-stage pre-treatment of lignocellulosic biomass for multi-product biorefinery: A review. Sustain. Energy Technol. 2022, 49, 101702. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.; Korstad, J. Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew. Sustain. Energy Rev. 2017, 73, 654–671. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Salmiaton, A.; Azlina, W.A.K.G.W.; Amran, M.S.M.; Fakhru’l-Razi, A.; Taufiq-Yap, Y.H. Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 1258–1270. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Cerqueira, M.A.; Silva, H.D.; Rodríguez-Jasso, R.M.; Vicente, A.A.; Teixeira, J.A. Biorefinery valorization of autohydrolysis wheat straw hemicellulose to be applied in a polymer-blend film. Carbohyd. Polym. 2013, 92, 2154–2162. [Google Scholar] [CrossRef]
- Loow, Y.L.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Recent advances in the application of inorganic salt pretreatment for transforming lignocellulosic biomass into reducing sugars. J. Agric. Food Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. Comment on processes for the direct conversion of cellulose or cellulosic biomass into levulinate esters. ChemSusChem 2010, 3, 1349–1351. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. High-yield conversion of plant biomass into the key value-added feedstocks 5-(hydroxymethyl)furfural, levulinic acid, and levulinic esters via 5-(chloromethyl)furfural. Green Chem. 2010, 12, 370–373. [Google Scholar] [CrossRef]
- Sun, Z.H.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Da Costa Lopes, A.M.; Łukasikd, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Guerra, A.; Filpponen, I.; Lucia, L.A.; Argyropoulos, D.S. Comparative evaluation of three lignin isolation protocols for various wood species. J. Agric. Food Chem. 2006, 54, 9696–9705. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Irvine, G.M. The significance of the glass transition of lignin in thermomechanical pulping. Wood Sci. Technol. 1985, 19, 139–149. [Google Scholar] [CrossRef]
- Petridis, L.; Schulz, R.; Smith, J.C. Simulation analysis of the temperature dependence of lignin structure and dynamics. J. Am. Chem. Soc. 2011, 133, 20277–20287. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Redhead, J.; Vanbergen, A.J.; Hulmes, L.; Hulmes, S.; Peyton, J.; Nowakowski, M.; Pywell, R.F.; Heard, M.S. Impact of habitat type and landscape structure on biomass, species richness and functional diversity of ground beetles. Agric. Ecosyst. Environ. 2010, 139, 181–186. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Han, B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural—A promising biomass-derived building block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef]
- Song, J.; Fan, H.; Ma, J.; Han, B. Conversion of glucose and cellulose into value-added products in water and ionic liquids. Green Chem. 2013, 15, 2619–2635. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Catalytic conversion of carbohydrate biomass in ionic liquids to 5-hydroxymethyl furfural and levulinic acid: A review. BioEnergy Res. 2020, 13, 693–736. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic liquids-solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Zhang, P.; Dong, S.J.; Ma, H.H. Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Ind. Crop. Prod. 2015, 76, 688–696. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Brandt, A.; Grasvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhao, Z.K. Efficient acid-catalyzed hydrolysis of cellulose in ionic liquid. Adv. Synth. Catal. 2007, 349, 1847–1850. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Palkovits, R.; Schueth, F. Depolymerization of cellulose using solid catalysts in ionic liquids. Angew. Chem. Int. Ed. 2008, 47, 8047–8050. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhou, Y.; Liu, L. Selective conversion of cellulose to levulinic acid via microwave-assisted synthesis in ionic liquids. Bioresour. Technol. 2013, 129, 616–619. [Google Scholar] [CrossRef] [PubMed]
- da Costa Lopes, A.M.; Bogel-Łukasik, R. Acidic ionic liquids as sustainable approach of cellulose and lignocellulosic biomass conversion without additional catalysts. ChemSusChem 2015, 8, 947–965. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Paraj’o, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Nargotra, P.; Sharma, V.; Gupta, M.; Kour, S.; Bajaj, B.K. Application of ionic liquid and alkali pre-treatment for enhancing saccharification of sunflower stalk biomass for potential biofuel-ethanol production. Bioresour. Technol. 2018, 267, 560–568. [Google Scholar] [CrossRef]
- Tam-Anh, D.N.; Kyoung-Rok, K.; Se Jong, H.; Young, C.H.; Woo, K.J.; Min, P.S. Pre-treatment of rice straw with ammonia and ionic liquid for lignocellulose conversion to fermentable sugars. Bioresour. Technol. 2010, 101, 7432–7438. [Google Scholar] [CrossRef]
- Lu, B.L.; Xu, A.; Wang, J.J. Cation does matter: How cationic structure affects the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 1326–1335. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Yan, Q.; Nowik, I.; Cheenkachorn, K.; Phusantisampan, T.; Modigell, M. Efficient pretreatment of rice straw by combination of screw press and ionic liquid to enhance enzymatic hydrolysis. Agric. Nat. Resour. 2015, 49, 146–154. [Google Scholar]
- Zhang, L.; Yu, H.; Wang, P.; Dong, H.; Peng, X. Conversion of xylan, D-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid. Bioresour. Technol. 2013, 130, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Matsagar, B.M.; Dhepe, P.L. Effects of cations, anions and H+ concentration of acidic ionic liquids on the valorization of polysaccharides into furfural. New J. Chem. 2017, 41, 6137–6144. [Google Scholar] [CrossRef]
- da Costa Lopes, A.M.; Lins, R.M.G.; Rebelo, R.A.; Lukasik, R.M. Biorefinery approach for lignocellulosic biomass valorisation with an acidic ionic liquid. Green Chem. 2018, 20, 4043–4057. [Google Scholar] [CrossRef]
- Alama, M.I.; De, D.E.; Khan, T.S.; Haider, M.A.; Saha, B. Acid functionalized ionic liquid catalyzed transformation of non-food biomass into platform chemical and fuel additive. Ind. Crop. Prod. 2018, 123, 629–637. [Google Scholar] [CrossRef]
- Chen, Z.J.; Ma, X.Y.; Xu, L.; Wang, Y.; Long, J.X. Catalytic conversion of duckweed to methyl levulinate in the presence of acidic ionic liquids. Bioresour. Technol. 2018, 268, 488–495. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Lin, H.; Chen, J.; Zhu, L.; Luo, Z. Conversion of C5 carbohydrates into furfural catalyzed by a Lewis acidic ionic liquid in renewable γ-valerolactone. Green Chem. 2017, 19, 3869–3879. [Google Scholar] [CrossRef]
- Naz, S.; Uroos, M.; Muhammad, N. Effect of molecular structure of cation and anions of ionic liquids and co-solvents on selectivity of 5-hydroxymethylfurfural from sugars, cellulose and real biomass. J. Mol. Liq. 2021, 334, 116523. [Google Scholar] [CrossRef]
- Naz, S.; Uroos, M.; Muhammad, N. One-pot production of 5-hydroxymethylfurfural and simultaneous lignin recovery from non-food lignocellulosic wastes using cost-effective ionic liquids. Biomass Convers. Bior. 2022. [Google Scholar] [CrossRef]
- Rajamani, S.; Santhosh, R.; Raghunath, R.; Jadhav, S.A. Value-added chemicals from sugarcane bagasse using ionic liquids. Chem. Pap. 2021, 75, 5605–5622. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Guo, Z.; Ling, Z.; Wang, C.; Zhang, X.; Xu, F. Integration of facile deep eutectic solvents pretreatment for enhanced enzymatic hydrolysis and lignin valorization from industrial xylose residue. Bioresour. Technol. 2018, 265, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents-solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.J.; Xue, Z.; Yuan, T.Q. Structure–function relationships of deep eutectic solvents for lignin extraction and chemical transformation. Green Chem. 2020, 22, 7219–7232. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Feng, Y.C.; Yan, G.H.; Wang, T.; Jia, W.L.; Zeng, X.H.; Sperry, J.; Sun, Y.; Tang, X.; Lei, T.Z.; Lin, L. Synthesis of MCM-41-supported metal catalysts in deep eutectic solvent for the conversion of carbohydrates into 5-hydroxymethylfurfural. ChemSusChem 2019, 12, 978–982. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Das, P.; Bhagia, S.; Pu, Y.Q.; Puri, S.K.; Ramakumar, S.S.V.; Ragauskas, A.J. Assessing the facile pretreatments of bagasse for efficient enzymatic conversion and their impacts on structural and chemical properties. ACS Sustain. Chem. Eng. 2018, 7, 1095–1104. [Google Scholar] [CrossRef]
- Xu, G.C.; Ding, J.C.; Han, R.Z.; Dong, J.J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, X.L.; Lusi, A.; Jacoby, W.A.; Wan, C.X. One-pot selective conversion of lignocellulosic biomass into furfural and co-products using aqueous choline chloride/methyl isobutyl ketone biphasic solvent system. Bioresour. Technol. 2019, 289, 121708. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for green-solvent design: From hydrophilic to hydrophobic (deep) eutectic solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2018, 26, 205–213. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H. Conversion of xylan and xylose into furfural in biorenewable deep eutectic solvent with trivalent metal chloride added. BioResources 2013, 8, 6014–6125. [Google Scholar] [CrossRef]
- da Silva Lacerda, V.; L’opez-Sotelo, J.B.; Correa-Guimar~aes, A.; Hern’andez-Navarro, S.; S’anchez-Bascones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; P’erez-Lebe~na, E.; Martín-Gil, J. A kinetic study on microwave-assisted conversion of cellulose and lignocellulosic waste into hydroxymethylfurfural/furfural. Bioresour. Technol. 2015, 180, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Hou, X.D.; Lin, K.P.; Zhang, X.; Fu, M.H. Rice straw pretreatment using deep eutectic solvents with different constituents molar ratios: Biomass fractionation, polysaccharides enzymatic digestion and solvent reuse. J. Biosci. Bioeng. 2018, 126, 346–354. [Google Scholar] [CrossRef]
- Wang, Z.K.; Shen, X.J.; Chen, J.J.; Jiang, Y.Q.; Hu, Z.Y.; Wang, X.; Liu, L. Lignocellulose fractionation into furfural and glucose by AlCl3-catalyzed DES/MIBK biphasic pretreatment. Int. J. Biol. Macromol. 2018, 117, 721–726. [Google Scholar] [CrossRef]
- Yu, H.T.; Xue, Z.M.; Lan, X.; Liu, Q.L.; Shi, R.F.; Mu, T.C. Highly efficient dissolution of xylan in ionic liquid-based deep eutectic solvents. Cellulose 2020, 27, 6175–6188. [Google Scholar] [CrossRef]
- Arora, S.; Gupta, N.; Singh, V. pH-Controlled Efficient conversion of hemicellulose to furfural using choline-based deep eutectic solvents as catalysts. ChemSusChem 2021, 14, 3953–3958. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Wang, J.; Hu, Q. Synthesis of furfural from d-xylose and corncob with chromium chloride as catalyst in biphasic system. Asian J. Chem. 2014, 26, 1717–1720. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.W.; Abu-Omar, M.M. Synthesis of furfural from xylose, xylan, and biomass using AlCl3⋅6H2O in biphasic media via xylose isomerization to xylulose. ChemSusChem 2012, 5, 405–410. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Zuo, Y.; Su, Z.; Hu, C. A simple two-step method for the selective conversion of hemicellulose in pubescens to furfural. ACS Sustain. Chem. Eng. 2017, 5, 8137–8147. [Google Scholar] [CrossRef]
- Hak, C.; Panchai, P.; Nutongkaew, T.; Grisdanurak, N.; Tulaphol, S. One-pot levulinic acid production from rice straw by acid hydrolysis in deep eutectic solvent. Chem. Eng. Commun. 2022. [Google Scholar] [CrossRef]
| Advantages | Disadvantages | |
|---|---|---|
| Single-stage | Easy to handle | Longer residence time, higher energy consumption, low yield, corrosion, failure to industrialize |
| Two-stage | Fast reaction rate, moderate yield | Partial not green, tedious industrialize |
| Multi-stage | Reduced sugar degradation, improved sugar yields, require less energy | Hard to manipulate |
| Component | Monomer | Reaction | Furanic Derivatives and Others |
|---|---|---|---|
| Cellulose | Glucose | Fermentation | H2, ethanol, lactic acid, succinic acid, acetic acid |
| Hemicellulose | Glucose, xylose, mannose, galactose, and arabinose | Hydrolysis | Reducing sugars |
| Hydrogenation | Xylitol, sorbitol | ||
| Lignin | Coniferyl, p-coumaryl, sinapyl alcohol | Isomerization | Fructose, xylulose |
| Dehydration | 5-hydroxymethylfurfural (HMF), furfural, 2,5-bishydroxymethylfuran, γ-valerolactone (GVL) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yu, D.; Luo, H.; Li, C.; Li, H. Efficient Reaction Systems for Lignocellulosic Biomass Conversion to Furan Derivatives: A Minireview. Polymers 2022, 14, 3671. https://doi.org/10.3390/polym14173671
Liu X, Yu D, Luo H, Li C, Li H. Efficient Reaction Systems for Lignocellulosic Biomass Conversion to Furan Derivatives: A Minireview. Polymers. 2022; 14(17):3671. https://doi.org/10.3390/polym14173671
Chicago/Turabian StyleLiu, Xiaofang, Dayong Yu, Hangyu Luo, Can Li, and Hu Li. 2022. "Efficient Reaction Systems for Lignocellulosic Biomass Conversion to Furan Derivatives: A Minireview" Polymers 14, no. 17: 3671. https://doi.org/10.3390/polym14173671
APA StyleLiu, X., Yu, D., Luo, H., Li, C., & Li, H. (2022). Efficient Reaction Systems for Lignocellulosic Biomass Conversion to Furan Derivatives: A Minireview. Polymers, 14(17), 3671. https://doi.org/10.3390/polym14173671








