Attenuation of Pseudomonas aeruginosa Quorum Sensing Virulence of Biofilm and Pyocyanin by mBTL-Loaded Calcium Alginate Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Growth Curve of Bacterial Isolates
2.3. Biofilm Formation Assay
2.4. Pyocyanin Production Assay
2.5. Cytotoxicity Studies
2.6. Bacterial Adhesion Assay
2.7. Statistical Analysis
3. Results
3.1. Growth Pattern of P. aeruginosa QS Mutants Challenged with Free mBTL and mBTL-Loaded CANPs
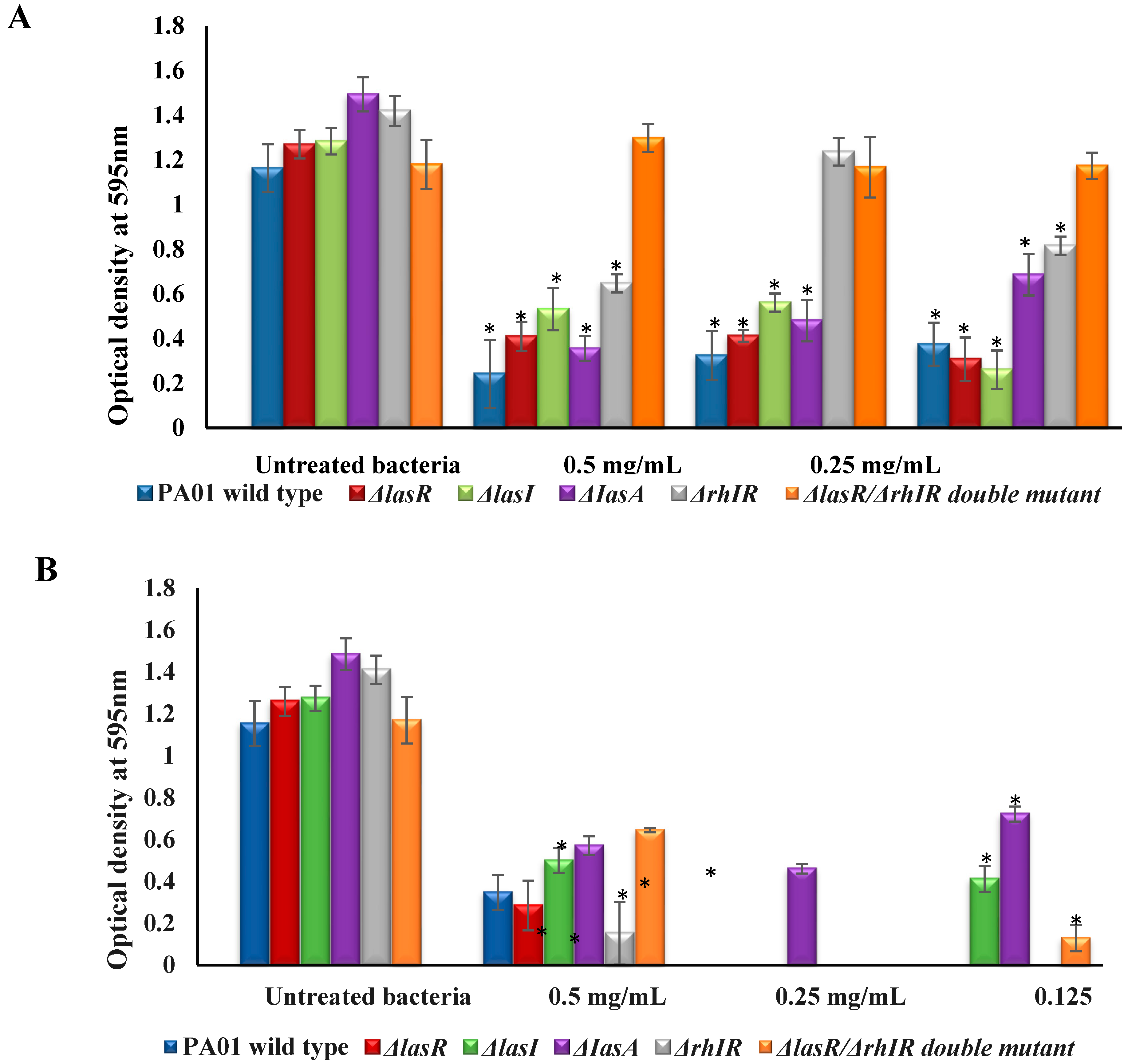
3.2. Effect of mBTL and mBTL-Loaded CANPs on Bacterial Biofilm Formation
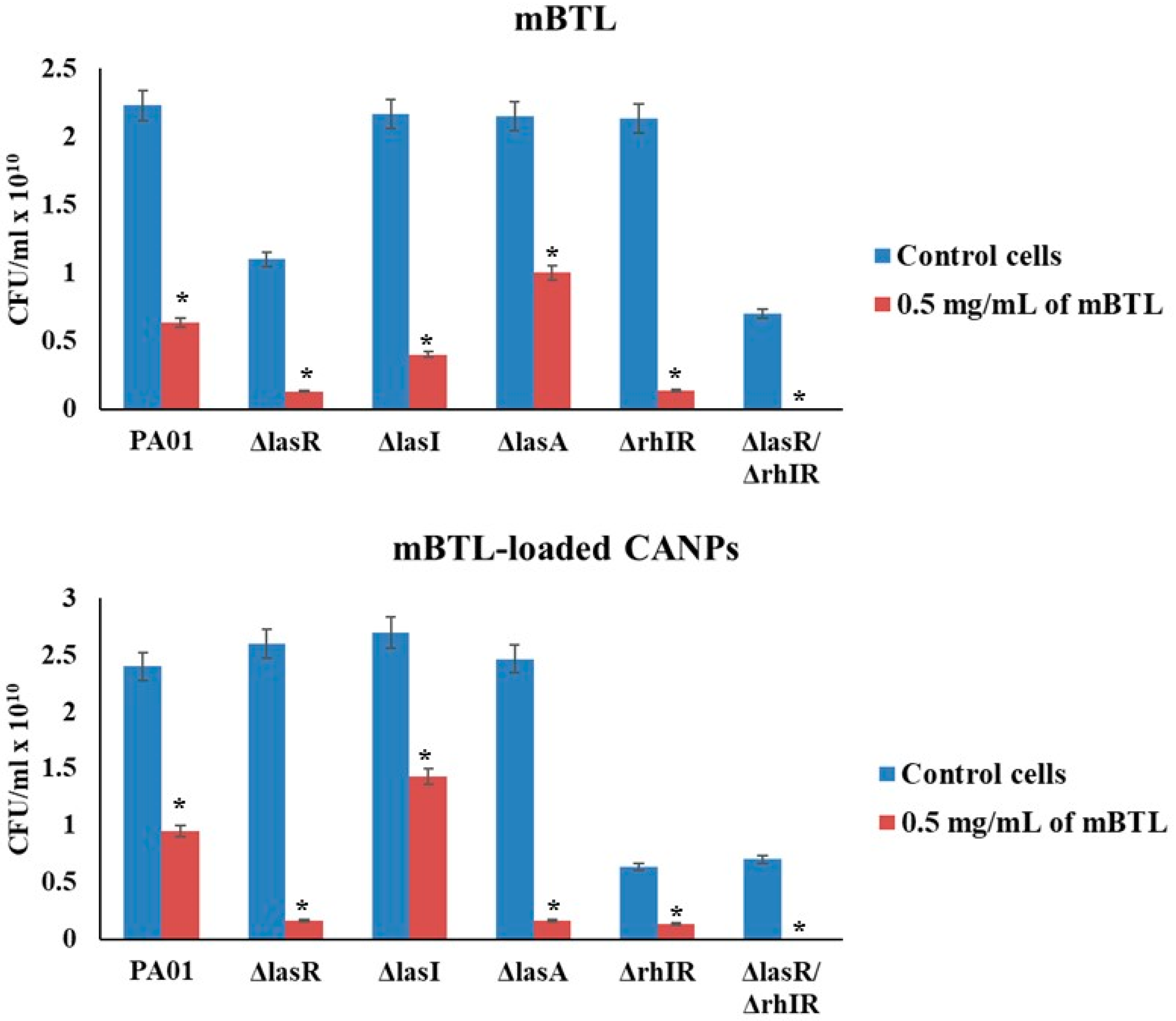
3.3. Effect of mBTL and mBTL-Loaded CANPs on Bacterial Pyocyanin Production
3.4. Cytotoxicity of Formulated CANPs
3.5. Effect of mBTL-Loaded CANPs on the Adhesion of P. aeruginosa to Human Lung A549 Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spaulding, C.N.; Klein, R.D.; Schreiber, H.L.T.; Janetka, J.W.; Hultgren, S.J. Precision antimicrobial therapeutics: The path of least resistance? NPJ Biofilms Microbiomes 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Singhal, L. Antibiotics and Antimicrobial Resistance; Springer: Singapore, 2018. [Google Scholar]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.S. A review of global initiatives to fight antibiotic resistance and recent antibiotics discovery. Acta Pharm. Sin. B 2016, 6, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Brooks, B.D.; Brooks, A.E. Therapeutic strategies to combat antibiotic resistance. Adv. Drug Deliv. Rev. 2014, 78, 14–27. [Google Scholar] [CrossRef]
- Lakemeyer, M.; Zhao, W.; Mandl, F.A.; Hammann, P.; Sieber, S.A. Thinking Outside the Box-Novel Antibacterials To Tackle the Resistance Crisis. Angew. Chem. Int. Ed. Engl. 2018, 57, 14440–14475. [Google Scholar] [CrossRef]
- Speck, P. Antibiotics: Avert an impending crisis. Nature 2013, 496, 169. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef]
- Williams, P. Strategies for inhibiting quorum sensing. Emerg. Top. Life Sci. 2017, 1, 23–30. [Google Scholar] [CrossRef]
- Defoirdt, T. Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends Microbiol. 2018, 26, 313–328. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef]
- Brackman, G.; Coenye, T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015, 21, 5–11. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Trespidi, G.; Chiarelli, L.R.; Barbieri, G.; Buroni, S. Quorum Sensing as Antivirulence Target in Cystic Fibrosis Pathogens. Int. J. Mol. Sci. 2019, 20, 1838. [Google Scholar] [CrossRef]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Krupa, I. Alginate-Mediated Synthesis of Hetero-Shaped Silver Nanoparticles and Their Hydrogen Peroxide Sensing Ability. Molecules 2020, 25, 435. [Google Scholar] [CrossRef]
- Schurks, N.; Wingender, J.; Flemming, H.C.; Mayer, C. Monomer composition and sequence of alginates from Pseudomonas aeruginosa. Int. J. Biol. Macromol. 2002, 30, 105–111. [Google Scholar] [CrossRef]
- Eltayb, E.K.; Aleanizy, F.S.; Alqahtani, F.Y.; Alkahtani, H.M.; Ansari, S.A.; Alsarra, I. Preparation and characterization of Meta-bromo-thiolactone calcium alginate nanoparticles. Saudi Pharm. J. 2022, 30, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Held, K.; Ramage, E.; Jacobs, M.; Gallagher, L.; Manoil, C. Sequence-verified two-allele transposon mutant library for Pseudomonas aeruginosa PAO1. J. Bacteriol. 2012, 194, 6387–6389. [Google Scholar] [CrossRef] [Green Version]
- Clinical Laboratory Institute, C.a.L.S. Performance Standards for Antimicrobial Susceptibility Testing. In CLSI Supplement M100, 30th ed.; Weinstein, M.P., Ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Aleanizy, F.S.; Alqahtani, F.Y.; Shazly, G.; Alfaraj, R.; Alsarra, I.; Alshamsan, A.; Gareeb Abdulhady, H. Measurement and evaluation of the effects of pH gradients on the antimicrobial and antivirulence activities of chitosan nanoparticles in Pseudomonas aeruginosa. Saudi Pharm. J. 2018, 26, 79–83. [Google Scholar] [CrossRef]
- Aleanizy, F.S.; Alqahtani, F.Y.; Eltayb, E.K.; Alrumikan, N.; Almebki, R.; Alhossan, A.; Almangour, T.A.; AlQahtani, H. Evaluating the effect of antibiotics sub-inhibitory dose on Pseudomonas aeruginosa quorum sensing dependent virulence and its phenotypes. Saudi J. Biol. Sci. 2021, 28, 550–559. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alowais, H.; Binkelaib, A.; Alwathlan, B.; Al-Bdrawy, A.; Hakansson, A.P.; Alsarra, I. Capsule Independent Antimicrobial Activity Induced by Nanochitosan against Streptococcus pneumoniae. Polymers 2021, 13, 2924. [Google Scholar] [CrossRef]
- Hawdon, N.A.; Aval, P.S.; Barnes, R.J.; Gravelle, S.K.; Rosengren, J.; Khan, S.; Ciofu, O.; Johansen, H.K.; Hoiby, N.; Ulanova, M. Cellular responses of A549 alveolar epithelial cells to serially collected Pseudomonas aeruginosa from cystic fibrosis patients at different stages of pulmonary infection. FEMS Immunol. Med. Microbiol. 2010, 59, 207–220. [Google Scholar] [CrossRef]
- Asfahl, K.L.; Schuster, M. Additive effects of quorum sensing anti-activators on Pseudomonas aeruginosa virulence traits and transcriptome. Front. Microbiol. 2018, 8, 2654. [Google Scholar] [CrossRef]
- Welsh, M.A.; Eibergen, N.R.; Moore, J.D.; Blackwell, H.E. Small molecule disruption of quorum sensing cross-regulation in pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J. Am. Chem. Soc. 2015, 137, 1510–1519. [Google Scholar] [CrossRef]
- Khan, S.; Tondervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Onsoyen, E.; Myrvold, R.; Howe, R.A.; Walsh, T.R.; Hill, K.E.; et al. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob. Agents Chemother. 2012, 56, 5134–5141. [Google Scholar] [CrossRef]
- Lamppa, J.W.; Ackerman, M.E.; Lai, J.I.; Scanlon, T.C.; Griswold, K.E. Genetically engineered alginate lyase-PEG conjugates exhibit enhanced catalytic function and reduced immunoreactivity. PLoS ONE 2011, 6, e17042. [Google Scholar] [CrossRef]
- Singh, N.; Romero, M.; Travanut, A.; Monteiro, P.F.; Jordana-Lluch, E.; Hardie, K.R.; Williams, P.; Alexander, M.R.; Alexander, C. Dual bioresponsive antibiotic and quorum sensing inhibitor combination nanoparticles for treatment of Pseudomonas aeruginosa biofilms in vitro and ex vivo. Biomater. Sci. 2019, 7, 4099–4111. [Google Scholar] [CrossRef]
- Zgheib, H.; Belguesmia, Y.; Boukherroub, R.; Drider, D. Alginate Nanoparticles Enhance Anti-Clostridium perfringens Activity of the Leaderless Two-Peptide Enterocin DD14 and Affect Expression of Some Virulence Factors. Probiotics Antimicrob. Proteins 2021, 13, 1213–1227. [Google Scholar] [CrossRef]
- Zohri, M.; Alavidjeh, M.S.; Haririan, I.; Ardestani, M.S.; Ebrahimi, S.E.; Sani, H.T.; Sadjadi, S.K. A Comparative Study Between the Antibacterial Effect of Nisin and Nisin-Loaded Chitosan/Alginate Nanoparticles on the Growth of Staphylococcus aureus in Raw and Pasteurized Milk Samples. Probiotics Antimicrob. Proteins 2010, 2, 258–266. [Google Scholar] [CrossRef]
- Singh, S.; Chopra, M.; Dilbaghi, N.; Manuja, B.K.; Kumar, S.; Kumar, R.; Rathore, N.S.; Yadav, S.C.; Manuja, A. Synthesis and evaluation of isometamidium-alginate nanoparticles on equine mononuclear and red blood cells. Int. J. Biol. Macromol. 2016, 92, 788–794. [Google Scholar] [CrossRef]
- Hassani, A.; Mahmood, S.; Enezei, H.H.; Hussain, S.A.; Hamad, H.A.; Aldoghachi, A.F.; Hagar, A.; Doolaanea, A.A.; Ibrahim, W.N. Formulation, Characterization and Biological Activity Screening of Sodium Alginate-Gum Arabic Nanoparticles Loaded with Curcumin. Molecules 2020, 25, 2244. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, K.M.; Park, S.; Cho, Y.; Lee, E.; Park, J.H.; Shin, O.S.; Son, J.; Yoon, S.S.; Yu, J.W. Bacterial Secretant from Pseudomonas aeruginosa Dampens Inflammasome Activation in a Quorum Sensing-Dependent Manner. Front. Immunol. 2017, 8, 333. [Google Scholar] [CrossRef] [Green Version]





| Bacterial Strain | Abbreviation | Description | Reference |
|---|---|---|---|
| PA01 | PA01 | PA01 wild-type | [21] |
| PA1430 | ΔlasR | Transcription regulator LasR | [21] |
| PA1432 | ΔlasI | Autoinducer synthesis protein LasI | [21] |
| PA1871 | ΔlasA | LasA protease precursor | [21] |
| PA3477 | ΔrhIR | Transcriptional regulator RhIR | [21] |
| D6A | ΔlasR ΔrhlR double mutant | ΔlasR ΔrhlR knockouts double mutant | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltayb, E.K.; Alqahtani, F.Y.; Alkahtani, H.M.; Alsarra, I.A.; Alfaraj, R.; Aleanizy, F.S. Attenuation of Pseudomonas aeruginosa Quorum Sensing Virulence of Biofilm and Pyocyanin by mBTL-Loaded Calcium Alginate Nanoparticles. Polymers 2022, 14, 3655. https://doi.org/10.3390/polym14173655
Eltayb EK, Alqahtani FY, Alkahtani HM, Alsarra IA, Alfaraj R, Aleanizy FS. Attenuation of Pseudomonas aeruginosa Quorum Sensing Virulence of Biofilm and Pyocyanin by mBTL-Loaded Calcium Alginate Nanoparticles. Polymers. 2022; 14(17):3655. https://doi.org/10.3390/polym14173655
Chicago/Turabian StyleEltayb, Esra Kamal, Fulwah Yahya Alqahtani, Hamad M. Alkahtani, Ibrahim A. Alsarra, Rihaf Alfaraj, and Fadilah Sfouq Aleanizy. 2022. "Attenuation of Pseudomonas aeruginosa Quorum Sensing Virulence of Biofilm and Pyocyanin by mBTL-Loaded Calcium Alginate Nanoparticles" Polymers 14, no. 17: 3655. https://doi.org/10.3390/polym14173655
APA StyleEltayb, E. K., Alqahtani, F. Y., Alkahtani, H. M., Alsarra, I. A., Alfaraj, R., & Aleanizy, F. S. (2022). Attenuation of Pseudomonas aeruginosa Quorum Sensing Virulence of Biofilm and Pyocyanin by mBTL-Loaded Calcium Alginate Nanoparticles. Polymers, 14(17), 3655. https://doi.org/10.3390/polym14173655








