Effect of CeO2–GO Nanocomposite on the Anticorrosion Properties of Epoxy Coating in Simulated Acid Rain Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of CeO2–GO (4:1) Nanocomposite Epoxy Coating
2.3. Preparation of Simulated Acid Rain Solution
2.4. Testing Procedures
2.4.1. Micromorphological Characterization
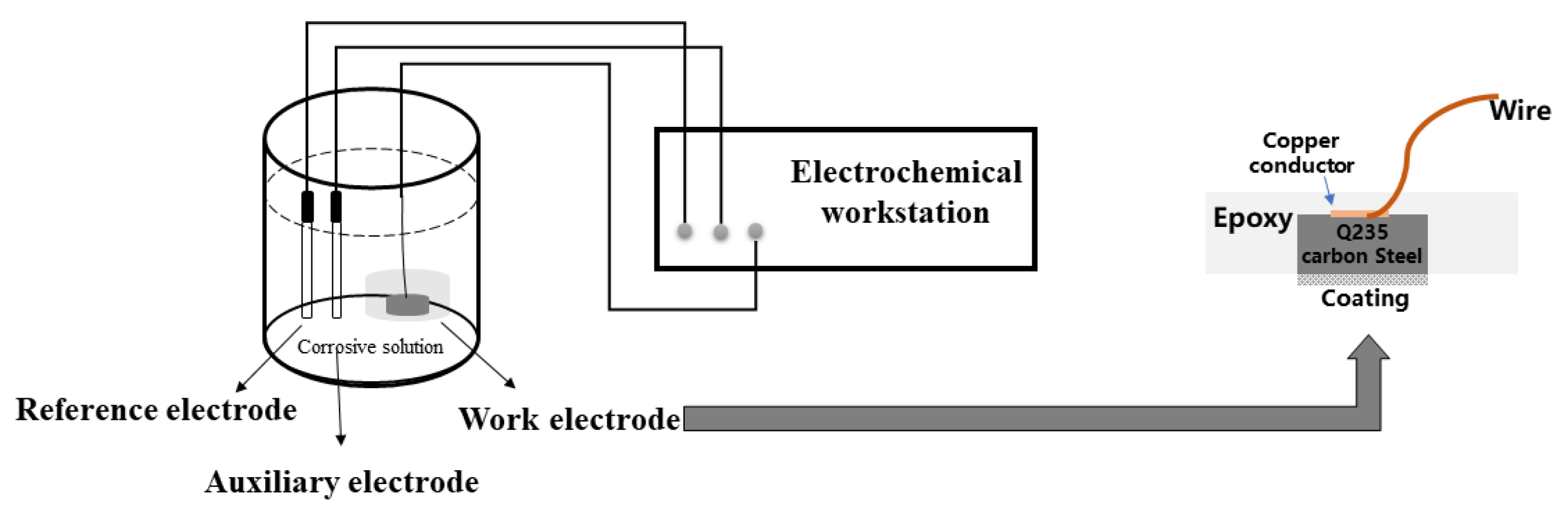
2.4.2. Electrochemical Testing
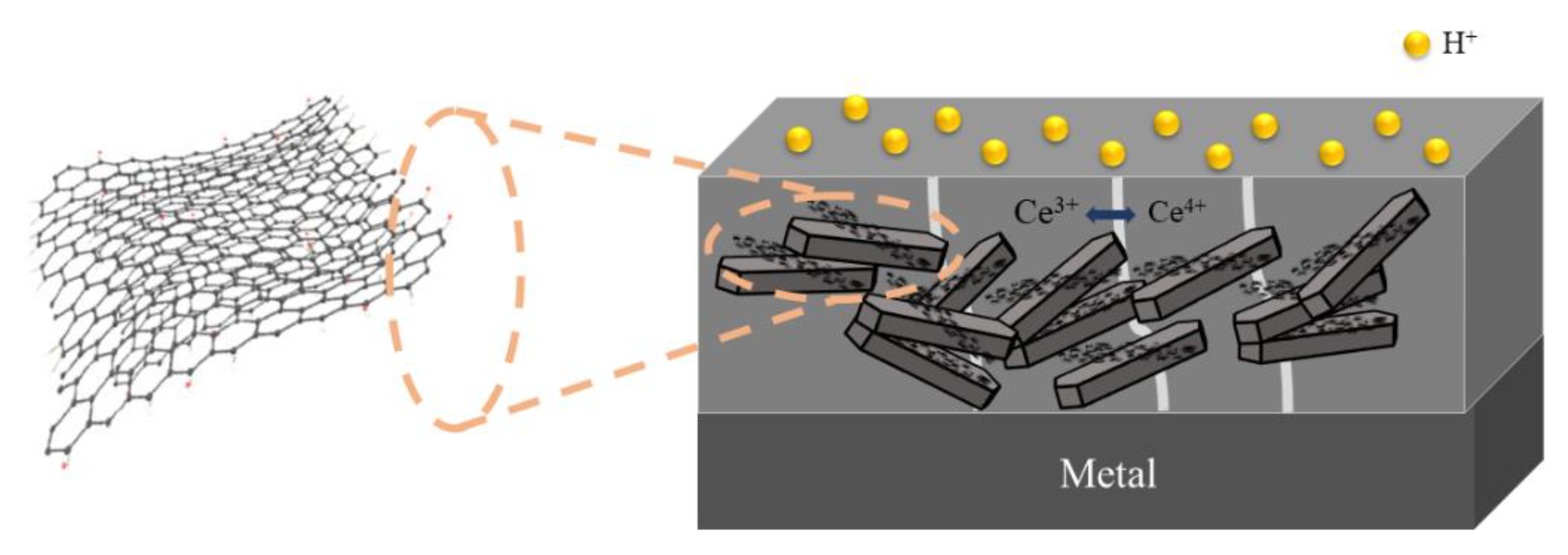
3. Anti-Corrosion Performance
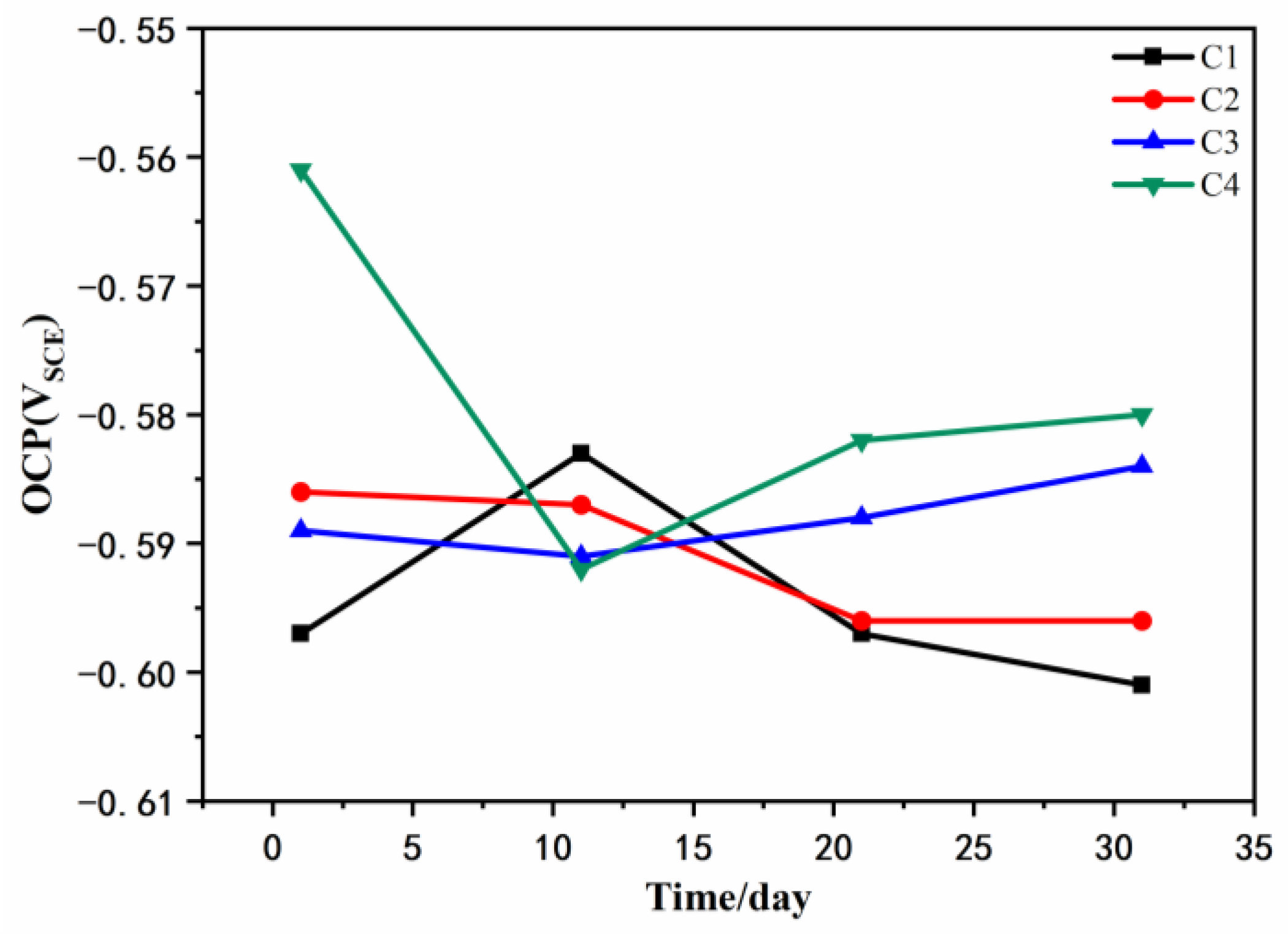
3.1. Open Circuit Potential (OCP) Analysis
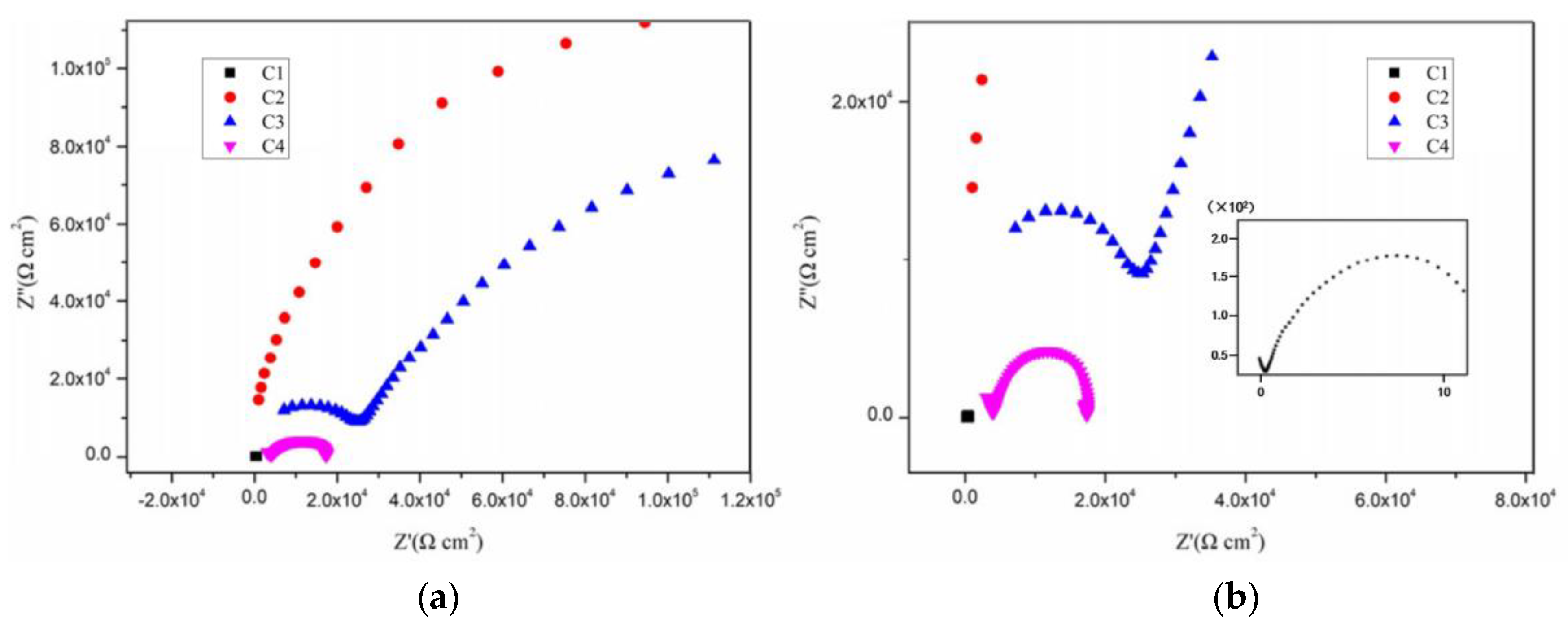
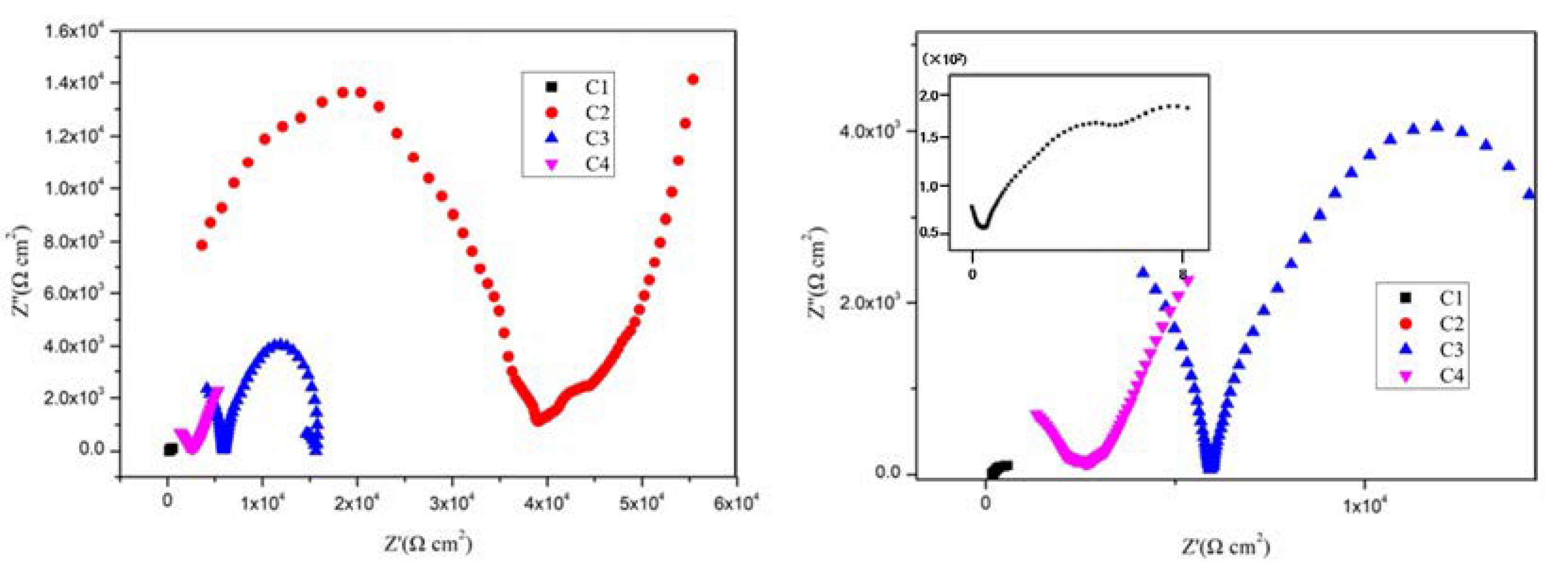
3.2. Electrochemical Alternating Current Impedance Spectroscopy (EIS) Analysis
3.3. Tafel Curve Analysis
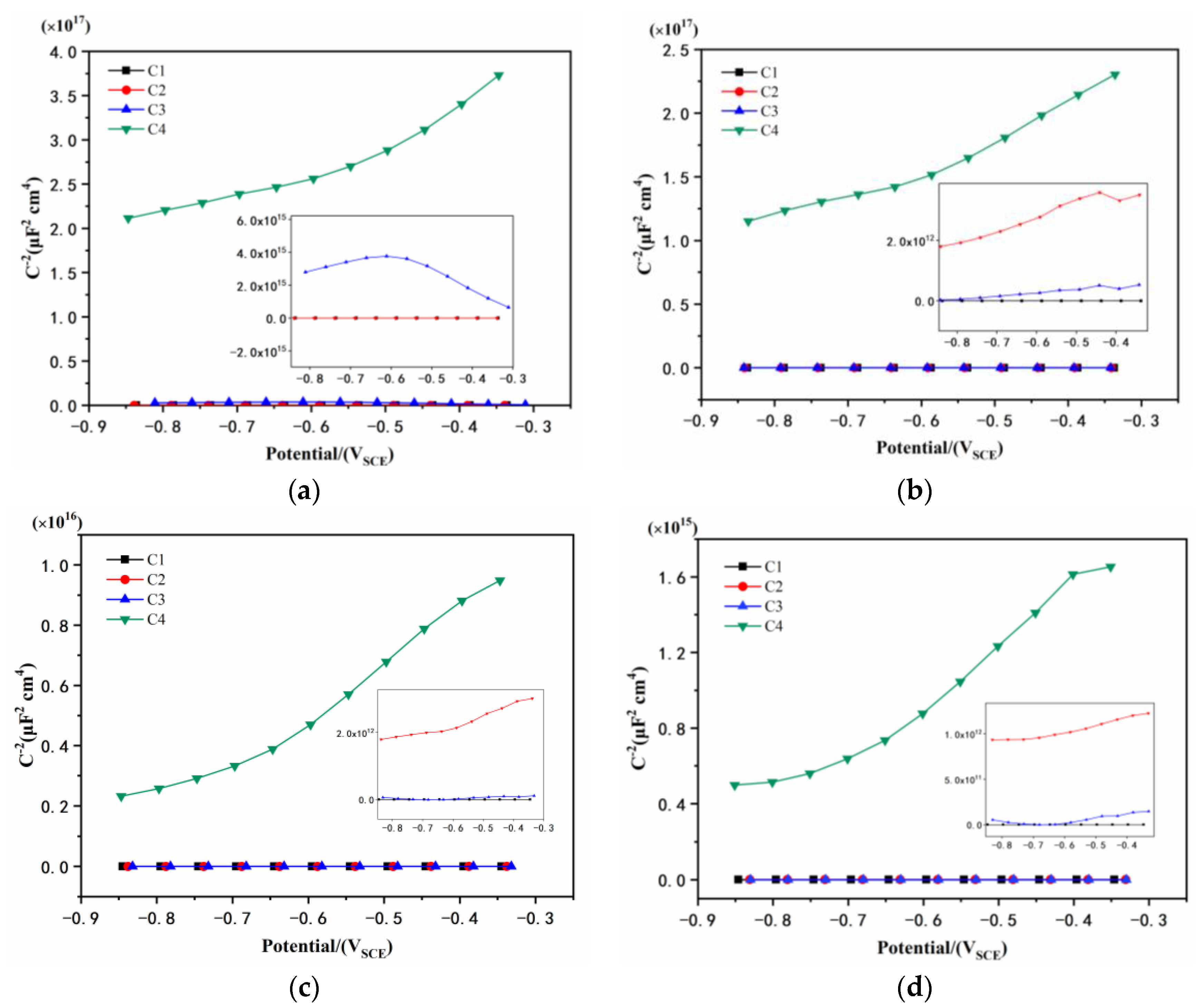
3.4. Mott–Schottky Curve Analysis
4. Corrosion Morphology Comparison
4.1. Corrosion Depth Analysis of Substrate
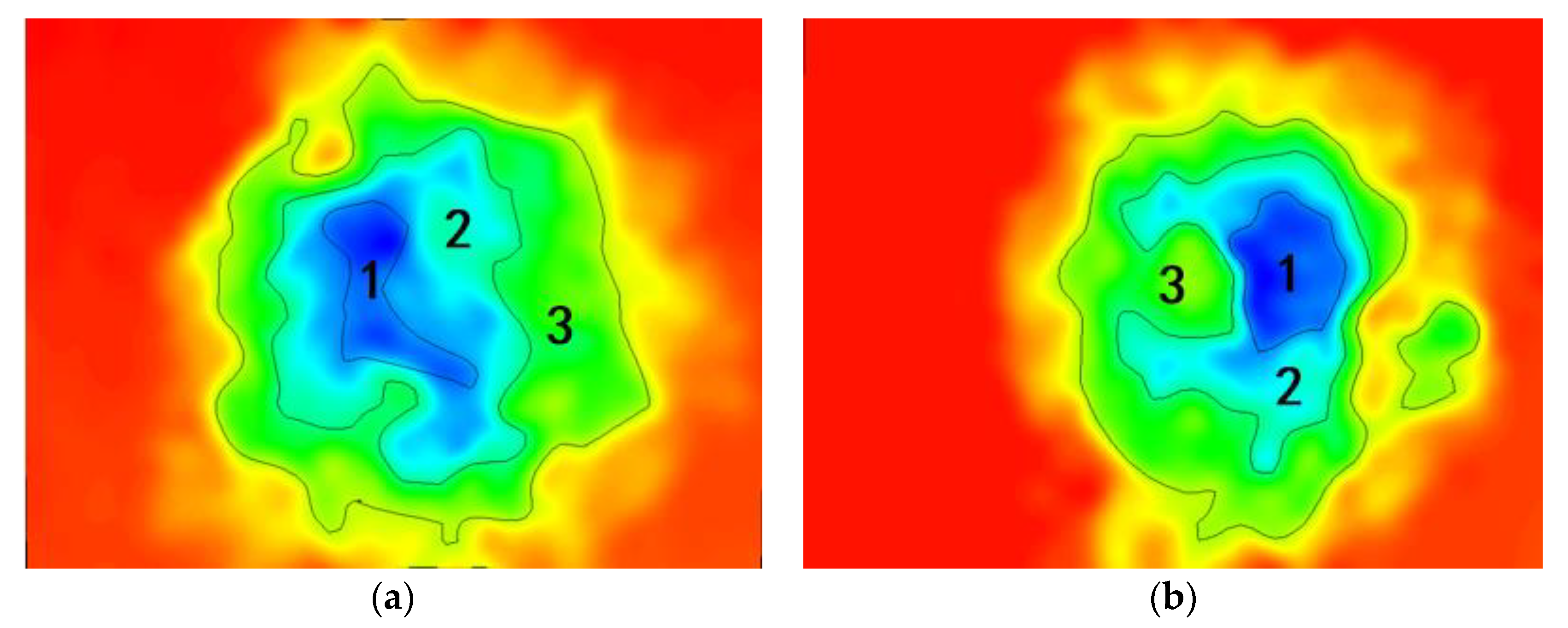
4.2. Corrosion Area Analysis of Substrate
5. Conclusions
- (1)
- The CeO2–GO (4:1)/EP coating induced a higher positive corrosion potential (Ecorr) and lower corrosion current density (Icorr) than those of the EP coating, GO/EP coating, and CeO2 coating in a simulated acid rain solution. The coating protection efficiency was as high as 98.62%.
- (2)
- The stereomicroscope analysis indicated that the CeO2–GO (4:1)/EP coating had the best barrier performance to the erosive medium. At the same time, the accumulation of corrosion products and the good compactness of the coating limited the further penetration of erosive ions and slowed the corrosion rate.
- (3)
- The resistance of the CeO2–GO (4:1)/EP coating to acidic corrosive medium was relatively poorer than that of neutral and saline-alkali corrosive medium. On the one hand, epoxy resin had a poor protection ability to the acidic medium. On the other hand, the hydrolysis product of CeO2 could not exist stably, and H+ constantly broke its hydrolysis balance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Tian, L.; Gao, M.; Zhao, J.; Ren, L. Recent advances in emerging integrated antifouling and anticorrosion coatings. Mater. Des. 2022, 213, 110307. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, Z.; Chen, G. Effects of different types of acid rain on water stability of asphalt pavement. Constr. Build. Mater. 2022, 322, 126308. [Google Scholar] [CrossRef]
- Liu, Z.; Sin, Z.; Wei, H.; Zhang, J. Acid rain reduces soil CO2 emission and promotes soil organic carbon accumulation in association with decreasing the biomass and biological activity of ecosystems: A meta-analysis. Catena 2022, 208, 105714. [Google Scholar] [CrossRef]
- Chang, H.; Wang, X.; Wang, Y.; Li, S.; Wang, J.; Liu, J.; Feng, P. Influence of low vacuum condition on mechanical performance and microstructure of hardened cement paste at early age. Constr. Build. Mater. 2022, 346, 128358. [Google Scholar] [CrossRef]
- Lyu, K.; Garboczi, E.; Gao, Y.; Miao, C.; Liu, X. Relationship between fine aggregate size and the air void system of six mortars: I. Air void content and diameter distribution. Cem. Concr. Compos. 2022, 131, 104599. [Google Scholar] [CrossRef]
- An, B. Corrosion Behavior of Typical Metal Materials in Acid Rain/Rainwater Environment. Ph.D. Thesis, Tianjin University, Tianjing, China, 2003. [Google Scholar]
- Zhu, X.; Zhao, H.; Wang, L.; Xue, Q. Bioinspired ultrathin graphene nanosheets sandwiched between epoxy layers for high performance of anticorrosion coatings. Chem. Eng. J. 2021, 410, 128301. [Google Scholar] [CrossRef]
- Li, B.-H.; Wang, S.-L.; Pal, S.; So, P.B.; Chen, G.-Y.; Huang, W.-J.; Hsu, Y.-L.; Kuo, S.-Y.; Yeh, J.M.; Lin, C.H. Versatile reactions on hydrophobic functionalization of metal-organic frameworks and anticorrosion application. Microporous Mesoporous Mater. 2021, 325, 111319. [Google Scholar] [CrossRef]
- Kumari, S.; Saini, A.; Dhayal, V. Metal oxide based epoxy coatings for corrosion protection of steel. Mater. Today Proc. 2021, 43, 3105–3109. [Google Scholar] [CrossRef]
- Samal, S.; Cibulková, J.; Čtvrtlík, R.; Tomáštík, J.; Václavek, L.; Kopeček, J.; Šittner, P. Tribological Behavior of NiTi Alloy Produced by Spark Plasma Sintering Method. Coatings 2021, 11, 1246. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, Z.L.; Hou, H.B.; Hao, L. Corrosion behaviour of electroless Ni–P coatings in simulated acid rain. Corros. Eng. Sci. Technol. 2013, 45, 277–281. [Google Scholar] [CrossRef]
- Yang, L.; Xin, L.; Yu, H.Z.; Ju, Z.Y. Preparation and Properties of Graphene Anticorrosive Coatings forTransmission Towers. IOP Conf. Ser.—Earth Environ. Sci. 2019, 295, 032105. [Google Scholar] [CrossRef]
- Nagai, T.; Hiratsuka, M.; Alanazi, A.; Nakamori, H.; Hirakuri, K. Anticorrosion of DLC coating in acid solutions. Appl. Surf. Sci. 2021, 552, 149373. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Meng, Z.; Xie, B.; Yu, X.; Su, G.; Wang, L. Epoxy coating with excellent anticorrosion and pH-responsive performances based on DEAEMA modified mesoporous silica nanomaterials. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127951. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Yang, M.; Wu, X.; Li, S.; Zhang, W.; Zhang, H. Modification effect of graphene oxide on oxidation coating of Ti-3Zr-2Sn-3Mo-25 Nb near-β titanium alloy. J. Alloys Compd. 2022, 901, 163561. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Z.; Wang, Q.; Zu, S.; Liu, M.; Yu, Z.; Zhang, Z.; Ren, L. Corrosion resistance and biocompatibility of graphene oxide coating on the surface of the additively manufactured NiTi alloy. Prog. Org. Coat. 2022, 164, 106722. [Google Scholar] [CrossRef]
- Araujo, A.F.; Ferreira, M.V.; Felisberto, M.D.; Sicupira, D.C.; Santos, L.A. Corrosion resistance of a superelastic NiTi alloy coated with graphene–based coatings. Prog. Org. Coat. 2022, 165, 106727. [Google Scholar] [CrossRef]
- Chunder, A.; Liu, J.; Zhai, L. Reduced Graphene Oxide/Poly(3-hexylthiophene) Supramolecular Composites. Macromol. Rapid Commun. 2010, 31, 380–384. [Google Scholar] [CrossRef]
- Ryu, S.; Kwon, Y.J.; Kim, Y.; Lee, J.U. Corrosion protection coating of three-dimensional metal structure by electrophoretic deposition of graphene oxide. Mater. Chem. Phys. 2020, 250, 123039. [Google Scholar] [CrossRef]
- Verma, R.; Sharma, S.; Mukherjee, B.; Singh, P.; Islam, A.; Keshri, A.K. Microstructural, mechanical and marine water tribological properties of plasma-sprayed graphene nanoplatelets reinforced Al2O3- 40 wt% TiO2 coating. J. Eur. Ceram. Soc. 2022, 42, 2892–2904. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Y.; Wu, S.; Zhang, D.; Oliver, S.; Chen, X.; Lei, L.; Shi, S. Covalently immobilization of modified graphene oxide with waterborne hydroxyl acrylic resin for anticorrosive reinforcement of its coatings. Prog. Org. Coat. 2022, 163, 106685. [Google Scholar] [CrossRef]
- Geng, Y.; Zhou, P.; Li, S.; Cao, J.; Zhou, Z.; Wu, Z.; Liu, A. Superior corrosion resistance of mild steel coated with graphene oxide modified silane coating in chlorinated simulated concrete solution. Prog. Org. Coat. 2022, 164, 106716. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, M.; Lu, P.; Zhou, W.; Shi, X.; Yang, K.; Miao, X. Mechanical and corrosion resistance of cold sprayed zinc (CSZ) nano composite coating enhanced by SiO2-GO hybrid material. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 632, 127824. [Google Scholar] [CrossRef]
- Meng, L.; Watson, B.W.; Qin, Y. Hybrid conjugated polymer/magnetic nanoparticle composite nanofibers through cooperative non-covalent interactions. Nanoscale Adv. 2020, 2, 2462–2470. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Tang, M.; Zhang, X.; Wu, K. Role of rare earth elements on the improvement of corrosion resistance of micro-alloyed steels in 3.5 wt.% NaCl solution. J. Mater. Res. Technol. 2021, 11, 519–534. [Google Scholar] [CrossRef]
- Cao, G.; Wang, Y.-H.; Ding, Z.-Y.; Yang, H.-L.; Liu, Z.-G.; Ouyang, J.-H.; Wang, Y.-M. Tunable corrosion resistance of rare-earth monosilicate to molten calcia-magnesia-aluminosilicate glass by RE-doping strategy. Corros. Sci. 2022, 202, 110319. [Google Scholar] [CrossRef]
- Wang, C.; Ma, R.; Zhou, Y.; Liu, Y.; Daniel, E.F.; Li, X.; Wang, P.; Dong, J.; Ke, W. Effects of rare earth modifying inclusions on the pitting corrosion of 13Cr4Ni martensitic stainless steel. J. Mater. Sci. Technol. 2021, 93, 232–243. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, X.; Chen, P.; Xiao, P.; Zhou, W.; Li, Y. Current research art of rare earth compound modified SiC-CMCs for enhanced wet-oxygen corrosion resistance. Ceram. Int. 2022, 48, 24131–24143. [Google Scholar] [CrossRef]
- Cai, G.; Xiao, S.; Deng, C.; Jiang, D.; Zhang, X.; Dong, Z. CeO2 grafted carbon nanotube via polydopamine wrapping to enhance corrosion barrier of polyurethane coating. Corros. Sci. 2021, 178, 109014. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, F.; Qiang, Y.; Zhao, W. Synthesizing a novel fluorinated reduced graphene oxide-CeO2 hybrid nanofiller to achieve highly corrosion protection for waterborne epoxy coatings. Carbon 2021, 176, 39–51. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Z.; Cui, X.; Feng, L.; Zhang, D.; Jin, G.; Liu, J.; Zheng, W. Effect of graphite/CeO2 on microstructure and tribological property of plasma cladded Co-based coatings. Mater. Chem. Phys. 2022, 280, 125756. [Google Scholar] [CrossRef]
- You, S.; Jiang, C.; Wang, L.; Xing, S.; Zhan, K. Effect of CeO2 nanoparticles on the microstructure and properties of the NiCo-CeO2 composite coatings. Vacuum 2022, 196, 110765. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Yang, J.; Zhang, J.; Ding, H. Large CeO2 nanoflakes modified by graphene as barriers in waterborne acrylic coatings and the improved anticorrosion performance. Prog. Org. Coat. 2020, 143, 105607. [Google Scholar] [CrossRef]
- An, K.; Sui, Y.; Qing, Y.; Yang, C.; Long, C.; Wang, L.; Liu, C. Synergistic reinforcement coating with anti-corrosion and UV aging resistance by filling modified CeO2 nanoflakes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126904. [Google Scholar] [CrossRef]
- Liu, X.; Jie, H.; Liu, R.; Liu, Y.; Li, T.; Lyu, K. Research on the Preparation and Anticorrosion Properties of EP/CeO2-GO Nanocomposite Coating. Polymers 2021, 13, 183. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Li, T.; Liu, Y.; Liu, L.; Lyu, K.; Shah, S. Research on the Anticorrosion Properties of CeO2-GO/EP Nanocomposite Coating in Simulated Sea Water. Polymers 2021, 13, 2072. [Google Scholar] [CrossRef]
- Lyu, K.; Liu, X.; Liu, R.; Yang, H.; Qiao, Y.; Shah, S.P. Evaluation of the Anticorrosion Performance of CeO2-Modified Graphene Oxide Nanocomposite Epoxy Coating Subjected to Simulated Saline-Alkali Solution. Polymers 2022, 14, 1412. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafer, J.; Othman, M.H.D.; Ismain, A.F.; Rahman, M.A.; Yusof, N.; Salleh, W.N.W.; Aziz, F.; Ajid, A.Z.A. Advanced ternary RGO/bimetallic Pt-Pd alloy/CeO2 nanocomposite electrocatalyst by one-step hydrothermal-assisted formic acid reduction reaction for methanol electrooxidation. J. Environ. Chem. Eng. 2021, 9, 104991. [Google Scholar] [CrossRef]
- Araneda, A.A.B.; Kappes, M.A.; Rodríguez, M.A.; Carranza, R.M. Pitting corrosion of Ni-Cr-Fe alloys at open circuit potential in chloride plus thiosulfate solutions. Corros. Sci. 2022, 198, 110121. [Google Scholar] [CrossRef]
- Reddy, I.N.; Manjunath, V.; Shim, J. Structural and optical properties, electrochemical impedance spectroscopy, and Mott–Schottky analysis of ZnFe2O4 nanoparticle-decorated V2O5 rectangular nanosheets for photoelectrochemical applications. J. Environ. Chem. Eng. 2021, 9, 106131. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Vafaeian, S. Comparison of electrochemical behavior between coarse-grained and fine-grained AISI 430 ferritic stainless steel by Mott–Schottky analysis and EIS measurements. J. Alloys Compd. 2015, 639, 301–307. [Google Scholar] [CrossRef]
- Hiromoto, S.; Itoh, S.; Doi, K.; Katayama, H.; Akashi, T. Short- and long-term corrosion behavior of carbonate apatite-coated Mg-4mass% Y-3mass% RE alloy in cell culture medium. Corros. Sci. 2022, 200, 110222. [Google Scholar] [CrossRef]
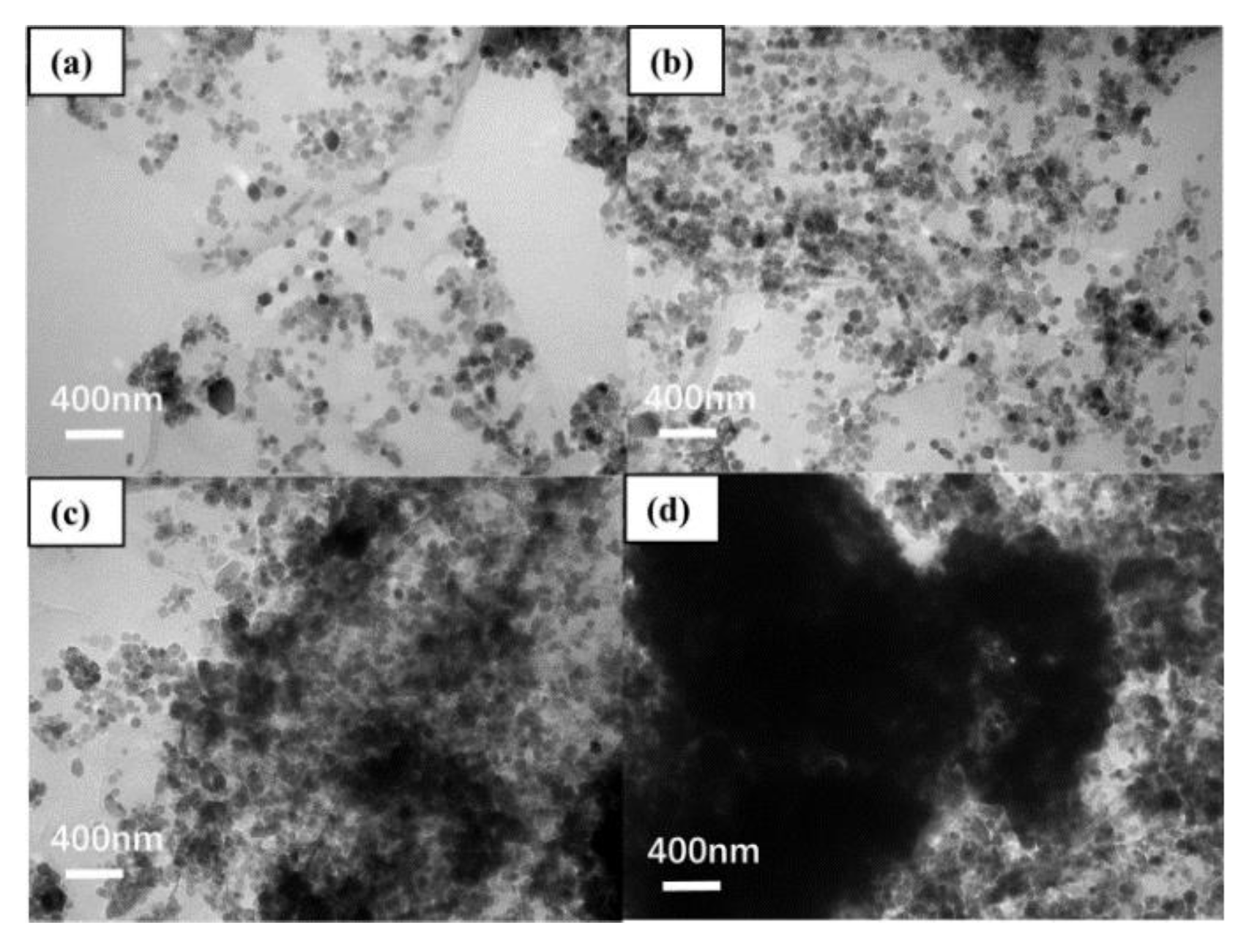






| Solid Content (%) | pH | Viscosity (mPa·S) | Mass Fraction of C (%) | Mass Fraction of O (%) | Mass Fraction of Cl (%) |
|---|---|---|---|---|---|
| 45 ± 5 | 1.7 ± 0.3 | ≥2500 | 47 ± 3 | 42 ± 4 | <1.0 |
| Component | C | Mn | Si | S | P |
|---|---|---|---|---|---|
| Mass fraction | 0.15 | 0.49 | 0.29 | 0.042 | 0.026 |
| Coating | Ecorr (V) | Icorr (A/cm2) | η (%) | Rp (ohm) |
|---|---|---|---|---|
| C1 | −0.566 | 9.196 × 10−5 | - | 5.090 × 102 |
| C2 | −0.525 | 1.328 × 10−5 | 85.56 | 3.345 × 103 |
| C3 | −0.561 | 8.510 × 10−6 | 90.75 | 1.112 × 104 |
| C4 | −0.518 | 1.265 × 10−6 | 98.62 | 2.305 × 104 |
| Coating | 1 d ND (cm−3) | 11 d ND (cm−3) | 21 d ND (cm−3) | 31 d ND (cm−3) |
|---|---|---|---|---|
| C1 | 7.507 × 1021 | 1.370 × 1022 | 2.156 × 1022 | 1.678 × 1022 |
| C2 | 2.309 × 1018 | 2.334 × 1018 | 3.525 × 1018 | 1.678 × 1019 |
| C3 | 1.919 × 1015 | 8.775 × 1018 | 5.234 × 1019 | 3.528 × 1019 |
| C4 | 2.997 × 1013 | 3.961 × 1013 | 5.888 × 1014 | 3.495 × 1014 |
| Coating | Corrosion Area/μm2 | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Total | |
| C1 | 666 | 1382 | 3596 | 5644 |
| C2 | 577 | 960 | 3558 | 5095 |
| C3 | 521 | 1367 | 2810 | 4698 |
| C4 | 338 | 816 | 3424 | 4578 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Liu, X.; Yang, H.; Jie, H.; Li, T.; Lyu, K.; Shah, S.P. Effect of CeO2–GO Nanocomposite on the Anticorrosion Properties of Epoxy Coating in Simulated Acid Rain Solution. Polymers 2022, 14, 3573. https://doi.org/10.3390/polym14173573
Liu R, Liu X, Yang H, Jie H, Li T, Lyu K, Shah SP. Effect of CeO2–GO Nanocomposite on the Anticorrosion Properties of Epoxy Coating in Simulated Acid Rain Solution. Polymers. 2022; 14(17):3573. https://doi.org/10.3390/polym14173573
Chicago/Turabian StyleLiu, Ruidan, Xiaoyan Liu, Heng Yang, Handuo Jie, Tianyu Li, Kai Lyu, and Surendra P. Shah. 2022. "Effect of CeO2–GO Nanocomposite on the Anticorrosion Properties of Epoxy Coating in Simulated Acid Rain Solution" Polymers 14, no. 17: 3573. https://doi.org/10.3390/polym14173573
APA StyleLiu, R., Liu, X., Yang, H., Jie, H., Li, T., Lyu, K., & Shah, S. P. (2022). Effect of CeO2–GO Nanocomposite on the Anticorrosion Properties of Epoxy Coating in Simulated Acid Rain Solution. Polymers, 14(17), 3573. https://doi.org/10.3390/polym14173573







