A Brief Review of Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Fuel Cells
Abstract
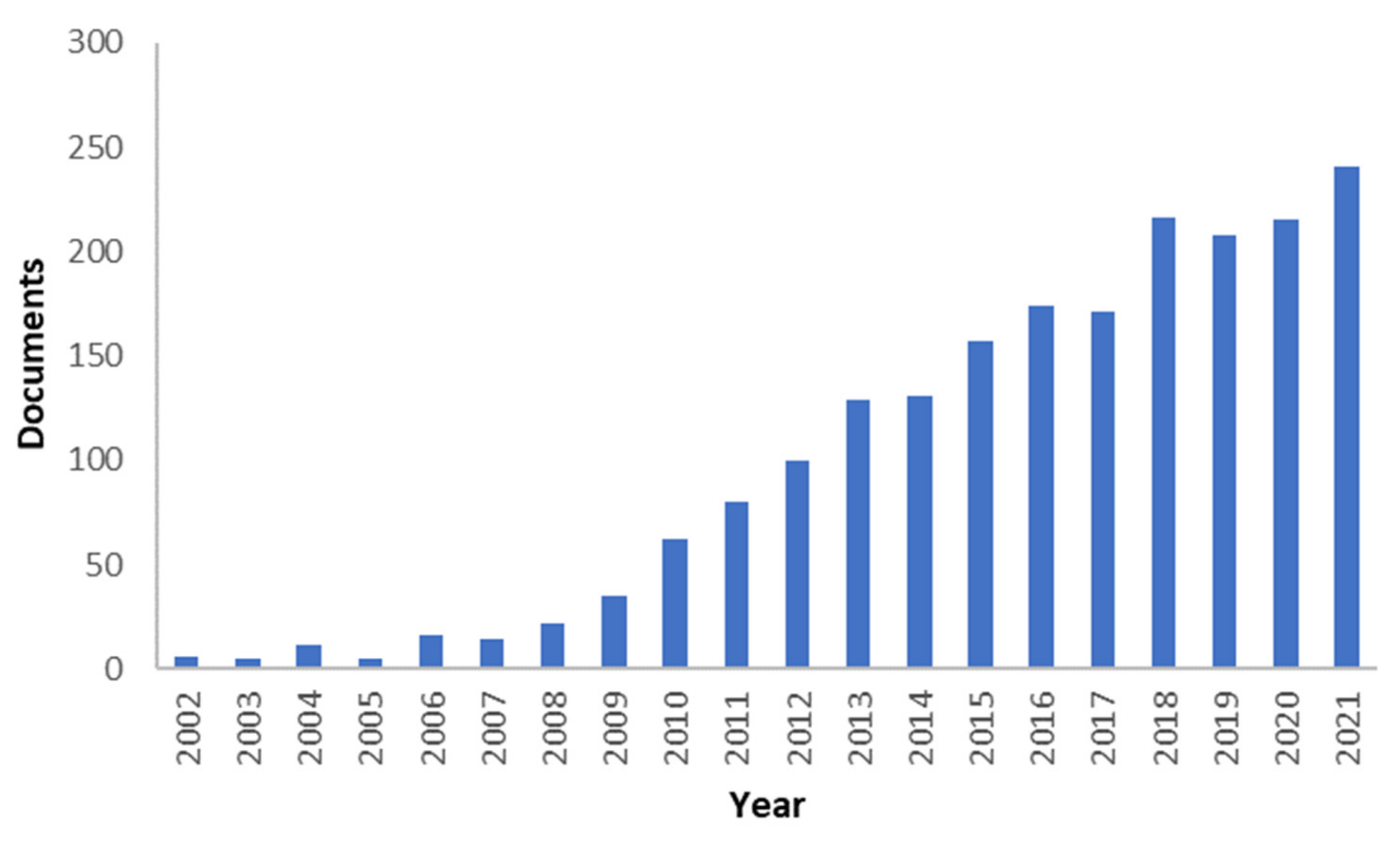
1. Introduction
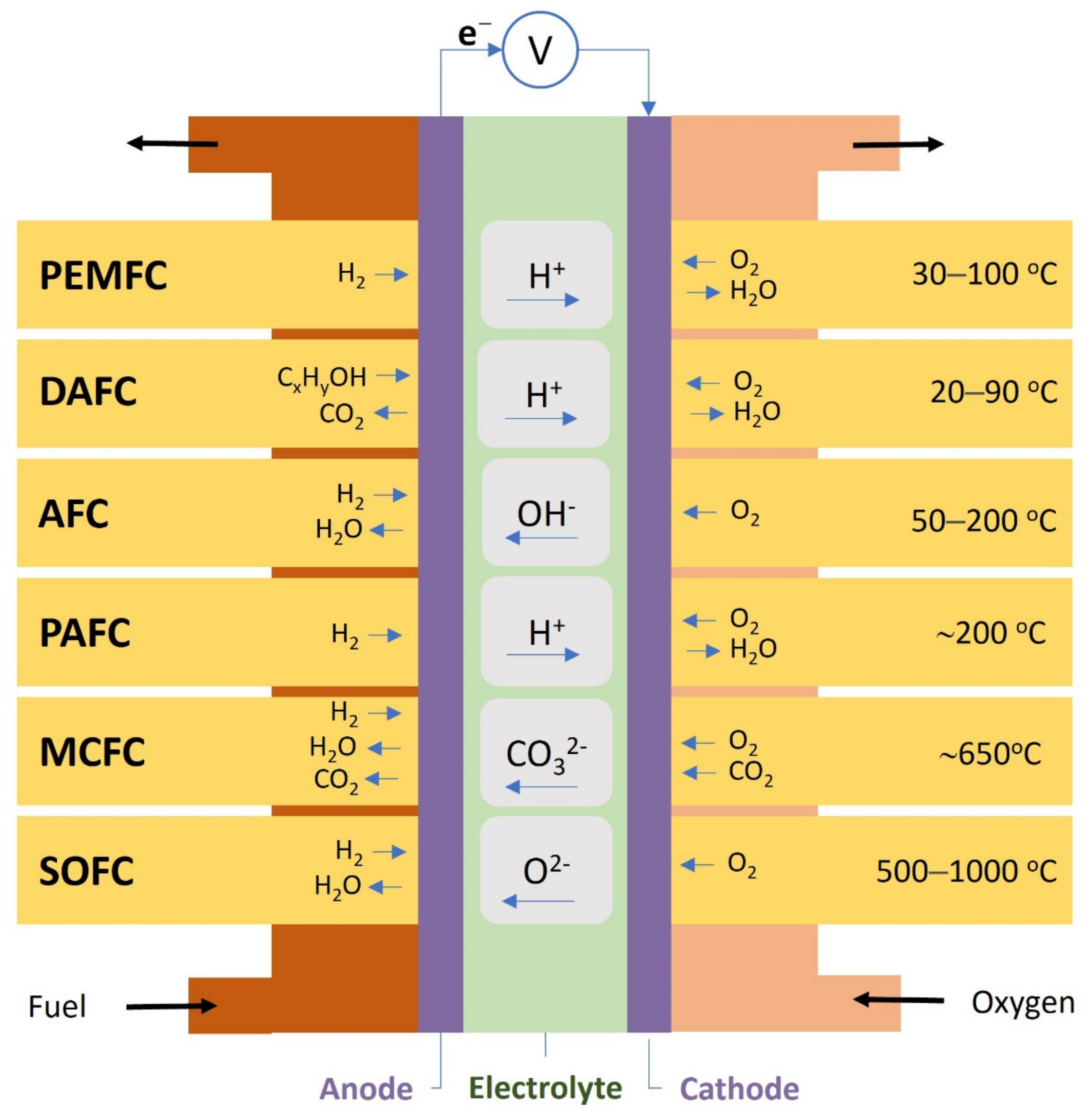
2. Fuel Cell
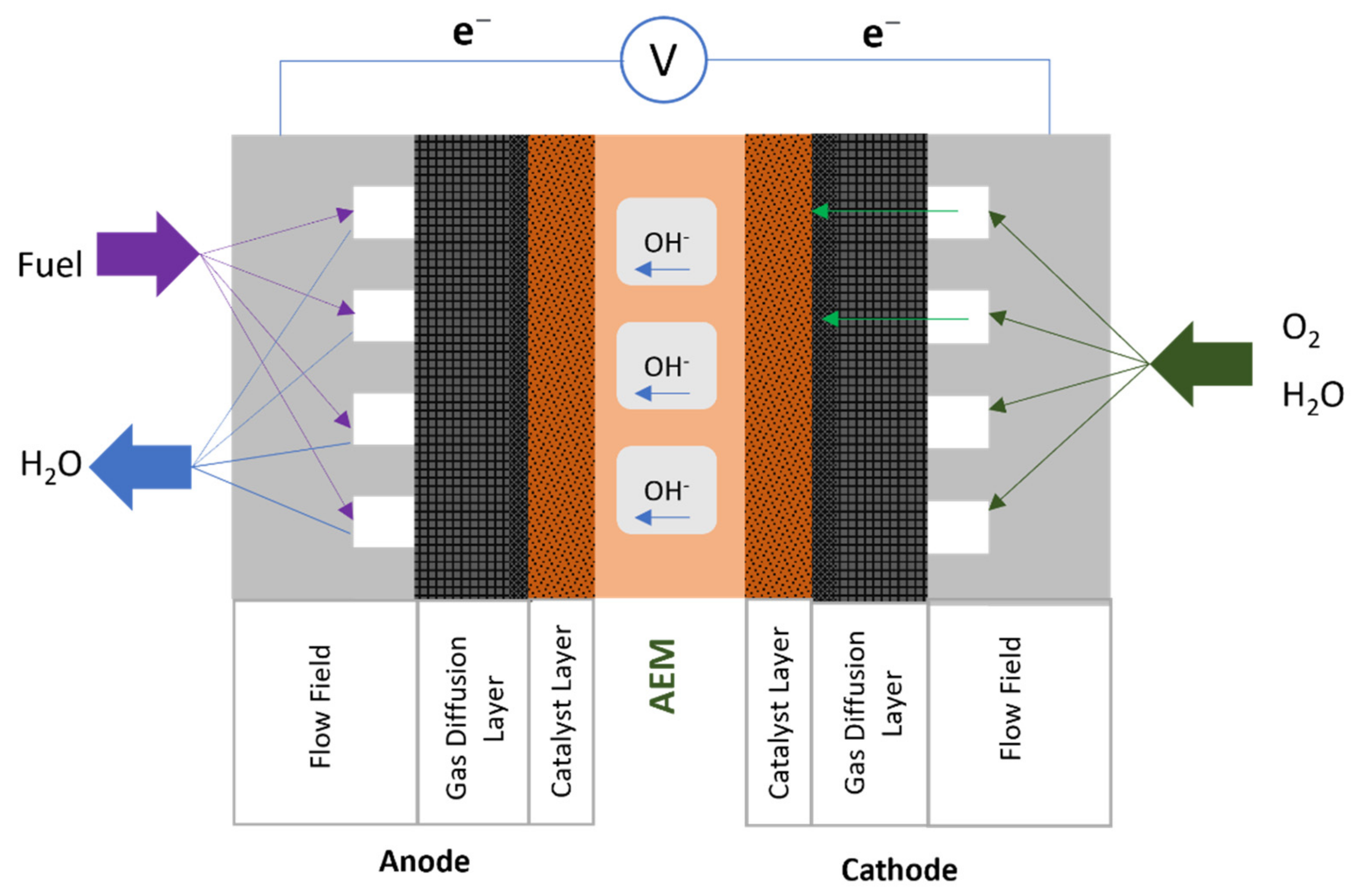
3. Anion Exchange Membrane Fuel Cell
4. Anion Exchange Membrane
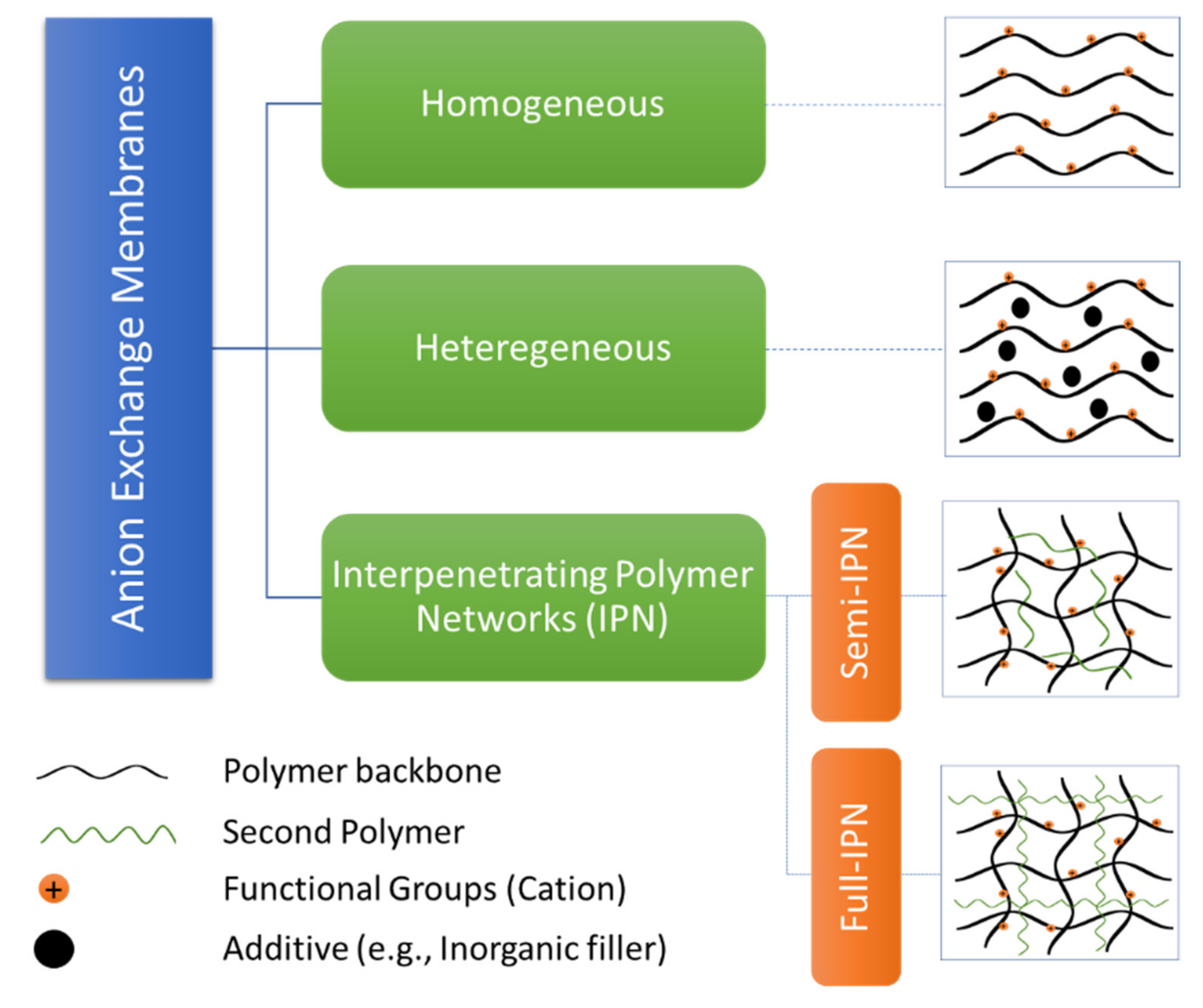
4.1. Types of Anion Exchange Membrane
4.1.1. Homogeneous Anion Exchange Membrane
4.1.2. Heterogeneous Anion Exchange Membrane
4.1.3. Interpenetrating Polymer Networks (IPNs)
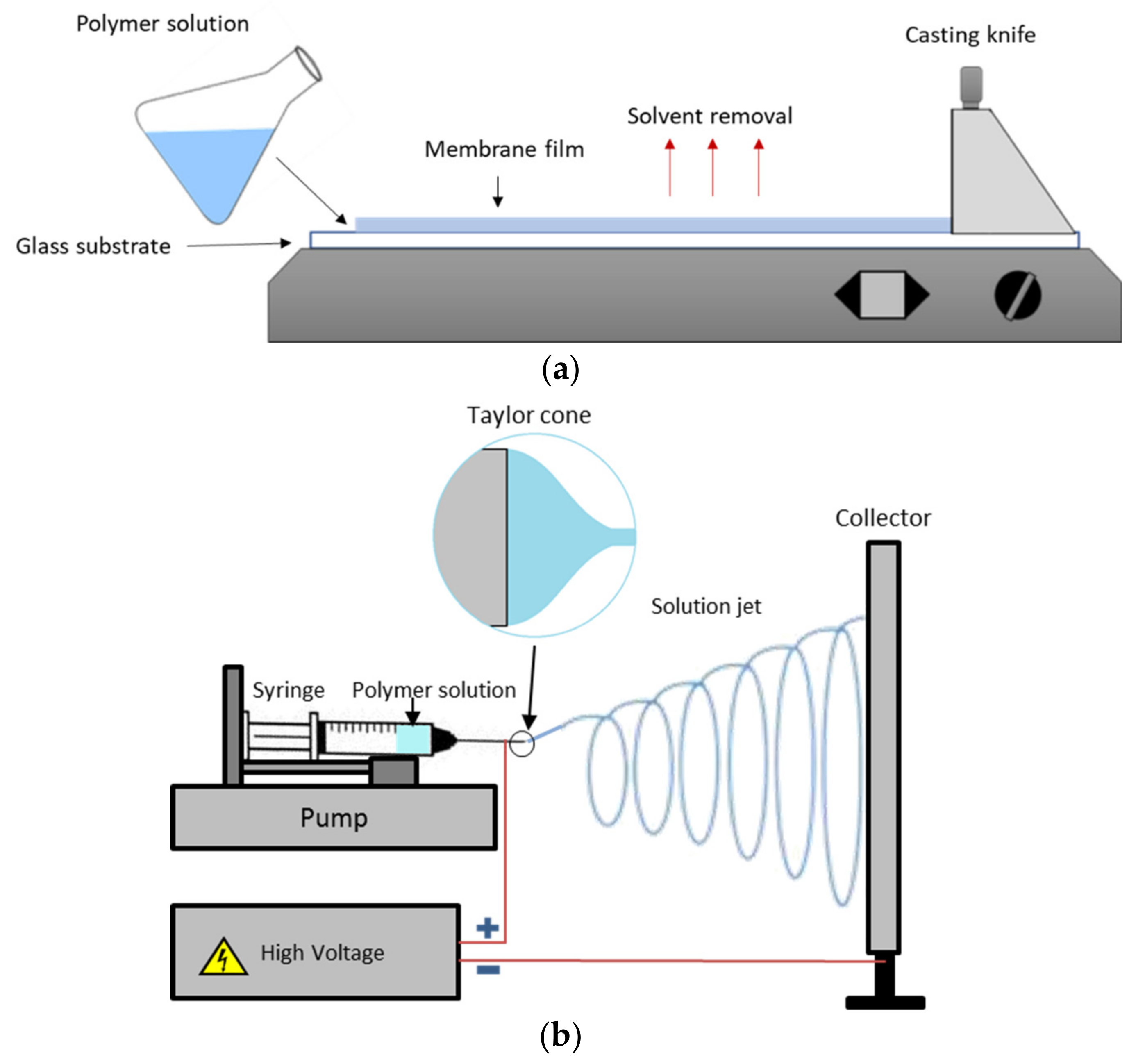
4.2. Fabrication of Anion Exchange Membrane
4.3. Materials for Anion-Exchange Membrane
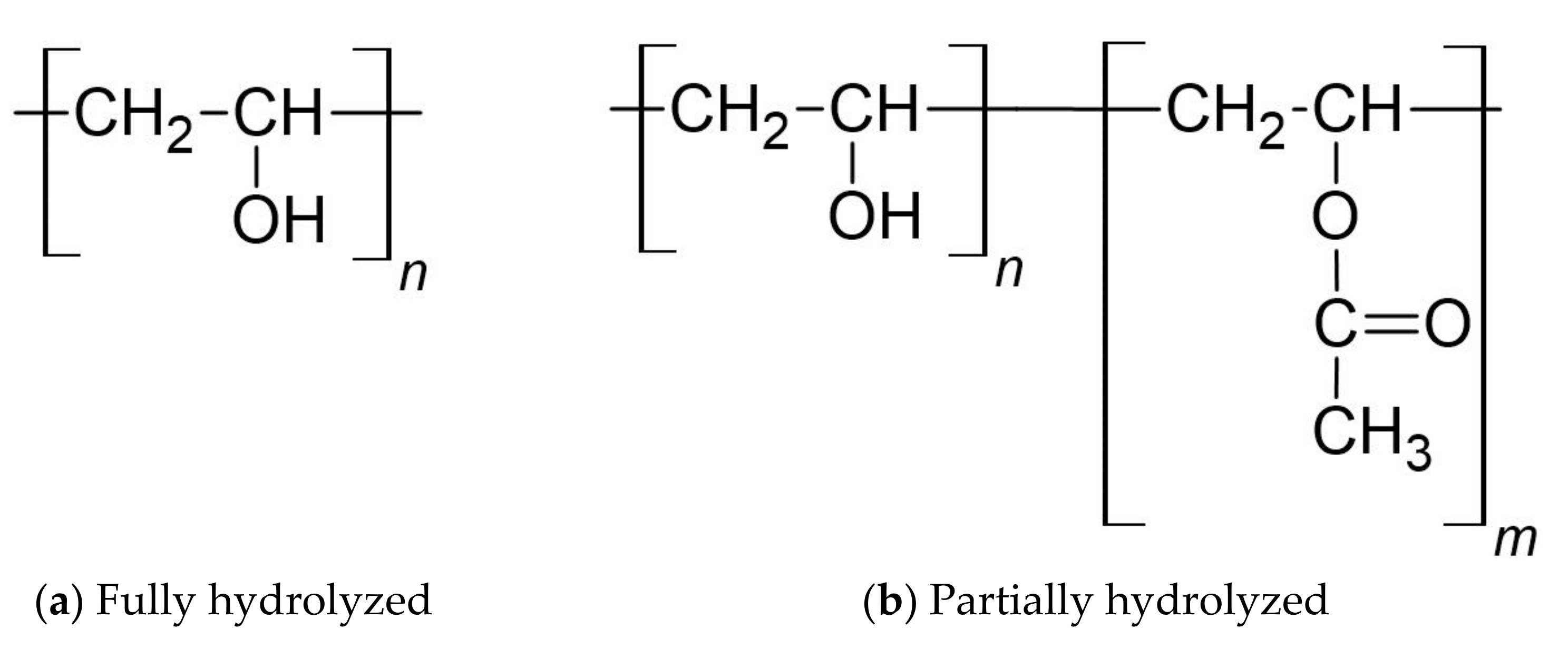
5. Poly(Vinyl Alcohol)
6. Additives for AEM Preparation
6.1. Crosslinkers
6.2. Inorganic Fillers
6.3. Plasticizers
6.4. Ionic Liquids (ILs)
7. Characteristics and Performance of PVA-Based Anions Exchange Membranes
7.1. High OH− Conductivity
7.2. Excellent Mechanical and Thermal Stability
7.3. Electron Insulator and Reactant Barrier
7.4. Fabrication Cost
8. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Megia, P.J.; Vizcaino, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Owusu, A.P.; Asumadu-Sarkodie, S. A Review of Renewable Energy Sources, Sustainability Issues and Climate Change Mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid Inorganic-Organic Proton-Conducting Membranes Based on SPEEK Doped with WO3 Nanoparticles for Application in Vanadium Redox Flow Batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.M.; de Boer, I.J.M.; Ripoll-Bosch, R. The Battle for Biomass: A Systematic Review of Food-Feed-Fuel Competition. Glob. Food Sec. 2020, 25, 100330. [Google Scholar] [CrossRef]
- Voitic, G.; Pichler, B.; Basile, A.; Iulianelli, A.; Malli, K.; Bock, S.; Hacker, V. Hydrogen Production; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128114599. [Google Scholar]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from Solar Energy, a Clean Energy Carrier from a Sustainable Source of Energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An Efficient Barrier toward Vanadium Crossover in Redox Flow Batteries: The Bilayer [Nafion/(WO3)x] Hybrid Inorganic-Organic Membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Dai, W.; Wang, H.; Yuan, X.; Martin, J.J.; Yang, D. A Review on Water Balance in the Membrane Electrode Assembly of Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2009, 34, 9461–9478. [Google Scholar] [CrossRef]
- Fang, J.; Wu, Y.; Zhang, Y.; Lyu, M.; Zhao, J. Novel Anion Exchange Membranes Based on Pyridinium Groups and Fluoroacrylate for Alkaline Anion Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2015, 40, 12392–12399. [Google Scholar] [CrossRef]
- Otsuji, K.; Shirase, Y.; Asakawa, T.; Yokota, N.; Nagase, K.; Xu, W.; Song, P.; Wang, S.; Tryk, D.A.; Kakinuma, K.; et al. Effect of Water Management in Membrane and Cathode Catalyst Layers on Suppressing the Performance Hysteresis Phenomenon in Anion-Exchange Membrane Fuel Cells. J. Power Sources 2022, 522, 230997. [Google Scholar] [CrossRef]
- Iravaninia, M.; Rowshanzamir, S. Polysulfone-Based Anion Exchange Membranes for Potential Application in Solid Alkaline Fuel Cells. J. Renew. Energy Environ. 2015, 2, 59–65. [Google Scholar]
- Das, G.; Choi, J.-H.; Nguyen, P.K.T.; Kim, D.-J.; Yoon, Y.S. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers 2022, 14, 1197. [Google Scholar] [CrossRef] [PubMed]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric Materials as Anion-Exchange Membranes for Alkaline Fuel Cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Qiao, J.; Baker, R.; Zhang, J. Alkaline Polymer Electrolyte Membranes for Fuel Cell Applications. Chem. Soc. Rev. 2013, 42, 5768–5787. [Google Scholar] [CrossRef]
- Ding, C.; Qiao, Z. A Review of the Application of Polyvinyl Alcohol Membranes for Fuel Cells. Ionics 2022, 28, 1–13. [Google Scholar] [CrossRef]
- Hren, M.; Bozic, M.; Fakin, D.; Kleinschek, K.S.; Gorgieva, S. Alkaline Membrane Fuel Cells: Anion Exchange Membranes and Fuels. Sustain. Energy Fuels 2021, 5, 604–637. [Google Scholar] [CrossRef]
- Mandal, M. Recent Advancement on Anion Exchange Membranes for Fuel Cell and Water Electrolysis. ChemElectroChem 2021, 8, 36–45. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Alkaline Anion-Exchange Membrane Fuel Cells: Challenges in Electrocatalysis and Interfacial Charge Transfer. Chem. Rev. 2019, 119, 11945–11979. [Google Scholar] [CrossRef]
- Pan, J.; Lu, S.; Li, Y.; Huang, A.; Zhuang, L.; Lu, J. High-Performance Alkaline Polymer Electrolyte for Fuel Cell Applications. Adv. Funct. Mater. 2010, 20, 312–319. [Google Scholar] [CrossRef]
- Jeong, S.K.; Lee, J.S.; Woo, S.H.; Seo, J.A.; Min, B.R. Characterization of Anion Exchange Membrane Containing Epoxy Ring and C-Cl Bond Quaternized by Various Amine Groups for Application in Fuel Cells. Energies 2015, 8, 7084–7099. [Google Scholar] [CrossRef]
- Gong, X.; He, G.; Yan, X.; Wu, Y.; Chen, W.; Wu, X. Electrospun Nanofiber Enhanced Imidazolium-Functionalized Polysulfone Composite Anion Exchange Membranes. RSC Adv. 2015, 5, 95118–95125. [Google Scholar] [CrossRef]
- Park, A.M.; Wycisk, R.J.; Ren, X.; Turley, F.E.; Pintauro, P.N. Crosslinked Poly(Phenylene Oxide)-Based Nanofiber Composite Membranes for Alkaline Fuel Cells. J. Mater. Chem. A 2016, 4, 132–141. [Google Scholar] [CrossRef]
- Lin, X.; Wu, L.; Liu, Y.; Ong, A.L.; Poynton, S.D.; Varcoe, J.R.; Xu, T. Alkali Resistant and Conductive Guanidinium-Based Anion-Exchange Membranes for Alkaline Polymer Electrolyte Fuel Cells. J. Power Sources 2012, 217, 373–380. [Google Scholar] [CrossRef]
- Sajjad, S.D.; Hong, Y.; Liu, F. Synthesis of Guanidinium-Based Anion Exchange Membranes and Their Stability Assessment. Polym. Adv. Technol. 2014, 25, 108–116. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, X.; Ran, J.; Li, C.; Wu, L.; Xu, T. Synthesis and Properties of Quaternary Phosphonium-Based Anion Exchange Membrane for Fuel Cells. Chin. J. Chem. 2012, 30, 2241–2246. [Google Scholar] [CrossRef]
- Vöge, A.; Deimede, V.; Kallitsis, J.K. Synthesis and Properties of Alkaline Stable Pyridinium Containing Anion Exchange Membranes. RSC Adv. 2014, 4, 45040–45049. [Google Scholar] [CrossRef]
- Hossain, M.A.; Jang, H.; Sutradhar, S.C.; Ha, J.; Yoo, J.; Lee, C.; Lee, S.; Kim, W. Novel Hydroxide Conducting Sulfonium-Based Anion Exchange Membrane for Alkaline Fuel Cell Applications. Int. J. Hydrogen Energy 2016, 41, 10458–10465. [Google Scholar] [CrossRef]
- Xu, T. Ion Exchange Membranes: State of Their Development and Perspective. J. Memb. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; An, L.; Zhao, G.; Yan, X.H. Physicochemical Properties of Alkaline Doped Polybenzimidazole Membranes for Anion Exchange Membrane Fuel Cells. J. Memb. Sci. 2015, 493, 340–348. [Google Scholar] [CrossRef]
- Fu, J.; Qiao, J.; Wang, X.; Ma, J.; Okada, T. Alkali Doped Poly(Vinyl Alcohol) for Potential Fuel Cell Applications. Synth. Met. 2010, 160, 193–199. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Memb. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Arı, G.A.; Özcan, Z. A Novel Approach for Stable Anion Exchange Membrane: Self-Assembled Multilayer Formation on the Membrane via LbL Method. Synth. Met. 2016, 220, 269–275. [Google Scholar] [CrossRef]
- Zhou, T.; Ao, B.; Wei, Y.; Chen, S.; Lian, K.; Qiao, J. Fabricating Hydroxyl Anion Conducting Membranes Based on Poly(Vinyl Alcohol) and Bis(2-Chloroethyl) Ether-1,3-Bis[3-(Dimethylamino)Propyl] Urea Copolymer with Linear Anion-Exchange Sites for Polymer Electrolyte Membrane Fuel Cell. Solid State Ion. 2017, 308, 112–120. [Google Scholar] [CrossRef]
- Movil, O.; Frank, L.; Staser, J.A. Graphene Oxide-Polymer Nanocomposite Anion-Exchange Membranes. J. Electrochem. Soc. 2015, 162, F419–F426. [Google Scholar] [CrossRef]
- Feketefoldi, B.; Cermenek, B. Chitosan-Based Anion Exchange Membranes for Direct Ethanol Fuel Cells. J. Membr. Sci. Technol. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Luo, X.; Xiong, J.; Liu, Z.; Cai, W. Effect of Nano-Size of Functionalized Silica on Overall Performance of Swelling-Filling Modified Nafion Membrane for Direct Methanol Fuel Cell Application. Appl. Energy 2018, 213, 408–414. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, G.; Shi, J. Poly (Vinyl Alcohol)/Sulfosuccinic Acid (PVA/SSA) as Proton-Conducting Membranes for Fuel Cells: Effect of Cross-Linking and Plasticizer Addition. ECS Trans. 2013, 53, 29–34. [Google Scholar] [CrossRef]
- Dicks, A.L.; Rand, D.A.J. Introducing Fuel Cells. In Fuel Cell Systems Explained; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 1–26. ISBN 9781118706992. [Google Scholar]
- Dincer, I.; Rosen, M.A. Exergy Analyses of Fuel Cell Systems. In Exergy; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 479–514. [Google Scholar] [CrossRef]
- Jiao, K.; Wang, B.; Du, Q.; Wang, Y.; Zhang, G.; Yang, Z.; Deng, H.; Xie, X. Introduction. In Water and Thermal Management of Proton Exchange Membrane Fuel Cells; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 1–23. ISBN 9780323911160. [Google Scholar]
- Ferriday, T.B.; Middleton, P.H. ScienceDirect Alkaline Fuel Cell Technology—A Review. Int. J. Hydrogen Energy 2021, 46, 18489–18510. [Google Scholar] [CrossRef]
- Dicks, A.L.; Rand, D.A.J. Alkaline Fuel Cells. In Fuel Cell Systems Explained; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 135–156. ISBN 9781118706992. [Google Scholar]
- Shin, M.S.; Lim, S.; Park, J.H.; Kim, H.J.; Chae, S.; Park, J.S. Thermally Crosslinked and Quaternized Polybenzimidazole Ionomer Binders for Solid Alkaline Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 11773–11783. [Google Scholar] [CrossRef]
- Hari Gopi, K.; Dhavale, V.M.; Bhat, S.D. Development of Polyvinyl Alcohol/Chitosan Blend Anion Exchange Membrane with Mono and Di Quaternizing Agents for Application in Alkaline Polymer Electrolyte Fuel Cells. Mater. Sci. Energy Technol. 2019, 2, 194–202. [Google Scholar] [CrossRef]
- Kim, E.; Lee, S.; Woo, S.; Park, S.H.; Yim, S.D.; Shin, D.; Bae, B. Synthesis and Characterization of Anion Exchange Multi-Block Copolymer Membranes with a Fluorine Moiety as Alkaline Membrane Fuel Cells. J. Power Sources 2017, 359, 568–576. [Google Scholar] [CrossRef]
- Cermenek, B.; Ranninger, J.; Hacker, V. Alkaline Direct Ethanol Fuel Cell. In Ethanol: Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 383–405. ISBN 9780128114582. [Google Scholar]
- Jiang, S.; Sun, H.; Wang, H.; Ladewig, B.P.; Yao, Z. A Comprehensive Review on the Synthesis and Applications of Ion Exchange Membranes. Chemosphere 2021, 282, 130817. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion Exchange Membranes: New Developments and Applications. J. Memb. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Pourzare, K.; Mansourpanah, Y.; Farhadi, S. Advanced Nanocomposite Membranes for Fuel Cell Applications: A Comprehensive Review. Biofuel Res. J. 2016, 3, 496–513. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Liang, S.; Dong, Q.; Jin, B.; Bai, R. Preparation and Characterization of Composite Membranes with Ionic Liquid Polymer-Functionalized Multiwalled Carbon Nanotubes for Alkaline Fuel Cells. RSC Adv. 2013, 3, 13477. [Google Scholar] [CrossRef]
- Sood, R.; Cavaliere, S.; Jones, D.J.; Rozière, J. Electrospun Nanofibre Composite Polymer Electrolyte Fuel Cell and Electrolysis Membranes. Nano Energy 2016, 26, 729–745. [Google Scholar] [CrossRef]
- Fennessey, S.F.; Farris, R.J. Fabrication of Aligned and Molecularly Oriented Electrospun Polyacrylonitrile Nanofibers and the Mechanical Behavior of Their Twisted Yarns. Polymer 2004, 45, 4217–4225. [Google Scholar] [CrossRef]
- Tamura, T.; Kawakami, H. Aligned Electrospun Nanofiber Composite Membranes for Fuel Cell Electrolytes. Nano Lett. 2010, 10, 1324–1328. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, B.; Yan, F. Anion Exchange Membranes for Alkaline Fuel Cell Applications: The Effects of Cations. ChemSusChem 2017, 11, 58–70. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef]
- Lin, B.; Qiu, L.; Qiu, B.; Peng, Y.; Yan, F. A Soluble and Conductive Polyfluorene Ionomer with Pendant Imidazolium Groups for Alkaline Fuel Cell Applications. Macromolecules 2011, 44, 9642–9649. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Kinsinger, C.L.; Yang, Y.; Seifert, S.; Yan, Y.; Mark Maupin, C.; Liberatore, M.W.; Herring, A.M. Anion Exchange Membranes Composed of a Poly(2,6-Dimethyl-1,4-Phenylene Oxide) Random Copolymer Functionalized with a Bulky Phosphonium Cation. J. Memb. Sci. 2016, 506, 50–59. [Google Scholar] [CrossRef]
- Hsu, P.-Y.; Hu, T.-Y.; Kumar, S.; Chang, C.-H.; Wu, K.; Tung, K.-L.; Lue, S. Highly Zeolite-Loaded Polyvinyl Alcohol Composite Membranes for Alkaline Fuel-Cell Electrolytes. Polymers 2018, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.F.; An, L.; Zhao, T.S.; Tang, Z.K. Advances and Challenges in Alkaline Anion Exchange Membrane Fuel Cells. Prog. Energy Combust. Sci. 2018, 66, 141–175. [Google Scholar] [CrossRef]
- Hari Gopi, K.; Bhat, S.D. Anion Exchange Membrane from Polyvinyl Alcohol Functionalized with Quaternary Ammonium Groups via Alkyl Spacers. Ionics 2018, 24, 1097–1109. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Y.; Zhang, H.; Hou, L. Preparation and Characterization of Quaternized Poly(Vinyl Alcohol)/Chitosan/MoS2composite Anion Exchange Membranes with High Selectivity. Carbohydr. Polym. 2018, 180, 96–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, H.; Xing, D.; Lu, W.; Shao, Z.; Yi, B. Preparation and Characterization of PTFE Based Composite Anion Exchange Membranes for Alkaline Fuel Cells. J. Memb. Sci. 2012, 421–422, 311–317. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.T.; Lam How Yee, E.; Poynton, S.D.; Driscoll, D.J.; Apperley, D.C. Poly(Ethylene-Co-Tetrafluoroethylene)-Derived Radiation-Grafted Anion-Exchange Membrane with Properties Specifically Tailored for Application in Metal-Cation-Free Alkaline Polymer Electrolyte Fuel Cells. Chem. Mater. 2007, 19, 2686–2693. [Google Scholar] [CrossRef]
- Omasta, T.J.; Wang, L.; Peng, X.; Lewis, C.A.; Varcoe, J.R.; Mustain, W.E. Importance of Balancing Membrane and Electrode Water in Anion Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 205–213. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Premakshi, H.G.; Kariduraganavar, M.Y. Synthesis and Characterization of GTMAC Grafted Chitosan Membranes for the Dehydration of Low Water Content Isopropanol by Pervaporation. J. Ind. Eng. Chem. 2015, 25, 151–161. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, C.; Chen, J.; Ren, X. Synthesis and Characterization of Cross-Linked Quaternized Chitosan/Poly(Diallyldimethylammonium Chloride) Blend Anion-Exchange Membranes. Ionics 2018, 24, 1180. [Google Scholar] [CrossRef]
- Wan, Y.; Peppley, B.; Creber, K.A.M.; Bui, V.T. Anion-Exchange Membranes Composed of Quaternized-Chitosan Derivatives for Alkaline Fuel Cells. J. Power Sources 2010, 195, 3785–3793. [Google Scholar] [CrossRef]
- Liao, X.; Ren, L.; Chen, D.; Liu, X.; Zhang, H. Nanocomposite Membranes Based on Quaternized Polysulfone and Functionalized Montmorillonite for Anion-Exchange Membranes. J. Power Sources 2015, 286, 258–263. [Google Scholar] [CrossRef]
- Miao, L.; Wang, X.; Fu, Y.; Hu, B.; Bai, Y.; Lü, C. Quaternized Polyhedral Oligomeric Silsesquioxanes (QPOSS) Modified Polysulfone-Based Composite Anion Exchange Membranes. Solid State Ion. 2017, 309, 170–179. [Google Scholar] [CrossRef]
- Lu, W.; Shao, Z.G.; Zhang, G.; Zhao, Y.; Li, J.; Yi, B. Preparation and Characterization of Imidazolium-Functionalized Poly (Ether Sulfone) as Anion Exchange Membrane and Ionomer for Fuel Cell Application. Int. J. Hydrogen Energy 2013, 38, 9285–9296. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Mei, J.; Xu, J.; Xu, L.; Han, H.; Ni, H.; Wang, S. A Facile Functionalized Routine for the Synthesis of Imidazolium-Based Anion-Exchange Membrane with Excellent Alkaline Stability. J. Memb. Sci. 2016, 505, 138–147. [Google Scholar] [CrossRef]
- Chen, W.; Yan, X.; Wu, X.; Huang, S.; Luo, Y.; Gong, X.; He, G. Tri-Quaternized Poly (Ether Sulfone) Anion Exchange Membranes with Improved Hydroxide Conductivity. J. Memb. Sci. 2016, 514, 613–621. [Google Scholar] [CrossRef]
- Lin, B.; Qiao, G.; Chu, F.; Wang, J.; Feng, T.; Yuan, N.; Zhang, S.; Zhang, X.; Ding, J. Preparation and Characterization of Imidazolium-Based Membranes for Anion Exchange Membrane Fuel Cell Applications. Int. J. Hydrogen Energy 2017, 42, 6988–6996. [Google Scholar] [CrossRef]
- Kim, D.J.; Park, C.H.; Nam, S.Y. Characterization of a Soluble Poly(Ether Ether Ketone) Anion Exchange Membrane for Fuel Cell Application. Int. J. Hydrogen Energy 2016, 41, 7649–7658. [Google Scholar] [CrossRef]
- Yan, X.; Gao, L.; Zheng, W.; Ruan, X.; Zhang, C.; Wu, X.; He, G. Long-Spacer-Chain Imidazolium Functionalized Poly(Ether Ether Ketone) as Hydroxide Exchange Membrane for Fuel Cell. Int. J. Hydrogen Energy 2016, 41, 14982–14990. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, N.; Li, R.; Zhang, Y.; Li, K.; Wang, F.; Zhu, H. A New Method for Improving the Conductivity of Alkaline Membrane by Incorporating TiO2- Ionic Liquid Composite Particles. Electrochim. Acta 2017, 255, 335–346. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Hao, Y.; He, X.; He, R. Preparation and Investigation of Various Imidazolium-Functionalized Poly(2,6-Dimethyl-1,4-Phenylene Oxide) Anion Exchange Membranes. Electrochim. Acta 2016, 207, 112–119. [Google Scholar] [CrossRef]
- Chen, N.; Long, C.; Li, Y.; Wang, D.; Zhu, H. A Hamburger-Structure Imidazolium-Modified Silica/Polyphenyl Ether Composite Membrane with Enhancing Comprehensive Performance for Anion Exchange Membrane Applications. Electrochim. Acta 2018, 268, 295–303. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl Alcohol: A Review of Research Status and Use of Polyvinyl Alcohol Based Nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Ben Halima, N. Poly(Vinyl Alcohol): Review of Its Promising Applications and Insights into Biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Susanto, H.; Samsudin, A.M.; Faz, M.W.; Rani, M.P.H. Impact of Post-Treatment on the Characteristics of Electrospun Poly (Vinyl Alcohol)/Chitosan Nanofibers. In AIP Conference Proceedings; Susanto, H., Suryana, R., Triyana, K., Eds.; AIP Publishing LLC: New York, NY, USA, 2016; Volume 1725, p. 020087. [Google Scholar]
- Kuraray Poval No Title. Available online: https://www.kuraray-poval.com/products/kuraray-poval (accessed on 24 April 2022).
- Marin, E.; Rojas, J.; Ciro, Y. A Review of Polyvinyl Alcohol Derivatives: Promising Materials for Pharmaceutical and Biomedical Applications. Afr. J. Pharm. Pharmacol. 2014, 8, 674–684. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A.; McPhee, D.J. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Feldman, D. Poly(Vinyl Alcohol) Recent Contributions to Engineering and Medicine. J. Compos. Sci. 2020, 4, 175. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, F.; Jiang, P.; Ge, M. The Experimental Study of Polyvinyl Alcohol (PVA) Textile Material Degradation by Ozone Oxidation Process. J. Text. Inst. 2021, 112, 117–122. [Google Scholar] [CrossRef]
- Moulay, S. Review: Poly(Vinyl Alcohol) Functionalizations and Applications. Polym.-Plast. Technol. Eng. 2015, 54, 1289–1319. [Google Scholar] [CrossRef]
- Thong, C.C.; Teo, D.C.L.; Ng, C.K. Application of Polyvinyl Alcohol (PVA) in Cement-Based Composite Materials: A Review of Its Engineering Properties and Microstructure Behavior. Constr. Build. Mater. 2016, 107, 172–180. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, D.; Zhang, W. Treatment of High-Strength Ethylene Glycol Waste Water in an Expanded Granular Sludge Blanket Reactor: Use of PVA-Gel Beads as a Biocarrier. Springerplus 2016, 5, 856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiao, J.; Jiang, G.; Liu, L.; Liu, Y. Cross-Linked Poly(Vinyl Alcohol)/Poly (Diallyldimethylammonium Chloride) as Anion-Exchange Membrane for Fuel Cell Applications. J. Power Sources 2013, 240, 359–367. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, W.; Xu, K.; Li, M.; Fan, M.; Wang, K. Preparation of Anion Exchange Membrane with Branch Polyethyleneimine as Main Skeleton Component. Mater. Des. 2018, 160, 698–707. [Google Scholar] [CrossRef]
- Herranz, D.; Escudero-Cid, R.; Montiel, M.; Palacio, C.; Fatás, E.; Ocón, P. Poly (Vinyl Alcohol) and Poly (Benzimidazole) Blend Membranes for High Performance Alkaline Direct Ethanol Fuel Cells. Renew. Energy 2018, 127, 883–895. [Google Scholar] [CrossRef]
- Wu, B.; Ge, L.; Yu, D.; Hou, L.; Li, Q.; Yang, Z.; Xu, T. Cationic Metal-Organic Framework Porous Membranes with High Hydroxide Conductivity and Alkaline Resistance for Fuel Cells. J. Mater. Chem. A 2016, 4, 14545–14549. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.S.; Kuo, S.C.; Liou, T.H. Fabrication of Anion-Exchange Composite Membranes for Alkaline Direct Methanol Fuel Cells. J. Power Sources 2012, 199, 37–45. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.J.; Chien, W.C.; Chiu, S.S. Quaternized Poly(Vinyl Alcohol)/Alumina Composite Polymer Membranes for Alkaline Direct Methanol Fuel Cells. J. Power Sources 2010, 195, 2212–2219. [Google Scholar] [CrossRef]
- Ye, Y.S.; Cheng, M.Y.; Xie, X.L.; Rick, J.; Huang, Y.J.; Chang, F.C.; Hwang, B.J. Alkali Doped Polyvinyl Alcohol/Graphene Electrolyte for Direct Methanol Alkaline Fuel Cells. J. Power Sources 2013, 239, 424–432. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Pepi, S.; Pardini, A.; Bonechi, C.; Tamasi, G.; Donati, A.; Rossi, C.; Magnani, A. Poly-Vinyl Alcohol (PVA) Crosslinked by Trisodium Trimetaphosphate (STMP) and Sodium Hexametaphosphate (SHMP): Effect of Molecular Weight, PH and Phosphorylating Agent on Length of Spacing Arms, Crosslinking Density and Water Interaction. J. Mol. Struct. 2020, 1202, 127264. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Effect of Glutaraldehyde on Thermal and Mechanical Properties of Starch and Polyvinyl Alcohol Blends. Des. Monomers Polym. 2019, 22, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shen, L.; Li, C.; Wang, Y. Properties and Pervaporation Performance of Poly(Vinyl Alcohol) Membranes Crosslinked with Various Dianhydrides. J. Appl. Polym. Sci. 2018, 135, 15–19. [Google Scholar] [CrossRef]
- Birck, C.; Degoutin, S.; Tabary, N.; Miri, V.; Bacquet, M. New Crosslinked Cast Films Based on Poly(Vinyl Alcohol): Preparation and Physico-Chemical Properties. Express Polym. Lett. 2014, 8, 941–952. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.C.; Edgren, D. Crosslinking Reaction of Poly(Vinyl Alcohol) with Glyoxal. J. Polym. Res. 2010, 17, 725–730. [Google Scholar] [CrossRef]
- Gorgieva, S.; Osmić, A.; Hribernik, S.; Božič, M.; Svete, J.; Hacker, V.; Wolf, S.; Genorio, B. Efficient Chitosan/Nitrogen-Doped Reduced Graphene Oxide Composite Membranes for Direct Alkaline Ethanol Fuel Cells. Int. J. Mol. Sci. 2021, 22, 1740. [Google Scholar] [CrossRef]
- Derbali, Z.; Fahs, A.; Chailan, J.F.; Ferrari, I.V.; Di Vona, M.L.; Knauth, P. Composite Anion Exchange Membranes with Functionalized Hydrophilic or Hydrophobic Titanium Dioxide. Int. J. Hydrogen Energy 2017, 42, 19178–19189. [Google Scholar] [CrossRef]
- Vinodh, R.; Sangeetha, D. Quaternized Poly(Styrene Ethylene Butylene Poly Styrene)/Multiwalled Carbon Nanotube Composites for Alkaline Fuel Cell Applications. J. Nanosci. Nanotechnol. 2013, 13, 5522–5533. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wang, J.; Wang, L. Synthesized Geminal-Imidazolium-Type Ionic Liquids Applying for PVA-FP/[DimL][OH] Anion Exchange Membranes for Fuel Cells. Polymer 2019, 170, 31–42. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, B.; Wang, H. Effects of [Bmim]OH on Structure and Conductive Properties of Alkaline PVA/[Bmim]OH Membranes. Polym. Bull. 2010, 65, 719–730. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A Mini-Review on Anion Exchange Membranes for Fuel Cell Applications: Stability Issue and Addressing Strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Zheng, J.; Dai, L.; Li, S.; Shi, C.; Li, Y.; Zhang, S.; Yang, H.; Sherazi, T.A. A Simple Self-Cross-Linking Strategy for Double-Layered Proton Exchange Membranes with Improved Methanol Resistance and Good Electrochemical Properties for Passive Direct Methanol Fuel Cells. ACS Appl. Energy Mater. 2018, 1, 941–947. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Y.; Ge, Q.; Yang, Z.; Wu, L.; Xu, T. Recyclable Cross-Linked Anion Exchange Membrane for Alkaline Fuel Cell Application. J. Power Sources 2018, 375, 404–411. [Google Scholar] [CrossRef]
- De Oliveira, P.N.; Mendes, A.M.M. Preparation and Characterization of an Eco-Friendly Polymer Electrolyte Membrane (PEM) Based in a Blend of Sulphonated Poly(Vinyl Alcohol)/Chitosan Mechanically Stabilised by Nylon 6,6. Mater. Res. 2016, 19, 954–962. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, J.; Jingfu, J.; Jiang, G.; Zhang, J.; Qiao, J. Poly(Ethylene Glycol) Plasticized Poly(Vinyl Alcohol)/Poly(Acrylamide-Co- Diallyldimethylammonium Chloride) as Alkaline Anion-Exchange Membrane for Potential Fuel Cell Applications. Synth. Met. 2013, 167, 43–50. [Google Scholar] [CrossRef]
- Chu, Y.; Chen, Y.; Chen, N.; Wang, F.; Zhu, H. A New Method for Improving the Ion Conductivity of Anion Exchange Membranes by Using TiO2 Nanoparticles Coated with Ionic Liquid. RSC Adv. 2016, 6, 96768–96777. [Google Scholar] [CrossRef]
- Lin, B.; Qiu, L.; Lu, J.; Yan, F. Cross-Linked Alkaline Ionic Liquid-Based Polymer Electrolytes for Alkaline Fuel Cell Applications. Chem. Mater. 2010, 22, 6718–6725. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Liu, L.; Liu, Y.; Sheng, J. Highly Stable Hydroxyl Anion Conducting Membranes Poly(Vinyl Alcohol)/Poly(Acrylamide-Co-Diallyldimethylammonium Chloride) (PVA/PAADDA) for Alkaline Fuel Cells: Effect of Cross-Linking. Int. J. Hydrogen Energy 2012, 37, 4580–4585. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, J.; Zhang, J. Anion Conducting Poly(Vinyl Alcohol)/Poly(Diallyldimethylammonium Chloride) Membranes with High Durable Alkaline Stability for Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2013, 237, 1–4. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, T.; Qiao, J.; Liu, Y.; Zhang, J. Hydroxyl Anion Conducting Membranes Poly(Vinyl Alcohol)/Poly(Diallyldimethylammonium Chloride) for Alkaline Fuel Cell Applications: Effect of Molecular Weight. Electrochim. Acta 2013, 111, 351–358. [Google Scholar] [CrossRef]
- Xu, P.Y.; Zhao, C.H.; Liu, Q.L. Poly(Vinyl Alcohol)/Cu(II) Complex Anion Exchange Membranes Prepared Using Chemical Fiber for Direct Methanol Fuel Cells. J. Appl. Polym. Sci. 2013, 130, 1172–1178. [Google Scholar] [CrossRef]
- Xu, P.Y.; Guo, T.Y.; Zhao, C.H.; Broadwell, I.; Zhang, Q.G.; Liu, Q.L. Anion Exchange Membranes Based on Poly(Vinyl Alcohol) and Quaternized Polyethyleneimine for Direct Methanol Fuel Cells. J. Appl. Polym. Sci. 2013, 128, 3853–3860. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, J.; Qiao, J.; Liu, L.; Jiang, G.; Zhang, J.; Liu, Y. High Durable Poly(Vinyl Alcohol)/Quaterized Hydroxyethylcellulose Ethoxylate Anion Exchange Membranes for Direct Methanol Alkaline Fuel Cells. J. Power Sources 2013, 227, 291–299. [Google Scholar] [CrossRef]
- Yang, C.C.; Lin, Y.T. Preparation of a Novel Composite Membrane and PtRu/Hollow Carbon Sphere (HCS) Anode Catalyst for Alkaline Direct Methanol Fuel Cell (ADMFC). Energy Procedia 2014, 61, 1410–1416. [Google Scholar] [CrossRef][Green Version]
- Yang, J.M.; Wang, N.C.; Chiu, H.C. Preparation and Characterization of Poly(Vinyl Alcohol)/Sodium Alginate Blended Membrane for Alkaline Solid Polymer Electrolytes Membrane. J. Memb. Sci. 2014, 457, 139–148. [Google Scholar] [CrossRef]
- Lu, W.; Shao, Z.G.; Zhang, G.; Zhao, Y.; Yi, B. Crosslinked Poly(Vinylbenzyl Chloride) with a Macromolecular Crosslinker for Anion Exchange Membrane Fuel Cells. J. Power Sources 2014, 248, 905–914. [Google Scholar] [CrossRef]
- Gao, Y.; Song, F.; Qiao, J.; Chen, S.; Zhao, X.; Zhang, J. Imidazolium-Functionalized Anion Exchange Polymer Electrolytes with High Tensile Strength and Stability for Alkaline Membrane Fuel Cells. Electrochim. Acta 2015, 177, 201–208. [Google Scholar] [CrossRef]
- Das, G.; Lee, S.H.; Lee, K.S.; Yoon, Y.S. A Unique Cross-Linking Strategy to Prepare Poly(Vinyl Alcohol)-Based Anion Conducting Membrane for Fuel Cell Applications. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 481–488. [Google Scholar] [CrossRef]
- Sharma, P.P.; Gahlot, S.; Bhil, B.M.; Gupta, H.; Kulshrestha, V. An Environmentally Friendly Process for the Synthesis of an FGO Modified Anion Exchange Membrane for Electro-Membrane Applications. RSC Adv. 2015, 5, 38712–38721. [Google Scholar] [CrossRef]
- Lu, Y.; Armentrout, A.A.; Li, J.; Tekinalp, H.L.; Nanda, J.; Ozcan, S. A Cellulose Nanocrystal-Based Composite Electrolyte with Superior Dimensional Stability for Alkaline Fuel Cell Membranes. J. Mater. Chem. A 2015, 3, 13350–13356. [Google Scholar] [CrossRef]
- Liao, G.M.; Yang, C.C.; Hu, C.C.; Pai, Y.L.; Lue, S.J. Novel Quaternized Polyvinyl Alcohol/Quaternized Chitosan Nano-Composite as an Effective Hydroxide-Conducting Electrolyte. J. Memb. Sci. 2015, 485, 17–29. [Google Scholar] [CrossRef]
- García-Cruz, L.; Casado-Coterillo, C.; Irabien, Á.; Montiel, V.; Iniesta, J. High Performance of Alkaline Anion-Exchange Membranes Based on Chitosan/Poly (Vinyl) Alcohol Doped with Graphene Oxide for the Electrooxidation of Primary Alcohols. C 2016, 2, 10. [Google Scholar] [CrossRef]
- Gong, Y.; Liao, X.; Xu, J.; Chen, D.; Zhang, H. Novel Anion-Conducting Interpenetrating Polymer Network of Quaternized Polysulfone and Poly(Vinyl Alcohol) for Alkaline Fuel Cells. Int. J. Hydrogen Energy 2016, 41, 5816–5823. [Google Scholar] [CrossRef]
- Liao, G.M.; Li, P.C.; Lin, J.S.; Ma, W.T.; Yu, B.C.; Li, H.Y.; Liu, Y.L.; Yang, C.C.; Shih, C.M.; Lue, S.J. Highly Conductive Quasi-Coaxial Electrospun Quaternized Polyvinyl Alcohol Nanofibers and Composite as High-Performance Solid Electrolytes. J. Power Sources 2016, 304, 136–145. [Google Scholar] [CrossRef]
- Li, P.-C.; Liao, G.; Kumar, S.R.; Shih, C.-M.; Yang, C.-C.; Wang, D.-M.; Lue, S.J. Fabrication and Characterization of Chitosan Nanoparticle-Incorporated Quaternized Poly(Vinyl Alcohol) Composite Membranes as Solid Electrolytes for Direct Methanol Alkaline Fuel Cells. Electrochim. Acta 2016, 187, 616–628. [Google Scholar] [CrossRef]
- Qin, H.; Hu, Y.; Zhu, C.; Chu, W.; Sheng, H.; Dong, Z.; He, Y.; Wang, J.; Li, A.; Chi, H.; et al. Functionalization of Polyvinyl Alcohol Composite Membrane by CoOOH for Direct Borohydride Fuel Cells. Electrochem. Commun. 2017, 77, 1–4. [Google Scholar] [CrossRef]
- Yang, J.M.; Fan, C.S.; Wang, N.C.; Chang, Y.H. Evaluation of Membrane Preparation Method on the Performance of Alkaline Polymer Electrolyte: Comparison between Poly(Vinyl Alcohol)/Chitosan Blended Membrane and Poly(Vinyl Alcohol)/Chitosan Electrospun Nanofiber Composite Membranes. Electrochim. Acta 2018, 266, 332–340. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Song, S.Y.; Yang, J.R.; Wang, J.L.; Wang, L. 4-Formyl Dibenzo-18-Crown-6 Grafted Polyvinyl Alcohol As Anion Exchange Membranes for Fuel Cell. Eur. Polym. J. 2019, 112, 581–590. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Hacker, V. Preparation and Characterization of PVA/PDDA/Nano-Zirconia Composite Anion Exchange Membranes for Fuel Cells. Polymers 2019, 11, 1399. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Zakaria, Z. Enhanced Alkaline Stability and Performance of Alkali-Doped Quaternized Poly(Vinyl Alcohol) Membranes for Passive Direct Ethanol Fuel Cell. Int. J. Energy Res. 2019, 43, 5252–5265. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, M.; He, X.; Qiao, J. Poly(Vinyl Alcohol)/Poly(Diallyldimethylammonium Chloride) Anion-Exchange Membrane Modified with Multiwalled Carbon Nanotubes for Alkaline Fuel Cells. J. Mater. 2019, 5, 286–295. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Performance of Quaternized Poly(Vinyl Alcohol)-Based Electrolyte Membrane in Passive Alkaline DEFCs Application: RSM Optimization Approach. J. Appl. Polym. Sci. 2019, 136, 1–18. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Wang, L. Preparation of Anion Exchange Membranes Based on Pyridine Functionalized Poly(Vinyl Alcohol) Crosslinked by 1,4-Dichlorobutane. J. Appl. Polym. Sci. 2019, 136, 1–9. [Google Scholar] [CrossRef]
- Duan, H.; Cheng, X.; Zeng, L.; Liao, Q.; Wang, J.; Wei, Z. Achieving High Conductivity at Low Ion Exchange Capacity for Anion Exchange Membranes with Electrospun Polyelectrolyte Nanofibers. ACS Appl. Energy Mater. 2020, 3, 10660–10668. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, M.; Xiao, Y.; Zhang, X.; Fan, M. Facile Fabrication of Poly(Vinyl Alcohol)/Polyquaternium-10 (PVA/PQ-10) Anion Exchange Membrane with Semi-Interpenetrating Network. Macromol. Mater. Eng. 2021, 306, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Wang, J.; Wang, L. Preparation and Characterization of a Sol-Gel Derived Silica/PVA-Py Hybrid Anion Exchange Membranes for Alkaline Fuel Cell Application. J. Electroanal. Chem. 2020, 873, 114342. [Google Scholar] [CrossRef]
- Chu, W.; He, Y.; Chu, Y.S.; Meng, L.; Liu, J.; Qin, H.; Tao, S. A Highly Stable Cu(OH)2-Poly(Vinyl Alcohol) Nanocomposite Membrane for Dramatically Enhanced Direct Borohydride Fuel Cell Performance. J. Power Sources 2020, 467, 228312. [Google Scholar] [CrossRef]
- Van Thuc, N.; Cam Ha, N.T.; Tho, N.H. Study on Synthesis and Characterization of Anion Exchange Membrane Based on Poly (Vinyl Alcohol) Modified by Free-Radical Polymerization. Int. J. Electrochem. Sci. 2020, 15, 8190–8199. [Google Scholar] [CrossRef]
- Arı, G.A.; Şimşek, Ö. Imidazolium Functionalized Poly(Vinyl Alcohol) Membranes for Direct Methanol Alkaline Fuel Cell Applications. Polym. Int. 2020, 69, 644–652. [Google Scholar] [CrossRef]
- Du, X.; Zhang, H.; Yuan, Y.; Wang, Z. Semi-Interpenetrating Network Anion Exchange Membranes Based on Quaternized Polyvinyl Alcohol/Poly(Diallyldimethylammonium Chloride). Green Energy Environ. 2020, 6, 743–750. [Google Scholar] [CrossRef]
- Yang, W.; Yan, J.; Liu, S.; Zhou, J.; Liu, J. Macromolecular Crosslink of Imidazole Functionalized Poly (Vinyl Alcohol) and Brominated Poly (Phenylene Oxide) for Anion Exchange Membrane with Enhanced Alkaline Stability and Ionic Conductivity. Int. J. Hydrogen Energy 2021, 46, 37007–37016. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Wolf, S.; Roschger, M.; Hacker, V. Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Polymer Electrolyte Fuel Cells. Int. J. Renew. Energy Dev. 2021, 10, 435–443. [Google Scholar] [CrossRef]
- Yang, W.; Liu, S.; Yan, J.; Zhong, F.; Jia, N.; Yan, Y.; Zhang, Q. Metallo-Polyelectrolyte-Based Robust Anion Exchange Membranes via Acetalation of a Commodity Polymer. Macromolecules 2021, 54, 9145–9154. [Google Scholar] [CrossRef]
- Yuan, C.; Li, P.; Zeng, L.; Duan, H.; Wang, J.; Wei, Z. Poly (Vinyl Alcohol) -Based Hydrogel Anion Exchange Membranes for Alkaline Fuel Cell. Macromolecules 2021, 54, 7900–7909. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Hacker, V. Effect of Crosslinking on the Properties of QPVA/PDDA Anion Exchange Membranes for Fuel Cells Application. J. Electrochem. Soc. 2021, 168, 044526. [Google Scholar] [CrossRef]
- Gaur, S.S.; Dhar, P.; Mehmood, K.; Srivastava, M.; Sakurai, S. Ion Transfer Channel Network Formed by Flower and Rod Shape Crystals of Hair Hydrolysate in Poly(Vinyl Alcohol) Matrix and Its Application as Anion Exchange Membrane in Fuel Cells. J. Colloid Interface Sci. 2021, 587, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Liao, Y.; Wang, J.; Wei, Z. Construction of Highly Efficient Ion Channel within Anion Exchange Membrane Based on Interpenetrating Polymer Network for H2/Air (CO2-Free) Alkaline Fuel Cell. J. Power Sources 2021, 486, 229377. [Google Scholar] [CrossRef]
- Merle, G.; Hosseiny, S.S.; Wessling, M.; Nijmeijer, K. New Cross-Linked PVA Based Polymer Electrolyte Membranes for Alkaline Fuel Cells. J. Memb. Sci. 2012, 409–410, 191–199. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Liu, L.; Zhang, J.; Xie, J.; Li, G. Synthesis and Properties of Chemically Cross-Linked Poly(Vinyl Alcohol)-Poly(Acrylamide-Co-Diallyldimethylammonium Chloride) (PVA-PAADDA) for Anion-Exchange Membranes. Solid State Ion. 2012, 214, 6–12. [Google Scholar] [CrossRef]
- Gouda, M.H.; Gouveia, W.; Afonso, M.L.; Šljukić, B.; El Essawy, N.A.; Nassr, A.B.A.A.; Santos, D.M.F. Poly(Vinyl Alcohol)-Based Crosslinked Ternary Polymer Blend Doped with Sulfonated Graphene Oxide as a Sustainable Composite Membrane for Direct Borohydride Fuel Cells. J. Power Sources 2019, 432, 92–101. [Google Scholar] [CrossRef]
- Anahidzade, N.; Dinari, M.; Abdolmaleki, A.; Tadavani, K.F.; Zhiani, M. Enhancement of Hydroxide Conduction by Incorporation of Metal-Organic Frameworks into a Semi-Interpenetrating Network. Energy Fuels 2019, 33, 5749–5760. [Google Scholar] [CrossRef]
- Caire, B.R. Mechanical Characterization of Anion Exchange Membranes under Controlled Environmental Conditions. J. Electrochem. Soc. 2015, 122, H677. [Google Scholar]
- Atifi, A.; Mounir, H.; El Marjani, A. Effect of Internal Current, Fuel Crossover, and Membrane Thickness on a PEMFC Performance. In Proceedings of the 2014 International Renewable and Sustainable Energy Conference (IRSEC), Ouarzazate, Morocco, 17–19 October 2014; pp. 907–912. [Google Scholar] [CrossRef]
- Pivovar, B.; Kim, Y.S. 2019 Anion Exchange Membrane Workshop Summary Report; National Renewable Energy Laboratory: Golden, CO, USA, 2020.
- Fumasep FAA-3-30. Available online: https://www.fuelcellstore.com/fumasep-faa-3-30?search=FAA-3-30 (accessed on 10 August 2022).
- Fumasep FAA-3-50. Available online: https://www.fuelcellstore.com/fumasep-faa-3-50?search=FAA-3-50 (accessed on 10 August 2022).
- Fumasep FAA-3-PK-75. Available online: https://www.fuelcellstore.com/fumasep-faa-3-pk-75?search=FAA-3-PK-75 (accessed on 10 August 2022).
- Sustainion® X37-50 Grade RT. Available online: https://www.fuelcellstore.com/sustainion-x37-50-grade-rt-membrane (accessed on 10 August 2022).
- Nafion® N-115. Available online: https://us.vwr.com/store/catalog/product.jsp?product_id=9880649 (accessed on 10 August 2022).
- Slade, S.; Campbell, S.A.; Ralph, T.R.; Walsh, F.C. Ionic Conductivity of an Extruded Nafion 1100 EW Series of Membranes. J. Electrochem. Soc. 2002, 149, A1556. [Google Scholar] [CrossRef]
- Nafion® N-117. Available online: https://us.vwr.com/store/catalog/product.jsp?product_id=9880650 (accessed on 10 August 2022).
- Aquivion® E98-05. Available online: https://www.fuelcellstore.com/solvay-aquivion-e98-05-72700003 (accessed on 10 August 2022).



| Fuel Types | |
|---|---|
| Hydrogen | |
| Anode: | 2H2 + 4OH− → 4H2O + 4e− |
| Cathode: | O2 + 2H2O + 4e− → 4OH− |
| Cell reaction: | H2 + O2 → 2H2O |
| Methanol | |
| Anode: | CH3OH + 6OH− → CO2 + 5H2O + 6e− |
| Cathode: | 3/2O2 + 3H2O + 6e− → 6OH− |
| Cell reaction: | CH3OH + 3/2O2 → CO2 + 5H2O |
| Ethanol | |
| Anode: | CH3CH2OH + 12OH− → 2CO2 + 9H2O + 12e− |
| Cathode: | 3O2 + 6H2O + 12e− → 12OH− |
| Cell reaction: | CH3CH2OH + 3O2 →2CO2 + 3H2O |
| Polymers | Synonyms | Structure | Characteristics | Challenges | Ref |
|---|---|---|---|---|---|
| Aliphatic backbones | |||||
| Poly(vinyl alcohol) (PVA) | 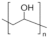 | Easy fabrication, biodegradability, hydrophilic, and good chemical stability. | Poor mechanical strength in wet state. | [59,60,61,62] | |
| Polytetrafluoroethylene (PTFE) | Teflon | 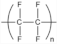 | Excellent chemical and thermal stability, low water uptake, and non-toxicity. | Limited synthetic route (impregnation of commercial PTFE film) | [63] |
| Poly(ethylene-co-tetrafluoroethylene) (ETFE) | 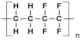 | Good mechanical strength, thermal, chemical stability, superior radiation resistance, and feasibility for graft polymerization. | Complex synthetic route, require high-energy electron beam for irradiation. | [64,65] | |
| Chitosan (CS) | 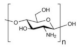 | Good film-forming characteristics, good mechanical strength, chemical resistance, low manufacturing cost, biocompatibility, biodegradability, and non-toxicity. | Low conductivity in pristine state | [66,67,68] | |
| Aromatic backbones | |||||
| Polysulfones (PSU/PSF) | Poly(arylene ether sulfone) | 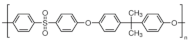 | Excellent mechanical and thermal stability, hydrolysis resistance, and wide temperature operating range. | Involves toxic chemicals in the synthesis | [60,69,70] |
| Poly(ether sulfone)(PES) | Poly(phenylene ether sulfone) | 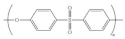 | Excellent solubility in an organic solvent, good thermal stability, good mechanical properties, and chemical resistance. | Using organic solvents which are mostly toxic and expensive | [71,72,73] |
| Poly(ether ether ketone) (PEEK) | 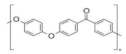 | Good mechanical properties, good chemical and thermo-oxidative stability, and low production cost. | Involves toxic chemicals in the synthesis | [74,75,76] | |
| Poly(2,6-dimethyl-1,4-phenyleneoxide) (PPO) | Poly(phenylene ether) (PPE) | 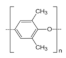 | Excellent mechanical properties, good dimensional stability, low moisture uptake, high thermal stability, low flammability, low dielectric constant, and low dielectric dissipation factor. | Involve carcinogen chloromethylation reagents, complex synthesis without nontoxic reagent | [60,77,78,79] |
| Cations | Structure | Cation Reagents |
|---|---|---|
| Quaternary Ammonium | 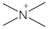 | Trialkylamine includes trimethylamine [20], triethylamine, and tripropylamine [21] |
| Imidazolium | 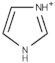 | 1-methylimidazole [22], 1,2-dimethylimidazole) [23] |
| Guanidinium | 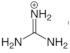 | 1,1,2,3,3-pentamethylguanidine (PMG) [24] Guanidinium hydrochloride [25] |
| Phosphonium | 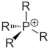 | Tris(2,4,6-tri-methoxyphenyl) phosphine (TTMPP) [26] |
| Pyridinium | 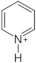 | 2,6- Bis(4-hydroxyphenyl)pyridine [27] 4-vinyl pyridine [10] |
| Sulfonium | 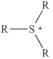 | Dimethyl sulfide sulfide [28] |
| Parameters | Description |
|---|---|
| Molecular weight | 30,000 to 200,000 g/mol [80] |
| Physical appearance | white to cream-colored, odorless, powder or granular [80] |
| Solubility | Soluble in water. Insoluble in oils, ketones, ester, aromatic and aliphatic hydrocarbons [80] |
| Density | 1.19–1.31 g/cm3 [80] |
| Melting point | 180–230 °C [83] |
| Thermal properties | Discoloration: ~150 °C [80] Darken: >150 °C [80] Decomposition: ~200 °C [80] |
| Viscosity | 2.5–110.0 mPa.s [83] |
| Glass transition temperature | 75–85 °C [80] |
| Structural formula | |
| Fully hydrolyzed | (-CH2CHOH-)-n- |
| Partially hydrolyzed | (-CH2CHOH-)-n-(-CH2CHOCOCH3-)-m- [80] |
| Empirical formula | |
| Fully hydrolyzed | (C2H4O)n |
| Partially hydrolyzed | (C2H4O)n(C4H6O2)m [80] |
| Degree of hydrolysis | |
| Fully hydrolyzed | 98.0–99.8% [80] |
| Partially hydrolyzed | 71.5–96.0% [80] |
| Additives | Objectives | Examples | Ref. |
|---|---|---|---|
| Cross-linker | Restraining membrane swelling and improving the membrane’s tensile strength and chemical stability |
| [98,99,100,101,102] |
| Inorganic filler | Enhance the thermal, mechanical, chemical, or additional electrochemical properties |
| [37,96,103,104,105] |
| Plasticizer | Improve mechanical properties by decreasing stiffness and thermal stability |
| [38] |
| Ionic liquids (ILs) | Increase conductivity of AEM |
| [106,107] |
| Properties | Unit | Description/Purposes | Method |
|---|---|---|---|
| Performances | |||
| Ion exchange capacity (IEC) | mmol g−1 or meq g−1 | Implies the milli-equivalents number of exchangeable ions in 1 g of the dry membrane | Back-titration |
| Ion conductivity | Measure the proton conductivity of PEMs or OH− conductivity of AEMs. | Electrochemical impedance spectroscopy (EIS) | |
| Water uptake (WU) | % or g/g | Investigate the changes in membrane mass when exposed to water | Gravimetric |
| Swelling degree (SD) | % or g/g | Investigate the dimensional change of the membranes when exposed to water | Length measurement |
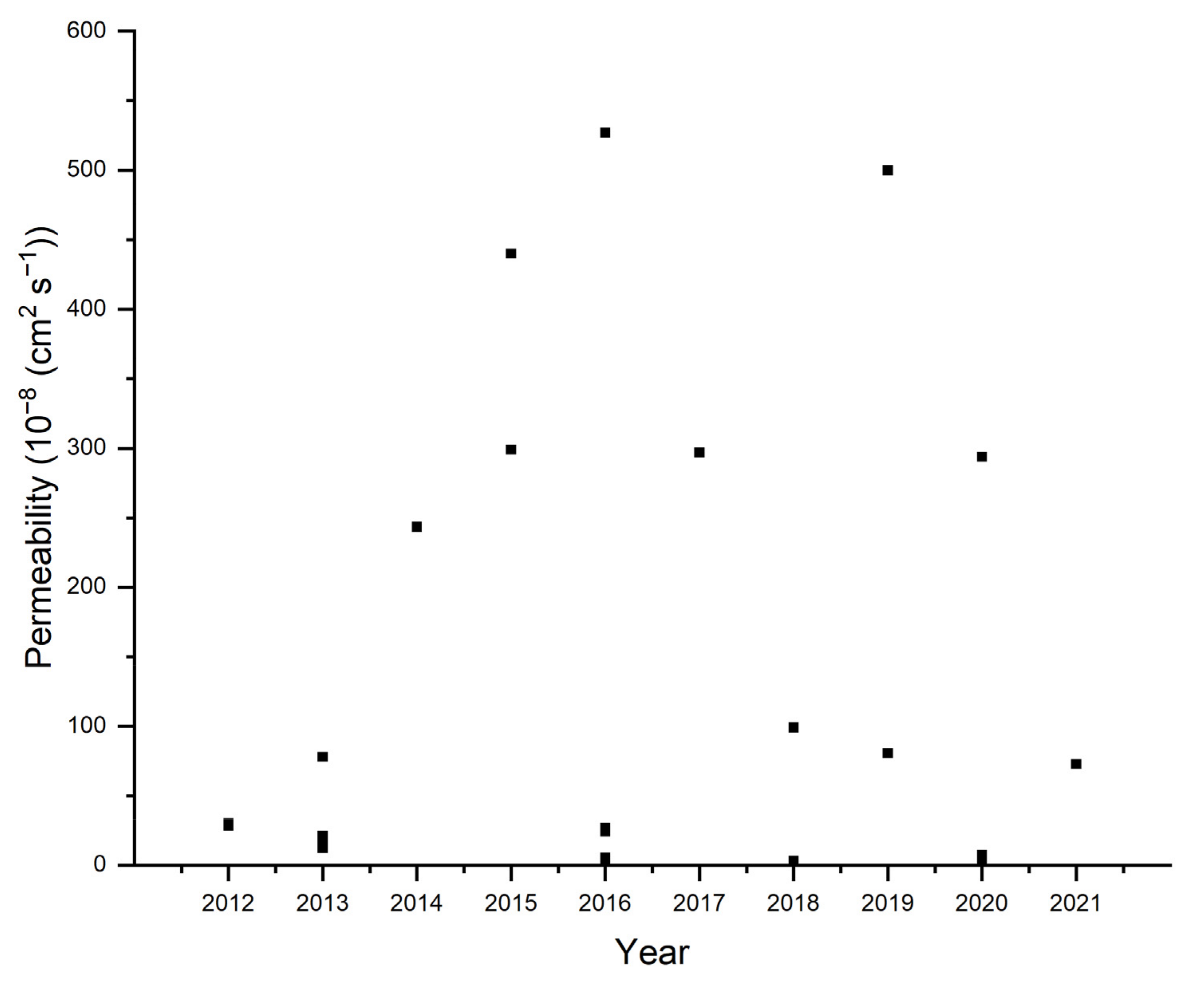
| Fuel permeability | cm2 s−1 | Investigate fuel crossover by diffusion due to the concentration gradient and by the electroosmotic drag as well. | Side-by-side cell |
| Thermal stability | % (weight) | Investigate the change in the weight of membrane temperature over a certain period. | Thermogravimetric analysis (TGA) |
| Chemical stability | % (conductivity) | Investigate the AEMs performance changes (ionic conductivity and IEC) over time when exposed to high pH environments at a specific temperature | Identical with IEC and ionic conductivity measurement |
| Oxidative stability | % (weight) | Investigate the oxidative stability of the membrane. | Gravimetric |
| Physical Structure | |||
| Crystallographic structures | % (crystallinity) | Investigate the crystallographic structure of inorganic materials in the membrane. | X-ray diffraction analysis (XRD) |
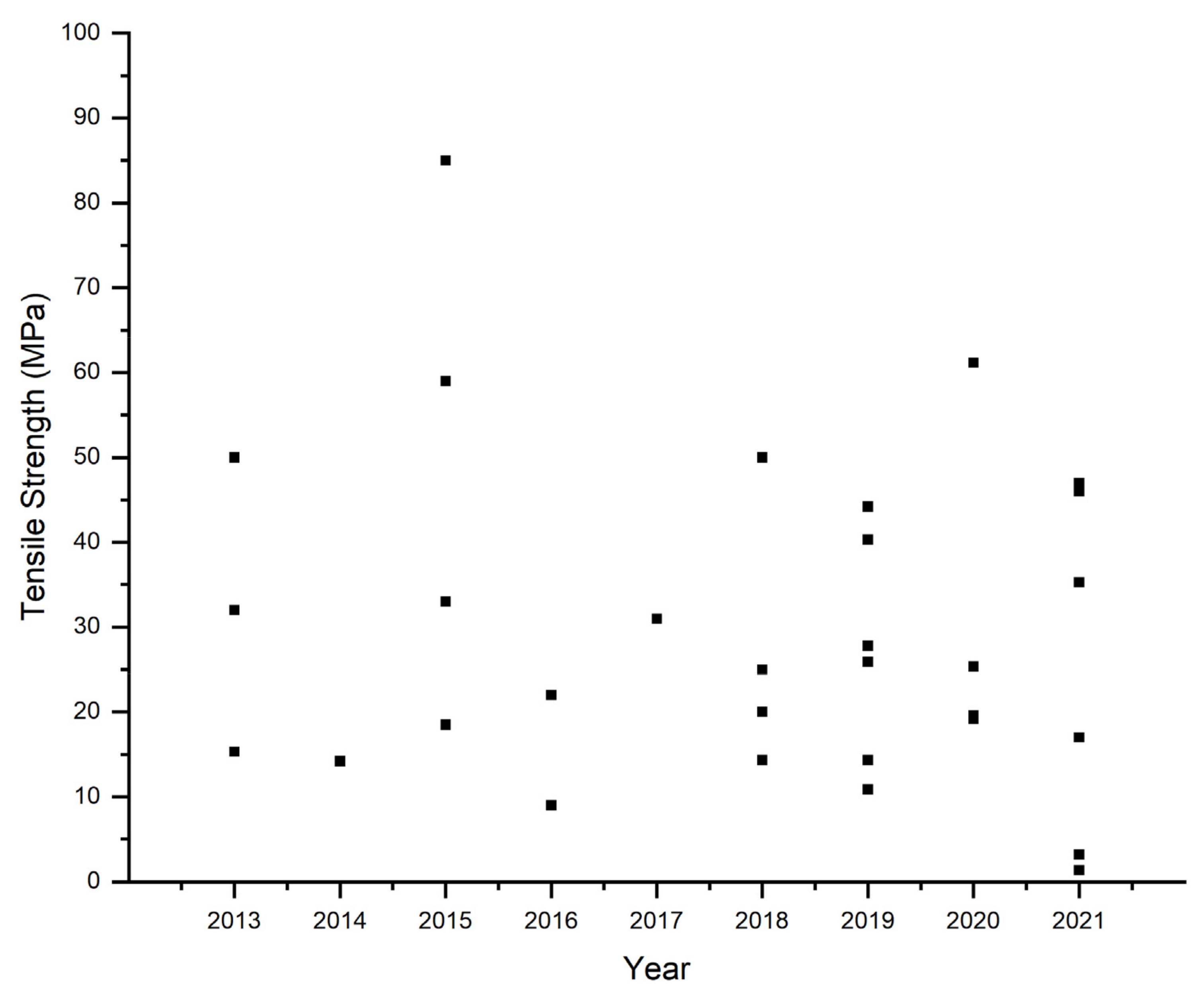
| Tensile strength | MPa | Investigate tensile strength of membranes. | Universal Testing machine |
| Elongation at break | % (length) | Investigate elongation at break of membranes. | Universal Testing machine |
| Morphology | - | Investigate the surface and cross-section morphology of membranes | Scanning electron microscopy (SEM) |
| Chemical structure | |||
| Polymer structure and chemical composition | - | Investigate changes in chemical structure due to chemical modification | FTIR spectroscopy 1H-NMR spectroscopy |
| Polymers | Cation | Additives * | Preparation | Ion Conductivity | Temp. | Water Uptake | Swelling Degree | Tensile Strength | Alcohol Permeability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| (mS cm−1) | (°C) | (wt%) | (%) | (MPa) | (cm2 s−1) | |||||
| QPVA | Ammonium | Glutaraldehyde (CL), QSiO2 (F) | Solution Casting | 2.4 | 25 | N/A | N/A | N/A | N/A | [95] |
| PVA/PAADDA | Ammonium | Glutaraldehyde (CL) | Solution Casting | 3.0 | 80 | 75 | N/A | N/A | 2.85 × 10−7 | [115] |
| PVA/PDDA | Ammonium | Glutaraldehyde (CL) | Solution Casting | 25.0 | 25 | 96 | N/A | N/A | N/A | [116] |
| PVA/PDDA | Ammonium | Glutaraldehyde (CL) | Solution Casting | 27.0 | 25 | 130 | 76 | 15.3 | N/A | [117] |
| PVA/Cu(II) complex | N/A | Glutaraldehyde (CL) | Chemical Fiber | 99.0 | 77 | 90 | 32 | N/A | 2.12 × 10−7 | [118] |
| PVA/PDDA | Ammonium | Glutaraldehyde (CL) | Solution Casting | 37.0 | 80 | 96 | N/A | N/A | N/A | [91] |
| PVA/PAADDA | Ammonium | Glutaraldehyde (CL), PEG (P) | Solution Casting | 9.0 | 80 | 113 | N/A | N/A | N/A | [112] |
| PVA/QPEI | N/A | Glutaraldehyde (CL) | Solution Casting | 45.0 | 80 | 82 | N/A | N/A | 7.80 × 10−7 | [119] |
| PVA | N/A | Glutaraldehyde (CL), Graphene (F) | Solution Casting | 21.3 | 80 | N/A | N/A | 50.0 | 1.91 × 10−7 | [97] |
| PVA/QHECE | Ammonium | Glutaraldehyde (CL) | Solution Casting | 7.5 | 90 | 82 | 8 | 32.0 | 1.26 × 10−7 | [120] |
| PVA/QASP/ TAMPFS-PET | Ammonium | Glutaraldehyde (CL), SiO2 (F) | Solution Casting | 65.9 | 70 | N/A | N/A | N/A | N/A | [121] |
| PVA/SA | N/A | Glutaraldehyde (CL) | Solution Casting | 91.0 | 25 | 314 | 330 | N/A | 2.43 × 10−6 | [122] |
| PVAc/PVBC | Ammonium, Imidazolium | PVAc macromolecul (CL) | Solution Casting | 54.0 | 80 | 139 | 26 | 14.2 | N/A | [123] |
| PVA/PMVIC-co-VP | Imidazolium | Glutaraldehyde (CL) | Solution Casting | 17.0 | 25 | 31 | N/A | 59.0 | N/A | [124] |
| PVA | N/A | CHDMG (CL) | Solution Casting | 4.7 | 25 | 50 | N/A | 18.5 | N/A | [125] |
| PVA/QPEI | Ammonium | Glutaraldehyde (CL), f-GO (F) | Solution Casting | 72.0 | 30 | 61 | 33 | 85.0 | 4 × 10−7 | [126] |
| PVA | N/A | CNC (F) | Solution Casting | 65.0 | 60 | 80 | 5 | 33.0 | N/A | [127] |
| QPVA/QCS | Ammonium | Glutaraldehyde (CL) | Solution Casting | 21.0 | 60 | N/A | 42 | N/A | 2.99 × 10−6 | [128] |
| PVA/PDDA | Ammonium | Glutaraldehyde (CL), f-GO (F) | Solution Casting | 21.0 | 80 | N/A | N/A | N/A | N/A | [35] |
| CS/PVA | N/A | Glutaraldehyde (CL) | Solution Casting | 0.2 | 25 | 138 | N/A | N/A | 2.43 × 10−7 | [129] |
| QPVA/QCS | Ammonium | Glutaraldehyde, EGDGE (CL) | Solution Casting | 16.0 | 25 | 98 | N/A | N/A | 3.17 × 10−8 | [36] |
| PVA/BPPO | Ammonium | Glutaraldehyde (CL), MoF (F) | Solution Casting | 145.0 | 80 | 27 | N/A | 22.0 | 2.68 × 10−7 | [94] |
| QPSF/PVA | Ammonium | TMEDA (CL) | Solution Casting | 182.0 | 60 | 12 | 27 | 14.0 | N/A | [130] |
| PVA/PDDA | Ammonium | Glutaraldehyde, PEDGE (CL) | Solution Casting | 2.3 | 25 | N/A | N/A | N/A | N/A | [33] |
| QPVA | Ammonium | N/A | Electrospinning | 42.0 | 60 | 23 | N/A | 9.0 | 5.27 × 10−6 | [131] |
| QPVA | Ammonium | Glutaraldehyde (CL), nano-Chitosan (F) | Solution Casting | 40.0 | 70 | 19 | 3 | N/A | 5.41 × 10−8 | [132] |
| PVA/CoOOH | Ammonium | N/A | 32.0 | 30 | N/A | N/A | N/A | 2.97 × 10−6 | [133] | |
| PVA/PUB | Ammonium | Glutaraldehyde (CL) | Solution Casting | 9.0 | 80 | 50 | N/A | 31.0 | N/A | [34] |
| PVA/PBI | Imidazolium | N/A | Solution Casting | 103.0 | 90 | 85 | N/A | 50.0 | N/A | [93] |
| PVA/CS | N/A | Glutaraldehyde (CL) | Electrospinning | 19.0 | 25 | 160 | N/A | N/A | 9.92 × 10−7 | [134] |
| QPVA/CS | Ammonium | Glutaraldehyde (CL), MoS2 (F) | Solution Casting | 32.0 | 25 | 137 | 33 | 25.0 | 3 × 10−8 | [62] |
| PVA/BPEI | N/A | Glutaraldehyde (CL) | Solution Casting | 86.0 | 80 | 100 | 90 | 20.0 | N/A | [92] |
| QPVA | Ammonium | Glutaraldehyde (CL) | Solution Casting | 11.0 | 80 | N/A | N/A | N/A | N/A | [61] |
| PVA/FDB18C6 | Ammonium | N/A | Solution Casting | 25.0 | 70 | 25 | 14 | 14.3 | N/A | [135] |
| PVA/PDDA | Ammonium | Glutaraldehyde (CL), ZrO2 (F) | Solution Casting | 31.6 | 25 | 89 | 42 | 10.9 | N/A | [136] |
| QPVA/KOH | Ammonium | Glutaraldehyde (CL) | Solution Casting | 30.7 | 70 | 76 | 55 | 25.9 | 8.06 × 10−7 | [137] |
| PVA/PDDA | Ammonium | Glutaraldehyde (CL), MWCNTs (F) | Solution Casting | 45.0 | 80 | 98 | N/A | 40.3 | N/A | [138] |
| QPVA/KOH | Ammonium | Glutaraldehyde (CL) | Solution Casting | 18.2 | 25 | N/A | N/A | N/A | N/A | [139] |
| PVA-FP/[DimL][OH] | Imidazolium | Glutaraldehyde (CL) | Solution Casting | 58.0 | 70 | 107 | 55 | 27.8 | 5 × 10−6 | [106] |
| PVA-PY-DLx | Pyridinium | 1,4-dichlorobutane (CL) | Solution Casting | 10.5 | 70 | 110 | 130 | 44.2 | N/A | [140] |
| QPPONF/PVA | Ammonium | 4-chlorobenzaldehyde (CL) | Solution Casting | 51.5 | 60 | 45 | 11 | 19.2 | N/A | [141] |
| PVA/PQ-10 | Ammonium | Glutaraldehyde (CL) | Solution Casting | 79.4 | 80 | 59 | 8 | 61.2 | N/A | [142] |
| Silica/PVA-Py | Ammonium | Glutaraldehyde (CL) | Sol-gel | 96.3 | 80 | 69 | 35 | 25.4 | 7.57 × 10−8 | [143] |
| Cu(OH)2-PVA-AER | Ammonium | n-Cu(OH)2 (F) | Solution Casting | 28.0 | 25 | N/A | N/A | N/A | 2.94 × 10−6 | [144] |
| PVA-PVA (modified) | N/A | N/A | Coating | 6.9 | 25 | 65 | N/A | N/A | N/A | [145] |
| PVA-Im/PC | Imidazolium | Glutaraldehyde (CL) | Coating | 7.8 | 20 | 72 | 0 | N/A | 1.10 × 10−8 | [146] |
| QPVA/MGMC | Imidazolium | N/A | Solution Casting | 15.3 | 25 | N/A | N/A | N/A | N/A | [44] |
| QPVA/PDDA | Ammonium | Glutaraldehyde (CL) | Solution Casting | 36.5 | 60 | 128 | 115 | 19.6 | N/A | [147] |
| BPPO-PVAIm | Imidazolium | N/A | Solution Casting | 78.8 | 80 | 42 | 10 | 47.0 | N/A | [148] |
| QPVA/PDDA | Ammonium | Glutaraldehyde (CL) | Solution Casting | 54.5 | 25 | 55 | 51 | N/A | N/A | [149] |
| PVA-CoCp | Cobaltocenium | Glutaraldehyde (CL) | Solution Casting | 72.0 | 80 | 20 | 5 | 17.0 | N/A | [150] |
| PVA-TFBA-IM-MC | Imidazolium | TFBA (CL) | Solution Casting | 150.0 | 80 | 726 | 54 | 1.4 | N/A | [151] |
| QPVA/PDDA | Ammonium | Glutaraldehyde (CL) | Solution Casting | 82.9 | 80 | 91 | 30 | 46.0 | N/A | [152] |
| PVA-HH | Ammonium | Glutaraldehyde (CL) | Solution Casting | 6.2 | 70 | 144 | 30 | 35.3 | 7.29 × 10−7 | [153] |
| PVA-PQVBC | Ammonium | Divinylbenzene, Glutaraldehyde (CL) | Solution Casting | 141.9 | 80 | 124 | 22 | 3.2 | N/A | [154] |
| Brand | Company | Product | Thickness (μm) | IEC (mmol g−1) | σ (mS cm−1) | TS (mPa) | Price (USD/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Anion Exchange Membranes | ||||||||
| Fumasep® | Fumatech, Germany | FAA-3-30 | 26–34 | 1.67–2.04 | >5 (Cl−) | 25–40 | 950 | [162] |
| FAA-3-50 | 45–55 | 1.60–2.10 (Cl−) | 3–8 (Cl−) | 25–40 | 1050 | [163] | ||
| FAA-3-PK-75 | 70–80 | 1.20–1.40 (Cl−) | 4.5–6.5 (Cl−) | 30–60 | 1600 | [164] | ||
| Sustainion® | Dioxide Materials, USA | X37-50 | 50 | N/A | 80 | N/A | 4583 | [165] |
| Cation Exchange Membranes | ||||||||
| Nafion® | The Chemours Company, USA | Nafion® 115 | 125 | >0.9 | 74 | 32–43 | 3434 | [166,167] |
| Nafion® 117 | 180 | >0.9 | 70 | 32–43 | 3770 | [167,168] | ||
| Aquivion® | Solvay, Belgium | E98-05 | 50 | >1 | >160 | 30–40 | 1667 | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsudin, A.M.; Bodner, M.; Hacker, V. A Brief Review of Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Fuel Cells. Polymers 2022, 14, 3565. https://doi.org/10.3390/polym14173565
Samsudin AM, Bodner M, Hacker V. A Brief Review of Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Fuel Cells. Polymers. 2022; 14(17):3565. https://doi.org/10.3390/polym14173565
Chicago/Turabian StyleSamsudin, Asep Muhamad, Merit Bodner, and Viktor Hacker. 2022. "A Brief Review of Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Fuel Cells" Polymers 14, no. 17: 3565. https://doi.org/10.3390/polym14173565
APA StyleSamsudin, A. M., Bodner, M., & Hacker, V. (2022). A Brief Review of Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Fuel Cells. Polymers, 14(17), 3565. https://doi.org/10.3390/polym14173565








