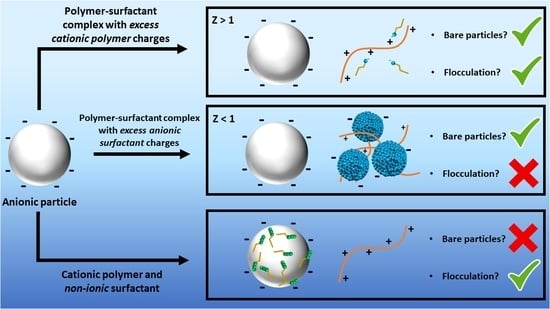
Using Polymer–Surfactant Charge Ratio to Control Synergistic Flocculation of Anionic Particulate Dispersions
Abstract
:1. Introduction
2. Materials and Methods
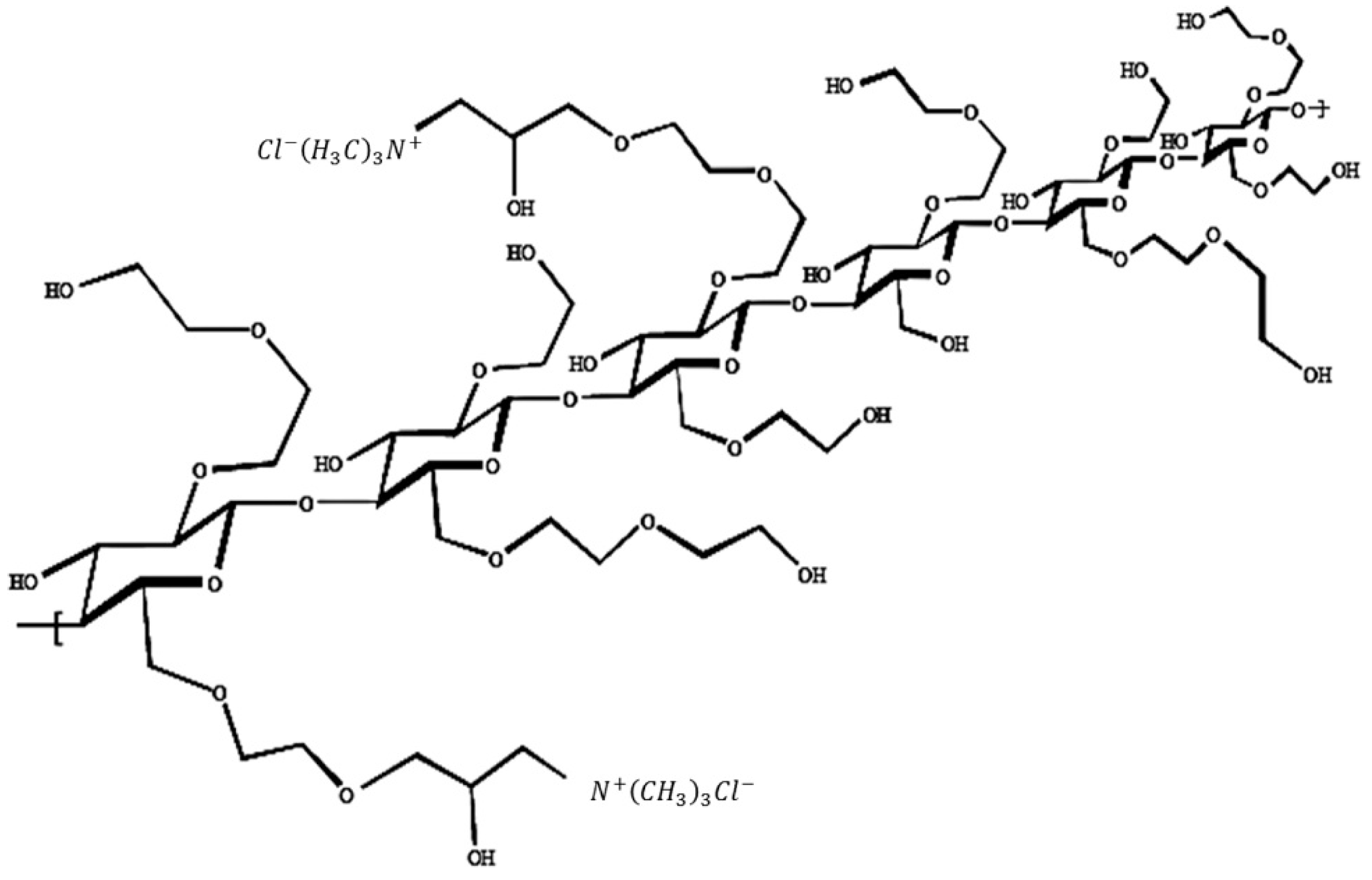
2.1. Materials
2.2. Methods-Solvent Relaxation NMR
3. Results and Discussion
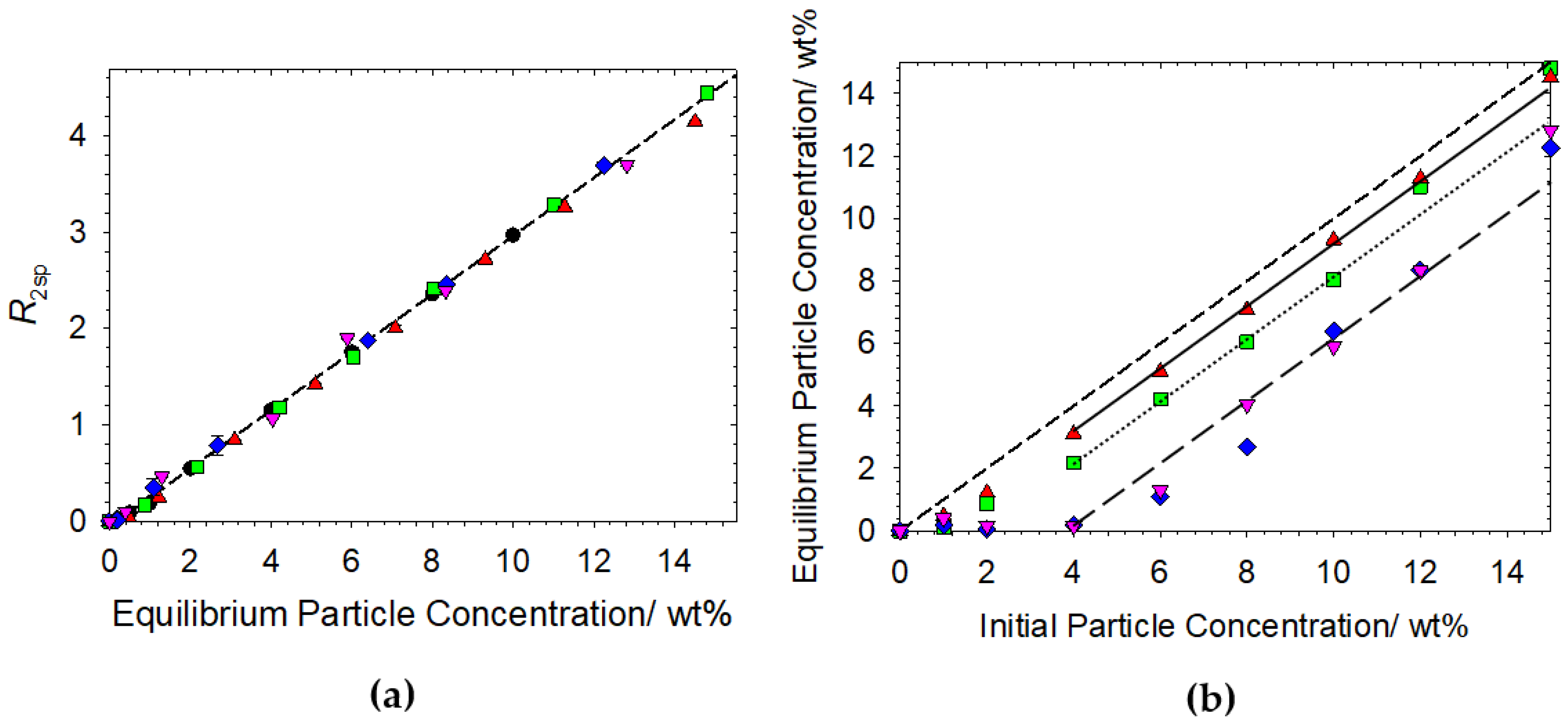
3.1. Particle Only Systems
3.2. Competitive Interactions within Particulate Based Dispersions–Oppositely Charged Polymer–Surfactant Complexes and Silica
3.3. Competitive Interactions within Particulate Based Dispersions–Oppositely Charged Polymer–Surfactant Complexes and Latex
3.4. Competitive Interactions–Non-Ionic Surfactant Effects
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goddard, E.D. Polymer/Surfactant Interaction—Its Relevance to Detergent Systems. J. Am. Oil Chem. Soc. 1994, 71, 1–16. [Google Scholar] [CrossRef]
- Chiappisi, L.; Hoffmann, I.; Gradzielski, M. Complexes of Oppositely Charged Polyelectrolytes and Surfactants—Recent Developments in the Field of Biologically Derived Polyelectrolytes. Soft Matter 2013, 9, 3896–3909. [Google Scholar] [CrossRef]
- Ristroph, K.D.; Prud’homme, R.K. Hydrophobic Ion Pairing: Encapsulating Small Molecules, Peptides, and Proteins into Nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.T.; Bronich, T.K.; Bromberg, L.; Hatton, T.A.; Kabanov, A.V. Block Ionomer Complexes as Prospective Nanocontainers for Drug Delivery. J. Control. Release 2006, 115, 9–17. [Google Scholar] [CrossRef]
- Wang, C.; Tam, K.C. Interaction between Polyelectrolyte and Oppositely Charged Surfactant: Effect of Charge Density. J. Phys. Chem. B 2004, 108, 8976–8982. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. PH-Responsive Polymers. Polym. Chem. 2016, 8, 144–176. [Google Scholar] [CrossRef]
- Benoît, M.; Ilias, I.; Raoul, Z.; Audebert, R. Mixed Micelles Formed by Cationic Surfactants and Anionic Hydrophobically Modified Polyelectrolytes. Langmuir 1996, 12, 2616. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Inaba, Y.; Kunieda, H.; Uchiyama, H. Anomalous Phase Behavior of Water-Soluble Polyelectrolyte and Oppositely Charged Surfactants. Colloid Polym. Sci. 1999, 277, 1117–1124. [Google Scholar] [CrossRef]
- Nylander, T.; Samoshina, Y.; Lindman, B. Formation of Polyelectrolyte–Surfactant Complexes on Surfaces. Adv. Colloid Interface Sci. 2006, 123–126, 105–123. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Abelenda-Nuñez, I.; Hernández-Rivas, M.; Ortega, F.; Rubio, R.G.; Guzmán, E. Impact of the Bulk Aggregation on the Adsorption of Oppositely Charged Polyelectrolyte-Surfactant Mixtures onto Solid Surfaces. Adv. Colloid Interface Sci. 2020, 282, 102203. [Google Scholar] [CrossRef]
- Hoffmann, I.; Theile, M.; Grätz, S.; Scholz, J.; Barreleiro, P.; Von Rybinski, W.; Gradzielski, M. On the Influence of Surfactants on the Adsorption of Polysaccharide-Based Polymers on Cotton Studied by Means of Fluorescence Spectroscopy. Langmuir 2012, 28, 11400–11409. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, Y.; Miyake, M. Creation of New Functions by Combination of Surfactant and Polymer—Complex Coacervation with Oppositely Charged Polymer and Surfactant for Shampoo and Body Wash. J. Oleo Sci. 2019, 68, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kimura, K.; Dubin, P.L.; Jaeger, W. Polyelectrolyte−Micelle Coacervation: Effects of Micelle Surface Charge Density, Polymer Molecular Weight, and Polymer/Surfactant Ratio. Macromolecules 2000, 33, 3324–3331. [Google Scholar] [CrossRef]
- Cooper, C.L.; Cosgrove, T.; Van Duijneveldt, J.S.; Murray, M.; Prescott, S.W. The Use of Solvent Relaxation NMR to Study Colloidal Suspensions. Soft Matter 2013, 9, 7211–7228. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Baghdadli, N.; Cazeneuve, C.; Luengo, G.S.; Rubio, R.G. Adsorption of Conditioning Polymers on Solid Substrates with Different Charge Density. ACS Appl. Mater. Interfaces 2011, 3, 3181–3188. [Google Scholar] [CrossRef]
- Cooper, C.L.; Cosgrove, T.; Van Duijneveldt, J.S.; Murray, M.; Prescott, S.W. Competition between Polymers for Adsorption on Silica: A Solvent Relaxation NMR and Small-Angle Neutron Scattering Study. Langmuir 2013, 29, 12670–12678. [Google Scholar] [CrossRef]
- Netz, R.R.; Andelman, D. Neutral and Charged Polymers at Interfaces. Phys. Rep. 2003, 380, 1–95. [Google Scholar] [CrossRef]
- Spruijt, E.; Biesheuvel, P.M.; De Vos, W.M. Adsorption of Charged and Neutral Polymer Chains on Silica Surfaces: The Role of Electrostatics, Volume Exclusion, and Hydrogen Bonding. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2015, 91, 012601. [Google Scholar] [CrossRef]
- Mears, S.J.; Cosgrove, T.; Thompson, L.; Howell, I. Solvent Relaxation NMR Measurements on Polymer, Particle, Surfactant Systems. Langmuir 1998, 14, 997–1001. [Google Scholar] [CrossRef]
- Cattoz, B.; Cosgrove, T.; Crossman, M.; Prescott, S.W. Surfactant-Mediated Desorption of Polymer from the Nanoparticle Interface. Langmuir 2012, 28, 2485–2492. [Google Scholar] [CrossRef]
- Terada, E.; Samoshina, Y.; Nylander, T.; Lindman, B. Adsorption of Cationic Cellulose Derivative/Anionic Surfactant Complexes onto Solid Surfaces. II. Hydrophobized Silica Surfaces. Langmuir 2004, 20, 6692–6701. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Crossman, M.; Griffths, P.C. Surfactant-Modulation of the Cationic-Polymer-Induced Aggregation of Anionic Particulate Dispersions. Polymers 2020, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Crossman, M.; Griffiths, P.C. Probing Selective Adsorption in Cationic-Polymer Induced Aggregation of Binary Anionic Particulate Dispersions Using Solvent Relaxation NMR. Polymers 2022, 14, 1875. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Abdullahi, W.; Dalgliesh, R.; Crossman, M.; Griffiths, P.C. Charge Modification as a Mechanism for Tunable Properties in Polymer–Surfactant Complexes. Polymers 2021, 13, 2800. [Google Scholar] [CrossRef]
- Patel, L.; Mansour, O.; Crossman, M.; Griffiths, P. Electrophoretic NMR Characterization of Charged Side Chain Cationic Polyelectrolytes and Their Interaction with the Anionic Surfactant, Sodium Dodecyl Sulfate. Langmuir 2019, 35, 9233–9238. [Google Scholar] [CrossRef]
- Roos, P.; Westling, Å.; Chronakis, I.S. Hydrophilic Monolayer Formation of Adsorbed Cationic Starch and Cationic Hydroxyethyl Cellulose Derivatives on Polyester Surfaces. Biosci. Biotechnol. Biochem. 2004, 68, 2247–2256. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Cooper, C.L.; Cosgrove, T.; Van Duijneveldt, J.S.; Murray, M.; Prescott, S.W. Colloidal Particles in Competition for Stabilizer: A Solvent Relaxation NMR Study of Polymer Adsorption and Desorption. Langmuir 2012, 28, 16588–16595. [Google Scholar] [CrossRef]
- Nelson, A.; Jack, K.S.; Cosgrove, T.; Kozak, D. NMR Solvent Relaxation in Studies of Multicomponent Polymer Adsorption. Langmuir 2002, 18, 2750–2755. [Google Scholar] [CrossRef]
- Fairhurst, D.; Sharma, R.; Takeda, S.-I.; Cosgrove, T.; Prescott, S.W. Fast NMR Relaxation, Powder Wettability and Hansen Solubility Parameter Analyses Applied to Particle Dispersibility. Powder Technol. 2021, 377, 545–552. [Google Scholar] [CrossRef]
- Beshah, K.; Izmitli, A.; Van Dyk, A.K.; Rabasco, J.J.; Bohling, J.; Fitzwater, S.J. Diffusion-Weighted PFGNMR Study of Molecular Level Interactions of Loops and Direct Bridges of HEURs on Latex Particles. Macromolecules 2013, 46, 2216–2227. [Google Scholar] [CrossRef]
- Quadrat, O.; Horský, J.; Šňupárek, J. Thickening Effect of Commercial Associative Thickeners on the Latices of Copolymers of Acrylic Monomers Carrying Hydrophilic Reactive Groups. J. Dispers. Sci. Technol. 2007, 24, 179–184. [Google Scholar] [CrossRef]
- Naderi, A.; Claesson, P.M.; Bergström, M.; Dedinaite, A. Trapped Non-Equilibrium States in Aqueous Solutions of Oppositely Charged Polyelectrolytes and Surfactants: Effects of Mixing Protocol and Salt Concentration. Colloids Surf. A Physicochem. Eng. Asp. 2005, 253, 83–93. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; Ortega, F.; Rubio, R.G. Equilibrium and Kinetically Trapped Aggregates in Polyelectrolyte–Oppositely Charged Surfactant Mixtures. Curr. Opin. Colloid Interface Sci. 2020, 48, 91–108. [Google Scholar] [CrossRef]
- van der Beek, G.P.; Cohen Stuart, M.A.; Cosgrove, T. Polymer Adsorption and Desorption Studies via Proton NMR Relaxation of the Solvent. Langmuir 2002, 7, 327–334. [Google Scholar] [CrossRef]
- Kumar, S.; Aswal, V.K.; Kohlbrecher, J. Size-Dependent Interaction of Silica Nanoparticles with Different Surfactants in Aqueous Solution. Langmuir 2012, 28, 9288–9297. [Google Scholar] [CrossRef]
- Mohr, A.; Nylander, T.; Piculell, L.; Lindman, B.; Boyko, V.; Bartels, F.W.; Liu, Y.; Kurkal-Siebert, V. Mixtures of Cationic Copolymers and Oppositely Charged Surfactants: Effect of Polymer Charge Density and Ionic Strength on the Adsorption Behavior at the Silica-Aqueous Interface. ACS Appl. Mater. Interfaces 2012, 4, 1500–1511. [Google Scholar] [CrossRef]
- Brown, W.; Zhao, J. Adsorption of Sodium Dodecyl Sulfate on Polystyrene Latex Particles Using Dynamic Light Scattering and Zeta Potential Measurements. Macromolecules 1993, 26, 2711–2715. [Google Scholar] [CrossRef]
- Beattie, J.K.; Hunter, R.J.; Zhang, H. Electroacoustic Detection of the Onset of Depletion Flocculation of Latex Dispersions by Hydroxyethyl Cellulose. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 83–86. [Google Scholar] [CrossRef]
- Ray, D.; Aswal, V.K. Observation of Adsorption versus Depletion Interaction for Charged Silica Nanoparticles in the Presence of Non-Ionic Surfactant. J. Phys. Condens. Matter 2013, 26, 035102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Brown, W. Dynamic Light Scattering Study of Nonionic Surfactant (C12E25) Adsorption on Polystyrene Latex Particles: Effect of Poly(Ethylene Oxide) Chain Size. J. Phys. Chem. 1996, 100, 5908–5912. [Google Scholar] [CrossRef]
- Colombié, D.; Landfester, K.; Sudol, E.D.; El-Aasser, M.S. Competitive Adsorption of the Anionic Surfactant SLS and the Nonionic Surfactant Triton X-405 on Polystyrene Latex Particles. Langmuir 2000, 16, 7905–7913. [Google Scholar] [CrossRef]
- Rossi, S.; Luckham, P.F.; Green, N.; Cosgrove, T. NMR Solvent Relaxation Studies of Na+-Montmorillonite Clay Suspensions Containing Non-Ionic Polymers. Colloids Surf. A Physicochem. Eng. Asp. 2003, 215, 11–24. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, C.; Abdullahi, W.; Crossman, M.; Griffiths, P.C. Using Polymer–Surfactant Charge Ratio to Control Synergistic Flocculation of Anionic Particulate Dispersions. Polymers 2022, 14, 3504. https://doi.org/10.3390/polym14173504
Hill C, Abdullahi W, Crossman M, Griffiths PC. Using Polymer–Surfactant Charge Ratio to Control Synergistic Flocculation of Anionic Particulate Dispersions. Polymers. 2022; 14(17):3504. https://doi.org/10.3390/polym14173504
Chicago/Turabian StyleHill, Christopher, Wasiu Abdullahi, Martin Crossman, and Peter Charles Griffiths. 2022. "Using Polymer–Surfactant Charge Ratio to Control Synergistic Flocculation of Anionic Particulate Dispersions" Polymers 14, no. 17: 3504. https://doi.org/10.3390/polym14173504
APA StyleHill, C., Abdullahi, W., Crossman, M., & Griffiths, P. C. (2022). Using Polymer–Surfactant Charge Ratio to Control Synergistic Flocculation of Anionic Particulate Dispersions. Polymers, 14(17), 3504. https://doi.org/10.3390/polym14173504