Advancements in MXene-Polymer Nanocomposites in Energy Storage and Biomedical Applications
Abstract
:1. Introduction
1.1. Biomedical
1.2. Energy Storage
1.3. Sensors
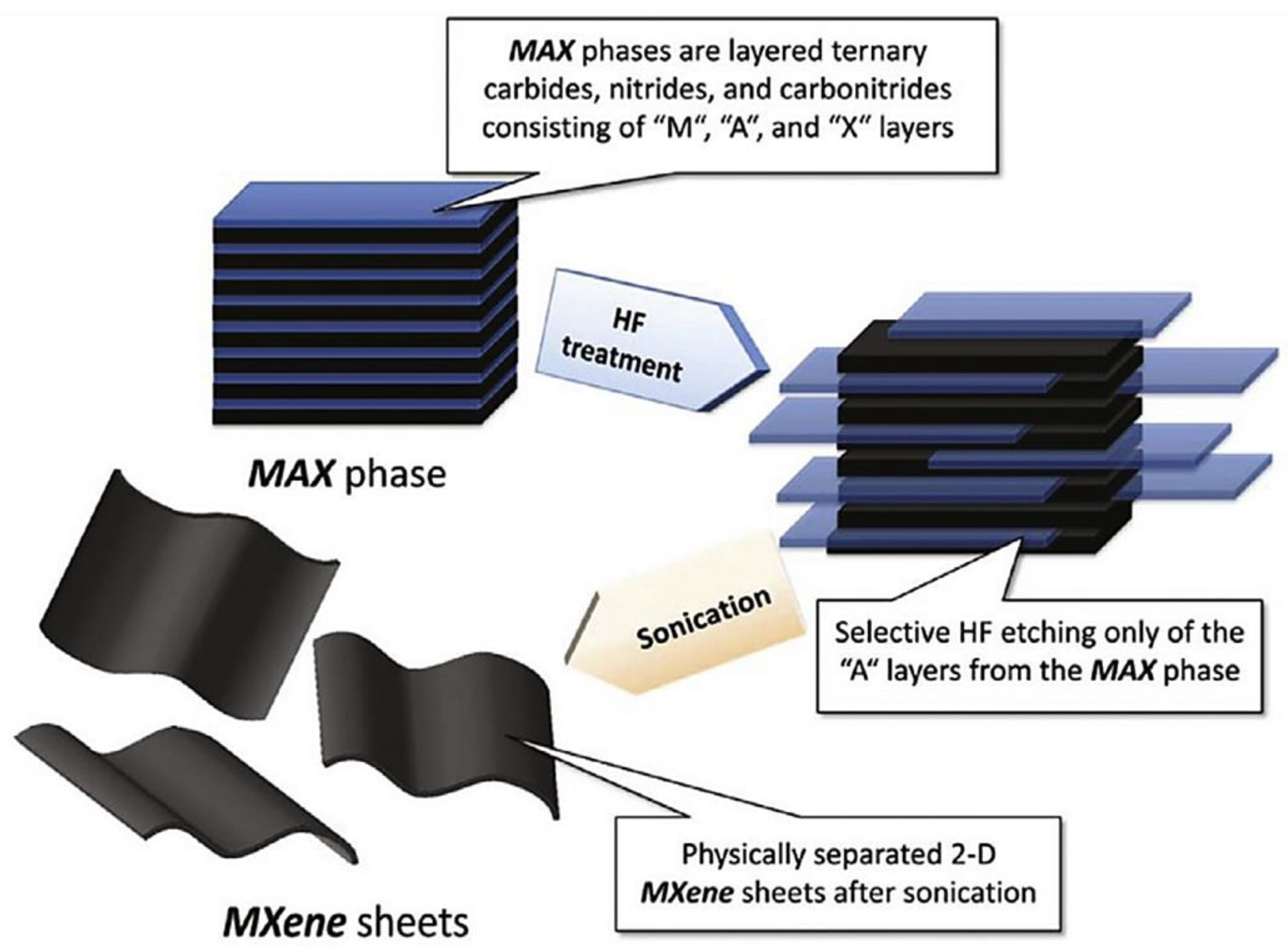
2. Synthesis and Structure of MXene
3. MXene Nanocomposites
3.1. MXene/Metals/Ceramics Composites
3.2. MXene/Carbon Nanocomposites
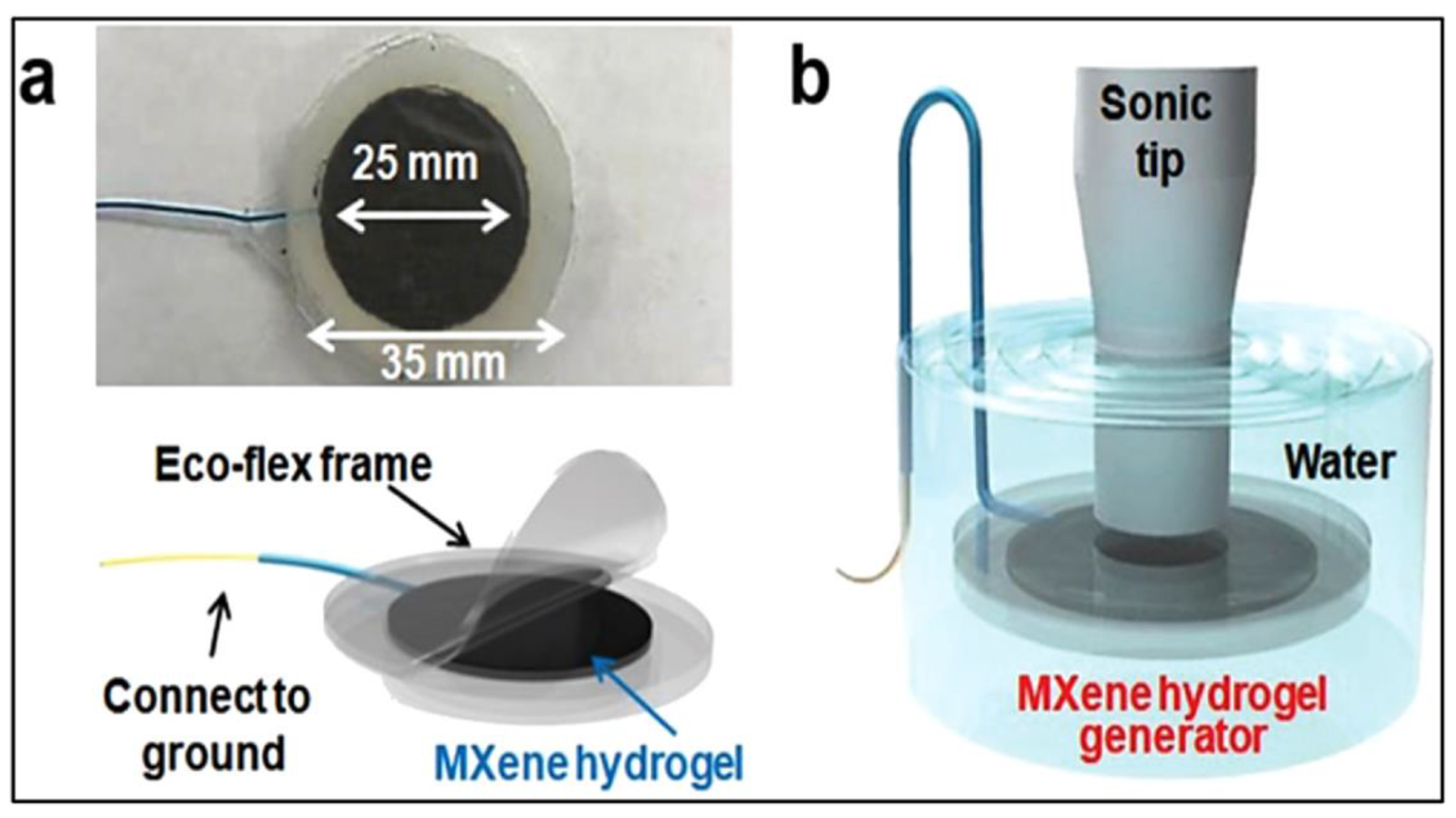
3.3. MXene Hydrogels
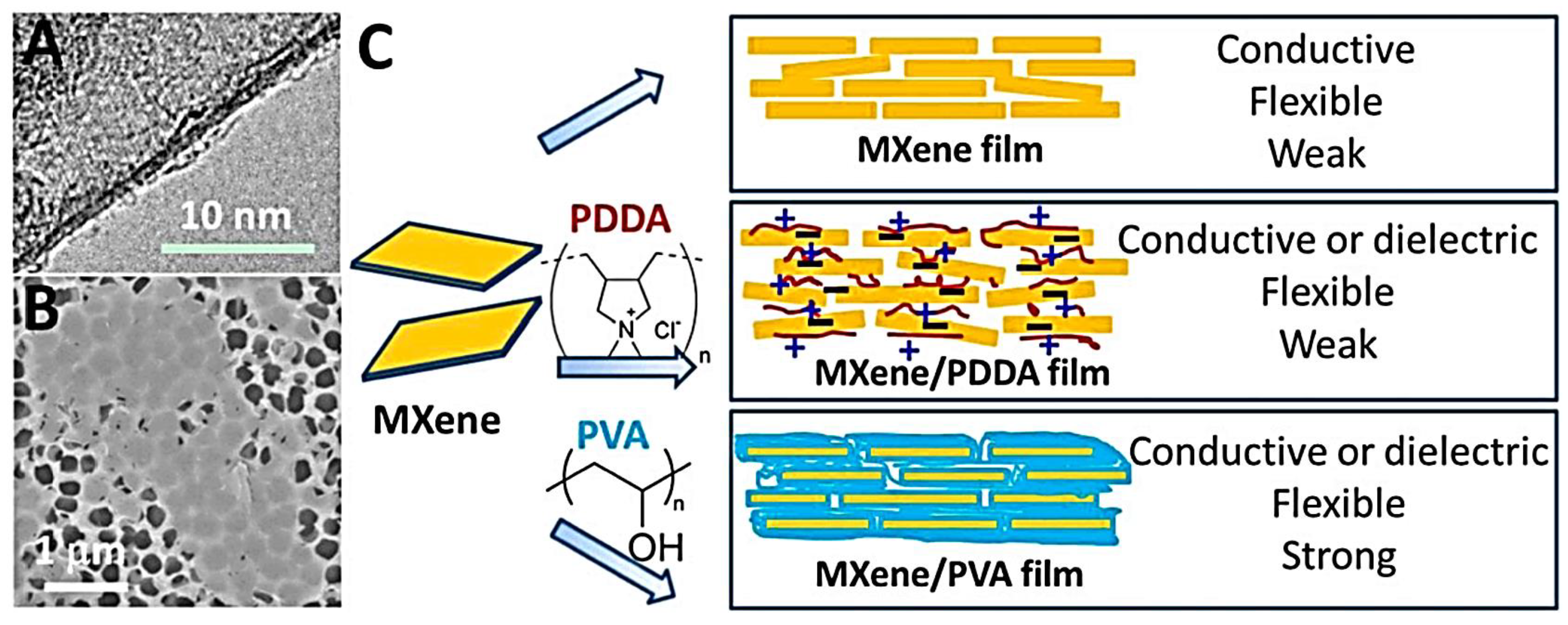
4. MXene-Polymer Composites
4.1. Polyvinyl Butyral Composite of MXene
4.2. UHMWPE Composite of MXene
4.3. PES Composite of MXene
4.4. CS Composite of MXene
4.5. CNF Composite of MXene
4.6. PS Composite of MXene
4.7. PVDF Composite of MXene
4.8. PPy Composite of MXene
4.9. P(VA)/P(AA) Composite of MXene
4.10. PDMAEMA Composite of MXene
4.11. PU Composite of MXene
4.12. PANI Composite of MXene
4.13. Poly-3,4-ethylene Dioxythiophene Composite of MXene
4.14. PE Composite of MXene
4.15. PEI Composite of MXene
4.16. PAM Composite of MXene
4.17. GdW10-Based Polyoxometalates Composite of MXene
4.18. PS Composite of MXene
4.19. PDDA-Ti3C2Tx Nanocomposite
4.20. ePTFE/MXene Composite
4.21. Alginate/MXene Composite
4.22. Ti3C2/Cellulose Composite
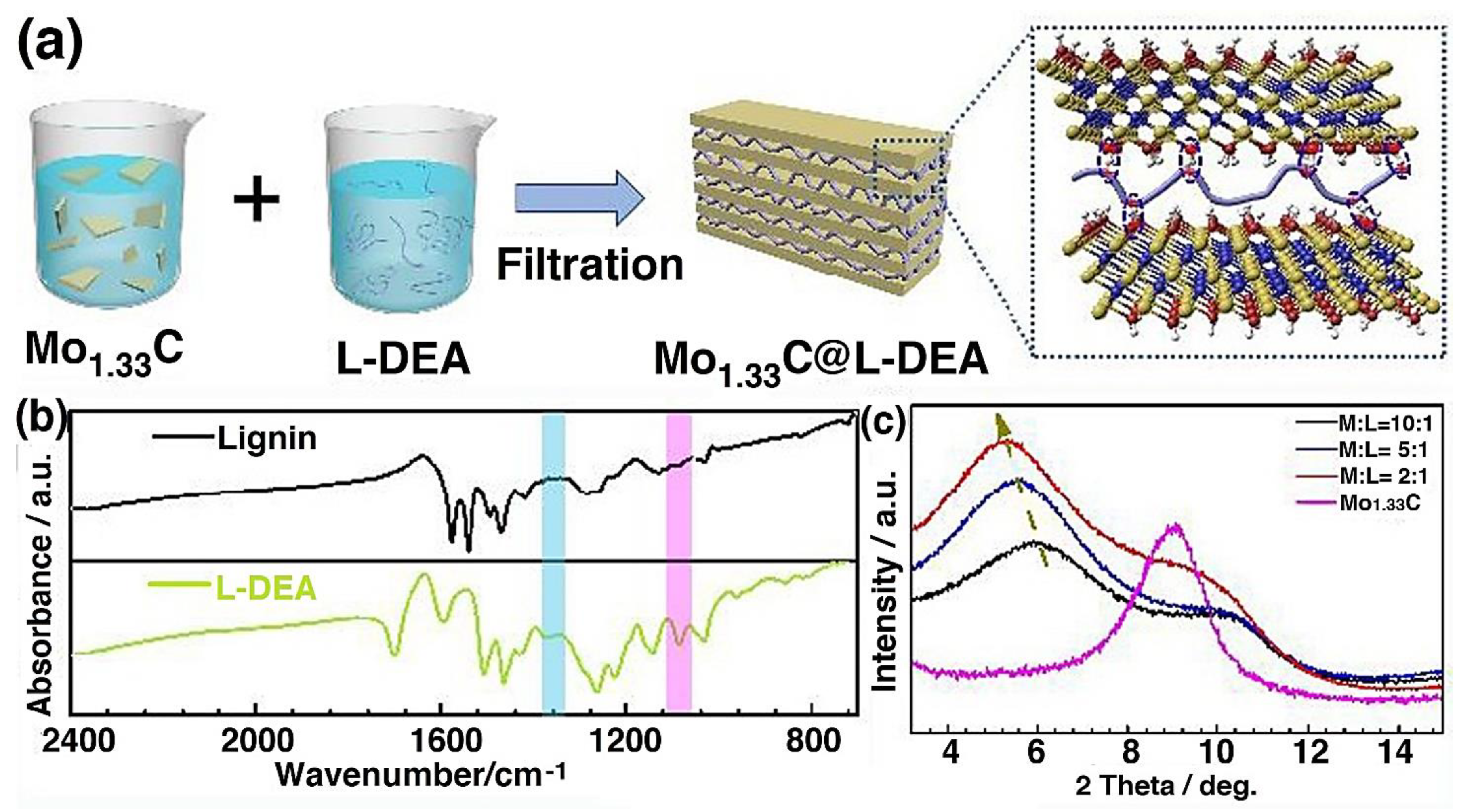
4.23. Lignin/MXene Composite
5. Applications
5.1. Biomedical Applications
5.1.1. Antibacterial: Antimicrobial Agent & Anticancer Activity
Antimicrobial Agent
Anticancer Activity
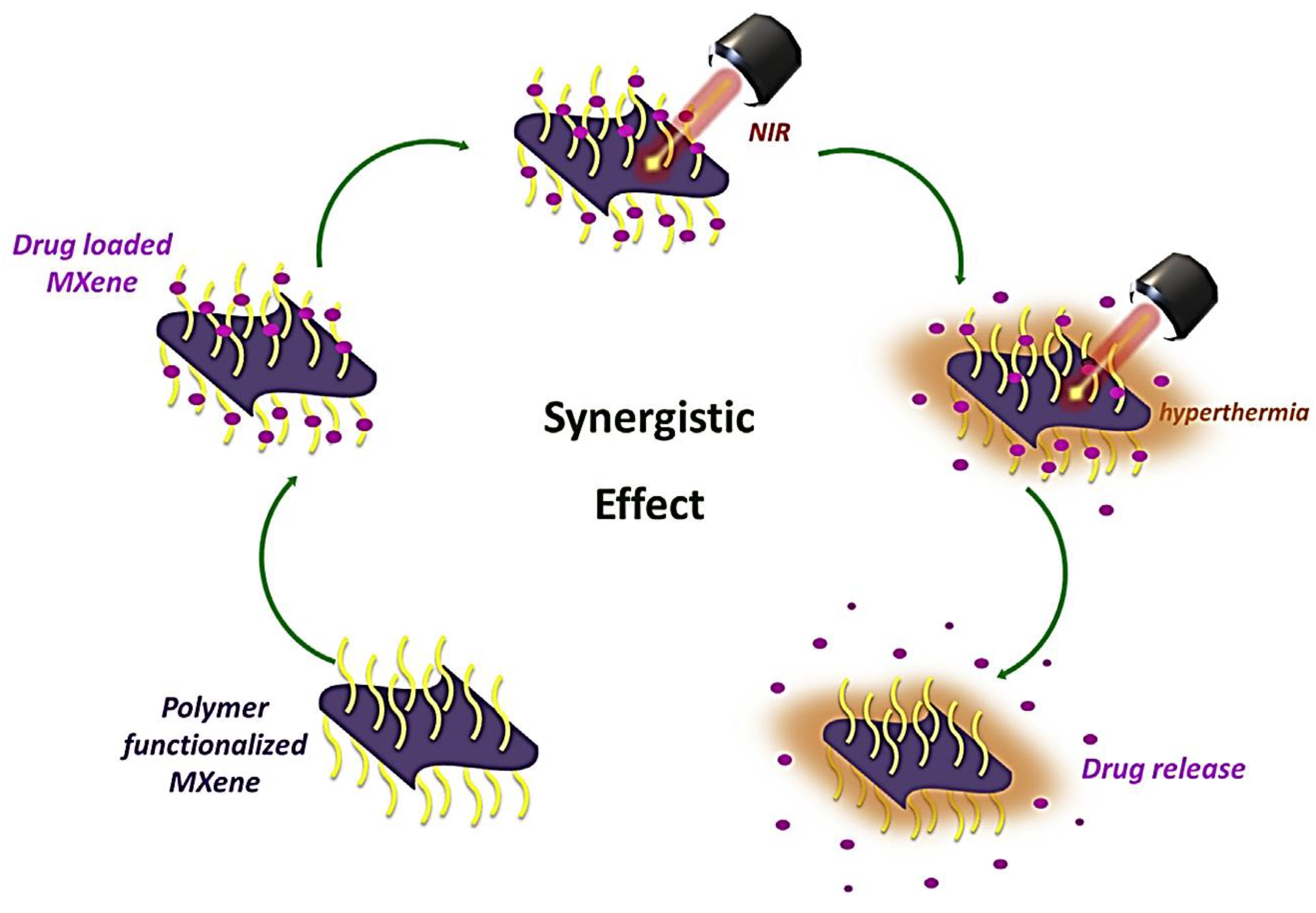
5.1.2. Drug Delivery System
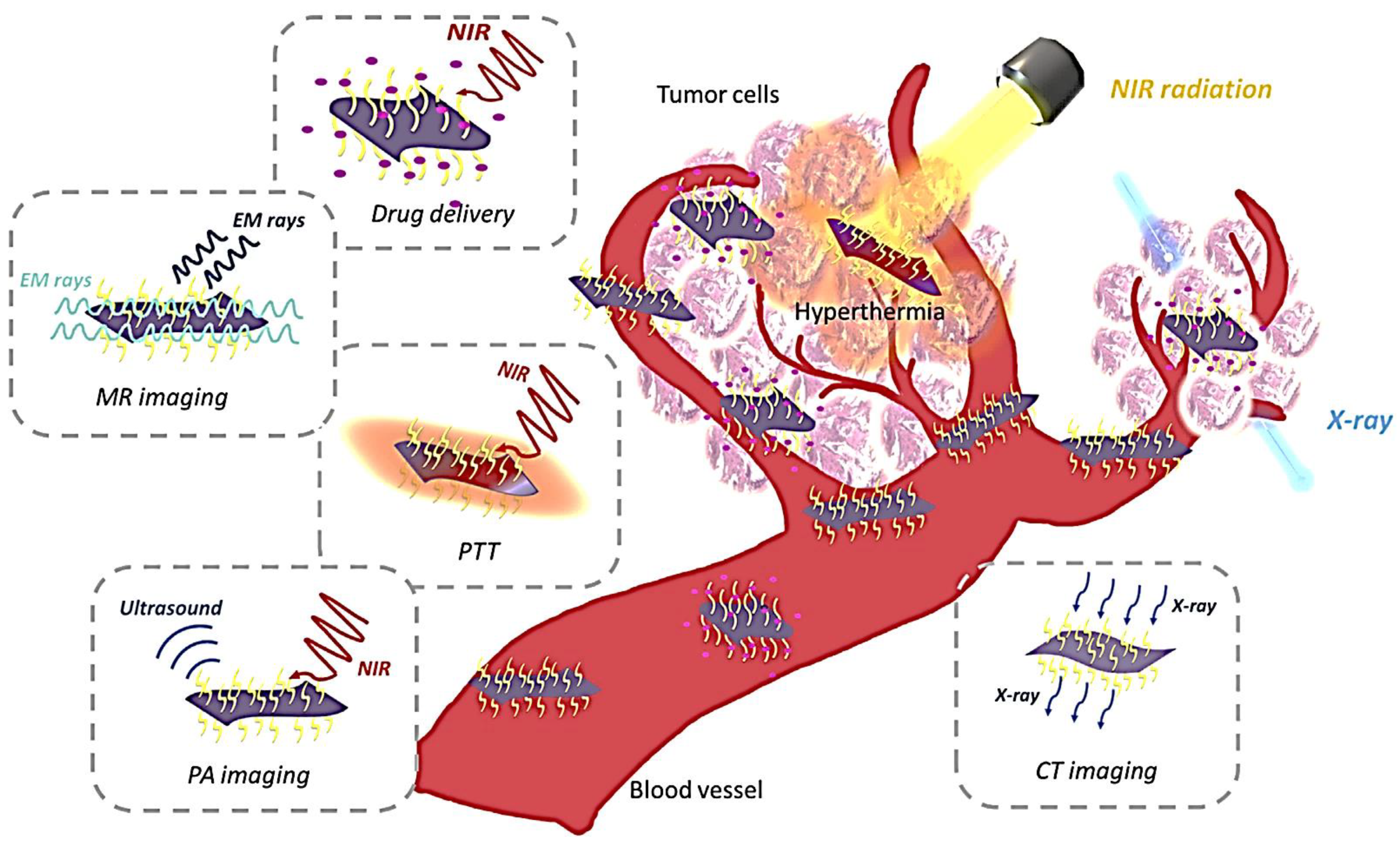
5.1.3. Bio-Imaging
Photoacoustic Imaging
Magnetic Resonance Imaging
X-ray Computed Tomography
Luminescence Imaging
5.1.4. Sensors
Biosensors
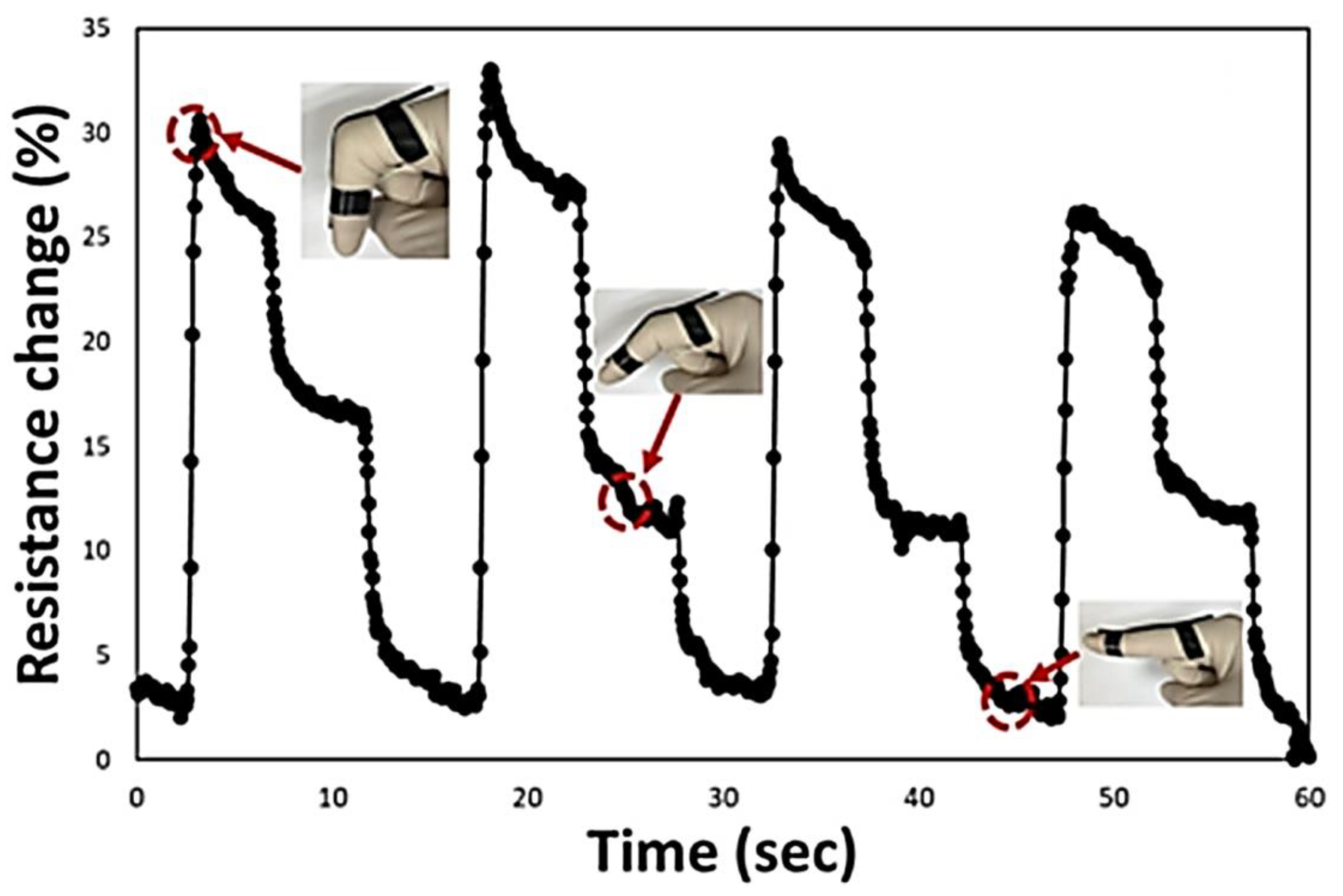
Physical Sensors
5.1.5. Tissue Engineering
5.1.6. Therapeutics
Photothermal Therapy
Photodynamic Therapy
Thermodynamic Therapy
6. Energy Applications
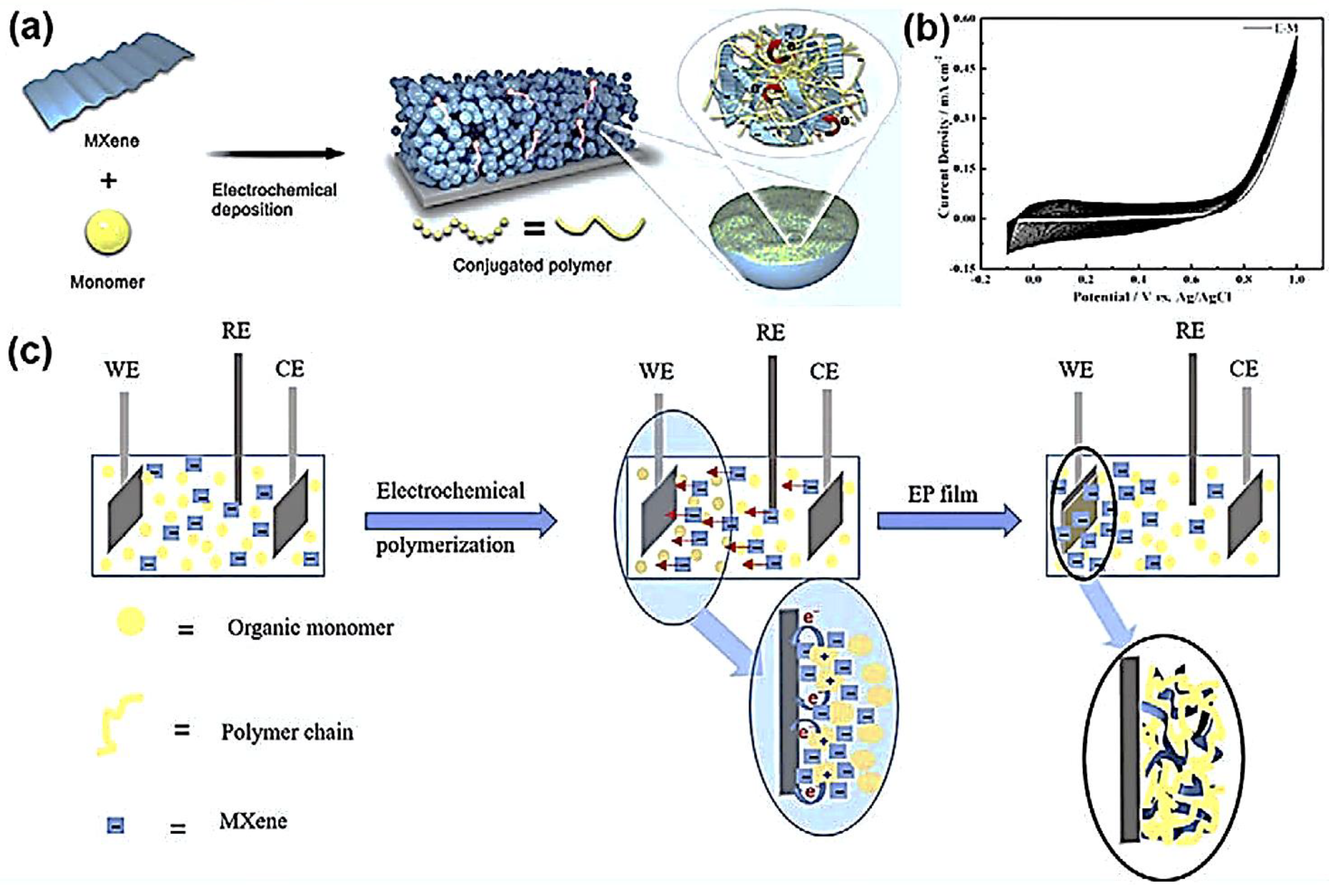
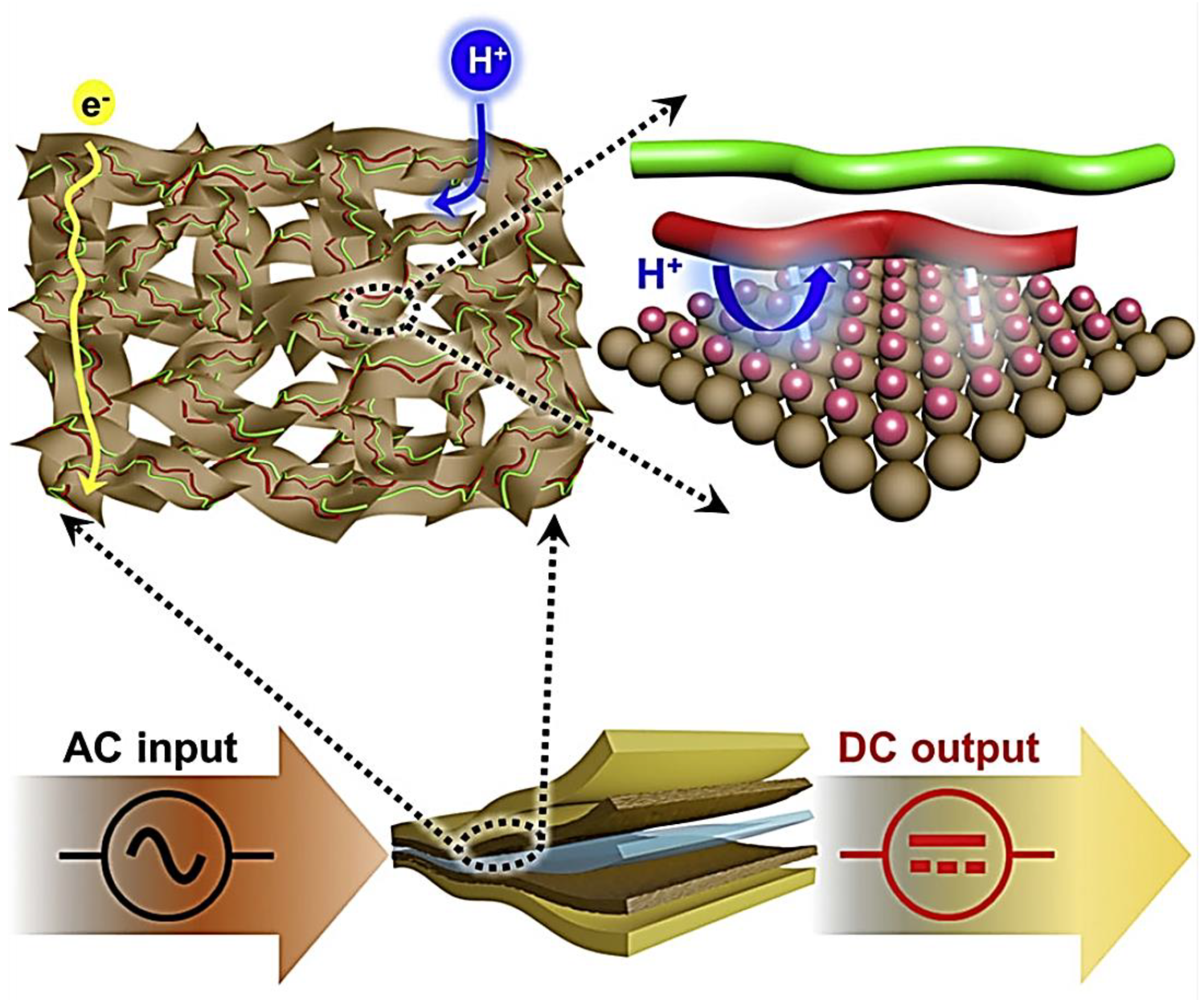
6.1. MXene-Polymer-Based Micro-Supercapacitors
6.2. Asymmetric Microsupercapacitors (AMSC)
7. Conducting Polymers Nanocomposites vs. MXene-Polymer Nanocomposites
8. Summary and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide “clay” with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Sinsawat, A.; Anderson, K.L.; Vaia, R.A.; Farmer, B.L. Influence of polymer matrix composition and architecture on polymer nanocomposite formation: Coarse-grained molecular dynamics simulation. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 3272–3284. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Twenty Years of Polymer-Clay Nanocomposites. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Parajuli, D.; Samatha, K. Topological properties of MXenes. Mxenes Compos. 2022, 171–199. [Google Scholar] [CrossRef]
- Polymer|Description, Examples, Types, Material, Uses, & Facts|Britannica. Available online: https://www.britannica.com/science/polymer (accessed on 28 July 2022).
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef]
- Carey, M.; Barsoum, M.W. MXene polymer nanocomposites: A review. Mater. Today Adv. 2021, 9, 100120. [Google Scholar] [CrossRef]
- George, S.M.; Kandasubramanian, B. Advancements in MXene-Polymer composites for various biomedical applications. Ceram. Int. 2020, 46, 8522–8535. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Gogotsi, Y.; Rosén, J.; Barsoum, M. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef]
- Markets and Markets, “Biomaterials Market by Type of Materials (Metallic, Ceramic, Polymers, Natural), Application (Cardiovascular, Orthopedic, Dental, Plastic Surgery, Wound Healing, Neurological Disorders, Tissue Engineering, Ophthalmology)—Global Forecast to 2025”. 2019. [Online]. Available online: https://www.marketsandmarkets.com/Market-Reports/biomaterials-393.html (accessed on 1 August 2022).
- Korde, J.M.; Kandasubramanian, B. Naturally biomimicked smart shape memory hydrogels for biomedical functions. Chem. Eng. J. 2020, 379, 122430. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Thomas, S. Inert ceramics. In Fundamental Biomaterials: Ceramics; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 117–127. [Google Scholar]
- Feng, W.; Wang, R.; Zhou, Y.; Ding, L.; Gao, X.; Zhou, B.; Hu, P.; Chen, Y. Ultrathin Molybdenum Carbide MXene with Fast Biodegradability for Highly Efficient Theory-Oriented Photonic Tumor Hyperthermia. Adv. Funct. Mater. 2019, 29, 1901942. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, H.; Zhao, M.; Dai, C.; Zhang, S.; Peng, W.; Chen, Y. 2D superparamagnetic tantalum carbide composite MXenes for efficient breast-cancer theranostics. Theranostics 2018, 8, 1648–1664. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpińska, D.; Dybko, A.; Jastrzębska, A.M.; Wojciechowski, T.; Rozmysłowska, A.; Chudy, M.; Grabowska-Jadach, I.; Ziemkowska, W.; Brzózka, Z.; et al. 2D Ti2C (MXene) as a novel highly efficient and selective agent for photothermal therapy. Mater. Sci. Eng. C 2019, 98, 874–886. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Liang, X.; Liu, Q.; Qu, M.; Zou, Q.; Li, J.; Tan, H.; Liu, L.; Fan, D.; et al. Two-Dimensional MXene (Ti3C2)-Integrated Cellulose Hydrogels: Toward Smart Three-Dimensional Network Nanoplatforms Exhibiting Light-Induced Swelling and Bimodal Photothermal/Chemotherapy Anticancer Activity. ACS Appl. Mater. Interfaces 2018, 10, 27631–27643. [Google Scholar] [CrossRef]
- Dai, C.; Chen, Y.; Jing, X.; Xiang, L.; Yang, D.; Lin, H.; Liu, Z.; Han, X.; Wu, R. Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation. ACS Nano 2017, 11, 12696–12712. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D Tantalum Carbide (MXene). Adv. Mater. 2018, 30, 1703284. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018, 7, 1701394. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zou, J.; Tang, Q.; Yang, X.; Zhang, Y.; Zhang, Q.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Surface Modified Ti3C2 MXene Nanosheets for Tumor Targeting Photothermal/Photodynamic/Chemo Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 40077–40086. [Google Scholar] [CrossRef]
- Zong, L.; Wu, H.; Lin, H.; Chen, Y. A polyoxometalate-functionalized two-dimensional titanium carbide composite MXene for effective cancer theranostics. Nano Res. 2018, 11, 4149–4168. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Han, J.; Chen, Y.; Lin, H.; Yang, T. Surface Nanopore Engineering of 2D MXenes for Targeted and Synergistic Multitherapies of Hepatocellular Carcinoma. Adv. Mater. 2018, 30, 1706981. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Geethamma, V.G.; Sreekala, M.S.; Thomas, S. Polymeric biomaterials: State-of-the-art and new challenges. In Fundamental Biomaterials: Polymers; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–20. [Google Scholar]
- Dai, C.; Lin, H.; Xu, G.; Liu, Z.; Wu, R.; Chen, Y. Biocompatible 2D Titanium Carbide (MXenes) Composite Nanosheets for pH-Responsive MRI-Guided Tumor Hyperthermia. Chem. Mater. 2017, 29, 8637–8652. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. Available online: www.nature.com/nature (accessed on 16 March 2021). [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef]
- Rasool, K.; Mahmoud, K.A.; Johnson, D.J.; Helal, M.; Berdiyorov, G.R.; Gogotsi, Y. Efficient Antibacterial Membrane based on Two-Dimensional Ti3C2Tx (MXene) Nanosheets. Sci. Rep. 2017, 7, 1598. [Google Scholar] [CrossRef]
- Warsi, A.-Z.; Aziz, F.; Zulfiqar, S.; Haider, S.; Shakir, I.; Agboola, P.O. Synthesis, Characterization, Photocatalysis, and Antibacterial Study of WO3, MXene and WO3/MXene Nanocomposite. Nanomaterials 2022, 12, 713. [Google Scholar] [CrossRef]
- Parajuli, D.; Vagolu, V.K.; Chandramoli, K.; Murali, N.; Samatha, K. Soft Chemical Synthesis of Nickel-Zinc-Cobalt-Ferrite Nanoparticles and their Structural, Morphological and Magnetic Study at Room Temperature. J. Nepal Phys. Soc. 2021, 7, 14–18. [Google Scholar] [CrossRef]
- Parajuli, D.; Taddesse, P.; Murali, N.; Samatha, K. Correlation between the structural, magnetic, and dc resistivity properties of Co0.5M0.5−xCuxFe2O4 (M = Mg, and Zn) nano ferrites. Appl. Phys. A Mater. Sci. Process. 2022, 128, 1–9. [Google Scholar] [CrossRef]
- Parajuli, D.; Kaphle, G.C.; Samatha, K. First-principles Study of Electronic and Magnetic Properties of Anatase and its role in Anatase-MXene Nanocomposite. J. Nepal Phys. Soc. 2019, 5, 42–53. [Google Scholar] [CrossRef]
- Parajuli, D.; Samatha, K. Structural analysis of Cu substituted Ni\Zn in Ni-Zn Ferrite. BIBECHANA 2021, 18, 128–133. [Google Scholar] [CrossRef]
- Parajuli, D.; Samatha, K. Morphological analysis of Cu substituted Ni\Zn in Ni-Zn ferrites. BIBECHANA 2021, 18, 80–86. [Google Scholar] [CrossRef]
- Parajuli, D.; Murali, N.; Rao, A.V.; Ramakrishna, A.; Yonatan Mulushoa, S.; Samatha, K. Structural, dc electrical resistivity and magnetic investigation of Mg, Ni, and Zn substituted Co-Cu nano spinel ferrites. S. Afr. J. Chem. Eng. 2022, 42, 106–114. [Google Scholar] [CrossRef]
- Parajuli, D.; Raghavendra, V.; Suryanarayana, B.; Rao, P.A.; Murali, N.; Varma, P.V.S.K.; Giri Prasad, R.; Ramakrishna, Y.; Chandramouli, K. Corrigendum to ‘Cadmium substitution effect on structural, electrical and magnetic properties of Ni-Zn nano ferrites’ [Results Phys. 19 (2020) 2211-379 103487]. Results Phys. 2021, 23, 103947. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef]
- Reese, C.; Roberts, M.; Ling, M.M.; Bao, Z. Organic thin film transistors. Mater. Today 2004, 7, 20–27. [Google Scholar] [CrossRef]
- Mortimer, R.J. Electrochromic materials. Chem. Soc. Rev. 1997, 26, 147. [Google Scholar] [CrossRef]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Meng, C.; Maeng, J.; John, S.W.M.; Irazoqui, P.P. Ultrasmall Integrated 3D Micro-Supercapacitors Solve Energy Storage for Miniature Devices. Adv. Energy Mater. 2014, 4, 1301269. [Google Scholar] [CrossRef]
- Kurra, N.; Hota, M.K.; Alshareef, H.N. Conducting polymer micro-supercapacitors for flexible energy storage and Ac line-filtering. Nano Energy 2015, 13, 500–508. [Google Scholar] [CrossRef]
- Gu, C.; Huang, N.; Chen, Y.; Qin, L.; Xu, H.; Zhang, S.; Li, F.; Ma, Y.; Jiang, D. π-Conjugated Microporous Polymer Films: Designed Synthesis, Conducting Properties, and Photoenergy Conversions. Angew. Chem. Int. Ed. 2015, 54, 13594–13598. [Google Scholar] [CrossRef]
- Gu, C.; Fei, T.; Lv, Y.; Feng, T.; Xue, S.; Lu, D.; Ma, Y. Color-stable White Electroluminescence Based on a Cross-linked Network Film Prepared by Electrochemical Copolymerization. Adv. Mater. 2010, 22, 2702–2705. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, Y.; Wu, X.; Nian, L.; Xie, Z.; Liu, L.; Ma, Y. In Situ Electrochemical Synthesis and Deposition of Discotic Hexa- peri -hexabenzocoronene Molecules on Electrodes: Self-Assembled Structure, Redox Properties, and Application for Supercapacitor. Small 2015, 11, 3028–3034. [Google Scholar] [CrossRef]
- Gu, C.; Chen, Y.; Zhang, Z.; Xue, S.; Sun, S.; Zhang, K.; Zhong, C.; Zhang, H.; Pan, Y.; Lv, Y.; et al. Electrochemical Route to Fabricate Film-Like Conjugated Microporous Polymers and Application for Organic Electronics. Adv. Mater. 2013, 25, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Fei, T.; Yao, L.; Lv, Y.; Lu, D.; Ma, Y. Multilayer Polymer Stacking by In Situ Electrochemical Polymerization for Color-Stable White Electroluminescence. Adv. Mater. 2011, 23, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, M.; Lu, J.; Meshkian, R.; Tao, Q.; Hultman, L.; Rosen, J. Prediction and synthesis of a family of atomic laminate phases with Kagomé-like and in-plane chemical ordering. Sci. Adv. 2017, 3, e1700642. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O.Å.; et al. Two-dimensional Mo1.33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering. Nat. Commun. 2017, 8, 14949. [Google Scholar] [CrossRef]
- Mahmood, M.; Zulfiqar, S.; Warsi, M.F.; Aadil, M.; Shakir, I.; Haider, S.; Agboola, P.O.; Shahid, M. Nanostructured V2O5 and its nanohybrid with MXene as an efficient electrode material for electrochemical capacitor applications. Ceram. Int. 2022, 48, 2345–2354. [Google Scholar] [CrossRef]
- Jang, D.; Farooq, S.Z.; Yoon, H.N.; Khalid, H.R. Design of a highly flexible and sensitive multi-functional polymeric sensor incorporating CNTs and carbonyl iron powder. Compos. Sci. Technol. 2021, 207, 108725. [Google Scholar] [CrossRef]
- MXene—What Is It, How It Is Made, What It Is Used for. Available online: https://www.nanowerk.com/mxene.php (accessed on 16 June 2022).
- Chen, X.; Zhao, Y.; Li, L.; Wang, Y.; Wang, J.; Xiong, J.; Du, S.; Zhang, P.; Shi, X.; Yu, J. MXene/Polymer Nanocomposites: Preparation, Properties, and Applications. Polym. Rev. 2020, 61, 80–115. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, W.; Wu, Y.; Zhang, P. Prediction of MXene based 2D tunable band gap semiconductors: GW quasiparticle calculations. Nanoscale 2019, 11, 3993–4000. [Google Scholar] [CrossRef]
- Parajuli, D.; Samatha, K. MXene as Topological Insulator. JETIR 2019, 6, 689–706. [Google Scholar] [CrossRef]
- Manawi, Y.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef]
- Yu, X.; Cai, X.; Cui, H.; Lee, S.W.; Yu, X.F.; Liu, B. Fluorine-free preparation of titanium carbide MXene quantum dots with high near-infrared photothermal performances for cancer therapy. Nanoscale 2017, 9, 17859–17864. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Huo, M.; Wang, M.; Lin, H.; Zhang, X.; Yin, J.; Chen, Y.; Chen, H. Highly catalytic niobium carbide (MXene) promotes hematopoietic recovery after radiation by free radical scavenging. ACS Nano 2019, 13, 6438–6454. [Google Scholar] [CrossRef] [PubMed]
- Malaki, M.; Xu, W.; Kasar, A.K.; Menezes, P.L.; Dieringa, H.; Varma, R.S.; Gupta, M. Advanced Metal Matrix Nanocomposites. Metals 2019, 9, 330. [Google Scholar] [CrossRef]
- Lu, Y.-W.; Zhang, X.; Cheng, J.; Luo, P.; Fei, M.; Lin, R.; Lu, Y.; Bian, R.; Xu, C.; Cai, D. MXene-reinforced alumina ceramic composites. Ceram. Int. 2017, 43, 17206–17210. [Google Scholar] [CrossRef]
- Yang, J.; Bao, W.; Jaumaux, P.; Zhang, S.; Wang, C.; Wang, G. MXene-Based Composites: Synthesis and Applications in Rechargeable Batteries and Supercapacitors. Adv. Mater. Interfaces 2019, 6, 1802004. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Park, J.; Ahn, H.; Wang, G. In situ synthesis of Co3O4/graphene nanocomposite material for lithium-ion batteries and supercapacitors with high capacity and supercapacitance. J. Alloys Compd. 2011, 29, 7778–7783. [Google Scholar] [CrossRef]
- Xu, C.; Song, S.; Liu, Z.; Chen, L.; Wang, L.; Fan, D.; Kang, N.; Ma, X.; Cheng, H.M.; Ren, W. Strongly Coupled High-Quality Graphene/2D Superconducting Mo2C Vertical Heterostructures with Aligned Orientation. ACS Nano 2017, 11, 5906–5914. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Xie, X.; Wu, Z.; Zhang, N. Robust and easily retrievable Pd/Ti3C2Tx⊂graphene hydrogels for efficient catalytic hydrogenation of nitroaromatic compounds. Chin. Chem. Lett. 2020, 31, 1014–1017. [Google Scholar] [CrossRef]
- Lee, K.H.; Zhang, Y.Z.; Jiang, Q.; Kim, H.; Alkenawi, A.A.; Alshareef, H.N. Ultrasound-Driven Two-Dimensional Ti3C2Tx MXene Hydrogel Generator. ACS Nano 2020, 14, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, D.W.; Kammermeyer, K. Separation of Gases by Means of Permeable Membranes. Permeability of Plastic Membranes to Gases. Ind. Eng. Chem. 1952, 44, 1465–1474. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, M.K. Element and Processing. Interface Sci. Technol. 2011, 18, 431–499. [Google Scholar]
- Kato, A.; Ikeda, Y.; Kohjiya, S. Carbon Black-Filled Natural Rubber Composites: Physical Chemistry and Reinforcing Mechanism. Polym. Compos. 2012, 1, 515–543. [Google Scholar] [CrossRef]
- Shen, C.; Wang, L.; Zhou, A.; Wang, B.; Wang, X.; Lian, W.; Hu, Q.; Qin, G.; Liu, X. Synthesis and electrochemical properties of two-dimensional RGO/Ti3C2 Tx nanocomposites. Nanomaterials 2018, 8, 80. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Song, H.; Tang, H.; Li, C. Synthesis, characterization, and tribological properties of two-dimensional Ti3C2. Cryst. Res. Technol. 2014, 49, 926–932. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, M.; Yang, X.; Wang, Z.; Luo, G.; Huang, Z.; Sui, X.; Li, C. Preparation and tribological properties of Ti3C2(OH)2 nanosheets as additives in base oil. RSC Adv. 2015, 5, 2762–2767. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical Characteristics and Functional Properties of Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 3–31. [Google Scholar]
- Ansari, F.; Berglund, L.A. Tensile Properties of Wood Cellulose Nanopaper and Nanocomposite Films. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 115–130. [Google Scholar]
- Suñer, S.; Joffe, R.; Tipper, J.L.; Emami, N. Ultra high molecular weight polyethylene/graphene oxide nanocomposites: Thermal, mechanical and wettability characterisation. Compos. Part B Eng. 2015, 78, 185–191. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Li, L.; Chen, P.; Wang, Z.; Gu, Q. Electrostatic adsorption method for preparing electrically conducting ultrahigh molecular weight polyethylene/graphene nanosheets composites with a segregated network. Compos. Sci. Technol. 2013, 89, 180–185. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, G.; Xiao, L.; Wang, H.; Zhang, Q.; Zhao, Z. Preparation and electrical conductivity of graphene/ultrahigh molecular weight polyethylene composites with a segregated structure. Carbon N. Y. 2012, 50, 4596–4599. [Google Scholar] [CrossRef]
- McKeen, L.W. High-Temperature/High-Performance Polymers. In Film Properties of Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 389–417. [Google Scholar]
- Wenten, I.G. Khoiruddin Reverse osmosis applications: Prospect and challenges. Desalination 2016, 391, 112–125. [Google Scholar] [CrossRef]
- Han, R.; Ma, X.; Xie, Y.; Teng, D.; Zhang, S. Preparation of a new 2D MXene/PES composite membrane with excellent hydrophilicity and high flux. RSC Adv. 2017, 7, 56204–56210. [Google Scholar] [CrossRef]
- Chawla, S.P.; Kanatt, S.R.; Sharma, A.K. Chitosan. In Polysaccharides: Bioactivity and Biotechnology; Springer International Publishing: Cham, Switzerland, 2015; pp. 219–246. [Google Scholar]
- Panesar, D.; Leung, R.; Sain, M.; Panthapulakkal, S. The effect of sodium hydroxide surface treatment on the tensile strength and elastic modulus of cellulose nanofiber. In Sustainable and Nonconventional Construction Materials Using Inorganic Bonded Fiber Composites; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 17–26. [Google Scholar]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary Strengthening and Toughening of MXene/Cellulose Nanofiber Composite Paper with Nacre-Inspired Structure and Superior Electromagnetic Interference Shielding Properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef] [PubMed]
- Ebnesajjad, S. Characteristics of adhesive materials. In Handbook of Adhesives and Surface Preparation; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 137–183. [Google Scholar]
- Zhao, Y.; Zhao, J. Functional group-dependent anchoring effect of titanium carbide-based MXenes for lithium-sulfur batteries: A computational study. Appl. Surf. Sci. 2017, 412, 591–598. [Google Scholar] [CrossRef]
- Liang, X.; Garsuch, A.; Nazar, L.F. Sulfur cathodes based on conductive MXene nanosheets for high-performance lithium-sulfur batteries. Angew. Chem. Int. Ed. 2015, 54, 3907–3911. [Google Scholar] [CrossRef]
- Drobny, J.G. Fluorine-Containing Polymers. In Brydson’s Plastics Materials: Eighth Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 389–425. [Google Scholar]
- Li, L.R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef]
- Tu, S.; Jiang, Q.; Zhang, X.; Alshareef, H.N. Large Dielectric Constant Enhancement in MXene Percolative Polymer Composites. ACS Nano 2018, 12, 3369–3377. [Google Scholar] [CrossRef]
- Kurosu, H. Chapter 16 Electrically-Conducting Polymers. In Studies in Physical and Theoretical Chemistry; Elsevier: Amsterdam, The Netherlands, 1998; Volume 84, pp. 589–611. [Google Scholar]
- De Leon, A.; Advincula, R.C. Conducting Polymers with Superhydrophobic Effects as Anticorrosion Coating. In Intelligent Coatings for Corrosion Control; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 409–430. [Google Scholar]
- Wu, W.; Wei, D.; Zhu, J.; Niu, D.; Wang, F.; Wang, L.; Yang, L.; Yang, P.; Wang, C. Enhanced electrochemical performances of organ-like Ti3C2 MXenes/polypyrrole composites as supercapacitors electrode materials. Ceram. Int. 2019, 45, 7328–7337. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Deng, Q.; Zhou, J.; Pei, Z.; Xue, Q.; Huang, Y.; Wang, Z.; Li, H.; Huang, Q.; et al. Highly Flexible, Freestanding Supercapacitor Electrode with Enhanced Performance Obtained by Hybridizing Polypyrrole Chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]
- Huang, X.; Wang, R.; Jiao, T.; Zou, G.; Zhan, F.; Yin, J.; Zhang, L.; Zhou, J.; Peng, Q. Facile Preparation of Hierarchical AgNP-Loaded MXene/Fe3O4 Polymer Nanocomposites by Electrospinning with Enhanced Catalytic Performance for Wastewater Treatment. ACS Omega 2019, 4, 1897–1906. [Google Scholar] [CrossRef]
- Sonia, T.A.; Sharma, C.P. Polymers in oral insulin delivery. In Oral Delivery of Insulin; Elsevier: Amsterdam, The Netherlands, 2014; pp. 257–310. [Google Scholar]
- Liu, Y.; He, Q. The Route of Nanomaterials Entering Brain. In Neurotoxicity of Nanomaterials and Nanomedicine; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 33–57. [Google Scholar]
- Chen, J.; Chen, K.; Tong, D.; Huang, Y.; Zhang, J.; Xue, J.; Huang, Q.; Chen, T. CO2 and temperature dual responsive “smart” MXene phases. Chem. Commun. 2015, 51, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Introduction—Biomaterials Science: An Evolving, Multidisciplinary Endeavor. Biomaterials Science: An Introduction to Materials, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. xxv–xxxix. [Google Scholar] [CrossRef]
- Janik, H.; Sienkiewicz, M.; Kucinska-Lipka, J. Polyurethanes. In Handbook of Thermoset Plastics; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 253–295. [Google Scholar]
- Yu, B.; Tawiah, B.; Wang, L.Q.; Yin Yuen, A.C.; Zhang, Z.C.; Shen, L.L.; Lin, B.; Fei, B.; Yang, W.; Li, A.; et al. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhi, W.; Xiang, S.; Bian, R.; Lin, R.; Wu, K.; Wang, T.; Cai, D. Study of MXene-filled polyurethane nanocomposites prepared via an emulsion method. Compos. Sci. Technol. 2018, 168, 404–411. [Google Scholar] [CrossRef]
- Jalal Uddin, A. Coatings for technical textile yarns. In Technical Textile Yarns; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 140–184. [Google Scholar]
- D’Arrigo, J. Early Work with Aqueous Carbohydrate Gels. Stud. Interface Sci. 2011, 25, 29–44. [Google Scholar]
- Wei, H.; Dong, J.; Fang, X.; Zheng, W.; Sun, Y.; Qian, Y.; Jiang, Z.; Huang, Y. Ti3C2Tx MXene/polyaniline (PANI) sandwich intercalation structure composites constructed for microwave absorption. Compos. Sci. Technol. 2019, 169, 52–59. [Google Scholar] [CrossRef]
- Li, L.; Zhang, N.; Zhang, M.; Zhang, X.; Zhang, Z. Flexible Ti3C2Tx/PEDOT:PSS films with outstanding volumetric capacitance for asymmetric supercapacitors. Dalt. Trans. 2019, 48, 1747–1756. [Google Scholar] [CrossRef]
- Zhang, J.; Seyedin, S.; Qin, S.; Wang, Z.; Moradi, S.; Yang, F.; Lynch, P.A.; Yang, W.; Liu, J.; Wang, X.; et al. Highly Conductive Ti3C2 T x MXene Hybrid Fibers for Flexible and Elastic Fiber-Shaped Supercapacitors. Small 2019, 15, 1804732. [Google Scholar] [CrossRef]
- Emblem, A. Plastics properties for packaging materials. In Packaging Technology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 287–309. [Google Scholar]
- Cao, X.; Wu, M.; Zhou, A.; Wang, Y.; He, X.; Wang, L. Non-isothermal crystallization and thermal degradation kinetics of MXene/linear low-density polyethylene nanocomposites. E-Polymers 2017, 17, 373–381. [Google Scholar] [CrossRef]
- Chen, C.; Boota, M.; Xie, X.; Zhao, M.; Anasori, B.; Ren, C.E.; Miao, L.; Jiang, J.; Gogotsi, Y. Charge transfer induced polymerization of EDOT confined between 2D titanium carbide layers. J. Mater. Chem. A 2017, 5, 5260–5265. [Google Scholar] [CrossRef]
- Parajuli, D.; Murali, N.; Samatha, K.; Veeraiah, V. Thermal, structural, morphological, functional group and first cycle charge/discharge study of Co substituted LiNi1−x-0.02Mg0.02CoxO2 (x = 0.00, 0.02, 0.04, 0.06, and 0.08) cathode material for LIBs. AIP Adv. 2022, 12, 085010. [Google Scholar] [CrossRef]
- Grishanov, S. Structure and properties of textile materials. In Handbook of Textile and Industrial Dyeing: Principles, Processes and Types of Dyes; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 1, pp. 28–63. [Google Scholar]
- Shao, W.; Tebyetekerwa, M.; Marriam, I.; Li, W.; Wu, Y.; Peng, S.; Ramakrishna, S.; Yang, S.; Zhu, M. Polyester@MXene nanofibers-based yarn electrodes. J. Power Sources 2018, 396, 683–690. [Google Scholar] [CrossRef]
- Wu, X.; Hao, L.; Zhang, J.; Zhang, X.; Wang, J.; Liu, J. Polymer-Ti3C2Tx composite membranes to overcome the trade-off in solvent resistant nanofiltration for alcohol-based system. J. Memb. Sci. 2016, 515, 175–188. [Google Scholar] [CrossRef]
- Hadjesfandiari, N.; Parambath, A. Stealth coatings for nanoparticles: Polyethylene glycol alternatives. In Engineering of Biomaterials for Drug Delivery Systems: Beyond Polyethylene Glycol; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 345–361. [Google Scholar]
- Hermanson, G.T. Immobilization of Ligands on Chromatography Supports. In Bioconjugate Techniques; Elsevier: Amsterdam, The Netherlands, 2013; pp. 589–740. [Google Scholar]
- Naguib, M.; Saito, T.; Lai, S.; Rager, M.S.; Aytug, T.; Parans Paranthaman, M.; Zhao, M.Q.; Gogotsi, Y. Ti3C2TX (MXene)-polyacrylamide nanocomposite films. RSC Adv. 2016, 6, 72069–72073. [Google Scholar] [CrossRef]
- Goswami, T.K.; Mangaraj, S. Advances in polymeric materials for modified atmosphere packaging (MAP). In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 163–242. [Google Scholar]
- McKeen, L.W. Plastics Used in Medical Devices. In Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 21–53. [Google Scholar]
- Si, J.Y.; Tawiah, B.; Sun, W.L.; Lin, B.; Wang, C.; Yuen, A.C.Y.; Yu, B.; Li, A.; Yang, W.; Lu, H.D.; et al. Functionalization of MXene nanosheets for polystyrene towards high thermal stability and flame retardant properties. Polymers 2019, 11, 976. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible solid-state supercapacitors: Design, fabrication and applications. Energy Environ. Sci. 2014, 7, 2160–2181. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Xie, S.; Ling, Y.; Liang, C.; Tong, Y.; Li, Y. Stabilized TiN nanowire arrays for high-performance and flexible supercapacitors. Nano Lett. 2012, 12, 5376–5381. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Liu, Y.; Xu, Y.; Chang, Z.; Wang, D.; Li, Q. Expanded Polytetrafluoroethylene/Mxene Nanosheet Composites with Hydrophilicity and Lipophilicity for Purification of Oil Spills and Wastewater. ACS Appl. Nano Mater. 2022, 5, 2483–2491. [Google Scholar] [CrossRef]
- Minakshi, M.; Barmi, M.J.; Jones, R.T. Rescaling metal molybdate nanostructures with biopolymer for energy storage having high capacitance with robust cycle stability. Dalt. Trans. 2017, 46, 3588–3600. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Sundaram, M.M.; Henry, D.J.; Gao, X. Alginate Biopolymer Effect on the Electrodeposition of Manganese Dioxide on Electrodes for Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 7040–7051. [Google Scholar] [CrossRef]
- Ramkumar, R.; Minakshi Sundaram, M. A biopolymer gel-decorated cobalt molybdate nanowafer: Effective graft polymer cross-linked with an organic acid for better energy storage. New J. Chem. 2016, 40, 2863–2877. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; Liu, L.; Jiang, J.; Liu, X.; Fahlman, M.; Hou, L.; Rosen, J.; Zhang, F. Flexible Solid-State Asymmetric Supercapacitors with Enhanced Performance Enabled by Free-Standing MXene-Biopolymer Nanocomposites and Hierarchical Graphene-RuOx Paper Electrodes. Batter. Supercaps 2020, 3, 604–610. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Street, R.M.; McDaniel, R.M.; Barsoum, M.W.; Schauer, C.L. Antibacterial properties of electrospun Ti3C2Tz (MXene)/chitosan nanofibers. RSC Adv. 2018, 8, 35386–35394. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Qiu, N.; Deng, Q.; Kang, M.H.; Yang, H.; Baek, J.U.; Koh, Y.H.; Du, S.; Huang, Q.; Kim, H.E. Cytocompatibility of Ti3AlC2, Ti3SiC2, and Ti2AlN: In Vitro Tests and First-Principles Calculations. ACS Biomater. Sci. Eng. 2017, 3, 2293–2301. [Google Scholar] [CrossRef]
- Chen, K.; Chen, Y.; Deng, Q.; Jeong, S.H.; Jang, T.S.; Du, S.; Kim, H.E.; Huang, Q.; Han, C.M. Strong and biocompatible poly(lactic acid) membrane enhanced by Ti3C2Tz (MXene) nanosheets for Guided bone regeneration. Mater. Lett. 2018, 229, 114–117. [Google Scholar] [CrossRef]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Habib, T.; Shah, S.; Gao, H.; Patel, A.; Echols, I.; Zhao, X.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L.; et al. Water Sorption in MXene/Polyelectrolyte Multilayers for Ultrafast Humidity Sensing. ACS Appl. Nano Mater. 2019, 2, 43. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H.N. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4, eaat0098. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Karwowska, E.; Wojciechowski, T.; Ziemkowska, W.; Rozmysłowska, A.; Chlubny, L.; Olszyna, A. The Atomic Structure of Ti2C and Ti3C2 MXenes is Responsible for Their Antibacterial Activity Toward E. coli Bacteria. J. Mater. Eng. Perform. 2019, 28, 1272–1277. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Insights into 2D MXenes for Versatile Biomedical Applications: Current Advances and Challenges Ahead. Adv. Sci. 2018, 5, 1800518. [Google Scholar] [CrossRef]
- Feng, H.; Wang, W.; Zhang, M.; Zhu, S.; Wang, Q.; Liu, J.; Chen, S. 2D titanium carbide-based nanocomposites for photocatalytic bacteriostatic applications. Appl. Catal. B Environ. 2020, 266, 118609. Available online: https://www.sciencedirect.com/science/article/pii/S0926337320300242 (accessed on 20 June 2022). [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Yin, H.; Guan, X.; Lin, H.; Pu, Y.; Fang, Y.; Yue, W.; Zhou, B.; Wang, Q.; Chen, Y.; Xu, H. Nanomedicine-Enabled Photonic Thermogaseous Cancer Therapy. Adv. Sci. 2020, 7, 1901954. [Google Scholar] [CrossRef] [PubMed]
- Soleymaniha, M.; Shahbazi, M.A.; Rafieerad, A.R.; Maleki, A.; Amiri, A. Promoting Role of MXene Nanosheets in Biomedical Sciences: Therapeutic and Biosensing Innovations. Adv. Healthc. Mater. 2019, 8, 1801137. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Huang, Y.; Deng, Q.; Zhou, J.; et al. Photoluminescent Ti3C2 MXene Quantum Dots for Multicolor Cellular Imaging. Adv. Mater. 2017, 29, 1604847. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, D.G.; Kandasubramanian, B. Progress in the Development of Intrinsically Conducting Polymer Composites as Biosensors. Macromol. Chem. Phys. 2019, 220, 1800561. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nayuk, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef]
- Harper, A.; Anderson, M.R. Electrochemical Glucose Sensors—Developments Using Electrostatic Assembly and Carbon Nanotubes for Biosensor Construction. Sensors 2010, 10, 8248–8274. [Google Scholar] [CrossRef]
- Ma, Y.; Yue, Y.; Zhang, H.; Cheng, F.; Zhao, W.; Rao, J.; Luo, S.; Wang, J.; Jiang, X.; Liu, Z.; et al. 3D Synergistical MXene/Reduced Graphene Oxide Aerogel for a Piezoresistive Sensor. ACS Nano 2018, 12, 3209–3216. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Li, L.; Hu, X.; Zou, Z.; Wang, J.; Luo, S.; Gao, Y. A highly flexible and sensitive piezoresistive sensor based on MXene with greatly changed interlayer distances. Nat. Commun. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, J.; Ge, G.; Zhang, Y.; Jin, W.; Huang, W.; Shao, J.; Yang, J.; Dong, X. Stretchable Ti3C2Tx MXene/Carbon Nanotube Composite Based Strain Sensor with Ultrahigh Sensitivity and Tunable Sensing Range. ACS Nano 2018, 12, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhong, M.; Fang, Z.; Wan, P.; Yu, G. A Wearable Transient Pressure Sensor Made with MXene Nanosheets for Sensitive Broad-Range Human-Machine Interfacing. Nano Lett. 2019, 19, 1143–1150. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, C.; Zhou, F.; Dong, Y.; Shi, X.; Nicolosi, V.; Wu, Z.S.; Bao, X. Ionic liquid pre-intercalated MXene films for ionogel-based flexible micro-supercapacitors with high volumetric energy density. J. Mater. Chem. A 2019, 7, 9478–9485. [Google Scholar] [CrossRef]
- Ciou, J.H.; Li, S.; Lee, P.S. Ti3C2 MXene Paper for the Effective Adsorption and Controllable Release of Aroma Molecules. Small 2019, 15, 1903281. [Google Scholar] [CrossRef]
- Zhang, C.J.; Anasori, B.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Shmeliov, A.; Duesberg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, Flexible, and Conductive 2D Titanium Carbide (MXene) Films with High Volumetric Capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhang, H.; Wu, X.; Zhang, J.; Wang, J.; Li, Y. Novel thin-film nanocomposite membranes filled with multi-functional Ti3C2Tx nanosheets for task-specific solvent transport. Compos. Part A Appl. Sci. Manuf. 2017, 100, 139–149. [Google Scholar] [CrossRef]
- Kim, H.B.; Sajid, M.; Kim, K.T.; Hoan Na, K.; Choi, K.H. Linear humidity sensor fabrication using bi-layered active region of transition metal carbide and polymer thin films. Sens. Actuators B 2017, 252, 725–734. [Google Scholar] [CrossRef]
- Sajid, M.; Kim, H.B.; Siddiqui, G.U.; Na, K.H.; Choi, K.H. Linear bi-layer humidity sensor with tunable response using combinations of molybdenum carbide with polymers. Sens. Actuators A Phys. 2017, 262, 68–77. [Google Scholar] [CrossRef]
- El-Denglawey, V.J.; Angadi, K.; Manjunatha, B.; Chethan, S.B. Somvanshi, Role of dysprosium in enhancing the humidity sensing performance in manganese zinc ferrites for sensor applications. J. Mater. Sci. Mater. Electron. 2021, 32, 23554–23565. [Google Scholar] [CrossRef]
- An, H.; Habib, T.; Shah, S.; Gao, H.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. Surface-agnostic highly stretchable and bendable conductive MXene multilayers. Sci. Adv. 2018, 4, eaaq0118. [Google Scholar] [CrossRef]
- Yuan, W.; Yang, K.; Peng, H.; Li, F.; Yin, F. A flexible VOCs sensor based on a 3D Mxene framework with a high sensing performance. J. Mater. Chem. A 2018, 6, 18116–18124. [Google Scholar] [CrossRef]
- Hu, Y.; Zhuo, H.; Luo, Q.; Wu, Y.; Wen, R.; Chen, Z.; Liu, L.; Zhong, L.; Peng, X.; Sun, R. Biomass polymer-assisted fabrication of aerogels from MXenes with ultrahigh compression elasticity and pressure sensitivity. J. Mater. Chem. A 2019, 7, 10273–10281. [Google Scholar] [CrossRef]
- Li, X.-P.; Li, Y.; Li, X.; Song, D.; Min, P.; Hu, C.; Zhang, H.-B.; Koratkar, N.; Yu, Z.-Z. Highly sensitive, reliable and flexible piezoresistive pressure sensors featuring polyurethane sponge coated with MXene sheets. J. Colloid Interface Sci. 2019, 542, 54–62. [Google Scholar] [CrossRef]
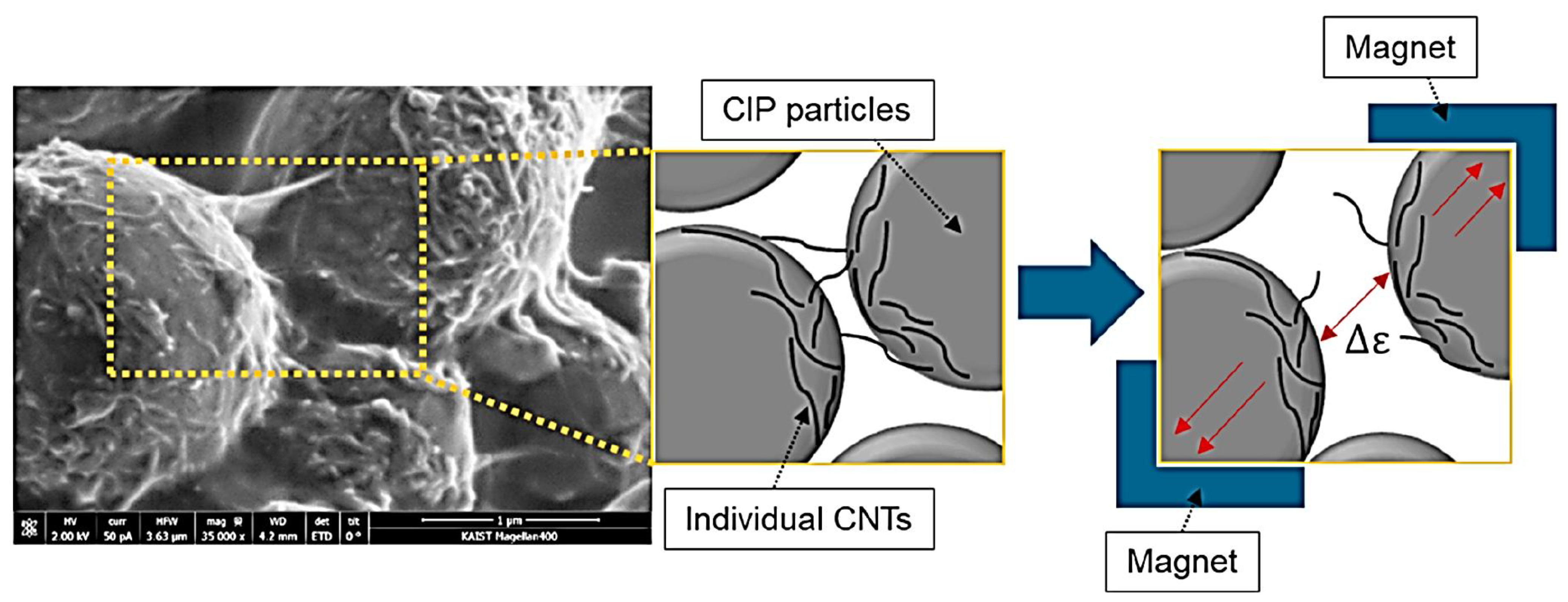
- Jang, D.; Park, J.E.; Kim, Y.K. Evaluation of (CNT@CIP)-Embedded Magneto-Resistive Sensor Based on Carbon Nanotube and Carbonyl Iron Powder Polymer Composites. Polymers 2022, 14, 542. [Google Scholar] [CrossRef]
- Riazi, H.; Taghizadeh, G.; Soroush, M. MXene-Based Nanocomposite Sensors. ACS Omega 2021, 6, 11103–11112. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, N.; Zhou, Z. Adsorptive environmental applications of MXene nanomaterials: A review. RSC Adv. 2018, 8, 19895–19905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Panatdasirisuk, W.; Mathis, T.S.; Anasori, B.; Lu, C.; Zhang, X.; Liao, Z.; Gogotsi, Y.; Yang, S. Layer-by-layer assembly of MXene and carbon nanotubes on electrospun polymer films for flexible energy storage. Nanoscale 2018, 10, 6005–6013. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Zada, S.; Dai, W.; Kai, Z.; Lu, H.; Meng, X.; Zhang, Y.; Cheng, Y.; Yan, F.; Fu, P.; Zhang, X.; et al. Algae extraction controllable delamination of vanadium carbide nanosheets with enhanced near-infrared photothermal performance. Angew. Chem. Int. Ed. 2020, 59, 6601–6606. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. Available online: https://pubs.rsc.org/en/content/articlehtml/2018/cs/c7cs00838d (accessed on 20 June 2022). [CrossRef]
- Xiang, H.; Lin, H.; Yu, L.; Chen, Y. Hypoxia-irrelevant photonic thermodynamic cancer nanomedicine. ACS Nano 2019, 13, 2223–2235. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Guo, X.; Wu, W.; Wang, G. 2D Metal Carbides and Nitrides (MXenes) as High-Performance Electrode Materials for Lithium-Based Batteries. Adv. Energy Mater. 2018, 8, 1801897. [Google Scholar] [CrossRef]
- Tang, H.; Hu, Q.; Zheng, M.; Chi, Y.; Qin, X.; Pang, H.; Xu, Q. MXene–2D layered electrode materials for energy storage. Prog. Nat. Sci. Mater. Int. 2018, 28, 133–147. [Google Scholar] [CrossRef]
- Okubo, M.; Sugahara, A.; Kajiyama, S.; Yamada, A. MXene as a Charge Storage Host. Acc. Chem. Res. 2018, 51, 591–599. [Google Scholar] [CrossRef]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z.; Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Energy Chem. 2018, 27, 73. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; El Ghazaly, A.; Fernandez-Rodriguez, J.; Persson, P.O.Å.; Rosen, J.; Zhang, F. High-Performance Ultrathin Flexible Solid-State Supercapacitors Based on Solution Processable Mo1.33C MXene and PEDOT:PSS. Adv. Funct. Mater. 2018, 28, 1703808. [Google Scholar] [CrossRef]
- Chen, Z.; Han, Y.; Li, T.; Zhang, X.; Wang, T.; Zhang, Z. Preparation and electrochemical performances of doped MXene/poly(3,4-ethylenedioxythiophene) composites. Mater. Lett. 2018, 220, 305–308. [Google Scholar] [CrossRef]
- Gund, G.S.; Park, J.H.; Harpalsinh, R.; Kota, M.; Shin, J.H.; Kim, T.; Gogotsi, Y.; Park, H.S. MXene/Polymer Hybrid Materials for Flexible AC-Filtering Electrochemical Capacitors. Joule 2019, 3, 164–176. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, J.; Wang, L.; Liu, H.; Liu, Y.; Wu, W.; Wang, F. Synthesis of polyaniline nanoparticles deposited on two-dimensional titanium carbide for high-performance supercapacitors. Mater. Lett. 2018, 214, 84–87. [Google Scholar] [CrossRef]
- Boota, M.; Pasini, M.; Galeotti, F.; Porzio, W.; Zhao, M.Q.; Halim, J.; Gogotsi, Y. Interaction of Polar and Nonpolar Polyfluorenes with Layers of Two-Dimensional Titanium Carbide (MXene): Intercalation and Pseudocapacitance. Chem. Mater. 2017, 29, 2731–2738. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; Liu, X.; Fahlman, M.; Halim, J.; Persson, P.O.Å.; Rosen, J.; Zhang, F. Polymer-MXene composite films formed by MXene-facilitated electrochemical polymerization for flexible solid-state microsupercapacitors. Nano Energy 2019, 60, 734–742. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, S.; Qin, J.; Zhao, X.; Shi, H.; Wang, X.; Chen, J.; Wu, Z.S. All-MXene-Based Integrated Electrode Constructed by Ti3C2 Nanoribbon Framework Host and Nanosheet Interlayer for High-Energy-Density Li-S Batteries. ACS Nano 2018, 12, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Su, D.; Xie, X.; Guo, X.; Bao, W.; Shao, G.; Wang, G. Immobilizing Polysulfides with MXene-Functionalized Separators for Stable Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2016, 8, 29427–29433. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zheng, Y.; Kota, S.; Huang, W.; Wang, S.; Qi, H.; Kim, S.; Tu, Y.; Barsoum, M.W.; Li, C.Y. 2D MXene-containing polymer electrolytes for all-solid-state lithium metal batteries. Nanoscale Adv. 2019, 1, 395–402. [Google Scholar] [CrossRef]
- Fei, M.; Lin, R.; Deng, Y.; Xian, H.; Bian, R.; Zhang, X.; Cheng, J.; Xu, C.; Cai, D. Polybenzimidazole/Mxene composite membranes for intermediate temperature polymer electrolyte membrane fuel cells. Nanotechnology 2018, 29, 035403. [Google Scholar] [CrossRef]
- Yang, C.; Xu, D.; Peng, W.C.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Ti2C3Tx nanosheets as photothermal agents for near-infrared responsive hydrogels. Nanoscale 2018, 10, 15387–15392. [Google Scholar] [CrossRef]
- Fan, X.; Ding, Y.; Liu, Y.; Liang, J.; Chen, Y. Plasmonic Ti3C2Tx MXene Enables Highly Efficient Photothermal Conversion for Healable and Transparent Wearable Device. ACS Nano 2019, 13, 8124–8134. [Google Scholar] [CrossRef]
- Park, T.H.; Yu, S.; Koo, M.; Kim, H.; Kim, E.H.; Park, J.E.; Ok, B.; Kim, B.; Noh, S.H.; Park, C.; et al. Shape-Adaptable 2D Titanium Carbide (MXene) Heater. ACS Nano 2019, 13, 6835–6844. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, L.; Jin, X.; Wang, W.; Zhang, S.; Tang, B. MXene Ti3C2Tx for phase change composite with superior photothermal storage capability. J. Mater. Chem. A 2019, 7, 14319–14327. [Google Scholar] [CrossRef]
- Shah, D.K.; KC, D.; Parajuli, D.; Akhtar, M.S.; Kim, C.Y.; Yang, O.-B. A computational study of carrier lifetime, doping concentration, and thickness of window layer for GaAs solar cell based on Al2O3 antireflection layer. Sol. Energy 2022, 234, 330–337. [Google Scholar] [CrossRef]
- Devendra, K.C.; Shah, D.K.; Wagle, R.; Shrivastava, A.; Parajuli, D. Ingap window layer for gallium arsenide (Gaas) based solar cell using pc1d simulation. J. Adv. Res. Dyn. Control Syst. 2020, 12, 2878–2885. [Google Scholar] [CrossRef]
- Parajuli, D.; Shah, D.K.; KC, D.; Kumar, S.; Park, M.; Pant, B. Influence of Doping Concentration and Thickness of Regions on the Performance of InGaN Single Junction-Based Solar Cells: A Simulation Approach. Electrochem 2022, 3, 407–415. [Google Scholar] [CrossRef]
- Sun, X.; Chen, K.; Liang, F.; Zhi, C.; Xue, D. Perspective on Micro-Supercapacitors. Front. Chem. 2022, 9, 1173. [Google Scholar] [CrossRef]
- Qin, L.; Ding, Z.; Hanif, M.; Jiang, J.; Liu, L.; Mo, Y.; Xie, Z.; Ma, Y. Poly(3,4-dioxythiophene) soft nano-network with a compatible ion transporting channel for improved electrochromic performance. Polym. Chem. 2016, 7, 6954–6963. [Google Scholar] [CrossRef]
- Asami, R.; Atobe, M.; Fuchigami, T. Electropolymerization of an immiscible monomer in aqueous electrolytes using acoustic emulsification. J. Am. Chem. Soc. 2005, 127, 13160–13161. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, L.; Gu, C.; Xu, Y.; Liu, D.; Lu, D.; Ma, Y. Electroactive Self-Assembled Monolayers for Enhanced Efficiency and Stability of Electropolymerized Luminescent Films and Devices. Adv. Funct. Mater. 2011, 21, 2896–2900. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Ihns, M.; Li, M.; Hwang, J.Y.; Mousavi, M.F.; Chaney, L.; Lech, A.T.; Kaner, R.B. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage. Proc. Natl. Acad. Sci. USA 2015, 112, 4233–4238. [Google Scholar] [CrossRef]
- Qin, K.; Kang, J.; Li, J.; Shi, C.; Li, Y.; Qiao, Z.; Zhao, N. Free-standing porous carbon nanofiber/ultrathin graphite hybrid for flexible solid-state supercapacitors. ACS Nano 2015, 9, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y. All Pseudocapacitive MXene-RuO 2 Asymmetric Supercapacitors MXene for supercapacitors View project Venture capital View project. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Wu, Z.S.; Parvez, K.; Feng, X.; Müllen, K. Graphene-based in-plane micro-supercapacitors with high power and energy densities. Nat. Commun. 2013, 4, 2487. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Parvez, K.; Feng, X.; Müllen, K. Photolithographic fabrication of high-performance all-solid-state graphene-based planar micro-supercapacitors with different interdigital fingers. J. Mater. Chem. A 2014, 2, 8288–8293. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zheng, Y.; Zheng, S.; Wang, S.; Sun, C.; Parvez, K.; Ikeda, T.; Bao, X.; Müllen, K.; Feng, X. Stacked-Layer Heterostructure Films of 2D Thiophene Nanosheets and Graphene for High-Rate All-Solid-State Pseudocapacitors with Enhanced Volumetric Capacitance. Adv. Mater. 2017, 29, 1602960. [Google Scholar] [CrossRef]
- Liu, W.; Lu, C.; Wang, X.; Tay, R.Y.; Tay, B.K. High-performance microsupercapacitors based on two-dimensional graphene/manganese dioxide/silver nanowire ternary hybrid film. ACS Nano 2015, 9, 1528–1542. [Google Scholar] [CrossRef]
- Pu, X.; Liu, M.; Li, L.; Han, S.; Li, X.; Jiang, C.; Du, C.; Luo, J.; Hu, W.; Wang, Z.L. Wearable Textile-Based In-Plane Microsupercapacitors. Adv. Energy Mater. 2016, 6, 1601254. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, Y.; Fan, Z. Printable Fabrication of Nanocoral-Structured Electrodes for High-Performance Flexible and Planar Supercapacitor with Artistic Design. Adv. Mater. 2017, 29, 1701736. [Google Scholar] [CrossRef]
- Dillon, A.D.; Ghidiu, M.J.; Krick, A.L.; Griggs, J.; May, S.J.; Gogotsi, Y.; Barsoum, M.W.; Fafarman, A.T. Highly Conductive Optical Quality Solution-Processed Films of 2D Titanium Carbide. Adv. Funct. Mater. 2016, 26, 4162–4168. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Akuzum, B.; Kurra, N.; Zhao, M.Q.; Alhabeb, M.; Anasori, B.; Kumbur, E.C.; Alshareef, H.N.; Ger, M.-D.; Gogotsi, Y. All-MXene (2D titanium carbide) solid-state microsupercapacitors for on-chip energy storage. Energy Environ. Sci. 2016, 9, 2847–2854. [Google Scholar] [CrossRef]
- Kurra, N.; Jiang, Q.; Alshareef, H.N. A general strategy for the fabrication of high performance microsupercapacitors. Nano Energy 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Guo, R.; Chen, J.; Yang, B.; Liu, L.; Su, L.; Shen, B.; Yan, X. In-Plane Micro-Supercapacitors for an Integrated Device on One Piece of Paper. Adv. Funct. Mater. 2017, 27, 1702394. [Google Scholar] [CrossRef]
- Kurra, N.; Alhebshi, N.A.; Alshareef, H.N. Microfabricated Pseudocapacitors Using Ni(OH)2 Electrodes Exhibit Remarkable Volumetric Capacitance and Energy Density. Adv. Energy Mater. 2015, 5, 1401303. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Huang, Y.; Li, H.; Wang, Z.; Pei, Z.; Xue, Q.; Geng, H.; Zhi, C. A Highly Durable, Transferable, and Substrate-Versatile High-Performance All-Polymer Micro-Supercapacitor with Plug-and-Play Function. Adv. Mater. 2017, 29, 1605137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Kurra, N.; Alshareef, H.N. Marker Pen Lithography for Flexible and Curvilinear On-Chip Energy Storage. Adv. Funct. Mater. 2015, 25, 4976–4984. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Iqbal, A.; Sambyal, P.; Koo, C.M. 2D MXenes for Electromagnetic Shielding: A Review. Adv. Funct. Mater. 2020, 30, 2000883. [Google Scholar] [CrossRef]
- Jimmy, J.; Kandasubramanian, B. Mxene functionalized polymer composites: Synthesis and applications. Eur. Polym. J. 2020, 122, 109367. [Google Scholar] [CrossRef]
- Yang, H.; Dai, J.; Liu, X.; Lin, Y.; Wang, J.; Wang, L.; Wang, F. Layered PVB/Ba3Co2Fe24O41/Ti3C2 Mxene composite: Enhanced electromagnetic wave absorption properties with high impedance match in a wide frequency range. Mater. Chem. Phys. 2017, 200, 179–186. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Chen, Q.; Li, P.; Zhou, A.; Cao, X.; Hu, Q. Preparation, mechanical and anti-friction performance of MXene/polymer composites. Mater. Des. 2016, 92, 682–689. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Ma, L.; Gao, J.; Jiang, Y. Acetylcholinesterase/chitosan-transition metal carbides nanocomposites-based biosensor for the organophosphate pesticides detection. Biochem. Eng. J. 2017, 128, 243–249. [Google Scholar] [CrossRef]
- Xu, H.; Holzwarth, J.M.; Yan, Y.; Xu, P.; Zheng, H.; Yin, Y.; Li, S.; Ma, P.X. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials 2014, 35, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.H.; Camp, J.P.; Hudson, T.W.; Schmidt, C.E. Synthesis and characterization of polypyrrole-hyaluronic acid composite biomaterials for tissue engineering applications. J. Biomed. Mater. Res. 2000, 50, 574–584. [Google Scholar] [CrossRef]
- Broda, C.R.; Lee, J.Y.; Sirivisoot, S.; Schmidt, C.E.; Harrison, B.S. A chemically polymerized electrically conducting composite of polypyrrole nanoparticles and polyurethane for tissue engineering. J. Biomed. Mater. Res. Part A 2011, 98A, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hobbs, H.L.; Wang, L.; Rutten, M.J.; Wamser, C.C. Biocompatible composites of polyaniline nanofibers and collagen. Synth. Met. 2009, 159, 1313–1318. [Google Scholar] [CrossRef]
- Kiefer, R.; Lee, R.J.; Temmer, R.; Tamm, T.; Aabloo, A. Chitosan Combined with Conducting Polymers for Novel Functionality: Antioxidant and Antibacterial Activity. Key Eng. Mater. 2014, 605, 428–431. [Google Scholar] [CrossRef]
- Stewart, E.M.; Fabretto, M.; Mueller, M.; Molino, P.J.; Griesser, H.J.; Short, R.D.; Wallace, G.G. Cell attachment and proliferation on high conductivity PEDOT–glycol composites produced by vapour phase polymerisation. Biomater. Sci. 2013, 1, 368–378. [Google Scholar] [CrossRef]
- Takano, T.; Mikazuki, A.; Kobayashi, T. Conductive polypyrrole composite films prepared using wet cast technique with a pyrrole-cellulose acetate solution. Polym. Eng. Sci. 2014, 54, 78–84. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Bajpai, J.; Soni, S.N. Designing Polyaniline (PANI) and Polyvinyl Alcohol (PVA) Based Electrically Conductive Nanocomposites: Preparation, Characterization and Blood Compatible Study. J. Macromol. Sci. Part A 2009, 46, 774–782. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Giannotti, M.I.; Armelin, E.; Sanz, F.; Alemán, C. Electronic, electric and electrochemical properties of bioactive nanomembranes made of polythiophene:thermoplastic polyurethane. Polym. Chem. 2014, 5, 1248–1257. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.G.; Kim, I. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ates, M.; Caliskan, S.; Özten, E. Supercapacitor study of reduced graphene oxide/Zn nanoparticle/polycarbazole electrode active materials and equivalent circuit models. J. Solid State Electrochem. 2018, 22, 3261–3271. [Google Scholar] [CrossRef]
- Pu, N.-W.; Chen, C.-Y.; Qiu, H.-X.; Liu, Y.-M.; Song, C.-H.; Lin, M.-H.; Ger, M.-D. Hydrothermal Synthesis of N-Doped Graphene/Fe2O3 Nanocomposite for Supercapacitors. Int. J. Electrochem. Sci 2018, 13, 6812–6823. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta 2006, 52, 1721–1726. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X. Synthesis, structure and electrochemical properties of lanthanum manganese nanofibers doped with Sr and Cu. J. Alloys Compd. 2015, 638, 204–213. [Google Scholar] [CrossRef]
- Wan, Y.; Li, J.; Yang, Z.; Ao, H.; Xiong, L.; Luo, H. Simultaneously depositing polyaniline onto bacterial cellulose nanofibers and graphene nanosheets toward electrically conductive nanocomposites. Curr. Appl. Phys. 2018, 18, 933–940. [Google Scholar] [CrossRef]
- Abraham, J.; Arif, P.M.; Xavier, P.; Bose, S.; George, S.C.; Kalarikkal, N.; Thomas, S. Investigation into dielectric behaviour and electromagnetic interference shielding effectiveness of conducting styrene butadiene rubber composites containing ionic liquid modified MWCNT. Polymer 2017, 112, 102–115. [Google Scholar] [CrossRef]
- Poothanari, M.A.; Abraham, J.; Kalarikkal, N.; Thomas, S. Excellent Electromagnetic Interference Shielding and High Electrical Conductivity of Compatibilized Polycarbonate/Polypropylene Carbon Nanotube Blend Nanocomposites. Ind. Eng. Chem. Res. 2018, 57, 4287–4297. [Google Scholar] [CrossRef]
- Du, X.; Zhao, W.; Wang, Y.; Wang, C.; Chen, M.; Qi, T.; Hua, C.; Ma, M. Preparation of activated carbon hollow fibers from ramie at low temperature for electric double-layer capacitor applications. Bioresour. Technol. 2013, 149, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Kim, S.; Lee, J.H.; Hwang, I.S.; Kim, I.D. Phase evolution of perovskite LaNiO3 nanofibers for supercapacitor application and p-type gas sensing properties of LaOCl-NiO composite nanofibers. J. Mater. Chem. 2011, 21, 1959–1965. [Google Scholar] [CrossRef]
- He, S.; Hu, X.; Chen, S.; Hu, H.; Hanif, M.; Hou, H. Needle-like polyaniline nanowires on graphite nanofibers: Hierarchical micro/nano-architecture for high performance supercapacitors. J. Mater. Chem. 2012, 22, 5114–5120. [Google Scholar] [CrossRef]
- Liao, C.; Wu, S. Pseudocapacitance behavior on Fe3O4-pillared SiOx microsphere wrapped by graphene as high performance anodes for lithium-ion batteries. Chem. Eng. J. 2019, 355, 805–814. [Google Scholar] [CrossRef]
- Naresh, N.; Narsimulu, D.; Jena, P.; Srinadhu, E.S.; Satyanarayana, N. Microwave-assisted hydrothermal synthesis of SnO2/reduced graphene-oxide nanocomposite as anode material for high performance lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 14723–14732. [Google Scholar] [CrossRef]






| SN | MXene | Polymer | Result | Application | Ref. |
|---|---|---|---|---|---|
| 1. | Ti3C2Tx | PVB | RLmax value of −46.3 dB at 5.8 GHz | EMIS | [224] |
| 2. | Ti3C2Tx | UHMWPE | Addition of Ti3C2 increases antifriction properties, mechanical strengths, and crystalline property | Improving mechanical properties | [225] |
| 3. | Ti3C2Tx | PES | Gentian Dye with flux 117.6 9 Lm−2h−1 rejects 80.3% and that with 114.9 Lm−2h−1 rejects 10.7% at pressure of 0.1 MPa. | Ultrafiltration membranes for purification | [83] |
| 4. | Ti3C2Tx | Chitosan | Recover 94–105% for malathion recovery in tap water. | Biosensor | [226] |
| 5. | Ti3C2Tx | Cellulose Nano fibers | EMIS ~25.8 dB at 12.4 GHz with 80% of d-Ti3C2Tx and ρ ~739.4 S m−1. | EMIS | [93] |
| 6. | Ti3C2Tx | PS | Improved electrochemical performance. | Immobilization of soluble PS | [95] |
| 7. | Ti3C2Tx | PS | Capacity reduced 0.05%/cycle, the SC of 1200 mAhg−1 over 5 h. C/DC current rate and a CRR of 80% attained over 400 cycles at 2 h. C/DC current rate. | Supercapacitor | [96] |
| 8. | Ti3C2Tx | PVDF | The antibacterial rate of the fresh membrane reached 67% and 73% compared to that of PVDF, while aged membranes exhibited over 99% growth inhibition. | Anti-fouling ultrafiltration membrane | [31] |
| 9. | Ti3C2Tx | PVDF/PDMS | Highly efficient light-to-heat conversion rates at nearly 100%. | Photothermal conversion | [98] |
| 10. | Ti3C2Tx | P(VDF-TFE-CFE) | ~15 wt.% MXene raised dielectric permittivity to 105 and 10 wt.%. MXene raised the dielectric constant 25 times. | Enhanced electric properties | [99] |
| 11. | Ti3C2Tx | Polypyrrole | Attained maximum SC of 184.36 Fg−1 at 2 mVs−1 with CRR of 83.33% after 4000 charging cycles at 1 Ag−1 | Supercapacitors | [102] |
| 12. | Ti3C2Tx | PVA/PAA | Composite nanofibers displayed excellent catalytic activity against 4-NP. | Wastewater treatment | [104] |
| 13. | V2C | PDMAEMA | Increasing temperature from 25 °C to 45 °C increases the transmittance from 15% to 75%, and further addition of CO2 increases conductivity from 2.8 to 33.7 mS cm−1. | Responsive polymers | [107] |
| 14. | Ti3C2Tx | Polyurethane | 0.5 wt.% of MXene addition increases the stress by ~70%, tensile strength by ~20%, Pus hardness by ~10%, breaking elongation reduction by ~17%, and water absorption reduction by 10%. | Mechanical properties improvement | [111] |
| 15. | Ti3C2Tx | Polyaniline | 1:3 mass ratio shows microwave absorption of −56.3 dB at 13.80 GHz with an efficiency of 99.9999%. | Microwave absorption | [114] |
| 16. | Ti3C2Tx | P (3,4 EDOT: PSS) | The addition of 1M H2SO4 gives an excellent result of 1065 F cm−3 volumetric capacitance at 2 mV s−1. | Increase in volumetric capacitance for ASC. | [115] |
| 17. | Ti3C2Tx | Low density polyethylene | Better thermal stability of composites after the incorporation of MXene. | Study of thermal stability | [118] |
| 18. | Ti3C2Tx | P-3,4 EDOT | The C/DCC in the first cycle is 575 and 307 mA h g−1. After 100 cycles of charging and discharging, the capacitance was maintained at 83% with respect to its first cycle | Upgrade in Li-ion battery technology | [119] |
| 19. | Ti3C2Tx | Polyester | Made yarn with SC of 18.39 m F cm−2 at 5 mV s−1, a power density of 0.39 mW cm−2, and a power density of 0.38 μW h cm−2. The retention performance was 98.2% over 6000 cycles. | Gave yarn for wearable electronics devices. | [122] |
| 20. | Ti3C2Tx | P(3,4-EDOT): PSS | 70 wt.% MXene made the fiber with 1489 S cm−1 conductivity, 7.13 Wh cm-3 energy density, and 8249 mW cm−3 power density. | Conductive fibers | [116] |
| 21. | Ti3C2Tx | Polyacrylamide | The conductivity is increased to 3.3 × 10−2 S m−1 after the addition of 6 wt.% MXene onto the membrane. | Improved flexibility and conductivity | [126] |
| 22. | Ti3C2Tx | PEA/P(DMS) | PDMS and PEI membranes are good for non-polar and polar solvent systems. Large-sized PEG addition will enhance their rejection ability. | Solvent resistant nanofiltration in alcohol-based mixtures | [123] |
| 23. | Ti3C2Tx | GdW10-based Polyoxometalates | Eradicated tumor cell with Ti3C2 NSs as a contrast agent for contrast-enhanced CT and MR imaging. | CT/MRI-guided precise PTT of tumors | [24] |
| F * | MXene | Other Polymer Nanocomposites |
|---|---|---|
| Biomedicine | Ta4C3-IONP-SP nanocomposites are one of the examples used for MRI [17]. Ti3C2-SP, Ta4C3-SP, and MnOx/Ti3C2-SP are used for a photoacoustic signal method with the help of stress waves received from the irradiated tissues by NIR. Ta4C3-IONP-SPs and Ta4C3-SP nanocomposites can attenuate X-rays and are used in computed tomography (CT) [17,20,21]. Ti3C2 +colloidal solution allows the growth of Gram (−) E. coli and Gram (+) Bacillus subtilis [67] antibacterial growth. Ti3C2Tz + PLA + octyltriethoxysilane (OTES) has good mechanical properties and biocompatibility help in tissue engineering [171,172]. (GOx/Au/Ti3C2/Nafion/GCE) an enzymatic biosensor that detects glucose [152]. Therapeutics: PLGA)/Ti3C2 [173] used in cancer treatment by photothermal ablation. Ti3C2/Al treats cancer under 808nm laser radiation [17]. V2C nanosheets are effective photothermal agents for photothermal treatment with PA and MRI [174]. AIPH@Nb2C@mSiO2 nanocomposites were used for thermodynamic therapy to kill cancer cells deficient in oxygen [176]. (Ti3C2-DOX) generate ROS in photodynamic therapy that kills cancerous cells [175]. They are also used in drug delivery. Nb2C/polymer nanocomposites ablate tumors by photothermal processes around the near-infrared region [140]. MnOx/Ti3C2-SP and MnOx/Ta4C3-SP MXene nanocomposites are used for acidic tumors [20,145]. Ag @ Ti3C2 @Cu2O nanocomposites has photo catalyst appropriate for antibacterial purposes [146]. | PPy/poly(D, L-lactic acid) with conductivity 5.65 × 10−3 to 15.56 × 10−3 S/cm is nerve tissue regeneration (in vivo rat), biocompatibility (PC12 cells) and is used for synthetic nerve conduits [227]. PPy/hyaluronic acid with a conductivity of 3.08 × 10−3 Scm−1 can support tissue growth, stimulate specific cell functions, and be used for tissue engineering and wound-healing applications [228]. PPy nanoparticles/PU with maximum conductivity of 2.3 × 10−6 Scm−1. Cytocompatible with C2C12 myoblast cells, elastomeric properties tissue engineering [229]. PAni nanofibers/collagen with a conductivity of 0.27 Scm−1 is well suited for culture and is used as Scaffold material for biomedical applications [230]. PPy/chitosan (10−3–10−7 Scm−1) have radical scavenger property and is used for food packaging and biomedical applications [231]. PEDOT/glycol (1486 Scm−1) and PPy/cellulose acetate (6.9 × 10−4 to 3.6 × 101 Scm−1) used as implantable devices [232,233]. Poly (acrylic acid)/polyvinyl alcohol (0.04–0.06 S/cm) with hydrogel, biocompatible, good mechanical strength, and good swelling properties [234]. Polythiophene derivative/PU with a conductivity of 2.23 × 10−5 (S/cm) is suitable for supporting electrically stimulated cell growth tissue engineering [235]. Hollow polymeric nanosphere-supported imidazolium-based ionic liquids (HPL-ILs) show enhanced antimicrobial activities [236]. Poly (-L-glutamic acid (PGA) based nanomaterials are highly efficient for drug delivery [237]. The porous organic polymers (POPs) are highly efficient for drug delivery [238]. |
| * Physical/Biosensors | AChE/CS/Ti3C2Tx biosensors detect organophosphates in water and food. AChE/CS-Ti3C2Tx/GCE biosensors indicated 94–105% malathion recovery [83] and detect the glucose level in diabetic patients, pollution monitoring, food processing, etc. CS/Ti3C2 [167] shows a fast response time (109.6 ms) with a recovery time of 110.6 ms and higher cycle stability even after 150,000 cycles. Similarly, Mo2C, Cr3C2, and polymer (PAM, PVA) composites [163], Ti3C2Tx/PDAC [141] were good humidity sensors. MXene/Polyelectrolyte [141] and Ti3C2Tx/polyimide nanocomposites were used as humidity sensors for breath checking for diagnosis. MXene/PVA hydrogel flexible sensor can detect diabetes indicated by the presence of Acetone and ammonia [142]. | CNT@CIP-based nanocomposites show a good sensing property at a low frequency (5, 10, and 20 Hz), showing 100% flexibility, repeatability of R2 > 0.98, and gauge factor 2.2 with the fractional change in resistance of 160% and excellent repeatability even after 500 cycles [169]. Pani/BC hydrogel-type composites have a conductivity of 10−2 (S/cm) and are applicable for biosensors and tissue engineering [88]. PEDOT: PSS/PU (aqueous dispersion) ~120 High pressure sensitivity electronic skin sensor [93]. PEDOT: PSS/PU/ionic liquid 8.8 × 10−5 (S/cm) are mechanically flexible, stretchable and actuating devices [91]. |
| Supercapacitors | Capacity reduced 0.05%/cycle, the SC of 1200 mAhg−1 over 5 h. C/DC current rate and a CRR of 80% reached over 400 cycles at 2 h. C/DC current rate [96]. Attained maximum SC of 184.36 Fg−1 at 2 mVs−1 with CRR of 83.33% after 4000 charging cycles at 1 Ag−1 [102]. Adding 1M H2SO4 gives an excellent result of 1065 F cm−3 volumetric capacitance at 2 mV s−1 [115]. | rGO/Zn/PCz nanocomposites have an improved capacitance of 33.88 F/g [239]. N-doped graphene/Fe2O3 nanocomposites exhibited a 354 F/g at a current density of 20 A/g [240]. PANI/SWCNT has a capacitance of 485 F/g [241]. LaxSr1−xCu0.1Mn0.9O3−δ (0.3 ≤ x ≤ 1) at 2 A/g current density displayed a capacitance of 464 F/g and that at 64.5 Wh/kg 2 A/g displayed a power density of 2 kW/kg [242]. |
| EMIS | PVB/Co2Z/Ti3C2 has RLmax of −46.3 dB at 5.8 GHz and below −10 dB at 1.6 GHz [224]. EMIS 25.8 dB at 12.4 GHz with 80% of d-Ti3C2Tx having ~739.4 S m−1 conductivity [93]. | The conductivity of BC/GE/PANI is 1.7 ± 0.1 S/cm [243]. MWCNT/SBR exhibited a shielding efficiency of 35.06 dB [244]. PP/PC/MWCNT shows shielding of 54.78 dB at 0.33 S/cm conductivity [245]. |
| Conducing Fibers | Made yarn with SC of 18.39 m Fcm−2 at 5 mV s−1, 0.39 mW cm−2 power density with 0.38 μW h cm−2 energy density. The retention performance was 98.2% over 6000 cycles [122]. 70 wt.% MXene made the fiber have ~1489 Scm−1 conductivity, 7.13 Wh cm−3 energy density, and 8249 mW cm−3 power density [116]. | Carbon hollow fibers show a 287 F/g capacitance at 50 mA/g with CRR 86.4% at 1 A/g [246]. LaNiO3 constituted nanofibers ~160 F/g capacitance at ~10 mV/s [247]. GE/PANI nanofiber 976 F/g capacitance at 0.4 A/g current density [248]. |
| Energy Storage | The C/DCC in the first cycle is 575 with 307 mA h g−1, of which 83% is maintained after 100 cycles [119]. V2O5/MXene shows SC of 768 F/g (at 1 A/g), a specific capacity of 93.3% after the 6000 GDC test, and increased current density from 1 to 5 A/g [59] | SiOx/Fe3O4/FLG has a CRR of 81.8% valued at 833.4 mAh/g (1550 mAh/cm3) at a current density of 0.5 A/g after 500 cycles [249]. SnO2/rGO nanocomposites show a CRR of 318 mAh/g at a current density of 500 mA/g after 300 cycles [250]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parajuli, D.; Murali, N.; K. C., D.; Karki, B.; Samatha, K.; Kim, A.A.; Park, M.; Pant, B. Advancements in MXene-Polymer Nanocomposites in Energy Storage and Biomedical Applications. Polymers 2022, 14, 3433. https://doi.org/10.3390/polym14163433
Parajuli D, Murali N, K. C. D, Karki B, Samatha K, Kim AA, Park M, Pant B. Advancements in MXene-Polymer Nanocomposites in Energy Storage and Biomedical Applications. Polymers. 2022; 14(16):3433. https://doi.org/10.3390/polym14163433
Chicago/Turabian StyleParajuli, D., N. Murali, Devendra K. C., Bhishma Karki, K. Samatha, Allison A Kim, Mira Park, and Bishweshwar Pant. 2022. "Advancements in MXene-Polymer Nanocomposites in Energy Storage and Biomedical Applications" Polymers 14, no. 16: 3433. https://doi.org/10.3390/polym14163433
APA StyleParajuli, D., Murali, N., K. C., D., Karki, B., Samatha, K., Kim, A. A., Park, M., & Pant, B. (2022). Advancements in MXene-Polymer Nanocomposites in Energy Storage and Biomedical Applications. Polymers, 14(16), 3433. https://doi.org/10.3390/polym14163433












