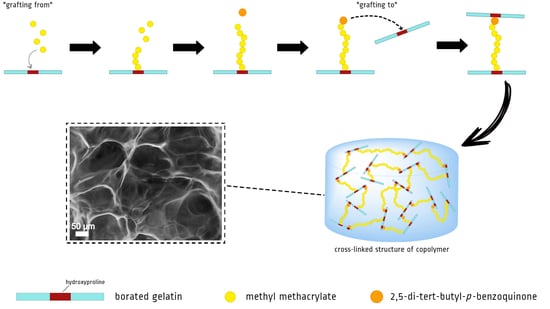
Grafting of Methyl Methacrylate onto Gelatin Initiated by Tri-Butylborane—2,5-Di-Tert-Butyl-p-Benzoquinone System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TBB
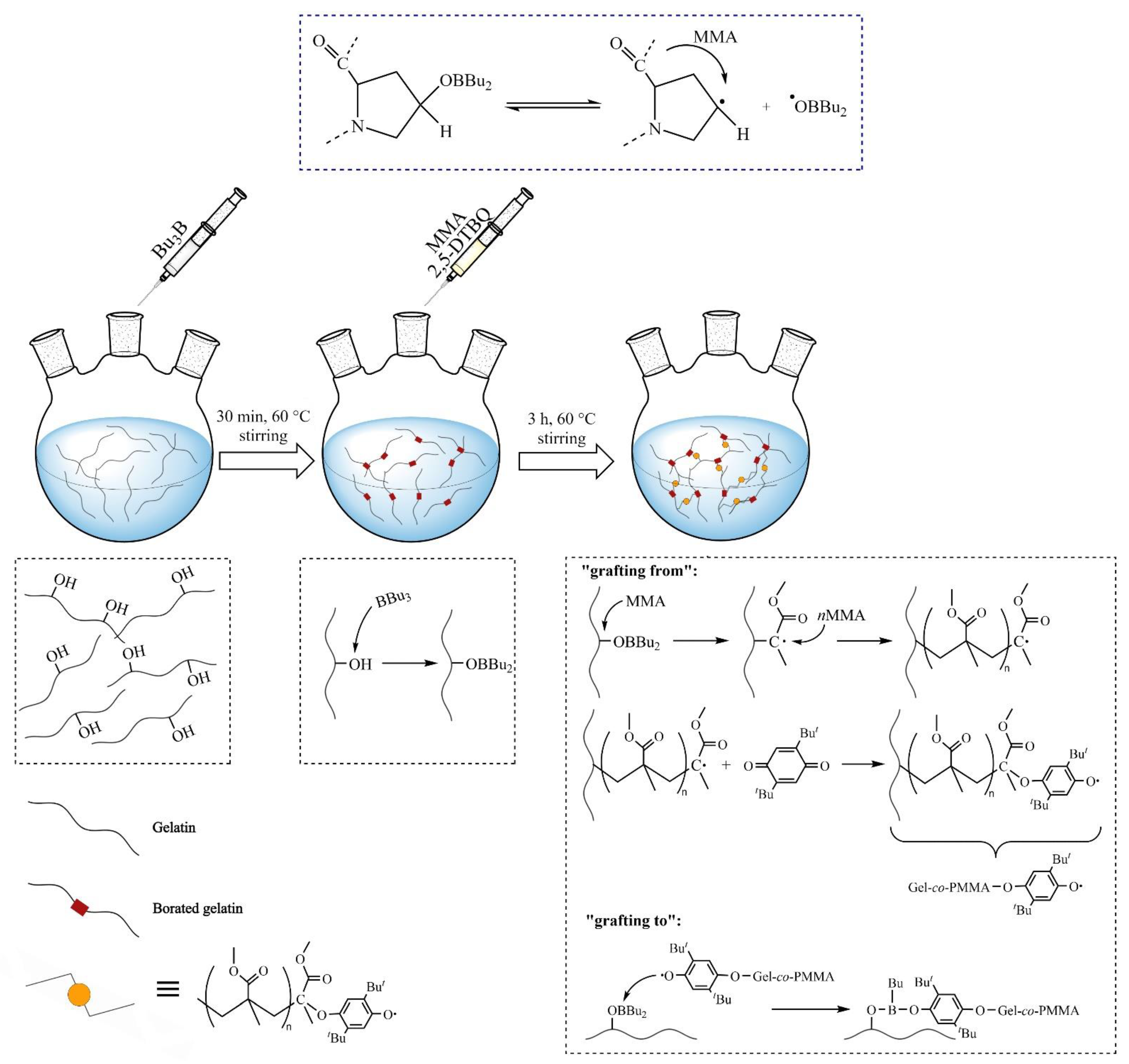
2.3. Polymerization Procedure
2.4. Determination of Unreacted Monomer
2.5. Enzymatic Hydrolysis
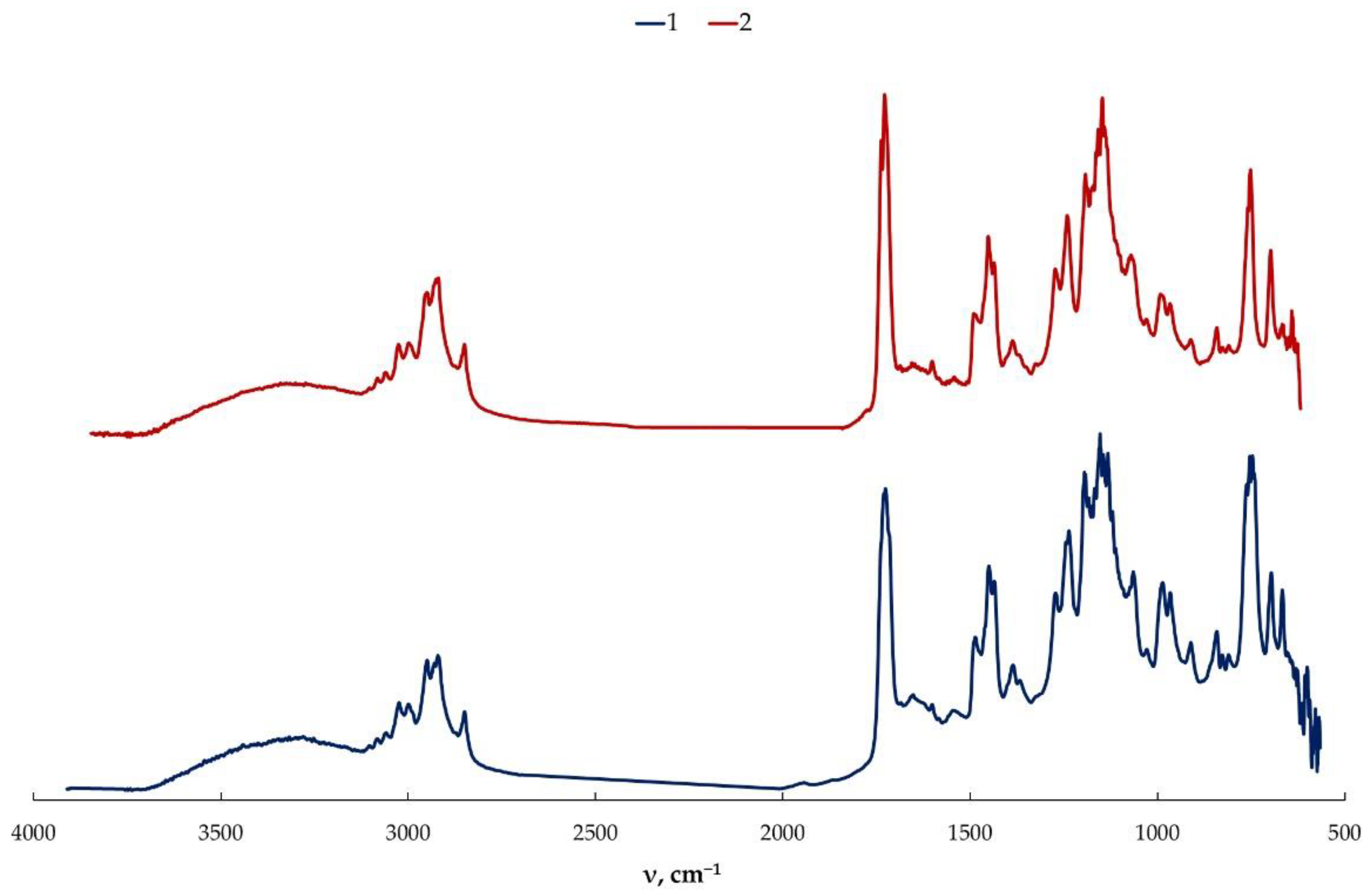
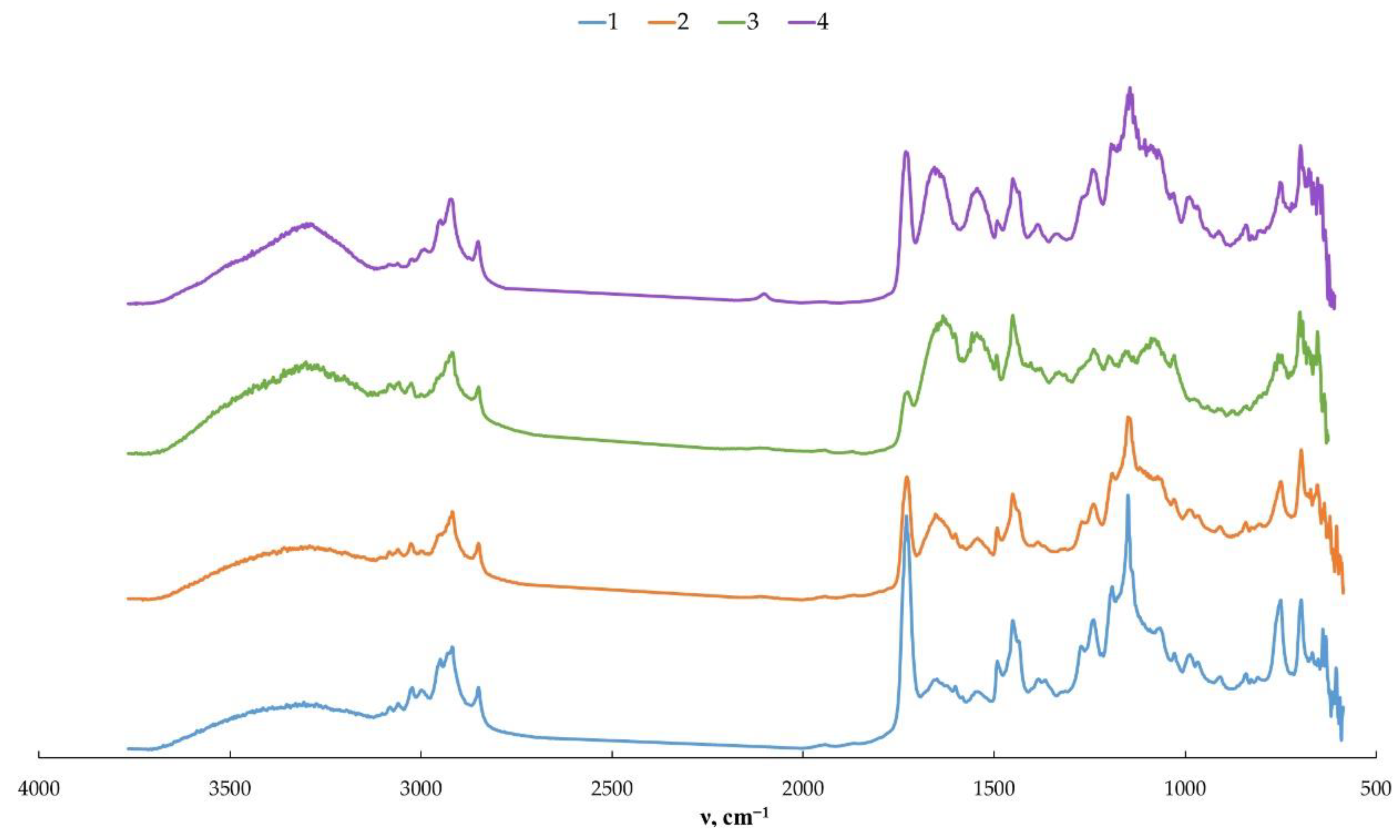
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
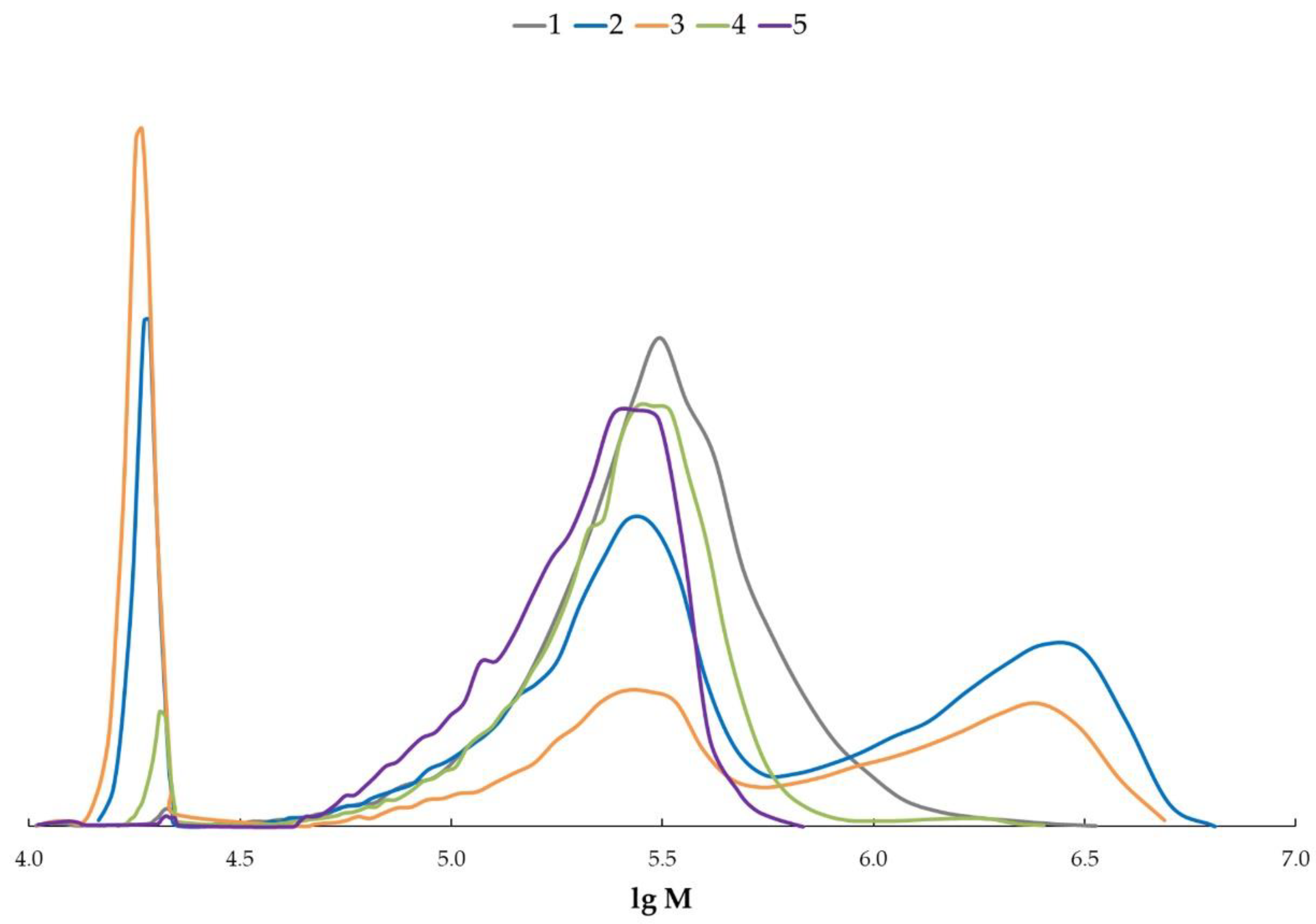
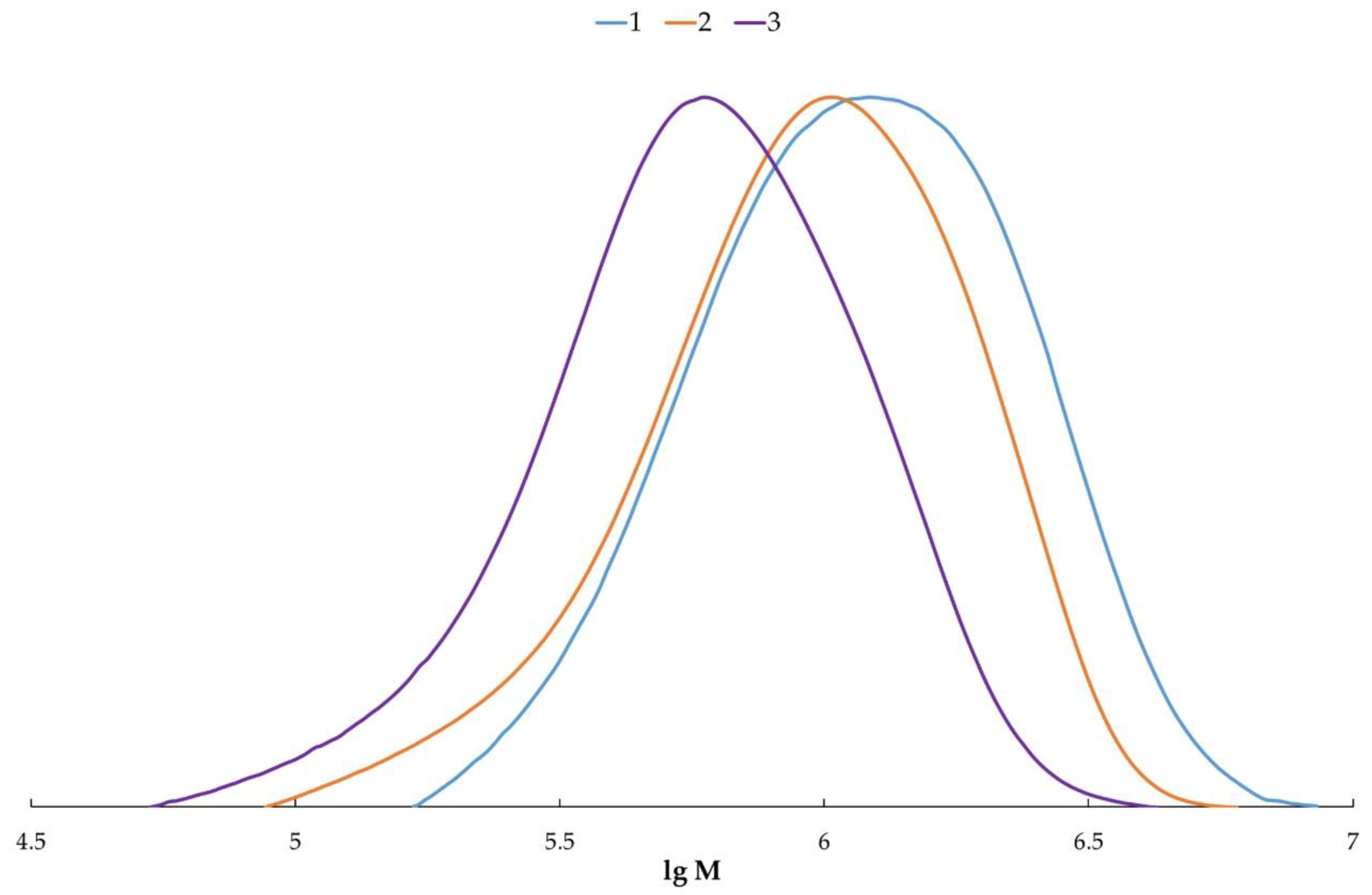
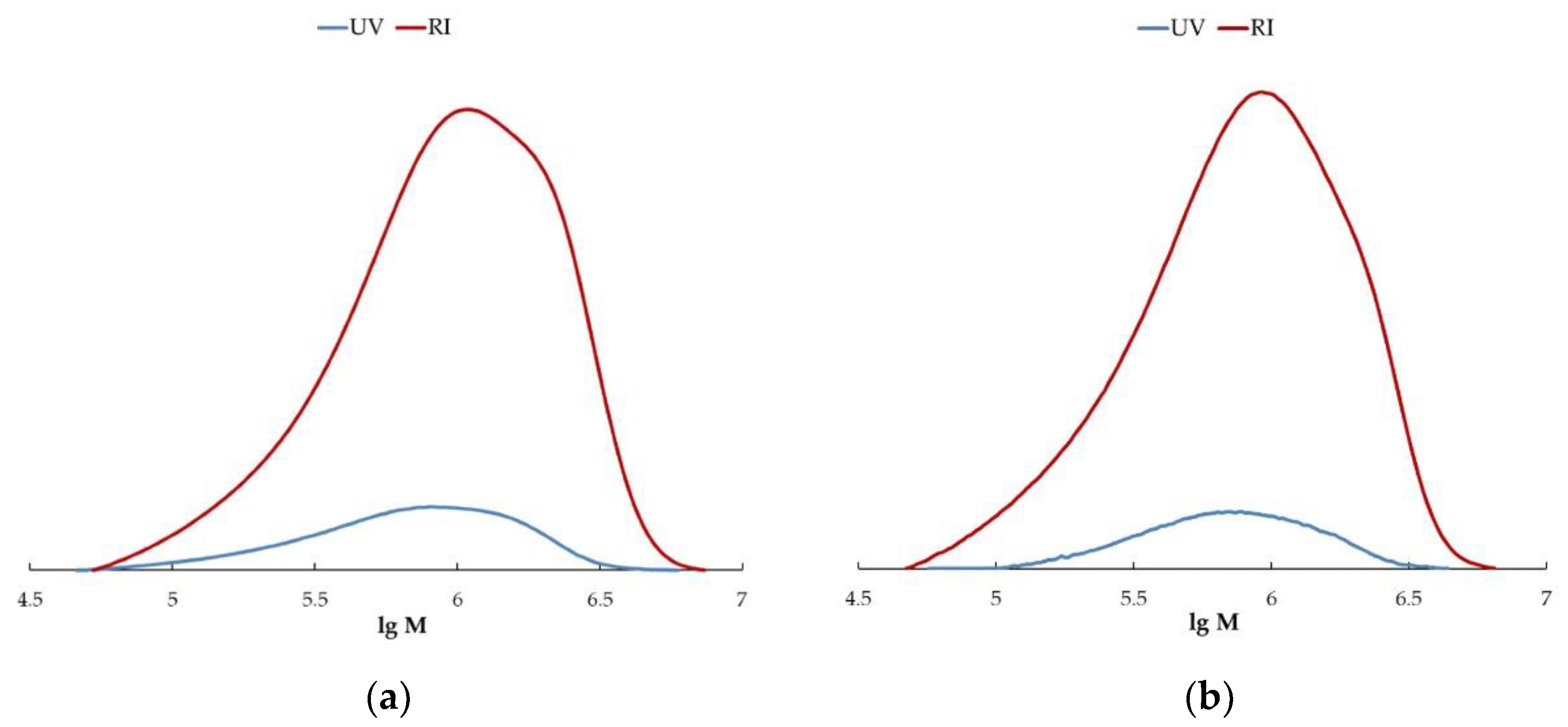
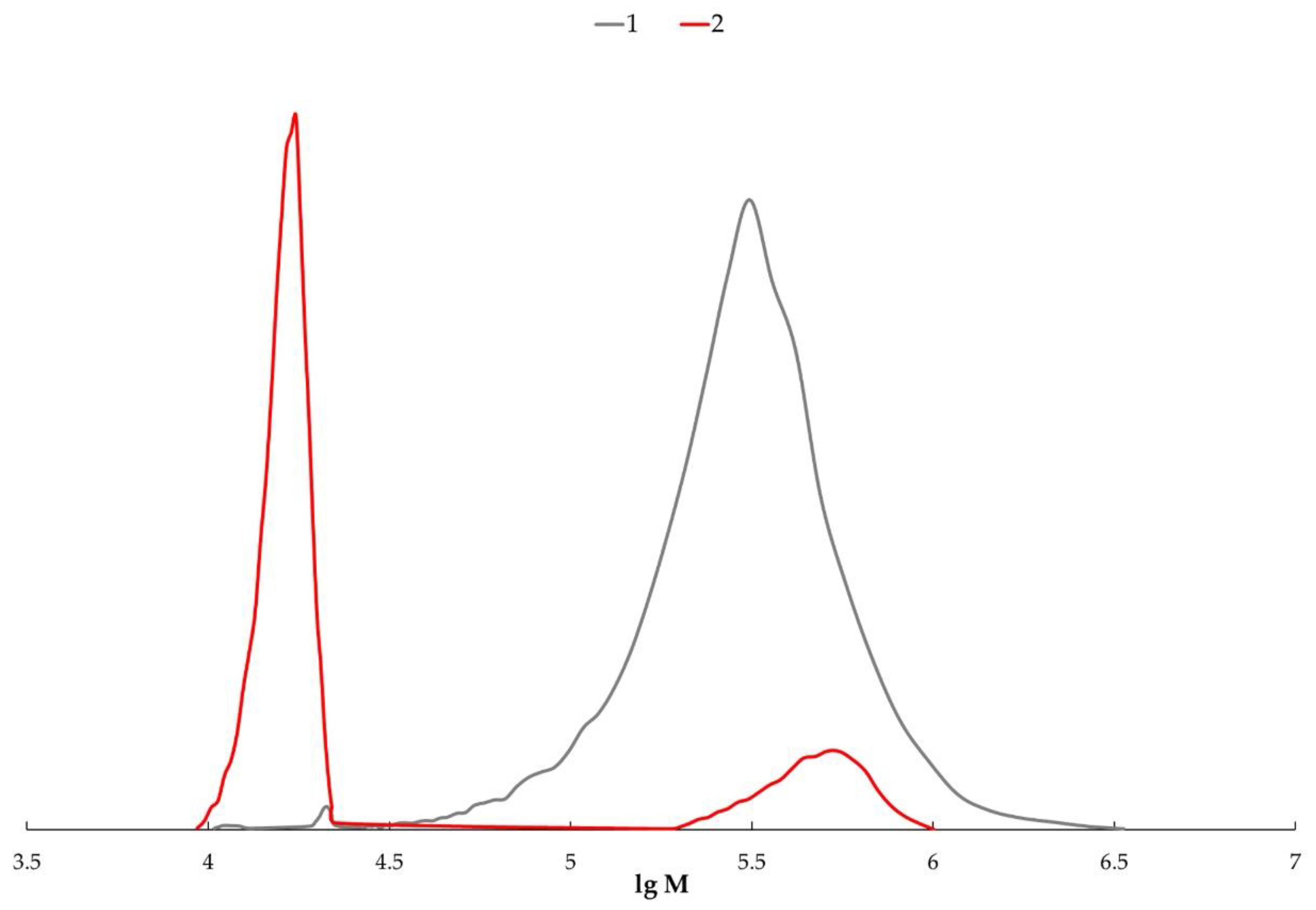
2.7. Size-Exclusion Chromatography
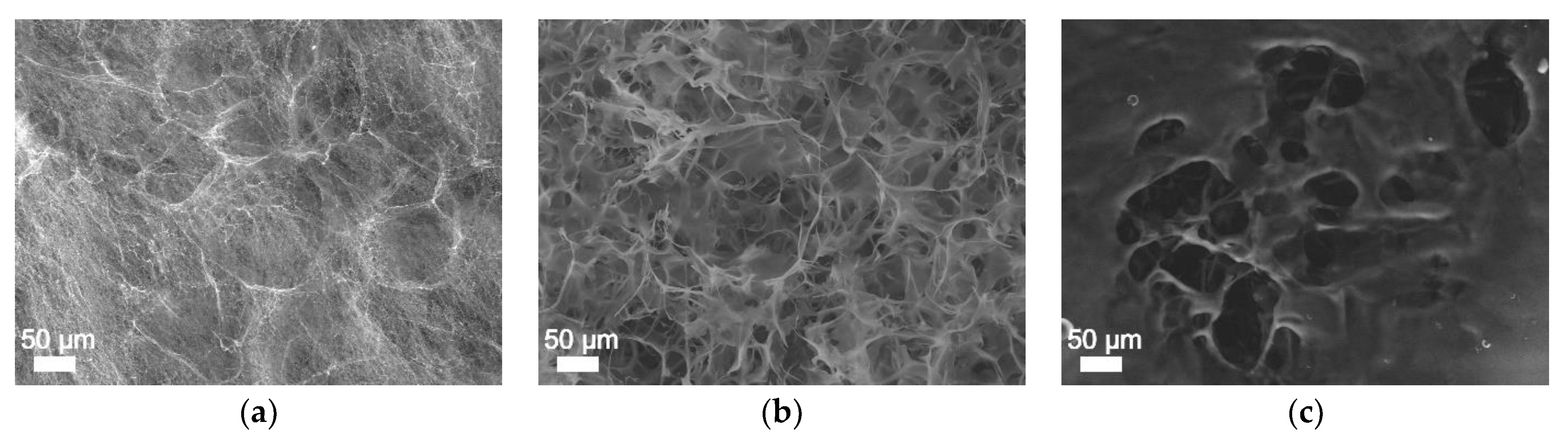
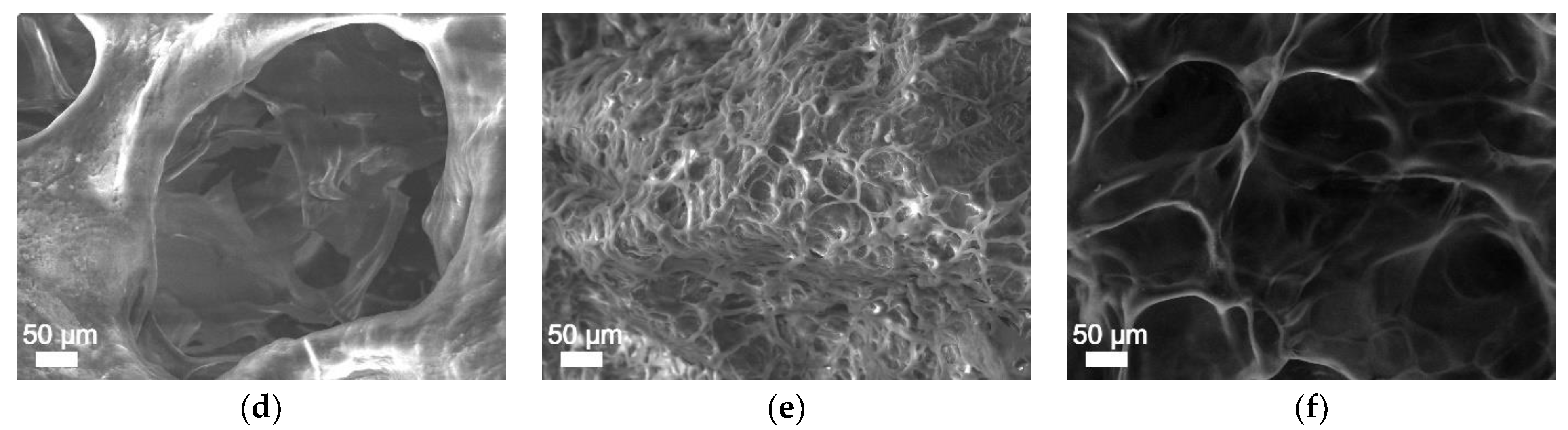
2.8. Surface Morphology Analysis
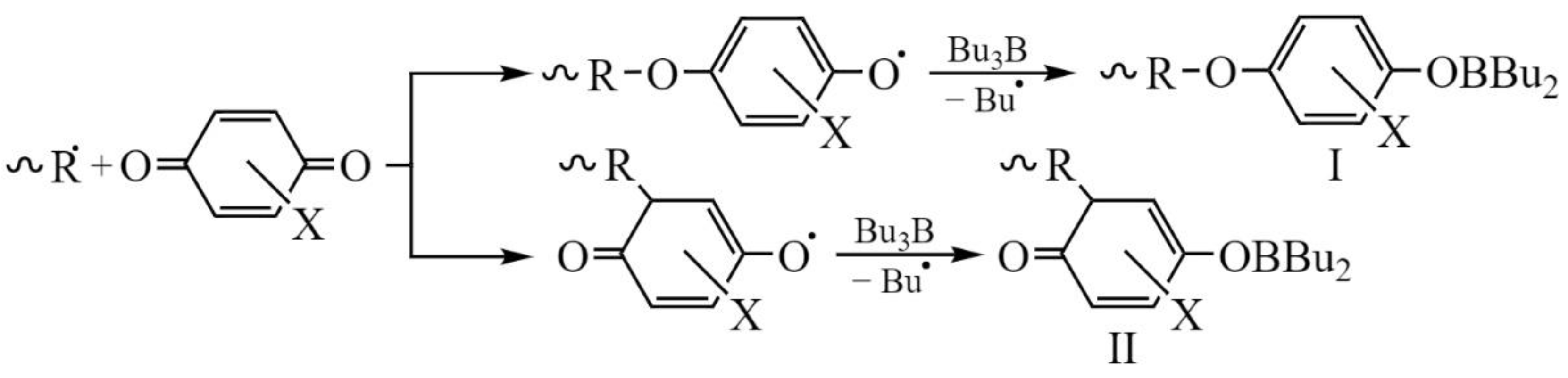
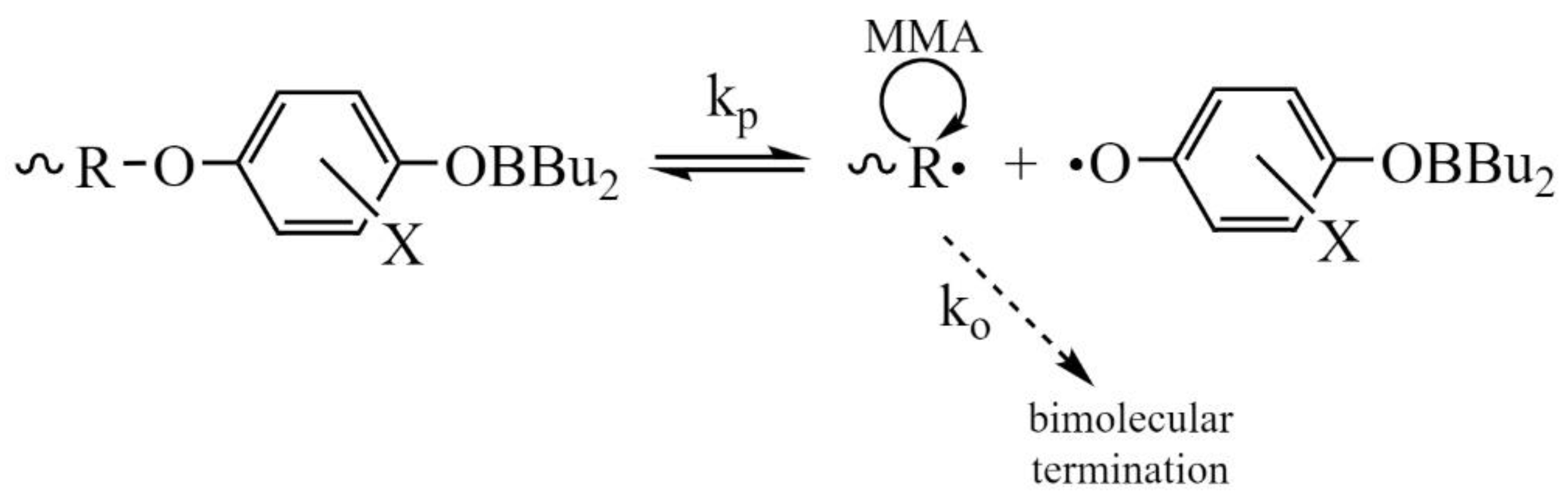
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schweizer, T.A.; Shambat, S.M.; Haunreiter, V.D.; Mestres, C.A.; Weber, A.; Maisano, F.; Zinkernagel, A.S.; Hasse, B. Polyester vascular graft material and risk for intracavitary thoracic vascular graft infection. Emerg. Infect. Dis. 2020, 26, 2448–2452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Q.; Lv, S.; Lu, J.; Jiang, S.; Regenstein, J.M.; Lin, L. Comparison of collagen and gelatin extracted from the skins of Nile tilapia (Oreochromis niloticus) and channel catfish (Ictalurus punctatus). Food Biosci. 2016, 13, 41–48. [Google Scholar] [CrossRef]
- Miele, D.; Catenacci, L.; Rossi, S.; Sandri, G.; Sorrenti, M.; Terzi, A.; Giannini, C.; Riva, F.; Ferrari, F.; Caramella, C.; et al. Collagen/PCL Nanofibers Electrospun in Green Solvent by DOE Assisted Process. An Insight into Collagen Contribution. Materials 2020, 13, 4698. [Google Scholar] [CrossRef]
- Cao, J.; Wang, P.; Liu, Y.; Zhu, C.; Fan, D. Double crosslinked HLC-CCS hydrogel tissue engineering scaffold for skin wound healing. Biol. Macromol. 2020, 155, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Borrego-González, S.; Dalby, M.J.; Díaz-Cuenca, A. Nanofibrous Gelatin-Based Biomaterial with Improved Biomimicry Using D-Periodic Self-Assembled Atelocollagen. Biomimetics 2021, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Guerrero, A.; Romero, A. Gelatin vs collagen-based sponges: Evaluation of concentration, additives and biocomposites. J. Polym. Res. 2019, 26, 190. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, Y.; Zheng, J.; Cao, Q.; Li, S.; He, L.; Wei, B.; Zhang, J.; Xu, C.; Wang, H. Refolding behavior of urea-induced denaturation collagen. Macromol. Res. 2020, 29, 402–410. [Google Scholar] [CrossRef]
- He, L.; Li, S.; Xu, C.; Wei, B.; Zhang, J.; Xu, Y.; Zhu, B.; Cao, Y.; Wu, X.; Xiong, Z.; et al. A new method of gelatin modified collagen and viscoelastic study of gelatin-collagen composite hydrogel. Macromol. Res. 2020, 28, 861–868. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chakraborty, E. Hydrogel based tissue engineering and its future applications in personalized disease modeling and regenerative therapy. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 3. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Reis, R.; Silva, T.H. Marine origin materials on biomaterials and advanced therapies to cartilage tissue engineering and regenerative medicine. Biomater. Sci. 2021, 9, 6718–6736. [Google Scholar] [CrossRef] [PubMed]
- Mahjoubnia, A.; Haghbin Nazarpak, M.; Karkhaneh, A. Polypyrrole-chitosan hydrogel reinforced with collagen-grafted PLA sub-micron fibers as an electrically responsive scaffold. Int. J. Polym. Mater. 2022, 71, 302–314. [Google Scholar] [CrossRef]
- León-Campos, M.I.; Claudio-Rizo, J.A.; Rodriguez-Fuentes, N.; Cabrera-Munguía, D.A.; Becerra-Rodriguez, J.J.; Herrera-Guerrero, A.; Soriano-Corral, F. Biocompatible interpenetrating polymeric networks in hydrogel state comprised from jellyfish collagen and polyurethane. J. Polym. Res. 2021, 28, 291. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Arif, M.M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Khan, R.U.; Awais, S.M.; Butt, M.A. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.M.; Palakurthi, S.S.; Khare, T.; Khare, S.; Palakurthi, S. Natural proteins and polysaccharides in the development of micro/nano delivery systems for the treatment of inflammatory bowel disease. Int. J. Biol. Macromol. 2020, 165, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; An, X.; Liu, L.; Tang, S.; Cao, H.; Xu, Q.; Liu, H. Cellulose, hemicellulose, lignin, and their derivatives as multi-components of bio-based feedstocks for 3D printing. Carbohydr. Polym. 2020, 250, 116881. [Google Scholar] [CrossRef]
- Levato, R.; Lim, K.S.; Li, W.; Asua, A.U.; Peña, L.B.; Wang, M.; Falandt, M.; Bernal, P.N.; Gawlitta, D.; Zhang, Y.S.; et al. High-resolution lithographic biofabrication of hydrogels with complex microchannels from low-temperature-soluble gelatin bioresins. Mater. Today Bio 2021, 12, 100162. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.A.; Popova, O.P.; Danilova, T.I.; Kuznetsova, A.V. Strategy of the selection and use of scaffolds in bioengineering. Biol. Bul. Rev. 2019, 139, 196–205. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2017, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Al Kayal, T.; Losi, P.; Pierozzi, S.; Soldani, G. A New Method for Fibrin-Based Electrospun/Sprayed Scaffold Fabrication. Sci. Rep. 2020, 10, 5111. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.O.; Martins, E.; Carvalho, D.N.; Alves, A.L.; Oliveira, C.; Duarte, A.R.; Silva, T.H.; Reis, R.L. Collagen from Atlantic cod (Gadus morhua) skins extracted using CO2 acidified water with potential application in healthcare. J. Polym. Res. 2020, 27, 73. [Google Scholar] [CrossRef]
- Castilho, M.; Hochleitner, G.; Wilson, W.; van Rietbergen, B.; Dalton, P.D.; Groll, J.; Malda, J.; Ito, K. Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds. Sci. Rep. 2018, 8, 1245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-J.; Xu, J.; Qiu, Z.-Y.; Ma, X.-L.; Zhang, Z.-Q.; Tan, X.-X.; Cui, Y.; Cui, F.-Z. Mechanical Properties and Cytocompatibility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen. Materials 2015, 8, 2616–2634. [Google Scholar] [CrossRef]
- Vedhanayagam, M.; Anandasadagopan, S.; Nair, B.U.; Sreeram, K.J. Polymethyl methacrylate (PMMA) grafted collagen scaffold reinforced by PdO–TiO2 nanocomposites. Mater. Sci. Eng. C 2020, 108, 110378. [Google Scholar] [CrossRef] [PubMed]
- Carrion, B.; Souzanchi, M.F.; Wang, V.T.; Tiruchinapally, G.; Shikanov, A.; Putnam, A.J.; Coleman, R.M. The Synergistic Effects of Matrix Stiffness and Composition on the Response of Chondroprogenitor Cells in a 3D Precondensation Microenvironment. Adv. Healthc. Mater. 2016, 5, 1192–1202. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Chasova, V.O.; Fukina, D.G.; Koryagin, A.V.; Valetova, N.B.; Suleimanov, E.V. Synthesis of Polymethyl-Methacrylate–Collagen-Graft Copolymer Using a Complex Oxide RbTe1.5W0.5O6 Photocatalyst. Polym. Sci. Ser. D 2022, 15, 110–117. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Xu, Z.; Li, S.; Jiang, Y.; Qu, G.; Li, Z.; Zhao, Y.; Wu, X.; Ren, J. Fabrication of gelatin-based printable inks with improved stiffness as well as antibacterial and UV-shielding properties. Int. J. Biol. Macromol. 2021, 186, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Ahmed, M.S.U.; Vig, K.; Vega Erramuspe, I.B.; Auad, M.L. Synthesis and characterization of chemically crosslinked gelatin and chitosan to produce hydrogels for biomedical applications. Polym. Adv. Technol. 2021, 32, 2229–2239. [Google Scholar] [CrossRef]
- Kumar, H.; Sakthivel, K.; Mohamed, M.G.; Boras, E.; Shin, S.R.; Kim, K. Designing gelatin methacryloyl (GelMA)-Based bioinks for visible light stereolithographic 3D biofabrication. Macromol. Biosci. 2021, 21, 2000317. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.R.; Shirzaei Sani, E.; Soucy, J.R.; Corbet, C.C.; Primbetova, A.; Koppes, R.A.; Annabi, N. Bioprinting of a cell-laden conductive hydrogel composite. ACS Appl. Mater. Interfaces 2019, 11, 30518–30533. [Google Scholar] [CrossRef]
- Lan, W.; Xu, M.; Zhang, X.; Zhao, L.; Huang, D.; Wei, X.; Chen, W. Biomimetic polyvinyl alcohol/type II collagen hydrogels for cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Uromicheva, M.A.; Kuznetsova, Y.L.; Valetova, N.B.; Mitin, A.V.; Semenycheva, L.L.; Smirnova, O.N. Synthesis of grafted polybutyl acrylate copolymer on fish collagen. Proc. Univ. Appl. Chem. Biotechnol. 2021, 11, 16–25. [Google Scholar] [CrossRef]
- Semenycheva, L.; Chasova, V.; Matkivskaya, J.; Fukina, D.; Koryagin, A.; Belaya, T.; Grigoreva, A.; Kursky, Y.; Suleimanov, E. Features of Polymerization of Methyl Methacrylate using a Photocatalyst—the Complex Oxide RbTe1.5W0.5O6. J. Inorg. Organomet. Polym. 2021, 31, 3572–3583. [Google Scholar] [CrossRef]
- Chasova, V.; Semenycheva, L.; Egorikhina, M.; Charykova, I.; Linkova, D.; Rubtsova, Y.; Fukina, D.; Koryagin, A.; Valetova, N.; Suleimanov, E. Cod Gelatin as an Alternative to Cod Collagen in Hybrid Materials for Regenerative Medicine. Macromol. Res. 2022, 30, 212–221. [Google Scholar] [CrossRef]
- Kuznetsova, Y.L.; Morozova, E.A.; Vavilova, A.S.; Markin, A.V.; Smirnova, O.N.; Zakharycheva, N.S.; Lyakaev, D.V.; Semenycheva, L.L. Synthesis of Biodegradable Grafted Copolymers of Gelatin and Polymethyl Methacrylate. Polym. Sci. Ser. D 2020, 13, 453–459. [Google Scholar] [CrossRef]
- Kuznetsova, Y.L.; Sustaeva, K.S.; Vavilova, A.S.; Markin, A.V.; Lyakaev, D.V.; Mitin, A.V.; Semenycheva, L.L. Tributylborane in the synthesis of graft-copolymers of gelatin and acrylamide. J. Organomet. Chem. 2020, 924, 121431. [Google Scholar] [CrossRef]
- Kuznetsova, Y.L.; Morozova, E.A.; Sustaeva, K.S.; Markin, A.V.; Mitin, A.V.; Baten’kin, M.A.; Salomatina, E.V.; Shurygina, M.P.; Gushchina, K.S.; Pryazhnikova, M.I.; et al. Tributylborane in the synthesis of graft copolymers of collagen and polymethyl methacrylate. Russ. Chem. Bull. 2022, 71, 389–398. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y. Tri-n-Butylborane/WaterComplex-Mediated Copolymerization of Methyl Methacrylate with Proteinaceous Materials and Proteins: A Review. Polymers 2010, 2, 575–595. [Google Scholar] [CrossRef]
- Kojima, K.; Iguchi, S.; Kajima, Y.; Yoshikuni, M. Grafting of methyl methacrylate onto collagen initiated by tributylborane. J. Appl. Polym. Sci. 1983, 28, 87–95. [Google Scholar] [CrossRef]
- Okamura, H.; Sudo, A.; Endo, T. Generation of radical species on polypropylene by alkylborane-oxygen system and its application to graft polymerization. J. Polym. Sci. A Polym. Chem. 2009, 47, 6163–6167. [Google Scholar] [CrossRef]
- Ludin, D.V.; Zaitsev, S.D.; Kuznetsova, Y.L.; Markin, A.V.; Mochalova, A.E.; Salomatina, E.V. Starch-graft-poly(methyl acrylate) copolymer: The new approach to synthesis and copolymer characterization. J. Polym. Res. 2017, 24, 117. [Google Scholar] [CrossRef]
- Grotewold, J.; Lissi, E.A.; Villa, A.E. Vinyl monomer polymerization mechanism in the presence of trialkylboranes. J. Polym. Sci. A Polym. Chem. 1968, 6, 3157–3162. [Google Scholar] [CrossRef]
- Arancibia, E.; Grotewold, J.; Lissi, E.A.; Villa, A.E. Mechanism of vinyl monomer polymerization in the presence of trialkylboranes and inhibitors. J. Polym. Sci. A Polym. Chem. 1969, 7, 3430–3433. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization; McGraw-Hill: New York, NY, USA, 1970; 615p. [Google Scholar]
- Dodonov, V.A.; Kuznetsova, Y.L.; Lopatin, M.A.; Skatova, A.A. Reactions of poly(methyl methacrylate) radicals with some p-quinones in the presence of tri-n-butylboron in methyl methacrylate polymerization. Russ. Chem. Bull. 2004, 53, 2209–2214. [Google Scholar] [CrossRef]
- Dodonov, V.A.; Kuznetsova, Y.L.; Vilkova, A.I.; Skuchilina, A.S.; Nevodchikov, V.I.; Beloded, L.N. Uncontrolled pseudoliving free-radical polymerization of methyl methacrylate in the presence of butyl-p-benzoquinones. Russ. Chem. Bull. 2007, 56, 1162–1165. [Google Scholar] [CrossRef]
- Kuznetsova, Y.L.; Chesnokov, S.A.; Zaitsev, S.D.; Ludin, D.V. The catalytic system tri-n-butyl boron-p-quinone in the free-radical polymerization of styrene. Polym. Sci. Ser. B 2012, 54, 434–442. [Google Scholar] [CrossRef]
- Ludin, D.V.; Kuznetsova, Y.L.; Zaitsev, S.D. Copolymerization of styrene with methyl methacrylate in the presence of the system tributylborane–p-quinone. Polym. Sci. Ser. B 2016, 58, 503–509. [Google Scholar] [CrossRef]
- Ludin, D.V.; Kuznetsova, Y.L.; Zamyshlyaeva, O.G.; Zaitsev, S.D. Controlled radical copolymerization of styrene and tert-butyl acrylate in the presence of tri-n-butylborane–p-quinone catalytic system. Polym. Sci. Ser. B 2017, 59, 7–15. [Google Scholar] [CrossRef]
- Ludin, D.V.; Kuznetsova, Y.L.; Zaitsev, S.D. Specific features of radical copolymerization of methyl methacrylate and n-butyl acrylate in the presence of the tributylborane–p-quinone system. Polym. Sci. Ser. B 2017, 59, 516–525. [Google Scholar] [CrossRef]
- Ludin, D.V.; Kuznetsova, Y.L.; Zaitsev, S.D. Tri-n-butylboron-p-quinone catalytic system in synthesis of block copolymers. Russ. J. Appl. Chem. 2015, 88, 295–301. [Google Scholar] [CrossRef]
- Ludin, D.; Voitovich, Y.; Salomatina, E.; Kuznetsova, Y.; Grishin, I.; Fedushkin, I.; Zaitsev, S. Polymerization with Borane Chemistry. Tributylborane/p-Quinone System as a New Method of Reversible-Deactivation Radical Copolymerization for Styrene and Methyl Acrylate. Macromol. Res. 2020, 28, 851–860. [Google Scholar] [CrossRef]
- Crosby, C.O.; Stern, B.; Kalkunte, N.; Pedahzur, S.; Ramesh, S.; Zoldan, J. Interpenetrating polymer network hydrogels as bioactive scaffolds for tissue engineering. Rev. Chem. Eng. 2022, 38, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.E.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Elsevier: Oxford, UK, 2012; 1024p. [Google Scholar]
- Sever, M.J.; Wilker, J.J. Synthesis of peptides containing DOPA (3,4-dihydroxyphenylalanine). Tetrahedron 2001, 57, 6139–6146. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Citric acid and heating on gelatin hydrolysis and gelation in confectionery gels. Food Hydrocoll. 2022, 129, 107642. [Google Scholar] [CrossRef]

| Initiating System | Homopolymer Yield (%) | The Percentage of Grafted PMMA in the Copolymer (%) |
|---|---|---|
| AIBN | 4.7 | 16 |
| AIBN, TBB | 10.9 | 80 |
| AIBN, TBB, 2,5-DTBQ | 21.3 | 74 |
| TBB | 30.4 * | 47 * |
| TBB, 2,5-DTBQ | 45.0 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, Y.; Gushchina, K.; Sustaeva, K.; Mitin, A.; Egorikhina, M.; Chasova, V.; Semenycheva, L. Grafting of Methyl Methacrylate onto Gelatin Initiated by Tri-Butylborane—2,5-Di-Tert-Butyl-p-Benzoquinone System. Polymers 2022, 14, 3290. https://doi.org/10.3390/polym14163290
Kuznetsova Y, Gushchina K, Sustaeva K, Mitin A, Egorikhina M, Chasova V, Semenycheva L. Grafting of Methyl Methacrylate onto Gelatin Initiated by Tri-Butylborane—2,5-Di-Tert-Butyl-p-Benzoquinone System. Polymers. 2022; 14(16):3290. https://doi.org/10.3390/polym14163290
Chicago/Turabian StyleKuznetsova, Yulia, Ksenya Gushchina, Karina Sustaeva, Alexander Mitin, Marfa Egorikhina, Victoria Chasova, and Lyudmila Semenycheva. 2022. "Grafting of Methyl Methacrylate onto Gelatin Initiated by Tri-Butylborane—2,5-Di-Tert-Butyl-p-Benzoquinone System" Polymers 14, no. 16: 3290. https://doi.org/10.3390/polym14163290
APA StyleKuznetsova, Y., Gushchina, K., Sustaeva, K., Mitin, A., Egorikhina, M., Chasova, V., & Semenycheva, L. (2022). Grafting of Methyl Methacrylate onto Gelatin Initiated by Tri-Butylborane—2,5-Di-Tert-Butyl-p-Benzoquinone System. Polymers, 14(16), 3290. https://doi.org/10.3390/polym14163290