Application of Water Hyacinth Biomass (Eichhornia crassipes) as an Adsorbent for Methylene Blue Dye from Aqueous Medium: Kinetic and Isothermal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Adsorbent Material
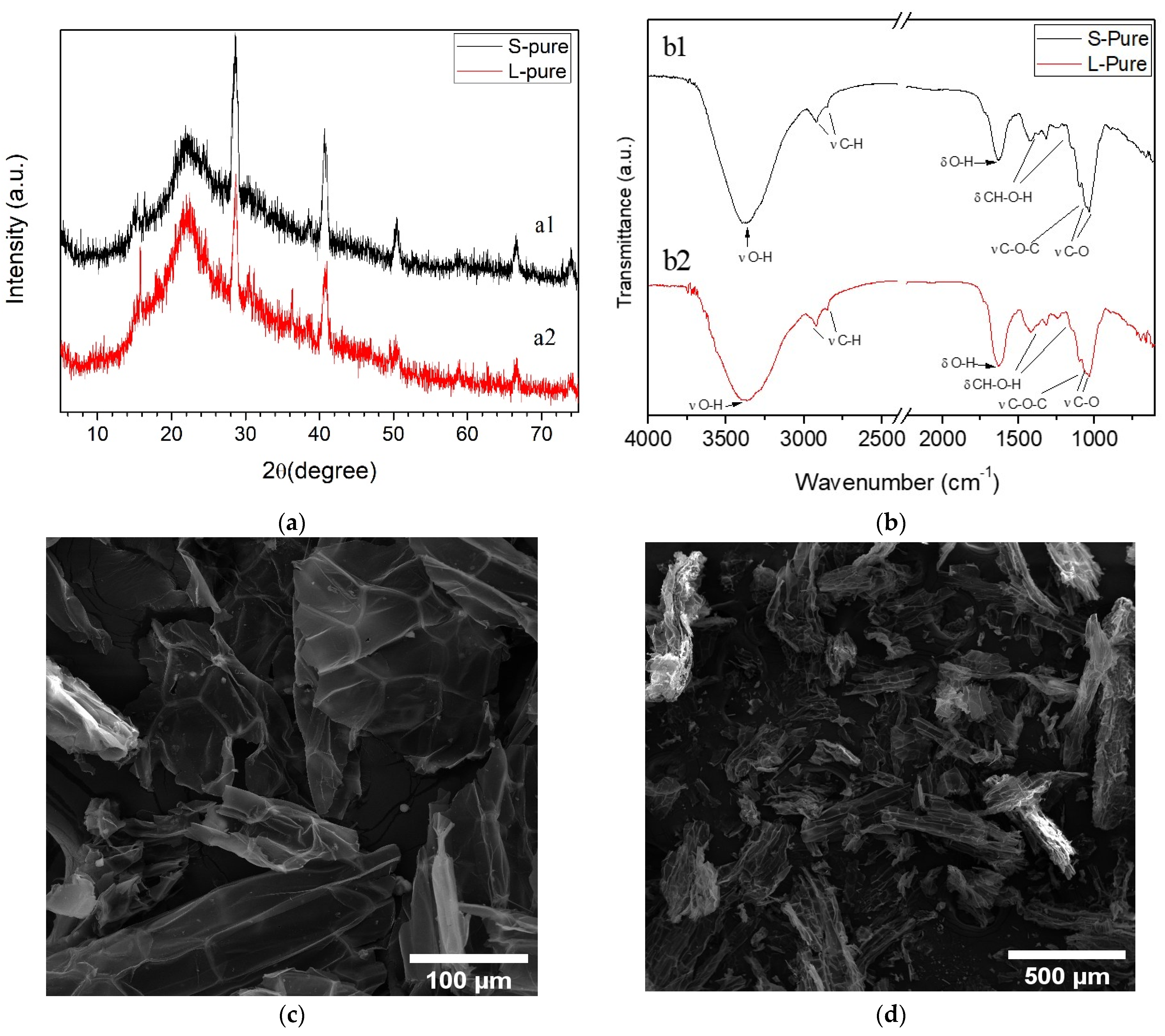
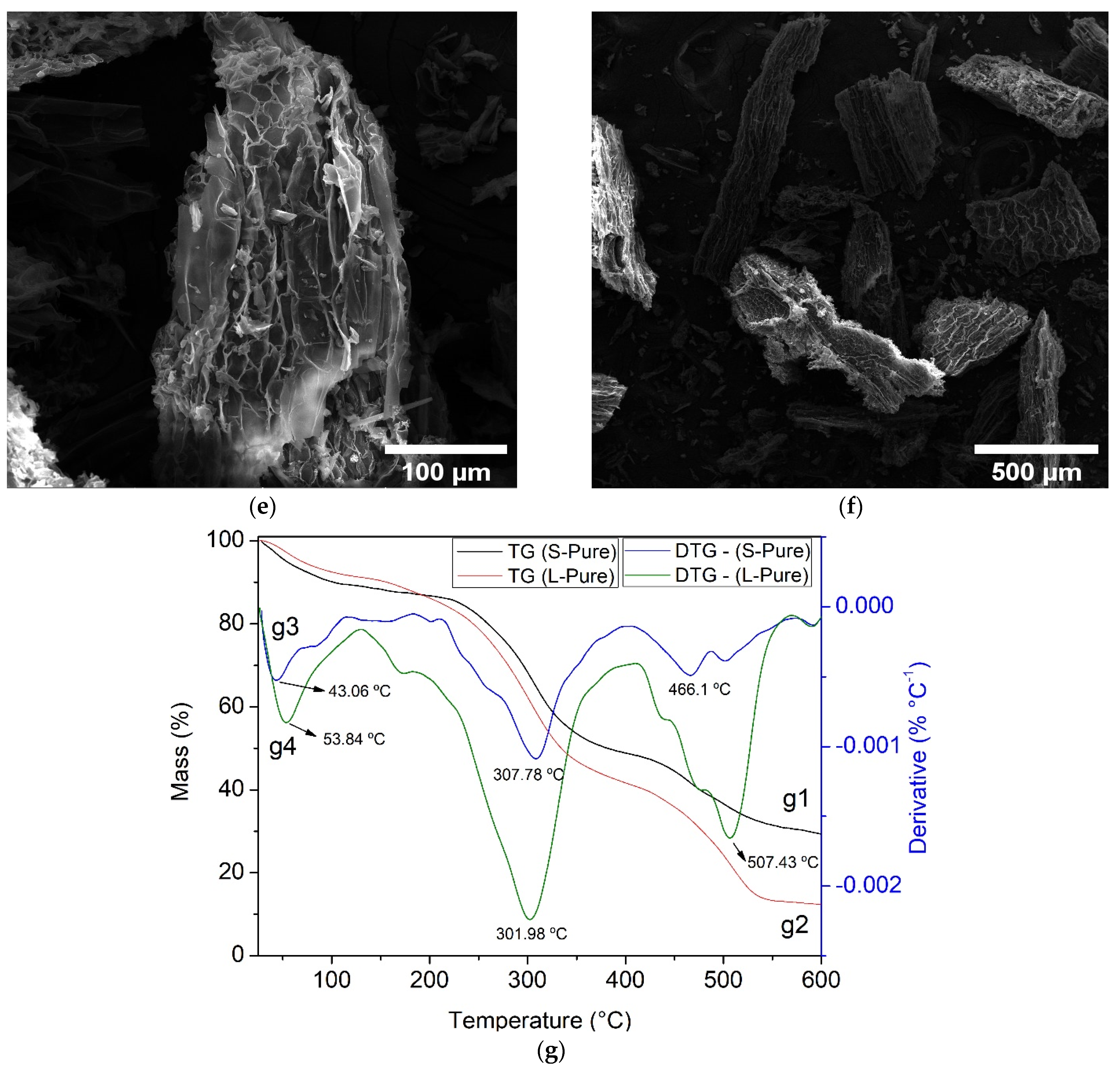
2.3. Biomass Characterization
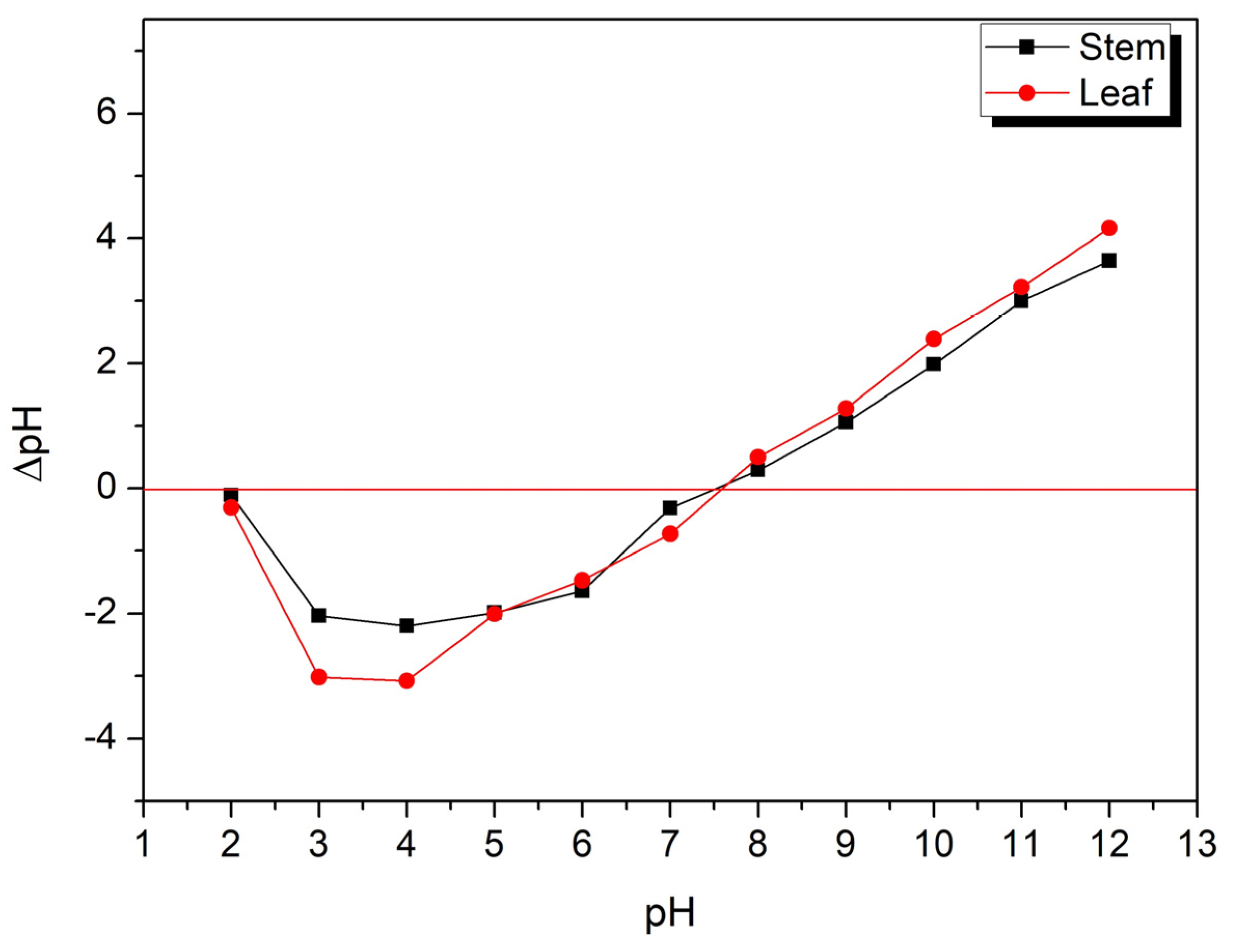
2.4. Point of Zero Charge (pHpzc)
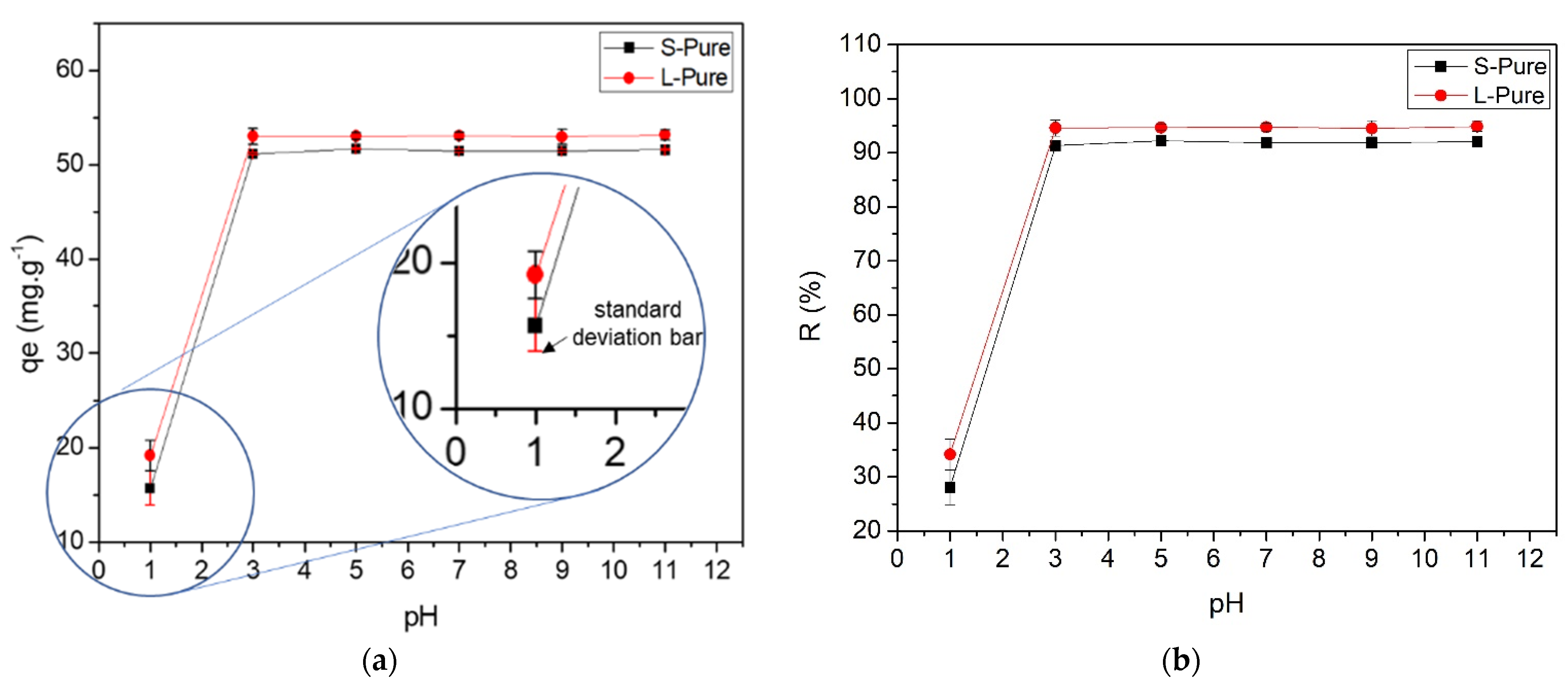
2.5. Influence of pH
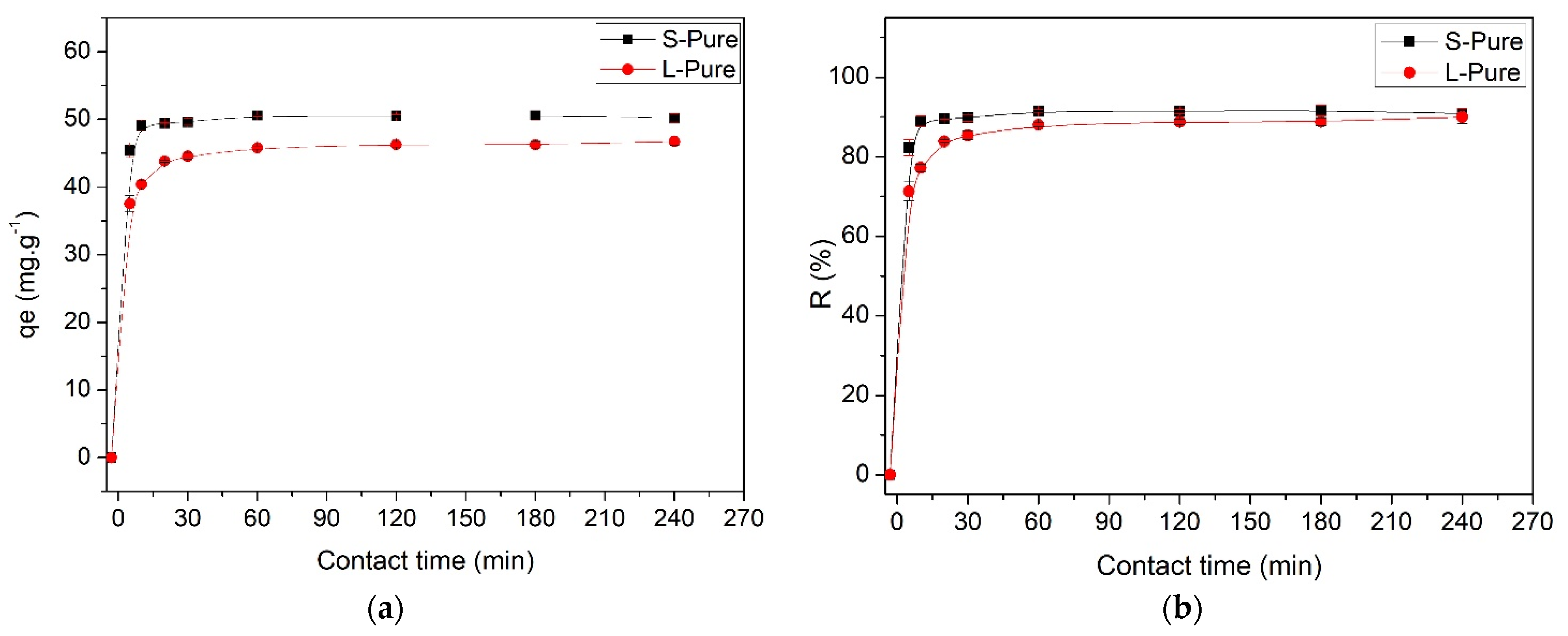
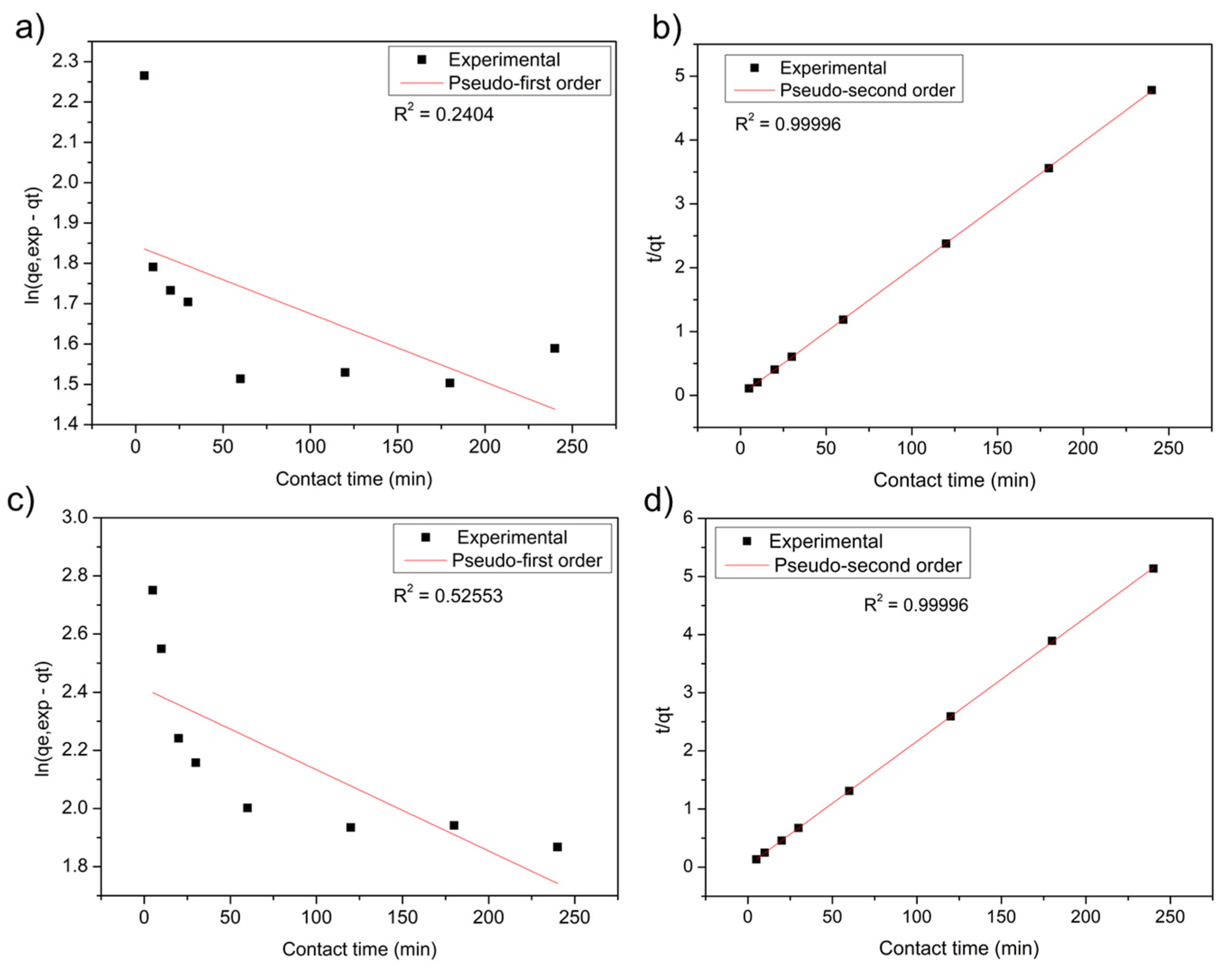
2.6. Adsorption Kinetics
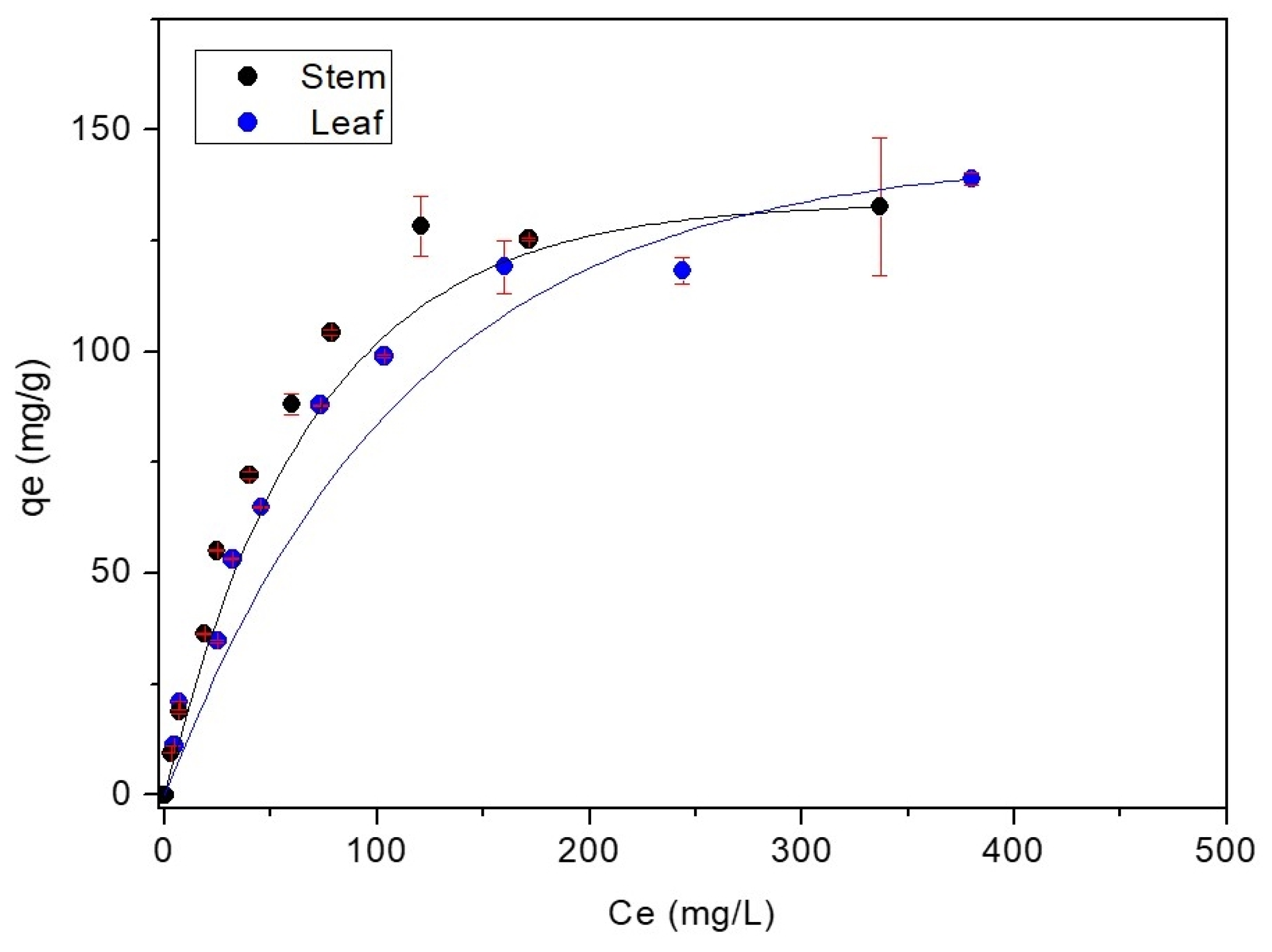
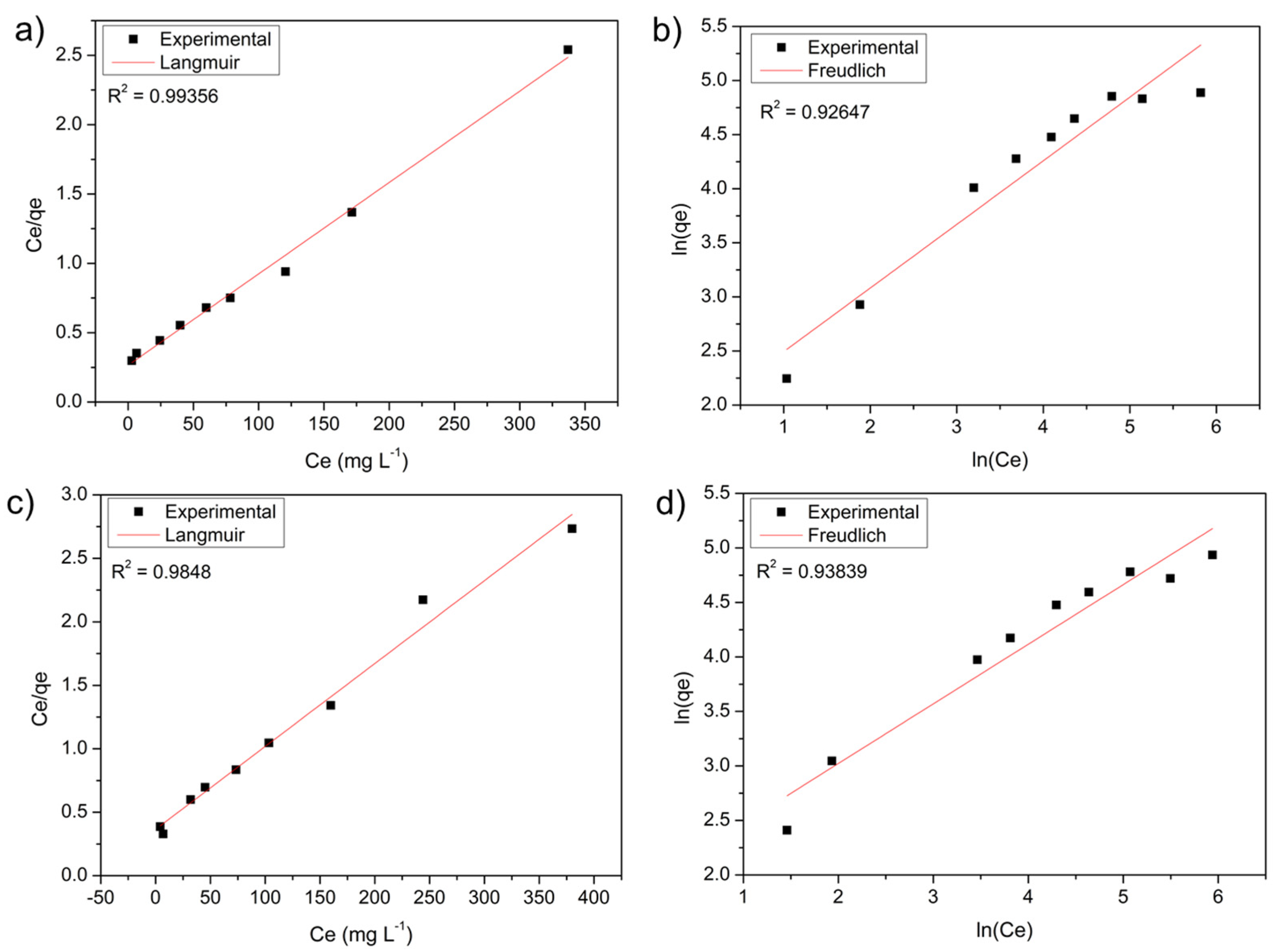
2.7. Adsorption Isotherms
2.8. Material Reuse
3. Results
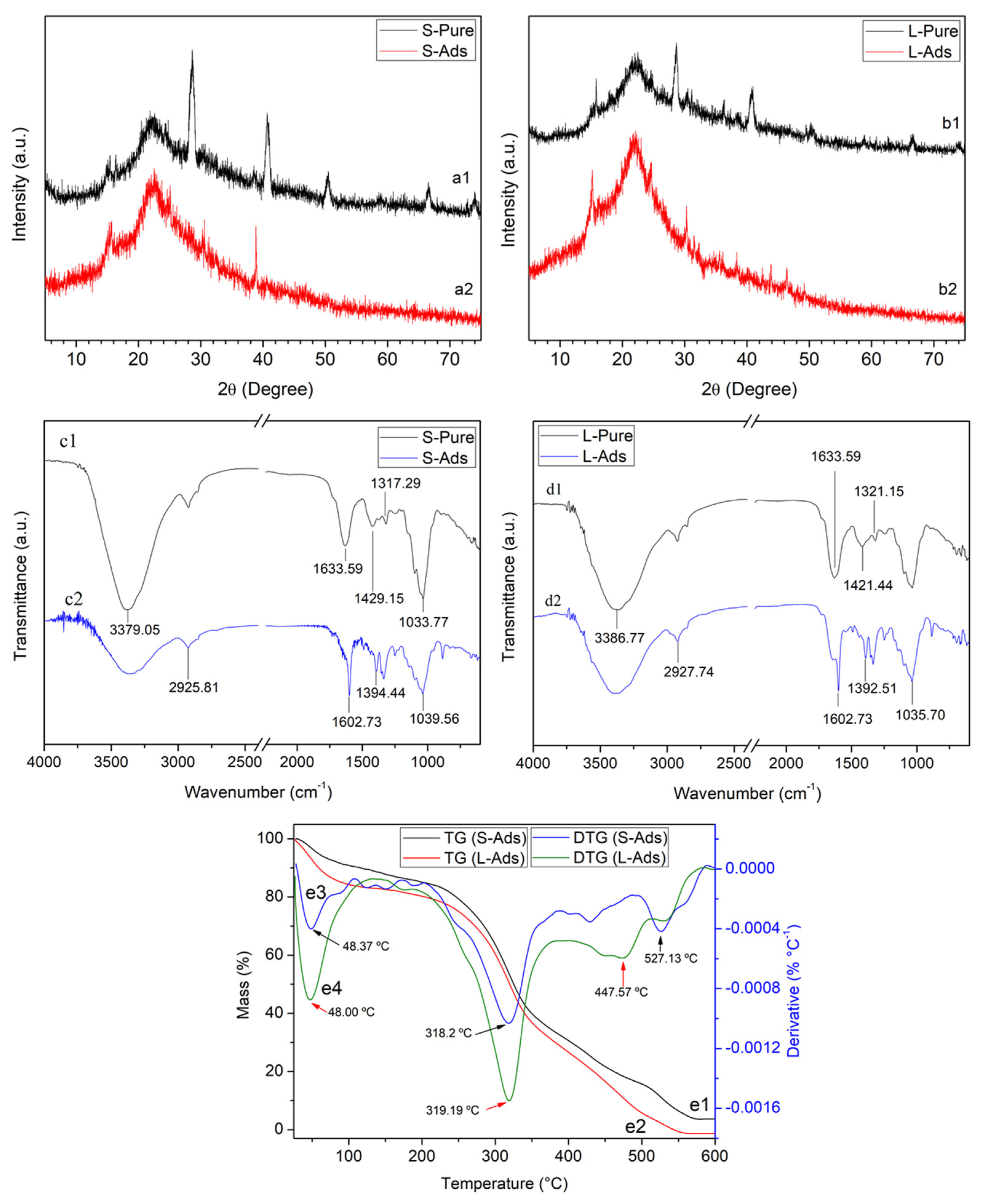
3.1. Characterization of Water Hyacinth Biomass
3.2. Adsorption Experiments
3.3. Characterization of Water Hyacinth Biomass after Adsorption
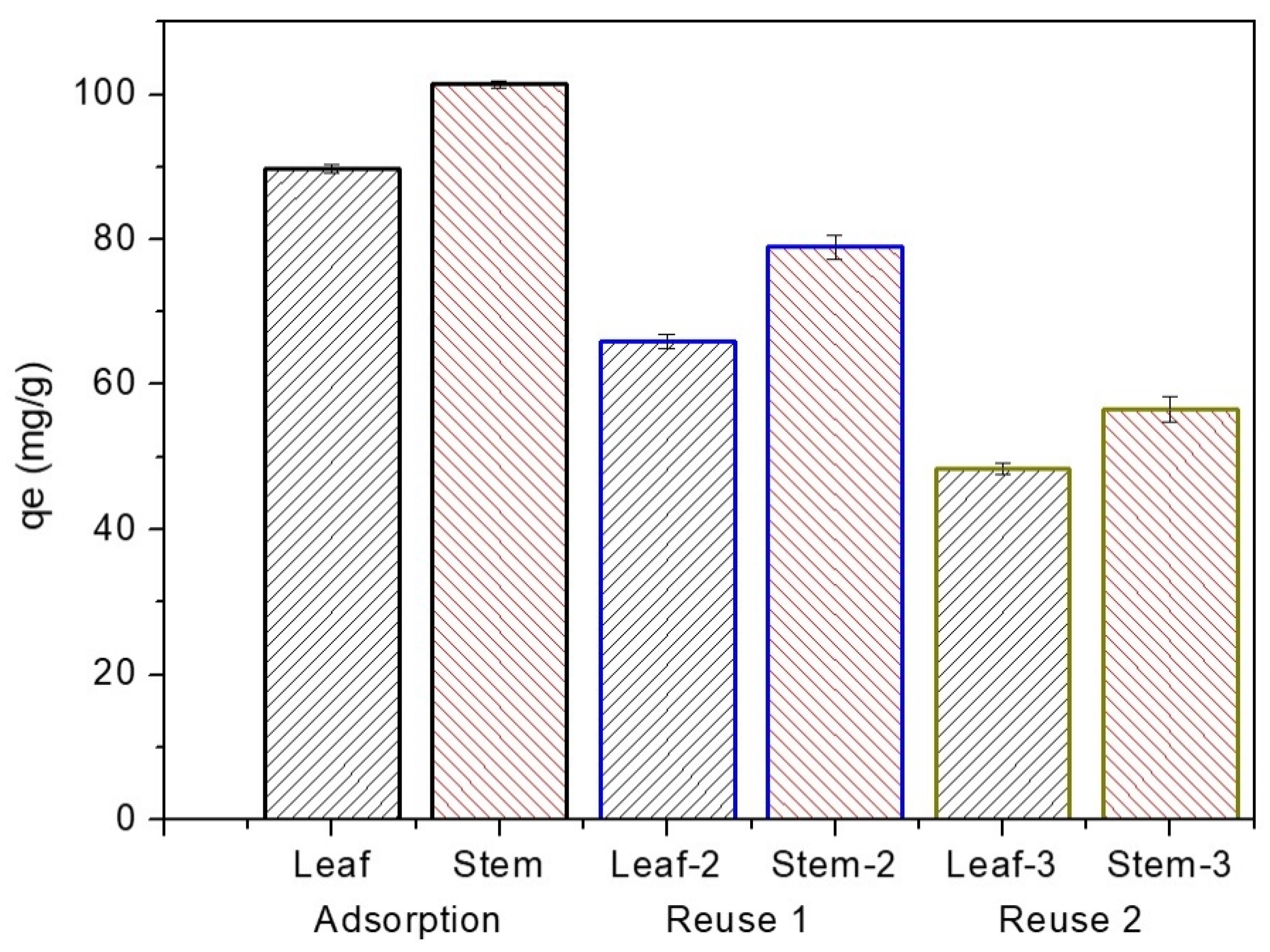
3.4. Material Reuse
3.5. Comparative Profile
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of Graphene Oxide-Based Hydrogels as Efficient Dye Adsorbents for Wastewater Treatment. Nanoscale Res. Lett. 2015, 10, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, L.; Wang, Y.H.; Liu, A.H. MoxPy nanoparticles supported on mesh structural carbon from biomass for rapid selective dyes adsorption. Talanta 2019, 196, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Doan, V.D.; Tran, T.K.N.; Nguyen, A.T.; Tran, V.A.; Nguyen, T.D. Comparative study on adsorption of cationic and anionic dyes by nanomagnetite supported on biochar derived from Eichhornia crassipes and Phragmites australis stems. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100569. [Google Scholar] [CrossRef]
- Dao, M.U.; Le, H.S.; Hoang, H.Y.; Tran, V.A.; Doan, V.D.; Le, T.T.N.; Sirotkin, A. Natural core-shell structure activated carbon beads derived from Litsea glutinosa seeds for removal of methylene blue: Facile preparation, characterization, and adsorption properties. Environ. Res. 2021, 198, 110481. [Google Scholar] [CrossRef] [PubMed]
- Basheer, A.A. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar] [CrossRef]
- Ferreira, F.J.L.; Silva, L.S.; Silva, M.S.; Osajima, J.A.; Meneguin, A.B.; Santagneli, S.H.; Barud, H.S.; Bezerra, R.D.S.; Silva-Filho, E.C. Understanding kinetics and thermodynamics of the interactions between amitriptyline or eosin yellow and aminosilane-modified cellulose. Carbohydr. Polym. 2019, 225, 115246. [Google Scholar] [CrossRef]
- Fu, J.; Lee, W.N.; Coleman, C.; Nowack, K.; Carter, J.; Huang, C.H. Removal of pharmaceuticals and personal care products by two-stage biofiltration for drinking water treatment. Sci. Total Environ. 2019, 664, 240–248. [Google Scholar] [CrossRef]
- Shattar, S.F.A.; Zakaria, N.A.; Foo, K.Y. Preparation of a montmorillonite-derived adsorbent for the practical treatment of ionic and nonionic pesticides. J. Mater. Res. Technol. 2019, 8, 4713–4724. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.M.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.C.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Borges, R.M.; Minillo, A.; Lemos, E.G.M.; Prado, H.F.A. Use of granular activated carbon filters associated with microorganisms to remove pharmaceuticals in drinking water treatment. Eng. Sanit. Ambient. 2016, 21, 709–720. [Google Scholar] [CrossRef] [Green Version]
- Mashkoor, F.; Nasar, A. Magsorbents: Potential candidates in wastewater treatment technology–A review on the removal of methylene blue dye. J. Magn. Magn. Mater. 2020, 500, 166408. [Google Scholar] [CrossRef]
- Siddiqui, S.; Zohra, F.; Chaudhry, S.A. Nigella sativa seed based nanohybrid composite-Fe2O3–SnO2/BC: A novel material for enhanced adsorptive removal of methylene blue from water. Environ. Res. 2019, 178, 108667. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; Hamad, H.A.; Ali, R.M. Journey from ceramic waste to highly efficient toxic dye adsorption from aqueous solutions via one-pot synthesis of CaSO4 rod-shape with silica. J. Mater. Res. Technol. 2020, 9, 16051–16063. [Google Scholar] [CrossRef]
- Yan, J.; Li, R. Simple and low-cost production of magnetite/graphene nanocomposites for heavy metal ions adsorption. Sci. Total Environ. 2022, 813, 152604. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.D.S.; Morais, A.I.S.; Osajima, J.A.; Nunes, L.C.C.; Silva Filho, E.C. Development of new phosphated cellulose for application as an efficient biomaterial for the incorporation/release of amitriptyline. Int. J. Biol. Macromol. 2016, 86, 362–375. [Google Scholar] [CrossRef]
- Silva, M.S.; Silva, L.S.; Ferreir, F.J.L.; Bezerra, R.D.S.; Marques, T.M.F.; Meneguin, A.B.; Barud, H.S.; Osajima, J.A.; Silva Filho, E.C. Study of interactions between organic contaminants and a new phosphated biopolymer derived from cellulose. Int. J. Biol. Macromol. 2020, 146, 668–677. [Google Scholar] [CrossRef]
- Sharma, G.; Khosla, A.; Kumar, A.; Kaushal, N.; Sharma, S.; Naushad, M.; Vo, D.V.N.; Iqbal, J.; Stadler, F.J. A comprehensive review on the removal of noxious pollutants using carrageenan based advanced adsorbents. Chemosphere 2022, 289, 133100. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Piccin, J.S.; Dettmer, A.; Rosseto, M.; Dotto, G.L.; Oliveira Schmitz, A.P.D.; Freitas, T.S.M.; Loss, R.A.; Geraldi, C.A.Q. Water hyacinth (Eichhornia crassipes) roots, an amazon natural waste, as an alternative biosorbent to uptake a reactive textile dye from aqueous solutions. Ecol. Eng. 2020, 150, 105817. [Google Scholar] [CrossRef]
- Madikizela, L.M. Removal of organic pollutants in water using water hyacinth (Eichhornia crassipes). J. Environ. Manag. 2021, 295, 113153. [Google Scholar] [CrossRef]
- Santos, L.O.; Silva, F.F.; Santos, L.C.; Carregosa, I.S.; Wisniewski, A., Jr. Potential bio-oil production from invasive aquatic plants by microscale pyrolysis studies. J. Braz. Chem. Soc. 2018, 29, 151–158. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 19th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Soest, P.V.; Wine, R.H. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Low, A.; Rabat, N.E. A review on recent developments in the adsorption of surfactants from wastewater. J. Environ. Manag. 2020, 254, 109797. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.D.S.; Leal, R.C.; Silva, M.S.; Morais, A.I.S.; Marques, T.H.C.; Osajima, J.A.; Meneguin, A.B.; Barud, H.D.S.; da Silva Filho, E.C. Direct Modification of Microcrystalline Cellulose with Ethylenediamine for Use as Adsorbent for Removal Amitriptyline Drug from Environment. Molecules 2017, 22, 2039. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.S.; Ferreira, F.J.L.; Silva, M.S.; Citó, A.M.G.L.; Meneguin, A.B.; Sabio, R.M.; Barud, H.S.; Bezerra, R.D.; Osajima, J.A.; Filho, E.C.S. Potential of amino-functionalized cellulose as an alternative sorbent intended to remove anionic dyes from aqueous solutions. Int. J. Biol. Macromol. 2018, 116, 1282–1295. [Google Scholar] [CrossRef]
- Queiroga, L.N.F.; Pereira, M.B.B.; Silva, L.S.; Silva Filho, E.C.; Santos, I.M.G.; Fonseca, M.G.; Georgelin, T.; Jaber, M. Microwave bentonite silylation for dye removal: Influence of the solvent. Appl. Clay Sci. 2019, 168, 478–487. [Google Scholar] [CrossRef]
- Rojas-Valencia, O.G.; Estrada-Flores, M.; Reza-San-Germán, C.M.; Torres-Santillán, E.; Hernández-Fuentes, J.; Ledezma-Martínez, J.L. Effect of thermal treatment of activated carbon fiber felt for reuse in removal of methylene blue from a synthetic wastewater. Rev. Mex. Ing. Química 2020, 19, 1515–1526. [Google Scholar] [CrossRef]
- You, H.; Huang, B.; Cao, C.; Liu, X.; Sun, X.; Xiao, L.; Qiu, J.; Luo, Y.; Qian, Q.; Chen, Q. Adsorption–desorption behavior of methylene blue onto aged polyethylene microplastics in aqueous environments. Mar. Pollut. Bull. 2021, 167, 112287. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Holanda, C.A.; Souza, J.L.; Santos, C.C.; Silva, H.A.S.; Santana, S.A.A.; Costa, M.C.P.; Schultz, M.S.; Brito Bezerra, C.W. Remoção do corante têxtil turquesa de remazol empregando aguapé (Eichhornia crassipes) como adsorvente. Orbital Electron. J. Chem. 2015, 7, 141–154. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Patil, M.R.; Shrivastava, V.S. Adsorptive removal of methylene blue from aqueous solution by polyaniline-nickel ferrite nanocomposite: A kinetic approach. Desalination Water Treat. 2016, 57, 5879–5887. [Google Scholar] [CrossRef]
- Mokhtar, M. Application of synthetic layered sodium silicate magadiite nanosheets for environmental remediation of methylene blue dye in water. Materials 2017, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, D.A.; Santos, A.S.; Pantoja, L.A.; Brito, P.L.; Costa, A.S.V. Production of second generation ethanol from water hyacinth: A review. Rev. Virtual Química 2019, 11, 127–143. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Magalhães, W.L.E.; Nisgoski, S.; Muniz, G.I.B.; Satyanarayana, K.G.; Lazzarotto, M. New and improved method of investigation using thermal tools for characterization of cellulose from eucalypts pulp. Thermochim. Acta 2016, 638, 44–51. [Google Scholar] [CrossRef]
- Lin, S.; Yang, H.; Na, Z.; Lin, K. A novel biodegradable arsenic adsorbent by immobilization of iron oxyhydroxide (FeOOH) on the root powder of long-root Eichhornia crassipes. Chemosphere 2018, 192, 258–566. [Google Scholar] [CrossRef]
- Bronzato, G.R.F.; Ziegler, S.M.; Silva, R.C.; Cesarino, I.; Leão, A.L. Characterization of the pre-treated biomass of Eichhornia crassipes (water hyacinth) for the second generation ethanol production. Mol. Cryst. Liq. Cryst. 2017, 655, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Lima, H.D.P.; Asencios, Y.J. Eichhornia crassipes (Mart.) Solms (natural or carbonized) as biosorbent to remove pollutants in water. SN Appl. Sci. 2021, 3, 750. [Google Scholar] [CrossRef]
- Pereira, M.B.B.; Honório, L.M.C.; Lima-Júnior, C.G.; Silva Filho, E.C.; Gaslain, F.; Rigaud, B.; Jaber, M. Modulating the structure of organofunctionalized hydroxyapatite/tripolyphosphate/chitosan spheres for dye removal. J. Environ. Chem. Eng. 2020, 8, 103980. [Google Scholar] [CrossRef]
- Mukaratirwa-Muchanyereyi, N.; Kugara, J.; Zaranyika, M.F. Surface composition and surface properties of water hyacinth (Eichhornia crassipes) root biomass: Effect of mineral acid and organic solvent treatment. Afr. J. Biotechnol. 2016, 15, 897–909. [Google Scholar] [CrossRef]
- Peng, H.; Wang, Y.; Tan, T.L.; Chen, Z. Exploring the phytoremediation potential of water hyacinth by FTIR Spectroscopy and ICP-OES for treatment of heavy metal contaminated water. Int. J. Phytoremediation 2020, 22, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Narendhirakannan, R.T. Bioethanol Production from Aquatic Weed Water Hyacinth (Eichhornia crassipes) by Yeast Fermentation. Waste Biomass Valorization 2015, 6, 209–216. [Google Scholar] [CrossRef]
- Prasad, R.; Sharma, D.; Yadav, K.D.; Ibrahim, H. Eichhornia crassipes as biosorbent for industrial wastewater treatment: Equilibrium and kinetic studies. Can. J. Chem. Eng. 2021, 100, 439–450. [Google Scholar] [CrossRef]
- Boniolo, M.R.; Yabuki, L.N.M.; Mortari, D.A.; Menegário, A.A.; Garcia, M.L. Biomassas brasileiras aplicadas à remoção de urânio de drenagem ácida de minas por processos de biossorção. Holos Environ. 2017, 17, 149–167. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues Sousa, H.; Lima, I.S.; Neris, L.M.L.; Silva, A.S.; Santos Nascimento, A.M.S.; Araújo, F.P.; Silva-Filho, E.C. Superabsorbent hydrogels based to polyacrylamide/cashew tree gum for the controlled release of water and plant nutrients. Molecules 2021, 26, 2680. [Google Scholar] [CrossRef]
- Oloo, C.M.; Onyari, J.M.; Wanyonyi, W.C.; Wabomba, J.N.; Muinde, V.M. Adsorptive removal of hazardous crystal violet dye form aqueous solution using Rhizophora mucronata stem-barks: Equilibrium and kinetics studies. Environ. Chem. Ecotoxicol. 2020, 2, 64–72. [Google Scholar] [CrossRef]
- Orozco, R.S.; Martínez-Juan, M.; García-Sánchez, J.J.; Ureña-Núñez, F. Removal of methylene blue from aqueous solution using Typha stems and leaves. BioResources 2018, 13, 1696–1710. [Google Scholar] [CrossRef] [Green Version]
- Emam, A.A.; Faraha, S.A.A.; Kamal, F.H.; Gamal, A.M.; Basseem, M. Modification and characterization of Nano cellulose crystalline from Eichhornia crassipes using citric acid: An adsorption study. Carbohydr. Polym. 2020, 240, 116202. [Google Scholar] [CrossRef]
- Das, S.; Gangly, A.; Dey, A.; Ting, Y.P.; Chatterjee, P.H. Characterization of water hyacinth biomass and microbial degradation of the biomass under solid state fermentation using a lignocellulolytic fungus (Alterneria spp. NITDS1). J. Chem. Biol. Phys. Sci. 2014, 4, 2279–2293. [Google Scholar]
- Zhou, W.; Zhu, D.; Langdon, A.; Li, L.; Liao, S.; Tan, L. The structure characterization of cellulose xanthogenate derived from the straw of Eichhornia crassipes. Bioresour. Technol. 2009, 100, 5366–5369. [Google Scholar] [CrossRef] [PubMed]
- Omondi, E.A.; Ndiba, P.K.; Njuru, P.G. Characterization of water hyacinth (E. crassipes) from Lake Victoria and ruminal slaughterhouse waste as co-substrates in biogas production. SN Appl. Sci. 2019, 1, 848. [Google Scholar] [CrossRef] [Green Version]
- Poomsawat, W.; Tsalidis, G.; Tsekos, C.; Jong, W. Experimental studies of furfural production from water hyacinth (Eichhornia crassipes). Energy Sci. Eng. 2019, 7, 2155–2164. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Coronilla, I.; Aranda-García, E.; Cristiani-Urbina, E. Biosorption of metanil yellow dye from aqueous solutions by the entire water hyacinth plant (Eichhornia crassipes) and its vegetative organs. Environ. Eng. Manag. J. 2019, 18, 1671–1682. [Google Scholar]
- Gaurav, G.K.; Mehmood, T.; Cheng, L.; Klemeš, J.J.; Shrivastava, D.K. Water hyacinth as a biomass: A review. J. Clean. Prod. 2020, 277, 122214. [Google Scholar] [CrossRef]
- Pal, D.; Maiti, S.K. Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study. J. Clean. Prod. 2019, 212, 986–996. [Google Scholar] [CrossRef]
- Marques, T.M.; Sales, D.A.; Silva, L.S.; Bezerra, R.D.; Silva, M.S.; Osajima, J.A.; Ferreira, O.P.; Ghosh, A.; Silva Filho, E.C.; Viana, B.C.; et al. Amino-functionalized titanate nanotubes for highly efficient removal of anionic dye from aqueous solution. Appl. Surf. Sci. 2020, 512, 145659. [Google Scholar] [CrossRef]
- Ngeno, E.C.; Orata, F.; Baraza, L.D.; Shikuku, V.O.; Kimosop, S.J. Adsorption of caffeine and ciprofloxacin onto pyrolitically derived water hyacinth biochar: Isothermal, kinetic and thermodynamic studies. J. Chem. Chem. Eng. 2016, 10, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Ibupoto, A.S.; Qureshi, U.A.; Ahmed, F.; Khatri, Z.; Khatri, M.; Maqsood, M.; Kim, I.S. Reusable carbon nanofibers for efficient removal of methylene blue from aqueous solution. Chem. Eng. Res. Des. 2018, 136, 744–752. [Google Scholar] [CrossRef]
- Sousa, H.R.; Silva, L.S.; Sousa, P.A.A.; Sousa, R.R.M.; Fonseca, M.G.; Osajima, J.A.; Silva-Filho, E.C. Evaluation of methylene blue removal by plasma activated palygorskites. J. Mater. Res. Technol. 2019, 8, 5432–5442. [Google Scholar] [CrossRef]
- Manna, S.; Roy, D.; Saha, P.; Gopakumar, D.; Thomas, S. Rapid methylene blue adsorption using modified lignocellulosic materials. Process Saf. Environ. Prot. 2017, 107, 346–356. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Li, T.; Zhu, J.; Pang, J.; Xu, J.; Wang, L. Study on Adsorption Characteristics of Heavy Metal Cd 2+ by Biochar Obtained from Water Hyacinth. Pol. J. Environ. Stud. 2022, 31, 2301–2316. [Google Scholar] [CrossRef]
- Extross, A.; Waknis, A.; Tagad, C.; Gedam, V.V.; Pathak, P.D. Adsorption of congo red using carbon from leaves and stem of water hyacinth: Equilibrium, kinetics, thermodynamic studies. Int. J. Environ. Sci. Technol. 2022, 1–38. [Google Scholar] [CrossRef]
- Carreño-Sayago, U.F. Development of microspheres using water hyacinth (Eichhornia crassipes) for treatment of contaminated water with Cr (VI). Environ. Dev. Sustain. 2021, 23, 4735–4746. [Google Scholar] [CrossRef]
- Jahangiri, F.M.; Moutushi, H.T.; Moniruzzaman, M.; Hoque, S.; Hossain, M.E. Removal of lead from aqueous solutions and wastewaters using water hyacinth (Eichhornia crassipes) roots. Water Pract. Technol. 2021, 16, 404–419. [Google Scholar] [CrossRef]
- Souza, J.L.; Holanda, C.A.; Aires, A.M.L.; Almeida, T.R.; Oliveira, E.S.; Rocha, M.B.C.; Costa, A.N. Potencialidades da Eichhornia azurea (aguapé) na remoção do corante têxtil turquesa remazol em meio aquoso: Estudo dos mecanismos cinéticos de adsorção. Braz. J. Dev. 2020, 61, 76037–76053. [Google Scholar] [CrossRef]
- Elboughdiri, N. Olive mill wastewater phenolic compounds adsorption onto active olive stones: Equilibrium isotherms and kinetics study. Int. J. Forensic Eng. 2018, 4, 31. [Google Scholar] [CrossRef]
- Ramirez-Muñoz, A.; Pérez, S.; Flórez, E.; Acelas, N. Recovering phosphorus from aqueous solutions using water hyacinth (Eichhornia crassipes) toward sustainability through its transformation to apatite. J. Environ. Chem. Eng. 2021, 9, 106225. [Google Scholar] [CrossRef]
- Saning, A.; Herou, S.; Dechtrirat, D.; Ieosakulrat, C.; Pakawatpanurut, P.; Kaowphong, S.; Chuenchom, L. Green and sustainable zero-waste conversion of water hyacinth (Eichhornia crassipes) into superior magnetic carbon composite adsorbents and supercapacitor electrodes. RSC Adv. 2019, 9, 24248–24258. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.M.; Vaidya, R.; Srinivas, S.; Anand, S.; Narayana, B. Application of water hyacinth root powder for Congo red dye removal in batch and continuous packed bed operation. Nanotechnol. Environ. Eng. 2021, 6, 31. [Google Scholar] [CrossRef]
- Saufi, H.; Alouani, M.E.; Aride, J.; Taibi, M. Rhodamine B biosorption from aqueous solution using Eichhornia crassipes powders: Isotherm, kinetic and thermodynamic studies. Chem. Data Collect. 2020, 25, 100330. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Effects of chemical treatments on hemp fibre structure. Appl. Surf. Sci. 2013, 276, 13–23. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Nejadebrahim, A.; Ebrahimi, M.; Allonas, X.; Croutxé-Barghorn, C. Methylene blue-clay nano-pigment as a new photosensitizer for preparing three-component photoinitiating systems with high thermal stability. Dye. Pigment. 2020, 180, 108475. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Cela-Dablanca, R.; Ferreira-Coelho, G.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Sulfadiazine, sulfamethazine and sulfachloropyridazine removal using three different porous materials: Pine bark, “oak ash” and mussel shell. Environ. Res. 2021, 195, 110814. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liang, S.; Tian, Q.H. Removal of heavy metal ions from aqueous solutions by adsorption using modified orange peel as adsorbent. Adv. Mater. Res. 2011, 236, 237–240. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2021, 265, 129087. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana peel as a biosorbent for the decontamination of water pollutants. A review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Delaqua, G.C.G.; Marvila, M.T.; Souza, D.; Rodriguez, R.J.S.; Colorado, H.A.; Vieira, C.M.F. Evaluation of the application of macrophyte biomass Salvinia auriculata Aublet in red ceramics. J. Environ. Manag. 2020, 275, 111253. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Rojas, O.J.; Wang, X.; Naebe, M. Kinetics and equilibrium adsorption of methylene blue onto cotton gin trash bioadsorbents. Cellulose 2020, 27, 6485–6504. [Google Scholar] [CrossRef]
- Choi, H.J.; Yu, S.W. Biosorption of methylene blue from aqueous solution by agricultural bioadsorbent corncob. Environ. Eng. Res. 2019, 24, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.B.; Priyantha, N.; Tennakoon, D.T.B.; Chieng, H.I.; Dahri, M.K.; Suklueng, M. Breadnut peel as a highly effective low-cost biosorbent for methylene blue: Equilibrium, thermodynamic and kinetic studies. Arab. J. Chem. 2017, 10, S3216–S3228. [Google Scholar] [CrossRef] [Green Version]
- Hevira, L.; Ighalo, J.O.; Aziz, H.; Zein, R. Terminalia catappa shell as low-cost biosorbent for the removal of methylene blue from aqueous solutions. J. Ind. Eng. Chem. 2021, 97, 188–199. [Google Scholar] [CrossRef]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Quesada, H.B.; Baptista, A.T.; Gomes, R.G.; Bergamasco, R. Soybean hulls as a low-cost biosorbent for removal of methylene blue contaminant. Environ. Prog. Sustain. Energy 2020, 39, e13328. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Utilization of Punica granatum peel as an eco-friendly biosorbent for the removal of methylene blue dye from aqueous solution. J. Appl. Biotechnol. Bioeng. 2018, 5, 242–249. [Google Scholar] [CrossRef] [Green Version]
- El Atouani, S.; Belattmania, Z.; Reani, A.; Tahiri, S.; Aarfane, A.; Bentiss, F.; Sabour, B. Brown seaweed Sargassum muticum as low-cost biosorbent of methylene blue. Int. J. Environ. Res. 2019, 13, 131–142. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Zhang, Y.; Pan, H.; Tao, L. Adsorption of methylene blue on organosolv lignin from rice straw. Procedia Environ. Sci. 2016, 31, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Velkova, Z.Y.; Kirova, G.K.; Stoytcheva, M.S.; Gochev, V. Biosorption of Congo Red and Methylene Blue by pretreated waste Streptomyces fradiae biomass–Equilibrium, kinetic and thermodynamic studies. J. Serb. Chem. Soc. 2018, 83, 107–120. [Google Scholar] [CrossRef]
- Jawad, A.H.; Kadhum, A.M.; Ngoh, Y.S. Applicability of dragon fruit (Hylocereus polyrhizus) peels as low-cost biosorbent for adsorption of methylene blue from aqueous solution: Kinetics, equilibrium and thermodynamics studies. Desalination Water Treat. 2018, 109, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Jawad, A.H.; Waheeb, A.S.; Rashid, R.A.; Nawawi, W.I.; Yousif, E. Equilibrium isotherms, kinetics, and thermodynamics studies of methylene blue adsorption on pomegranate (Punica granatum) peels as a natural low-cost biosorbent. Desalination Water Treat. 2018, 105, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Mosoarca, G.; Vancea, C.; Popa, S.; Gheju, M.; Boran, S. Syringa vulgaris leaves powder a novel low-cost adsorbent for methylene blue removal: Isotherms, kinetics, thermodynamic and optimization by Taguchi method. Sci. Rep. 2020, 10, 17676. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Nascimento, L.; Brito, M.; da Silva, K.; Paschoal, W.; Fujiyama, R. Biosorption of methylene blue dye using natural biosorbents made from weeds. Materials 2019, 12, 2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakoor, S.; Nasar, A. Adsorptive treatment of hazardous methylene blue dye from artificially contaminated water using cucumis sativus peel waste as a low-cost adsorbent. Groundw. Sustain. Dev. 2017, 5, 152–159. [Google Scholar] [CrossRef]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Khalil, A.; Zerrouq, F. Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surf. Interfaces 2018, 11, 74–81. [Google Scholar] [CrossRef]
| Fiber Fraction Composition | Eichhornia crassipes | |
|---|---|---|
| Stem | Leaf | |
| Dry matter-DM (% as fresh matter) | 91.63 ± 0.01 | 91.36 ± 0.02 |
| Neutral detergent fiber (%DM) | 68.91 ± 0.38 | 58.88 ± 0.91 |
| Acid detergent fiber (%DM) | 38.67 ± 1.06 | 29.17 ± 0.42 |
| Acid detergent lignin (%DM) | 10.88 ± 1.40 | 8.36 ± 0.66 |
| Cellulose (%DM) | 27.79 ± 0.20 | 20.81 ± 0.17 |
| Hemicelluloses (%DM) | 30.24 ± 0.32 | 29.71 ± 0.28 |
| Pseudo-First-Order | Pseudo-Second-Order | |||||||
|---|---|---|---|---|---|---|---|---|
| Adsorbent | qe (mg.g−1) | K1 (min−1) | Error | R2 | qe (mg.g−1) | K2 (mg.g−1 min−1) | Error | R2 |
| Stem | 06.29 | 0.0016 | 0.11028 | 0.24042 | 50.42 | 0.0811 | 0.00566 | 0.99996 |
| Leaf | 11.16 | 0.0027 | 0.11048 | 0.52553 | 46.88 | 0.0141 | 0.00565 | 0.99996 |
| Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|
| Adsorbent | qmax (mg.g−1) | KL (mg.L−1) | Error | R2 | 1/n | KF (mg.g−1) | Error | R2 |
| Stem | 153.84 | 0.024 | 0.025 | 0.9935 | 1.700 | 6.724 | 0.236 | 0.9264 |
| Leaf | 153.84 | 0.017 | 0.047 | 0.9848 | 1.828 | 6.881 | 0.210 | 0.9383 |
| Adsorbent | qmax (mg.g−1) | References |
|---|---|---|
| Cotton gin rubbish dust | 112.6 | [82] |
| Corn cob | 405.22 | [83] |
| Bread nutshell | 409.00 | [84] |
| Terminalia catappa (Indian almond) husks | 88.62 | [85] |
| White pine sawdust | 87 | [86] |
| Soy husk | 169.90 | [87] |
| Punica granatum bark | 10.7296 | [88] |
| Brown algae Sargassum muticum | 9.55 | [89] |
| Rice straw | 20.38 | [90] |
| Streptomyces fradiae biomass | 59.63 | [91] |
| Pitaya peels | 190.30 | [92] |
| Pomegranate peels | 200.0 | [93] |
| Syringa vulgaris leaf powder | 188.2 | [94] |
| Weeds | 41,67 | [95] |
| Cucumis sativus bark | 20.1410 | [96] |
| Walnut shell powder | 142.85 | [97] |
| Water hyacinth stem | 50.42 | This study |
| Water hyacinth leaf | 46.88 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, M.T.; Barros, A.Z.B.; Morais, A.I.S.; Carvalho Melo, A.L.F.; Bezerra, R.D.S.; Osajima, J.A.; Silva-Filho, E.C. Application of Water Hyacinth Biomass (Eichhornia crassipes) as an Adsorbent for Methylene Blue Dye from Aqueous Medium: Kinetic and Isothermal Study. Polymers 2022, 14, 2732. https://doi.org/10.3390/polym14132732
Carneiro MT, Barros AZB, Morais AIS, Carvalho Melo ALF, Bezerra RDS, Osajima JA, Silva-Filho EC. Application of Water Hyacinth Biomass (Eichhornia crassipes) as an Adsorbent for Methylene Blue Dye from Aqueous Medium: Kinetic and Isothermal Study. Polymers. 2022; 14(13):2732. https://doi.org/10.3390/polym14132732
Chicago/Turabian StyleCarneiro, Marcelo T., Ana Z. B. Barros, Alan I. S. Morais, André L. F. Carvalho Melo, Roosevelt D. S. Bezerra, Josy A. Osajima, and Edson C. Silva-Filho. 2022. "Application of Water Hyacinth Biomass (Eichhornia crassipes) as an Adsorbent for Methylene Blue Dye from Aqueous Medium: Kinetic and Isothermal Study" Polymers 14, no. 13: 2732. https://doi.org/10.3390/polym14132732
APA StyleCarneiro, M. T., Barros, A. Z. B., Morais, A. I. S., Carvalho Melo, A. L. F., Bezerra, R. D. S., Osajima, J. A., & Silva-Filho, E. C. (2022). Application of Water Hyacinth Biomass (Eichhornia crassipes) as an Adsorbent for Methylene Blue Dye from Aqueous Medium: Kinetic and Isothermal Study. Polymers, 14(13), 2732. https://doi.org/10.3390/polym14132732









