The Design of Sulfated Ce/HZSM-5 for Catalytic Decomposition of CF4
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.2. Catalytic Activity Test
2.3. Catalyst Characterization
3. Results and Discussion
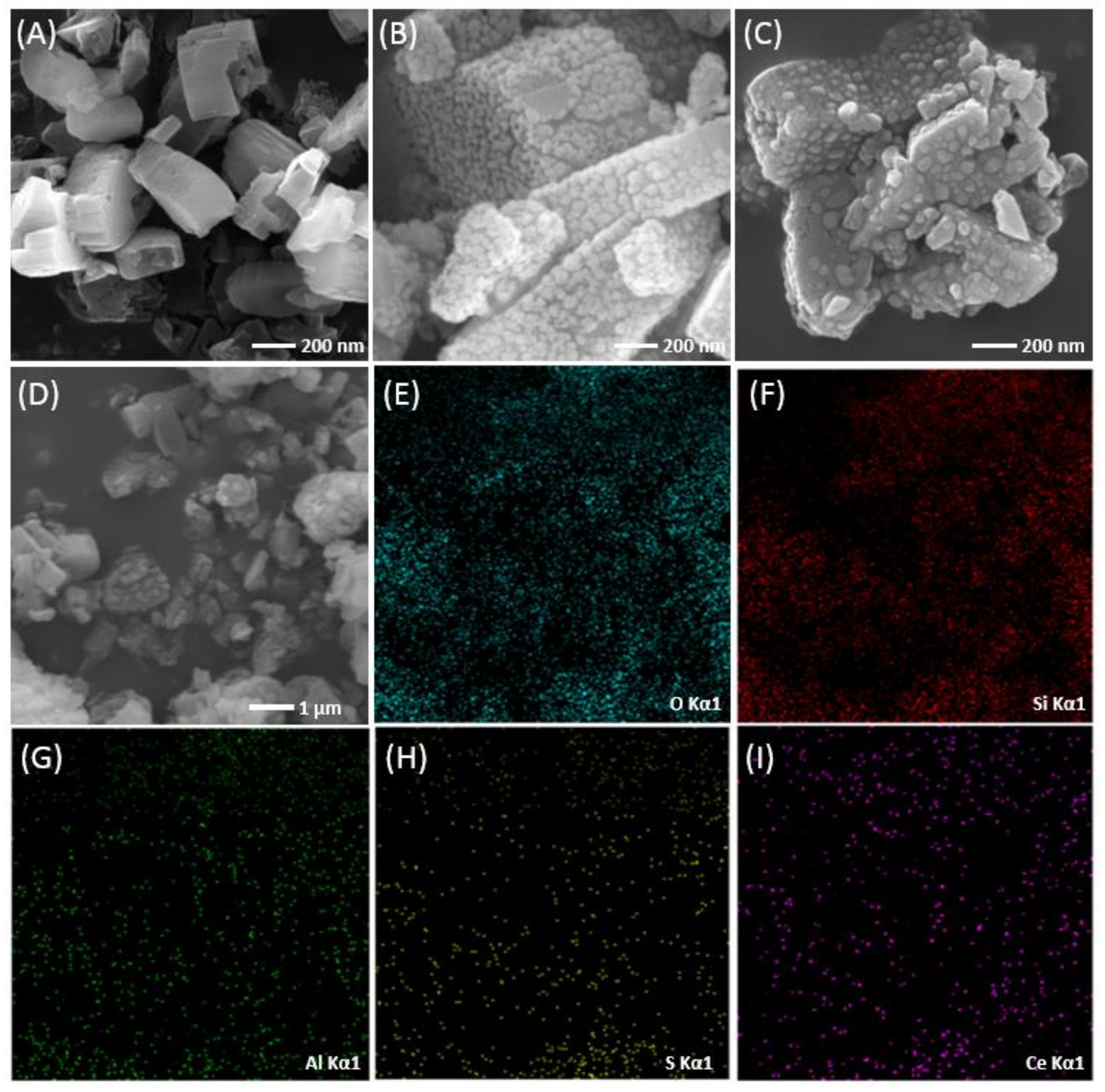
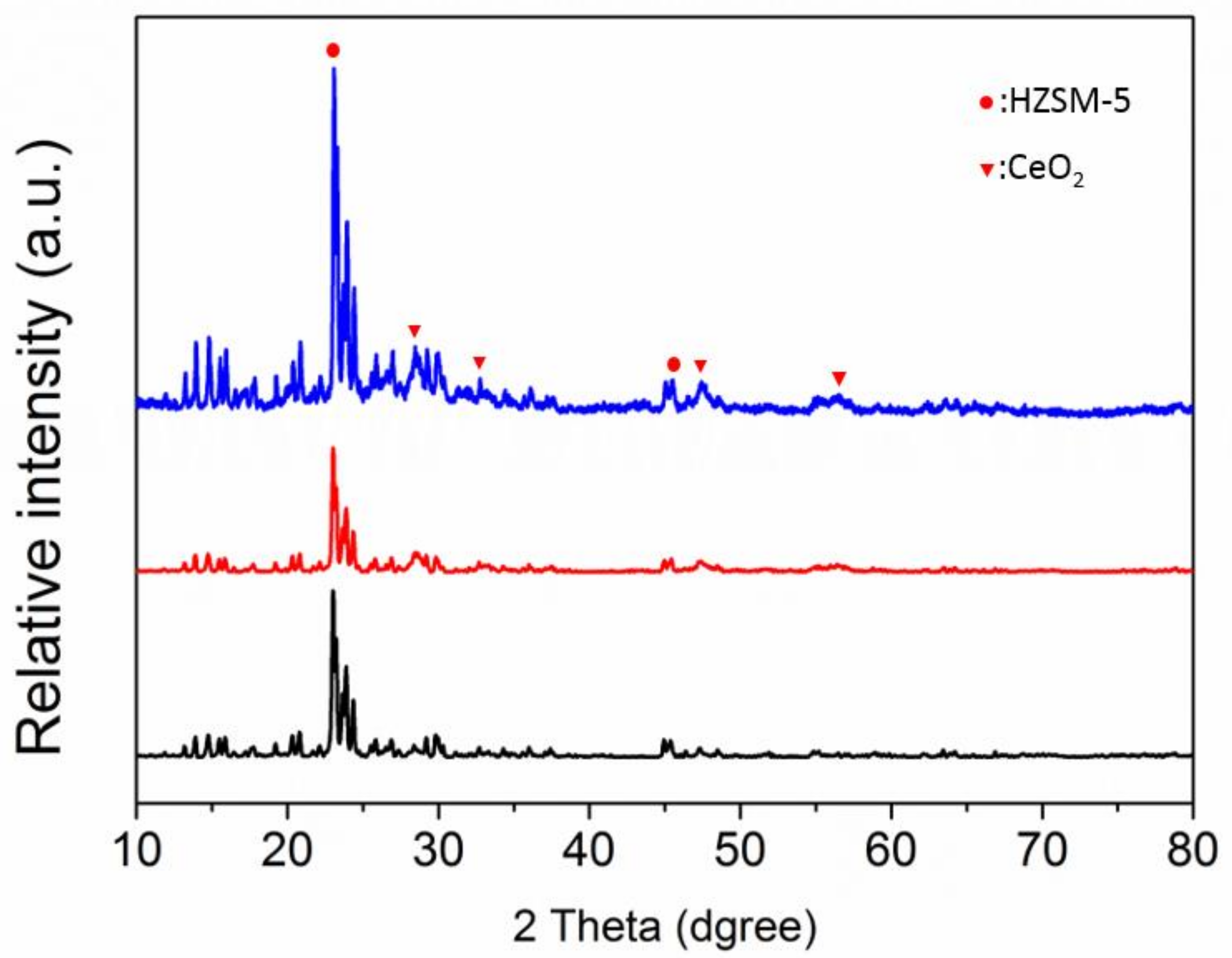
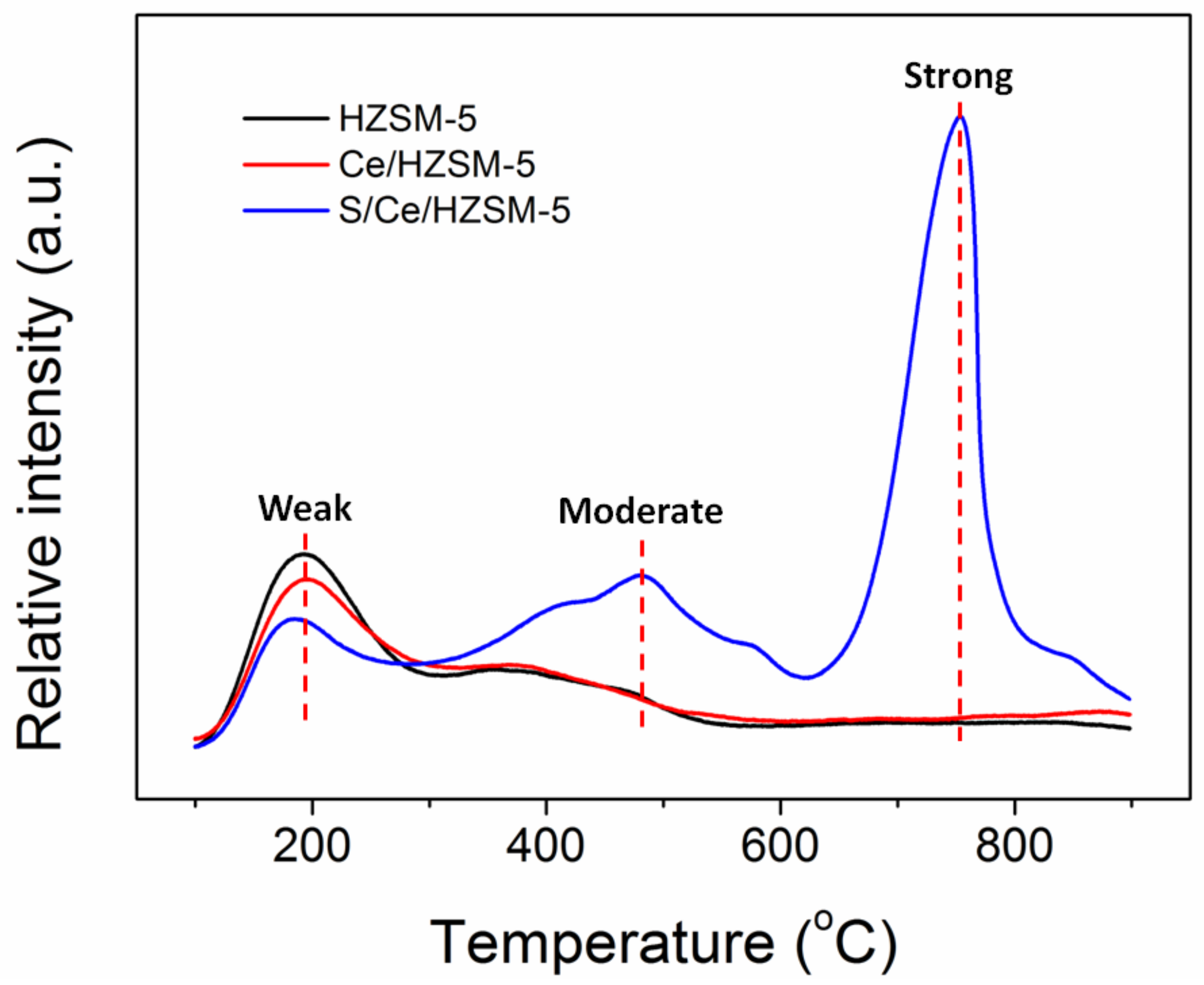
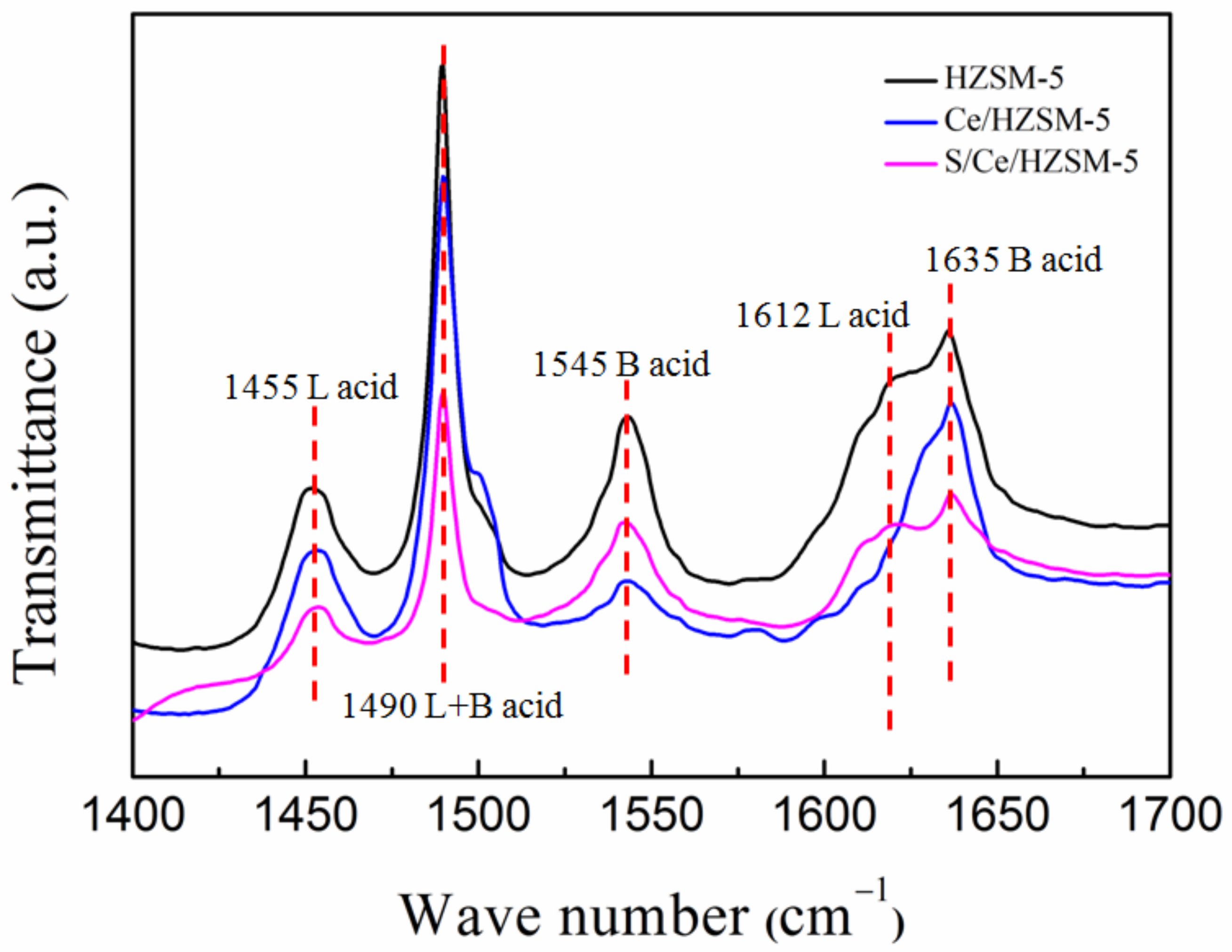
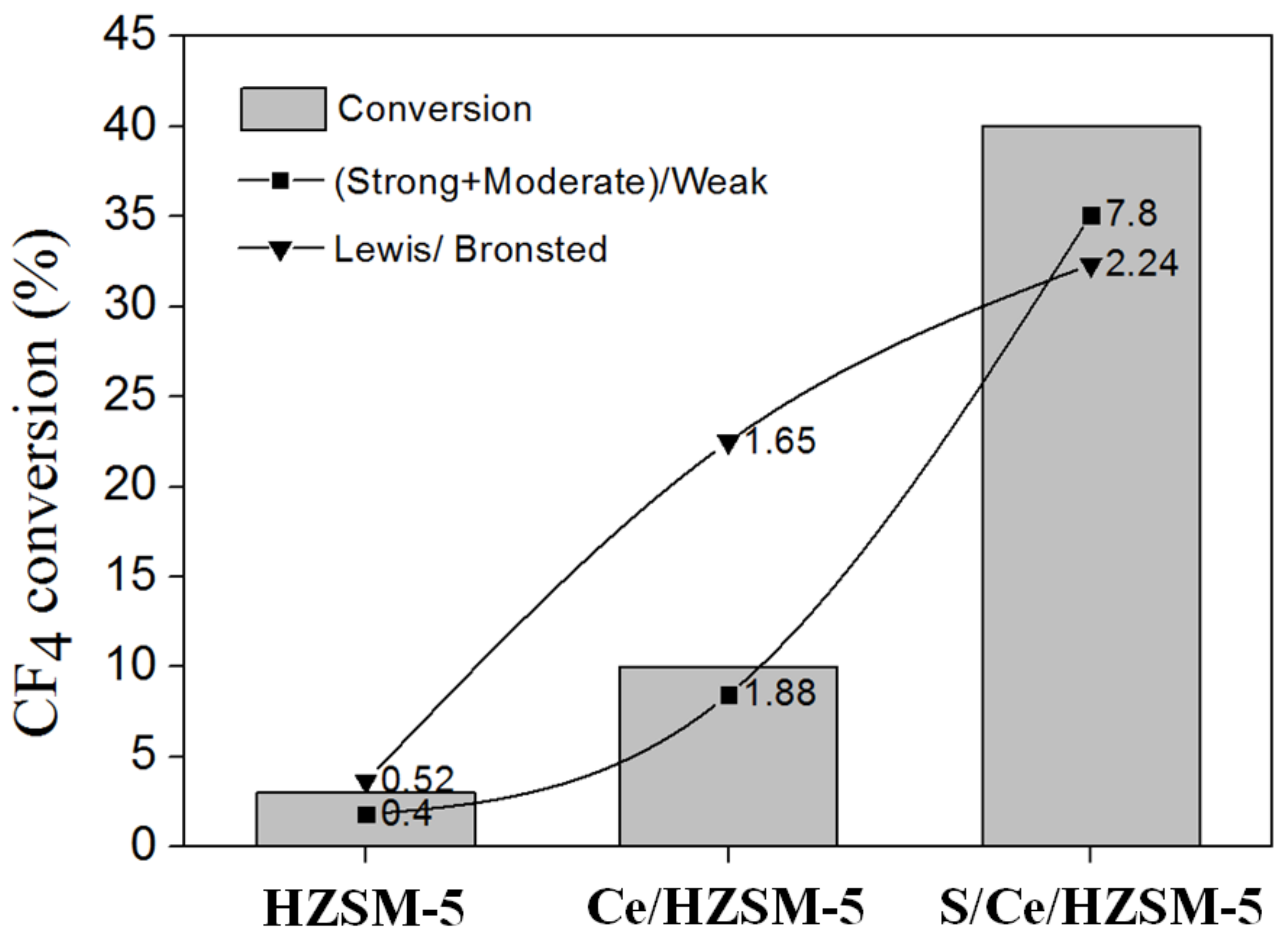
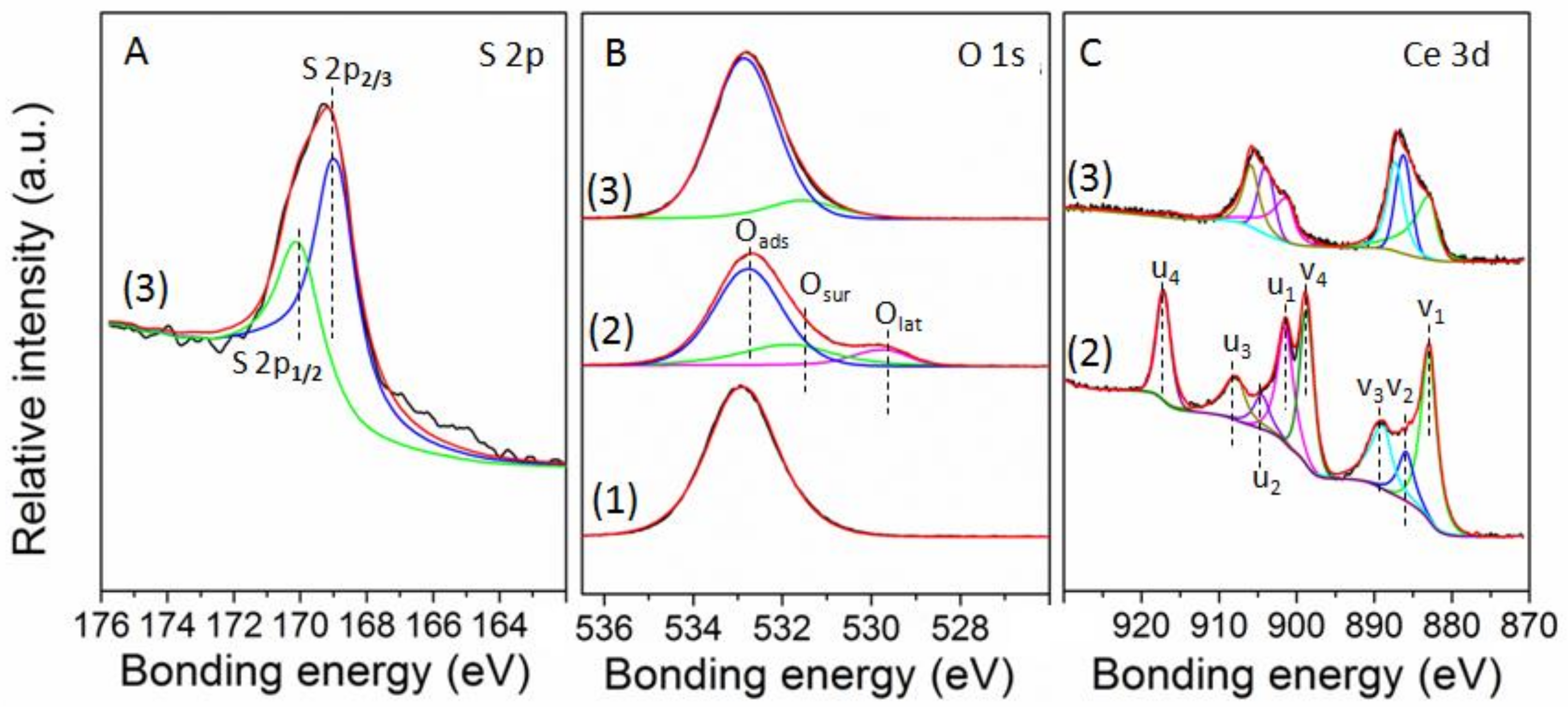
3.1. Characterization of Catalysts
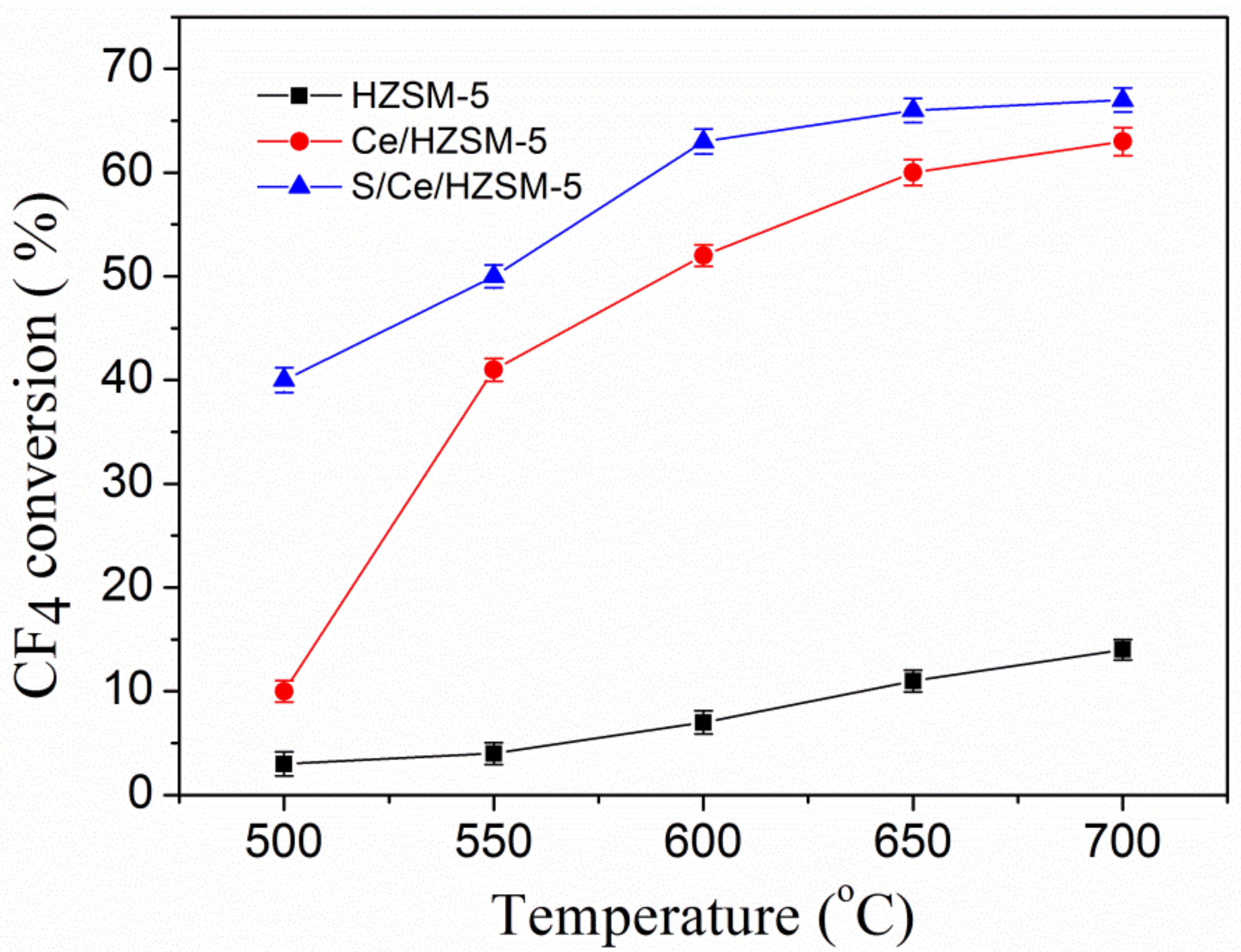
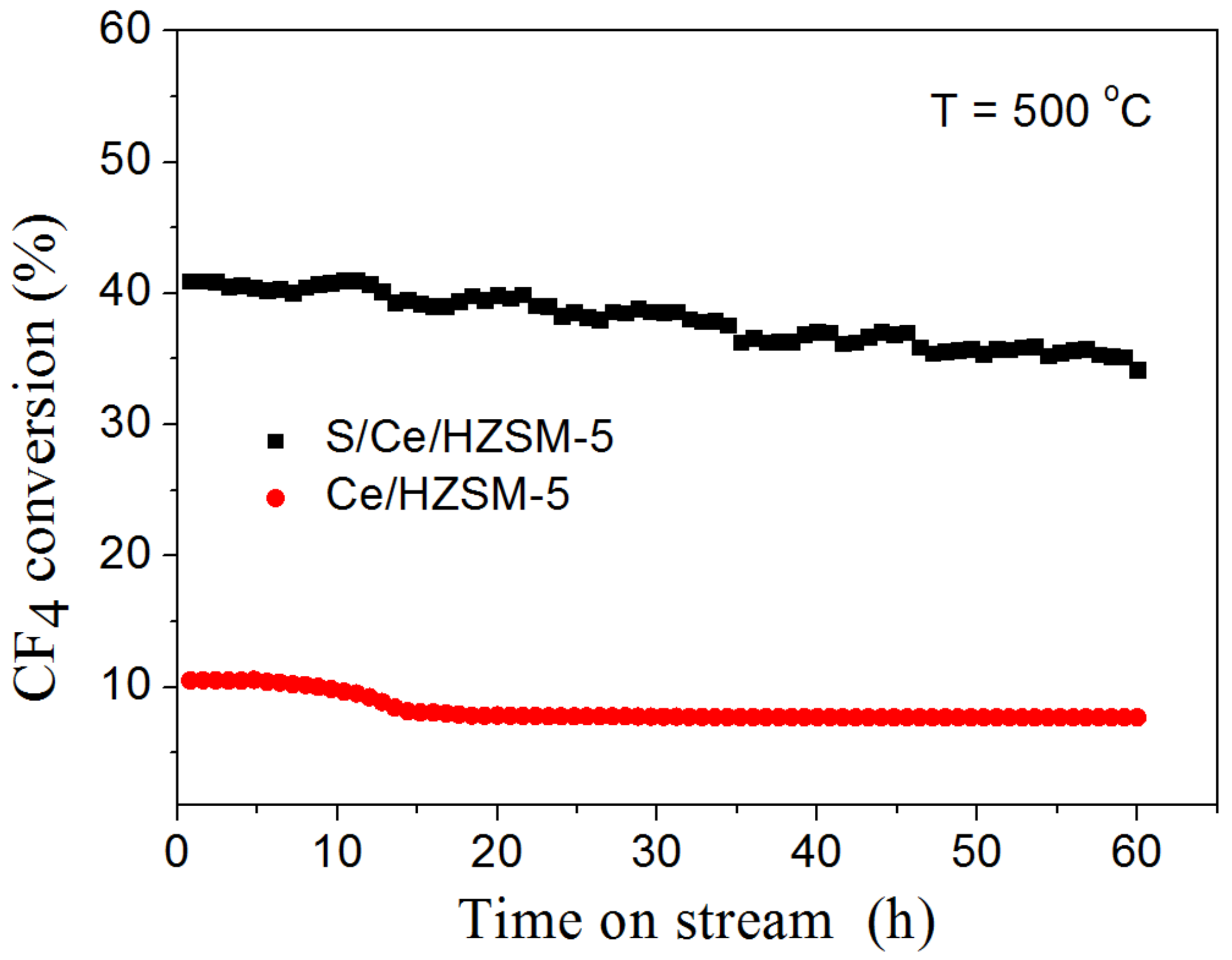
3.2. Catalytic Performance of CF4 Decomposition
3.3. Mechanism Analysis of Hydrolytic Decomposition of CF4
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Hannus, I. Adsorption and transformation of halogenated hydrocarbons over zeolites. Appl. Catal. A Gen. 1999, 189, 263–276. [Google Scholar] [CrossRef]
- Mühle, J.; Ganesan, A.L.; Miller, B.R.; Salameh, P.K.; Harth, C.M.; Greally, B.R.; Rigby, M.; Porter, L.W.; Steele, L.P.; Trudinger, C.M.; et al. Perfluorocarbons in the global atmosphere: Tetrafluoromethane, hexafluoroethane, and octafluoropropane. Atmos. Chem. Phys. 2010, 10, 5145–5164. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.T.; Chen, H.P.; Hsien, W.Y. A review of uses, environmental hazards and recovery/recycle technologies of perfluorocarbons (PFCs) emissions from the semiconductor manufacturing processes. J. Loss Prevent. Proc. Ind. 2002, 15, 65–75. [Google Scholar] [CrossRef]
- Tabereaux, A.T. Anode effects, PFCs, global warming, and the aluminum industry. J. Miner. Met. Mater. Soc. (TMS) 1994, 46, 30–34. [Google Scholar] [CrossRef]
- Perfluorocarbon (PFC) Emissions—International Aluminium Institute. Available online: https://international-aluminium.org/ (accessed on 5 June 2022).
- Zheng, X.; Zheng, X.; Xiang, K.; Shen, F.; Liu, H. The Zr Modified γ-Al2O3 Catalysts for Stable Hydrolytic Decomposition of CF4 at Low Temperature. Catalysts 2022, 12, 313. [Google Scholar] [CrossRef]
- Jia, L.; Ma, S. The Experimental Study on High Temperature Air Combustion and CF4 Decomposition. In Proceedings of the Heat Transfer Summer Conference, San Francisco, CA, USA, 17–22 July 2005; pp. 705–708. [Google Scholar]
- Efremov, A.; Lee, J.; Kim, J. On the Control of Plasma Parameters and Active Species Kinetics in CF4/O2/Ar Gas Mixture by CF4/O2 and O2/Ar Mixing Ratios. Plasma Chem. Plasma Process. 2017, 37, 1445–1462. [Google Scholar] [CrossRef]
- Hou, X.; Qiu, Y.; Yuan, E.; Zhang, X.; Liu, G. SO42−/TiO2 promotion on HZSM-5 for catalytic cracking of paraffin. Appl. Catal. A Gen. 2017, 537, 12–23. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, J.P.; Retn, X.Y.; Zhao, X.Y.; Liu, S.N.; Guo, Z.X.; Shen, W.Z.; Bai, J.; Wei, X.Y. Preparation of hierarchical HZSM-5 based sulfated zirconium solid acid catalyst for catalytic upgrading of pyrolysis vapors from lignite pyrolysis. Fuel 2019, 237, 1079–1085. [Google Scholar] [CrossRef]
- de Rivas, B.; Sampedro, C.; Ramos-Fernandez, E.V.; Lopez-Fonseca, R.; Gascon, J.; Makkee, M.; Gutiérrez-Ortiz, J.I. Influence of the synthesis route on the catalytic oxidation of 1,2-dichloroethane over CeO2/H-ZSM5 catalysts. Appl. Catal. A Gen. 2013, 456, 96–104. [Google Scholar] [CrossRef]
- Joshi, Y.V.; Thomson, K.T. The roles of gallium hydride and Brønsted acidity in light alkane dehydrogenation mechanisms using Ga-exchanged HZSM-5 catalysts: A DFT pathway analysis. Catal. Today 2005, 105, 106–121. [Google Scholar] [CrossRef]
- Zhan, W.; Guo, Y.; Gong, X.; Guo, Y.; Wang, Y. Current status and perspectives of rare earth catalytic materials and catalysis. Chin. J. Catal. 2014, 35, 1238–1250. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, W.; Wang, X.; Lu, W. Sandwich-structured CeO2@ZSM-5 hybrid composites for catalytic oxidation of 1,2-dichloroethane: An integrated solution to coking and chlorine poisoning deactivation. Appl. Catal. B Environ. 2017, 203, 31–42. [Google Scholar] [CrossRef]
- Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Jia, W.; Wu, Q.; Lang, X.; Hu, C.; Zhao, G.; Li, J.; Zhu, Z. Influence of Lewis Acidity on Catalytic Activity of the Porous Alumina for Dehydrofluorination of 1,1,1,2-Tetrafluoroethane to Trifluoroethylene. Catal. Lett. 2015, 145, 654–661. [Google Scholar] [CrossRef]
- Mekhemer, G.A.H.; Khalaf, H.A.; Mansour, S.A.A.; Nohman, A.K.H. Sulfated Alumina Catalysts: Consequences of Sulfate Content and Source. Mon. Für Chem./Chem. Mon. 2005, 136, 2007–2016. [Google Scholar] [CrossRef]
- Song, J.Y.; Chung, S.H.; Kim, M.S.; Seo, M.G.; Lee, Y.H.; Lee, K.Y.; Kim, J.S. The catalytic decomposition of CF4 over Ce/Al2O3 modified by a cerium sulfate precursor. J. Mol. Catal. A Chem. 2013, 370, 50–55. [Google Scholar] [CrossRef]
- Xu, X.; Jeon, J.Y.; Choi, M.H.; Kim, H.Y.; Choi, W.C.; Park, Y.K. A Strategy to Protect Al2O3-based PFC Decomposition Catalyst from Deactivation. Chem. Lett. 2005, 34, 364–365. [Google Scholar] [CrossRef]
- El-Bahy, Z.; Ohnishi, R.; Ichikawa, M. Hydrolysis of CF4 over alumina-based binary metal oxide catalysts. Appl. Catal. B Environ. 2003, 40, 81–91. [Google Scholar] [CrossRef]
- Takita, Y.; Morita, C.; Ninomiya, M.; Wakamatsu, H.; Nishiguchi, H.; Ishihara, T. Catalytic Decomposition of CF4 over AlPO4-Based Catalysts. Chem. Lett. 1999, 28, 417–418. [Google Scholar] [CrossRef]
- Dwivedi, U.; Naik, S.N.; Pant, K.K. High quality liquid fuel production from waste plastics via two-step cracking route in a bottom-up approach using bi-functional Fe/HZSM-5 catalyst. Waste Manag. 2021, 132, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, C.; Zhang, Q.; Pan, P.; Gu, L.; Xu, D.; Bao, J.; Dai, Z. 2D Electron Gas and Oxygen Vacancy Induced High Oxygen Evolution Performances for Advanced Co3O4/CeO2 Nanohybrids. Adv. Mater. 2019, 31, 1900062. [Google Scholar] [CrossRef] [PubMed]
- Takita, Y.; Ninomiya, M.; Miyake, H.; Wakamatsu, H.; Yoshinaga, Y.; Ishihara, T. Catalytic decomposition of perfluorocarbons Part II. Decomposition of CF4 over AlPO4-rare earth phosphate catalysts. Phys. Chem. Chem. Phys. 1999, 1, 4501–4504. [Google Scholar] [CrossRef]
- Panda, A.K.; Mishra, B.G.; Mishra, D.K.; Singh, R.K. Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surf. A Physicochem. Eng. Asp. 2010, 363, 98–104. [Google Scholar] [CrossRef]
- Stanciulescu, M.; Bulsink, P.; Caravaggio, G.; Nossova, L.; Burich, R. NH3-TPD-MS study of Ce effect on the surface of Mn- or Fe-exchanged zeolites for selective catalytic reduction of NOx by ammonia. Appl. Surf. Sci. 2014, 300, 201–207. [Google Scholar] [CrossRef]
- Benaliouche, F.; Boucheffa, Y.; Ayrault, P.; Mignard, S.; Magnoux, P. NH3-TPD and FTIR spectroscopy of pyridine adsorption studies for characterization of Ag- and Cu-exchanged X zeolites. Microporous Mesoporous Mater. 2008, 111, 80–88. [Google Scholar] [CrossRef]
- Luo, J.; Kamasamudram, K.; Currier, N.; Yezerets, A. NH3-TPD methodology for quantifying hydrothermal aging of Cu/SSZ-13 SCR catalysts. Chem. Eng. Sci. 2018, 190, 60–67. [Google Scholar] [CrossRef]
- Xu, X.; Jeon, J.Y.; Choi, M.H.; Kim, H.Y.; Choi, W.C.; Park, Y.K. The modification and stability of γ-Al2O3 based catalysts for hydrolytic decomposition of CF4. J. Mol. Catal. A Chem. 2007, 266, 131–138. [Google Scholar] [CrossRef]
- Damyanova, S.; Centeno, M.; Petrov, L.; Grange, P. Fourier transform infrared spectroscopic study of surface acidity by pyridine adsorption on Mo/ZrO2–SiO2(Al2O3) catalysts. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2495–2501. [Google Scholar] [CrossRef]
- Takita, Y.; Anabe, T.; Ito, M.; Ogura, M.; Muraya, T.; Yasuda, S.; Nishiguchi, H.; Ishihara, T. Decomposition of CH2FCF3 (134a) over Metal Phosphate Catalysts. Ind. Eng. Chem. Res. 2002, 41, 2585–2590. [Google Scholar] [CrossRef]
- Swamidoss, C.M.A.; Sheraz, M.; Anus, A.; Jeong, S.; Park, Y.-K.; Kim, Y.-M.; Kim, S. Effect of Mg/Al2O3 and Calcination Temperature on the Catalytic Decomposition of HFC-134a. Catalysts 2019, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Takita, Y.; Moriyama, J.-I.; Nishiguchi, H.; Ishihara, T.; Hayano, F.; Nakajo, T. Decomposition of CCl2F2 over metal sulfate catalysts. Catal. Today 2004, 88, 103–109. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Zhang, X.; Liu, J.; Zhang, Y.; Guo, Y.; Sun, B. Catalytic conversion of NO assisted by plasma over Mn-Ce/ZSM5-multi-walled carbon nanotubes composites: Investigation of acidity, activity and stability of catalyst in the synergic system. Appl. Surf. Sci. 2018, 457, 187–199. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, H.; Han, W.; Dong, F.; Tang, Z. Enhanced catalytic performance of Nb doping Ce supported on ordered mesoporous TiO2-SiO2 catalysts for catalytic elimination of 1,2-dichlorobenzene. Mol. Catal. 2019, 479, 110638. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Zhang, G.; Yao, X.; Chuang, S.S.C.; Li, Z. Oxygen Vacancy Promoting Dimethyl Carbonate Synthesis from CO2 and Methanol over Zr-Doped CeO2 Nanorods. ACS Catal. 2018, 8, 10446–10456. [Google Scholar] [CrossRef]
- Zong, L.; Zhang, G.; Zhao, H.; Zhang, J.; Tang, Z. One pot synthesized CeO2-WO3-TiO2 catalysts with enriched TiO2 (001) facets for selective catalytic reduction of NO with NH3 by evaporation-induced self-assembly method. Chem. Eng. J. 2018, 354, 295–303. [Google Scholar] [CrossRef]
- Han, W.; Zhao, H.; Dong, F.; Tang, Z. Morphology-controlled synthesis of 3D, mesoporous, rosette-like CeCoOx catalysts by pyrolysis of Ce[Co(CN)6] and application for the catalytic combustion of toluene. Nanoscale 2018, 10, 21307–21319. [Google Scholar] [CrossRef]
| Catalysts | BET Surface Area (m2 g−1) | Pore Diameter (nm) |
|---|---|---|
| HZSM-5 | 248 | 2.38 |
| Ce/HZSM-5 | 148 | 2.28 |
| S/Ce/HZSM-5 | 278 | 2.63 |
| Catalysts | Amount of Acid Site (µmol g−1) | ||||
|---|---|---|---|---|---|
| Weak | Moderate | Strong | Total | (Strong + Moderate)/Weak | |
| HZSM-5 | 663 | 267 | 0 | 930 | 0.40 |
| Ce/HZSM-5 | 290 | 544 | 0 | 834 | 1.88 |
| S/Ce/HZSM-5 | 273 | 855 | 1274 | 2402 | 7.80 |
| Catalysts | Amount of Acid Site (µmol g−1) | ||
|---|---|---|---|
| L-Acid Site | B-Acid Site | Lewis/Brønsted (L/B) | |
| HZSM-5 | 120.57 | 233.91 | 0.52 |
| Ce/HZSM-5 | 138.26 | 83.61 | 1.65 |
| S/Ce/HZSM-5 | 156.84 | 69.97 | 2.24 |
| Catalysts | Distribution (%) | |||
|---|---|---|---|---|
| Olat | Osur | Oads | Ce3+/Ce | |
| HZSM-5 | 0 | 0 | 100 | / |
| Ce/HZSM-5 | 10.5 | 22.0 | 67.5 | 25.9 |
| S/Ce/HZSM-5 | 0 | 10.1 | 89.9 | 31.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Chen, S.; Liu, W.; Xiang, K.; Liu, H. The Design of Sulfated Ce/HZSM-5 for Catalytic Decomposition of CF4. Polymers 2022, 14, 2717. https://doi.org/10.3390/polym14132717
Zheng X, Chen S, Liu W, Xiang K, Liu H. The Design of Sulfated Ce/HZSM-5 for Catalytic Decomposition of CF4. Polymers. 2022; 14(13):2717. https://doi.org/10.3390/polym14132717
Chicago/Turabian StyleZheng, Xie, Shijie Chen, Wanning Liu, Kaisong Xiang, and Hui Liu. 2022. "The Design of Sulfated Ce/HZSM-5 for Catalytic Decomposition of CF4" Polymers 14, no. 13: 2717. https://doi.org/10.3390/polym14132717
APA StyleZheng, X., Chen, S., Liu, W., Xiang, K., & Liu, H. (2022). The Design of Sulfated Ce/HZSM-5 for Catalytic Decomposition of CF4. Polymers, 14(13), 2717. https://doi.org/10.3390/polym14132717





