Characterization and Antibacterial Evaluation of Biodegradable Mannose-Conjugated Fe-MIL-88NH2 Composites Containing Vancomycin against Methicillin-Resistant Staphylococcus aureus Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
2.2. Surface Morphology
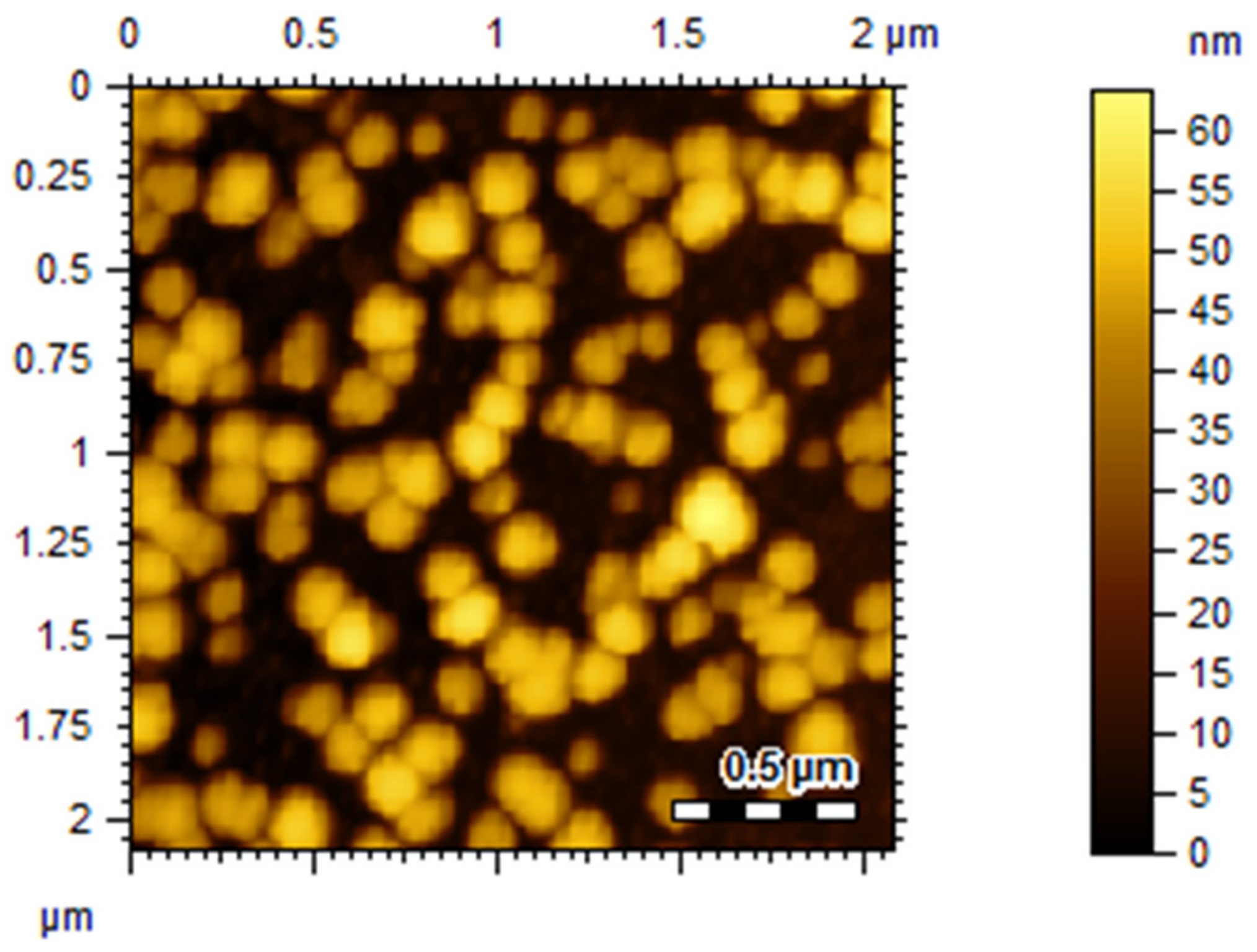
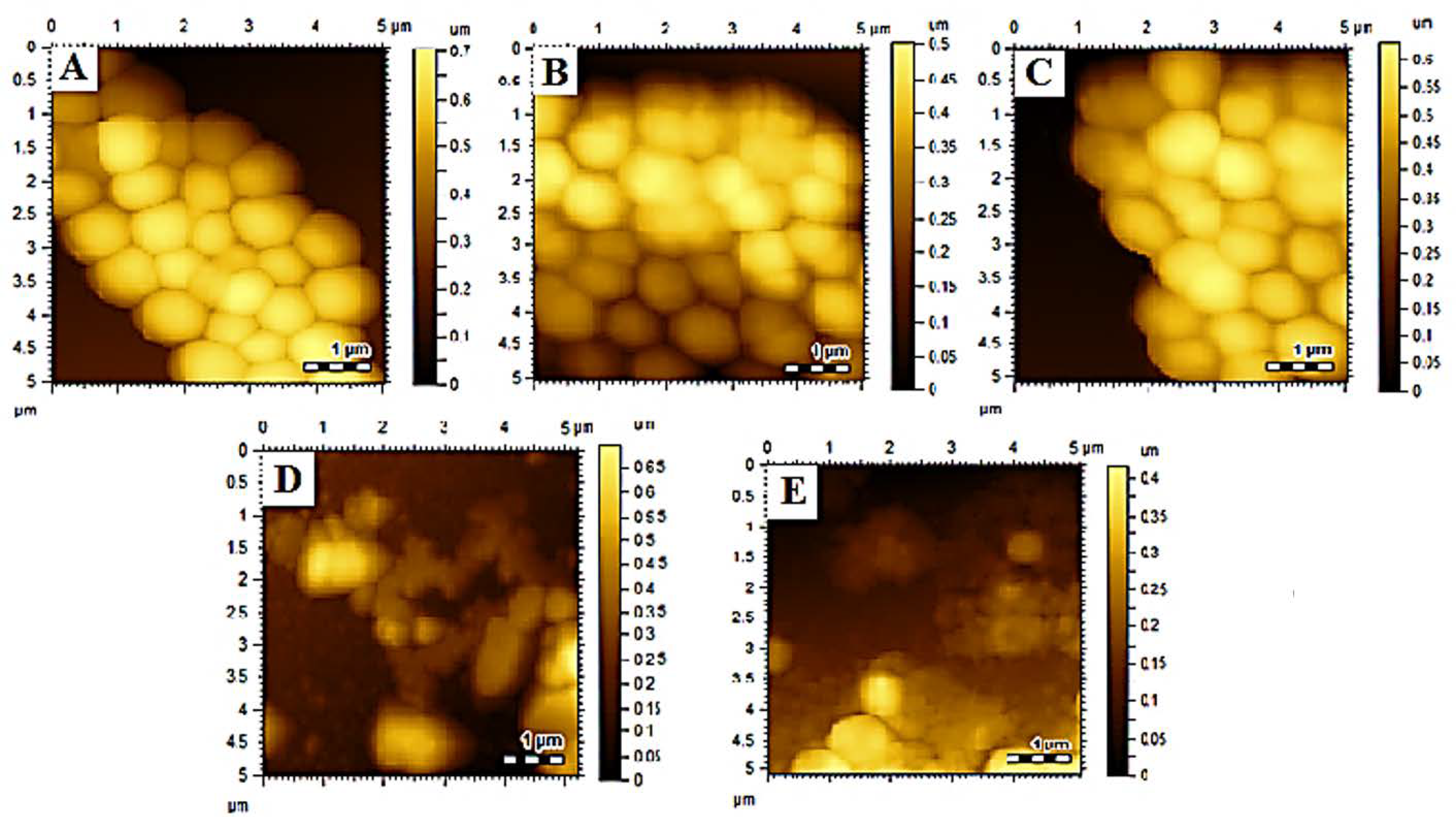
2.3. Atomic Force Microscopy (AFM) Analysis
2.4. Antibacterial Assay
2.5. Minimum Inhibitory Concentration Assay with Tetrazolium Microplates
2.6. Analyses of the Surface Morphology
2.7. Statistical Analysis
3. Results
3.1. FT-IR Analysis
3.2. Size, PDI, Zeta-Potential, and Surface Morphology
3.3. Drug Loading Efficiency
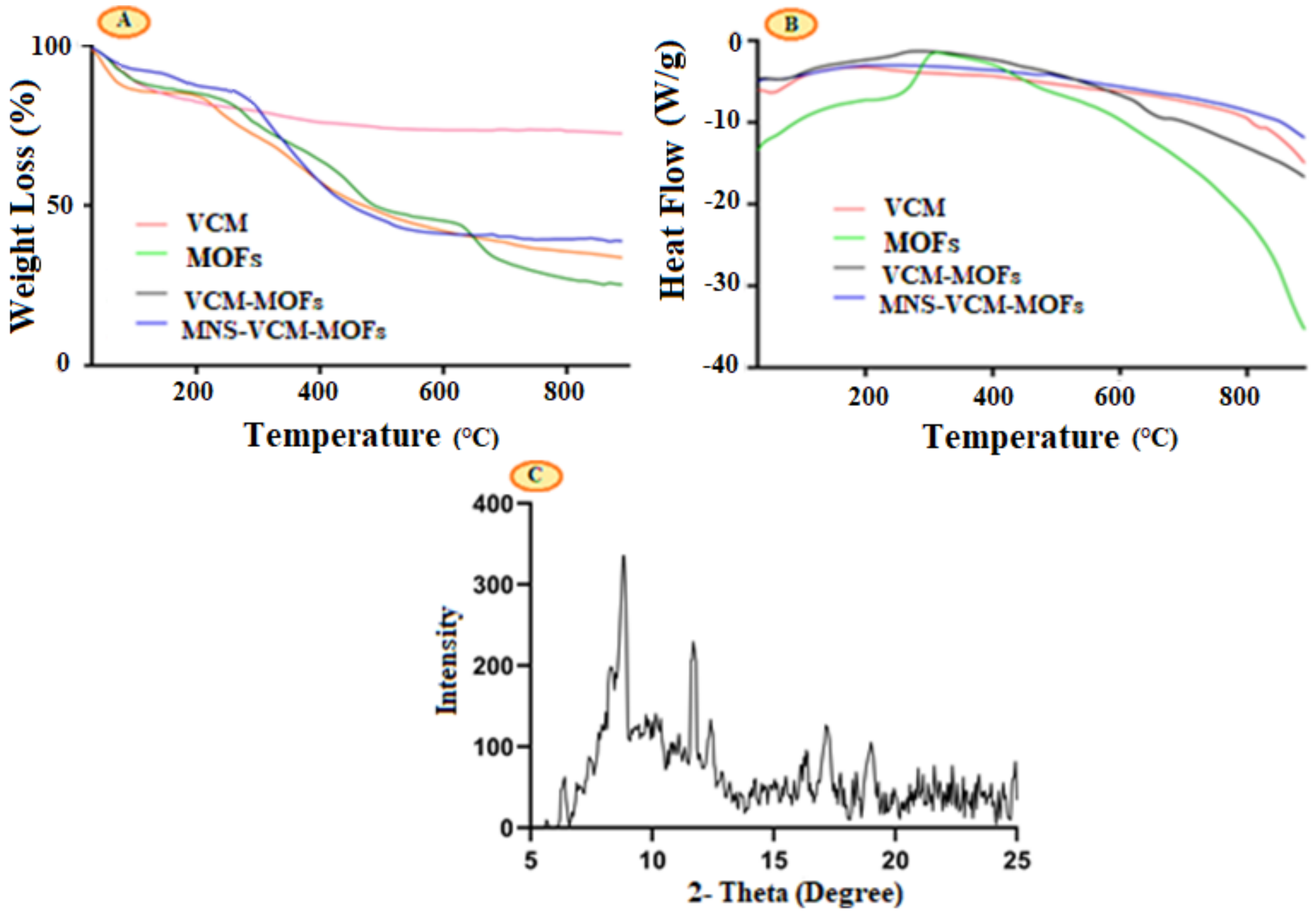
3.4. Thermogravimetric (TG) and Differential Scanning Calorimetry (DSC) Thermal Analysis
3.5. Powder-XRD
3.6. Anti-Bacterial Assay
3.6.1. Tetrazolium Microplate Assay
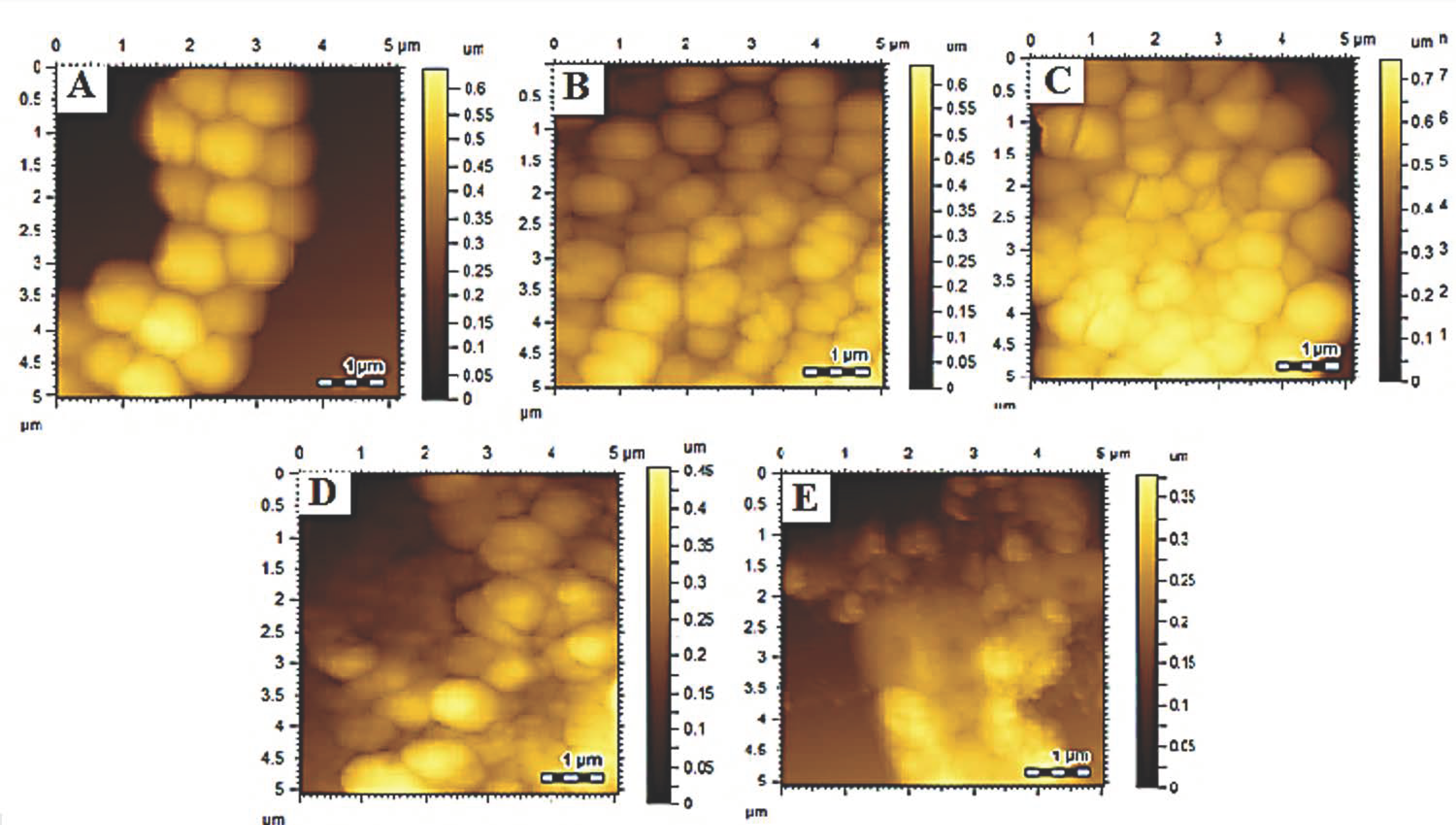
3.6.2. Morphological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, S.B. The challenge of antibiotic resistance. Sci. Am. 1998, 278, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Di Cerbo, A.; Aloisi, P.; Manelli, M.; Pellesi, V.; Provenzano, C.; Camellini, S.; Messi, P.; Sabia, C. In Vitro Activity of Essential Oils Against Planktonic and Biofilm Cells of Extended-Spectrum beta-Lactamase (ESBL)/Carbapenamase-Producing Gram-Negative Bacteria Involved in Human Nosocomial Infections. Antibiotics 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Di Cerbo, A.; Messi, P.; Sabia, C. Antibiotic Resistance and Virulence Traits in Vancomycin-Resistant Enterococci (VRE) and Extended-Spectrum beta-Lactamase/AmpC-producing (ESBL/AmpC) Enterobacteriaceae from Humans and Pets. Antibiotics 2020, 9, 152. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Canello, S.; Guidetti, G.; Fiore, F.; Corsi, L.; Rubattu, N.; Testa, C.; Cocco, R. Adverse food reactions in dogs due to antibiotic residues in pet food: A preliminary study. Vet. Ital. 2018, 54, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Canello, S.; Guidetti, G.; Laurino, C.; Palmieri, B. Unusual antibiotic presence in gym trained subjects with food intolerance; a case report. Nutr. Hosp. 2014, 30, 395–398. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Palatucci, A.T.; Rubino, V.; Centenaro, S.; Giovazzino, A.; Fraccaroli, E.; Cortese, L.; Ruggiero, G.; Guidetti, G.; Canello, S.; et al. Toxicological Implications and Inflammatory Response in Human Lymphocytes Challenged with Oxytetracycline. J. Biochem. Mol. Toxicol. 2016, 30, 170–177. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Guidetti, G.; Canello, S.; Corsi, L. Tetracyclines: Insights and updates of their use in human and animal pathology and their potential toxicity. Open Biochem. J. 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Scarano, A. Cytotoxic and Bacteriostatic Activity of Nanostructured TiO2 Coatings. Pol. J. Microbiol. 2016, 65, 225–229. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Scarano, A.; Guidetti, G.; Canello, S. The contradictory world of tetracyclines. Panminerva Med. 2020, 62, 116–117. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Rubino, V.; Morelli, F.; Ruggiero, G.; Landi, R.; Guidetti, G.; Canello, S.; Terrazzano, G.; Alessandrini, A. Mechanical phenotyping of K562 cells by the Micropipette Aspiration Technique allows identifying mechanical changes induced by drugs. Sci. Rep. 2018, 8, 1219. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Scarano, A.; Pezzuto, F.; Guidetti, G.; Canello, S.; Pinetti, D.; Genovese, F.; Corsi, L. Oxytetracycline-protein complex: The dark side of pet food. Open Public Health J. 2018, 11, 162–169. [Google Scholar] [CrossRef]
- Gallo, A.; Landi, R.; Rubino, V.; Di Cerbo, A.; Giovazzino, A.; Palatucci, A.T.; Centenaro, S.; Guidetti, G.; Canello, S.; Cortese, L.; et al. Oxytetracycline induces DNA damage and epigenetic changes: A possible risk for human and animal health? PeerJ 2017, 5, e3236. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, C.; Di Cerbo, A.; Lecce, L.; Piccoli, C.; Canello, S.; Guidetti, G.; Capitanio, N. Effect of Chicken Bone Extracts on Metabolic and Mitochondrial Functions of K562 Cell Line. Pharmaceuticals 2020, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Di Cerbo, A.; Laurino, C. Antibiotic treatments in zootechnology and effects induced on the food chain of domestic species and, comparatively, the human specie. Nutr. Hosp. 2014, 29, 1427–1433. [Google Scholar] [CrossRef]
- Yaseen, M.; Kamran, M.; Farid, A.; Ismail, S.; Muzammal, M.; Amir, K.A.; Rashid, S.A. Antibacterial, Hemagglutination, and Insecticidal Activity Studies on the Solvent Extracts of the Roots of Olea ferruginea. Makara J. Sci. 2022, 26, 8. [Google Scholar]
- Al Mohaini, M.; Farid, A.; Muzammal, M.; Dadrasnia, A.; Alsalman, A.J.; Al Hawaj, M.A.; Alhashem, Y.N.; Ismail, S. Pathological study of Pasteurella Multocida Recombinant Clone ABA392. Pak. J. Med. Health Sci. 2022, 16, 1112. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Chen, Y.X.; Qiu, H.C.; Jue, K.A. [Restriction map of E. coli shuttle plasmid (p# GTE5) with secretive function]. Wei Sheng Wu Xue Bao 1989, 29, 228–231. [Google Scholar]
- Weinstein, R.A.; Fridkin, S.K. Vancomycin-Intermediate and -Resistant Staphylococcus aureus: What the Infectious Disease Specialist Needs to Know. Clin. Infect. Dis. 2001, 32, 108–115. [Google Scholar] [CrossRef]
- Farid, A.; Shah, A.H.; Ayaz, M.; Amin, A.; Yaseen, M.; Ullah, H.; Haq, F. Comparative study of biological activity of glutathione, sodium tungstate and glutathione-tungstate mixture. Afr. J. Biotechnol. 2012, 11, 10431–10437. [Google Scholar]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Seeger, H.M.; Di Cerbo, A.; Caramaschi, T.; Facci, P. What do we really measure in AFM punch-through experiments on supported lipid bilayers? Soft Matter 2011, 7, 7054–7064. [Google Scholar] [CrossRef]
- Guildford, A.L.; Poletti, T.; Osbourne, L.H.; Di Cerbo, A.; Gatti, A.M.; Santin, M. Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response. J. R. Soc. Interface 2009, 6, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Seeger, H.M.; Di Cerbo, A.; Alessandrini, A.; Facci, P. Supported lipid bilayers on mica and silicon oxide: Comparison of the main phase transition behavior. J. Phys. Chem. B 2010, 114, 8926–8933. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A. The applications of metal-organic-frameworks in controlled release of drugs. Rev. J. Chem. 2017, 7, 1–22. [Google Scholar] [CrossRef]
- Cohen, S.M. Modifying MOFs: New chemistry, new materials. Chem. Sci. 2010, 1, 32–36. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Morris, R.E.; Brammer, L. Coordination change, lability and hemilability in metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 5444–5462. [Google Scholar] [CrossRef]
- Saadullah, M.; Asif, M.; Farid, A.; Naseem, F.; Rashid, S.A.; Ghazanfar, S.; Muzammal, M.; Ahmad, S.; Bin Jardan, Y.A.; Alshaya, H.; et al. A Novel Distachionate from Breynia distachia Treats Inflammations by Modulating COX-2 and Inflammatory Cytokines in Rat Liver Tissue. Molecules 2022, 27, 2596. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef]
- Dwek, R.A. Glycobiology: Toward Understanding the Function of Sugars. Chem. Rev. 1996, 96, 683–720. [Google Scholar] [CrossRef] [PubMed]
- Muzammal, M.; Khan, M.A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Farid, A. In Silico Analysis of Honeybee Venom Protein Interaction with Wild Type and Mutant (A82V + P375S) Ebola Virus Spike Protein. Biologics 2022, 2, 45–55. [Google Scholar] [CrossRef]
- Bucior, I.; Burger, M.M. Carbohydrate-carbohydrate interactions in cell recognition. Curr. Opin. Struct. Biol. 2004, 14, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.E.; Paulson, J.C. Cell surface biology mediated by low affinity multivalent protein-glycan interactions. Curr. Opin. Chem. Biol. 2004, 8, 617–625. [Google Scholar] [CrossRef]
- Kato, K.; Ishiwa, A. The role of carbohydrates in infection strategies of enteric pathogens. Trop. Med. Health 2015, 43, 41–52. [Google Scholar] [CrossRef]
- Disney, M.D.; Seeberger, P.H. The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens. Chem. Biol. 2004, 11, 1701–1707. [Google Scholar] [CrossRef]
- Hameed, A.; Condò, C.; Tauseef, I.; Idrees, M.; Ghazanfar, S.; Farid, A.; Muzammal, M.; Al Mohaini, M.; Alsalman, A.J.; Al Hawaj, M.A.; et al. Isolation and Characterization of a Cholesterol-Lowering Bacteria from Bubalus bubalis Raw Milk. Fermentation 2022, 8, 163. [Google Scholar] [CrossRef]
- Abid, S.; Farid, A.; Rammessha, A.; Rehman, M.U.; Walaa, F.A.; Alhomrani, M.; Alamri, A.S.; Asdaq, S.M.B.; Hefft, D.I.; Saqib, S.; et al. Identification, Biochemical Characterization, and Safety Attributes of Locally Isolated Lactobacillus fermentum from Bubalus bubalis (Buffalo) Milk as a Probiotic. Microorganisms 2022, 10, 954. [Google Scholar] [CrossRef]
- Al Mohaini, M.; Farid, A.; Muzammal, M.; Gazanffar, S.; Dadrasnia, A.; Alsalman, A.J.; Al Hawaj, M.A.; Alhashem, Y.N.; Ismail, S. Enhancing Lipase Production of Bacillus salmalay Strain 139SI Using Different Carbon Sources and Surfactants. Appl. Microbiol. 2022, 2, 237–247. [Google Scholar] [CrossRef]
- Piaru, S.P.; Perumal, S.; Cai, L.W.; Mahmud, R.; Majid, A.M.S.A.; Ismail, S.; Man, C.N. Chemical composition, anti-angiogenic and cytotoxicity activities of the essential oils of Cymbopogan citratus (lemon grass) against colorectal and breast carcinoma cell lines. J. Essent. Oil Res. 2012, 24, 453–459. [Google Scholar] [CrossRef]
- Al Mohaini, M.; Farid, A.; Alsalman, A.J.; Al Hawaj, M.A.; Alhashem, Y.N.; Ghazanfar, S. Screening of Anticancer and Immunomodulatory Properties of Recombinant pQE-HAS113 Clone Derived from Streptococcus Equi. Pak. J. Med. Health Sci. 2022, 16, 1100. [Google Scholar] [CrossRef]
- Ravikumar, L.; Saravanan, R.; Saravanamani, K.; Karunakaran, M. Synthesis and Characterization of New Polyamides with Substitutions in the Pendent Benzylidene Rings. Des. Monomers Polym. 2009, 12, 291–303. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.-Z.; Lu, Y.; Li, Y.-G.; Zhang, Z.; Chen, W.-L.; Fu, H.; Wang, E.-B. Metal–organic frameworks constructed from three kinds of new Fe-containing secondary building units. Inorg. Chim. Acta 2012, 384, 219–224. [Google Scholar] [CrossRef]
- Biemmi, E.; Bein, T.; Stock, N. Synthesis and characterization of a new metal organic framework structure with a 2D porous system: (H2NEt2)2[Zn3(BDC)4]⋅3DEF. Solid State Sci. 2006, 8, 363–370. [Google Scholar] [CrossRef]
- Al Hawaj, M.A.; Farid, A.; Al Mohaini, M.; Alsalman, A.J.; Muzammal, M.; Khan, M.H.; Dadrasnia, A.; Alhashem, Y.N.; Ghazanfar, S.; Almusalami, M.; et al. Biosurfactant Screening and Antibiotic Analysis of Bacillus salmalaya. Int. J. Curr. Res. Rev. 2022, 14, 56. [Google Scholar] [CrossRef]
- Guo, Y.-P.; Yao, Y.-B.; Guo, Y.-J.; Ning, C.-Q. Hydrothermal fabrication of mesoporous carbonated hydroxyapatite microspheres for a drug delivery system. Microporous Mesoporous Mater. 2012, 155, 245–251. [Google Scholar] [CrossRef]
- Saidykhan, L.; Abu Bakar, M.Z.; Rukayadi, Y.; Kura, A.U.; Latifah, S.Y. Development of nanoantibiotic delivery system using cockle shell-derived aragonite nanoparticles for treatment of osteomyelitis. Int. J. Nanomed. 2016, 11, 661–673. [Google Scholar] [CrossRef]
- Kacurakova, M.; Mathlouthi, M. FTIR and laser-Raman spectra of oligosaccharides in water: Characterization of the glycosidic bond. Carbohydr. Res. 1996, 284, 145–157. [Google Scholar] [CrossRef]
- Chalati, T.; Horcajada, P.; Gref, R.; Couvreur, P.; Serre, C. Optimisation of the synthesis of MOF nanoparticles made of flexible porous iron fumarate MIL-88A. J. Mater. Chem. 2011, 21, 2220–2227. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef]
- Li, F.-L.; Zhuang, M.-Y.; Shen, J.-J.; Fan, X.-M.; Choi, H.; Lee, J.-K.; Zhang, Y.-W. Specific Immobilization of Escherichia coli Expressing Recombinant Glycerol Dehydrogenase on Mannose-Functionalized Magnetic Nanoparticles. Catalysts 2019, 9, 7. [Google Scholar] [CrossRef]
- Rao, K.; Imran, M.; Jabri, T.; Ali, I.; Perveen, S.; Shafiullah; Ahmed, S.; Shah, M.R. Gum tragacanth stabilized green gold nanoparticles as cargos for Naringin loading: A morphological investigation through AFM. Carbohydr. Polym. 2017, 174, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Burgmann, H.; Sorum, H.; Norstrom, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Hu, F.; Ji, K.S.; Wu, W.B.; Ding, D.; Kong, D.L.; Liu, B. Metal–Organic-Framework-Assisted In Vivo Bacterial Metabolic Labeling and Precise Antibacterial Therapy. Adv. Mater. 2018, 30, e1706831. [Google Scholar] [CrossRef]
- Jelić, D. Thermal stability of amorphous solid dispersions. Molecules 2021, 26, 238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bisen, D.P.; Shukla, U.; Sharma, B.G. X-ray diffraction: A powerful method of characterizing nanomaterials. Recent Res. Sci. Technol. 2012, 4, 77–79. [Google Scholar]
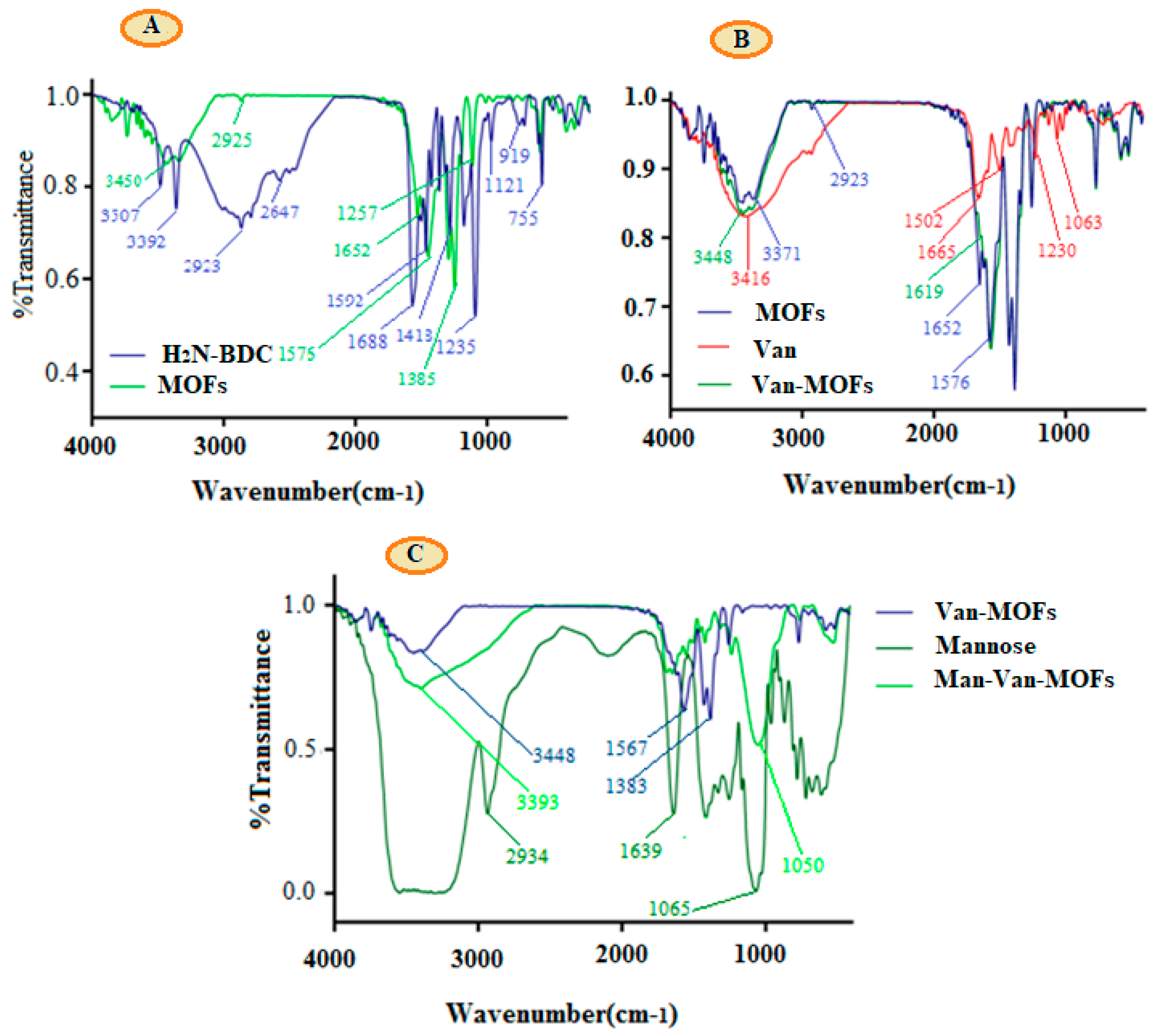


| FT-IR Peaks (cm−1) | ||||||
|---|---|---|---|---|---|---|
| NH2-BDC | MOFs | VCM | VCM-MOFs | Mannose | MNS-VCM-MOFs | Vibrational Mode |
| 3707 | - | 3416 | 3448 | 3600–3200 | 3393 | -OH stretching |
| 3392 | 3371 | - | - | - | - | -NH2 stretching |
| 2923 | - | - | 2934 | - | -CH | |
| 1688 | 1652 | 1665 | 1619 | - | - | -C=O |
| 1592 | 1576 | - | - | - | Disappeared | -NH2 |
| 1230 | 1383 | 1065 | 1050 | -C-O | ||
| - | 1385 and 1576 | - | - | - | Disappeared | Metal coordinated -COOH |
| Test Samples | Size (nm) | PDI | Zeta Potential (mV) | %EE |
|---|---|---|---|---|
| MOFs | 490.40 ± 0.63 | 0.61 ± 0.02 | −12.4 ± 2.94 | |
| VCM-MOFs | 564.03 ± 18.47 | 0.66 ± 0.04 | −15 ± 0.50 | 70.87 ± 2.65 |
| MNSVCMMOFs | 683.36 ± 21.42 | 0.68 ± 0.02 | −20 ± 1.30 | 65.21 ± 4.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haseena; Shah, M.; Rehman, K.; Khan, A.; Farid, A.; Marini, C.; Di Cerbo, A.; Shah, M.R. Characterization and Antibacterial Evaluation of Biodegradable Mannose-Conjugated Fe-MIL-88NH2 Composites Containing Vancomycin against Methicillin-Resistant Staphylococcus aureus Strains. Polymers 2022, 14, 2712. https://doi.org/10.3390/polym14132712
Haseena, Shah M, Rehman K, Khan A, Farid A, Marini C, Di Cerbo A, Shah MR. Characterization and Antibacterial Evaluation of Biodegradable Mannose-Conjugated Fe-MIL-88NH2 Composites Containing Vancomycin against Methicillin-Resistant Staphylococcus aureus Strains. Polymers. 2022; 14(13):2712. https://doi.org/10.3390/polym14132712
Chicago/Turabian StyleHaseena, Muddaser Shah, Khadija Rehman, Adnan Khan, Arshad Farid, Carlotta Marini, Alessandro Di Cerbo, and Muhammad Raza Shah. 2022. "Characterization and Antibacterial Evaluation of Biodegradable Mannose-Conjugated Fe-MIL-88NH2 Composites Containing Vancomycin against Methicillin-Resistant Staphylococcus aureus Strains" Polymers 14, no. 13: 2712. https://doi.org/10.3390/polym14132712
APA StyleHaseena, Shah, M., Rehman, K., Khan, A., Farid, A., Marini, C., Di Cerbo, A., & Shah, M. R. (2022). Characterization and Antibacterial Evaluation of Biodegradable Mannose-Conjugated Fe-MIL-88NH2 Composites Containing Vancomycin against Methicillin-Resistant Staphylococcus aureus Strains. Polymers, 14(13), 2712. https://doi.org/10.3390/polym14132712








