Preparation and Application of a Novel Slow-Releasing with Core-Shell Deicer in Asphalt Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Core-Shell Deicer Preparation
2.3. Deicing Asphalt Mastics Preparation
2.4. Asphalt Mixture with Deicers Preparation
2.5. Characterization
2.5.1. SEM-EDS Characterization
2.5.2. DSC Characterization
2.5.3. TG Characterization
2.5.4. FTIR Characterization
2.6. Performance Evaluation
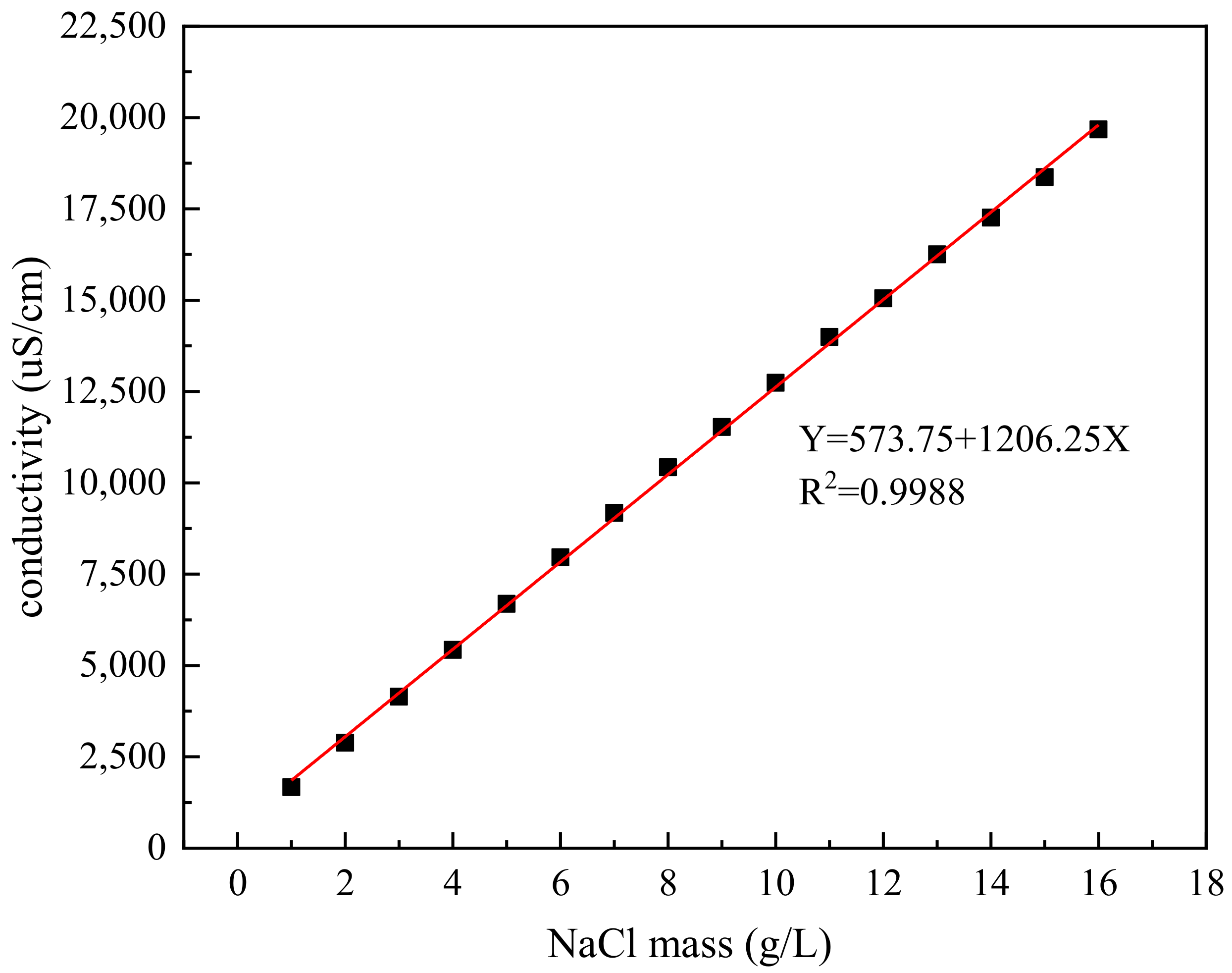
2.6.1. Conductivity Test
2.6.2. Moisture Absorption Rate Test
2.6.3. Ice- and Snow-Melting Performance Evaluation
Deicer
Deicing Asphalt Mixture
2.6.4. Road Performance
3. Results and Discussion
3.1. Core-Shell Deicer
3.1.1. SEM-EDS Characterization
3.1.2. DSC Study
3.1.3. TG/DTG Analysis
3.1.4. Salt Loaded and Coverage Rate Study
3.1.5. Moisture Absorption Rate Analysis
3.1.6. Ice Melting Performance Evaluation
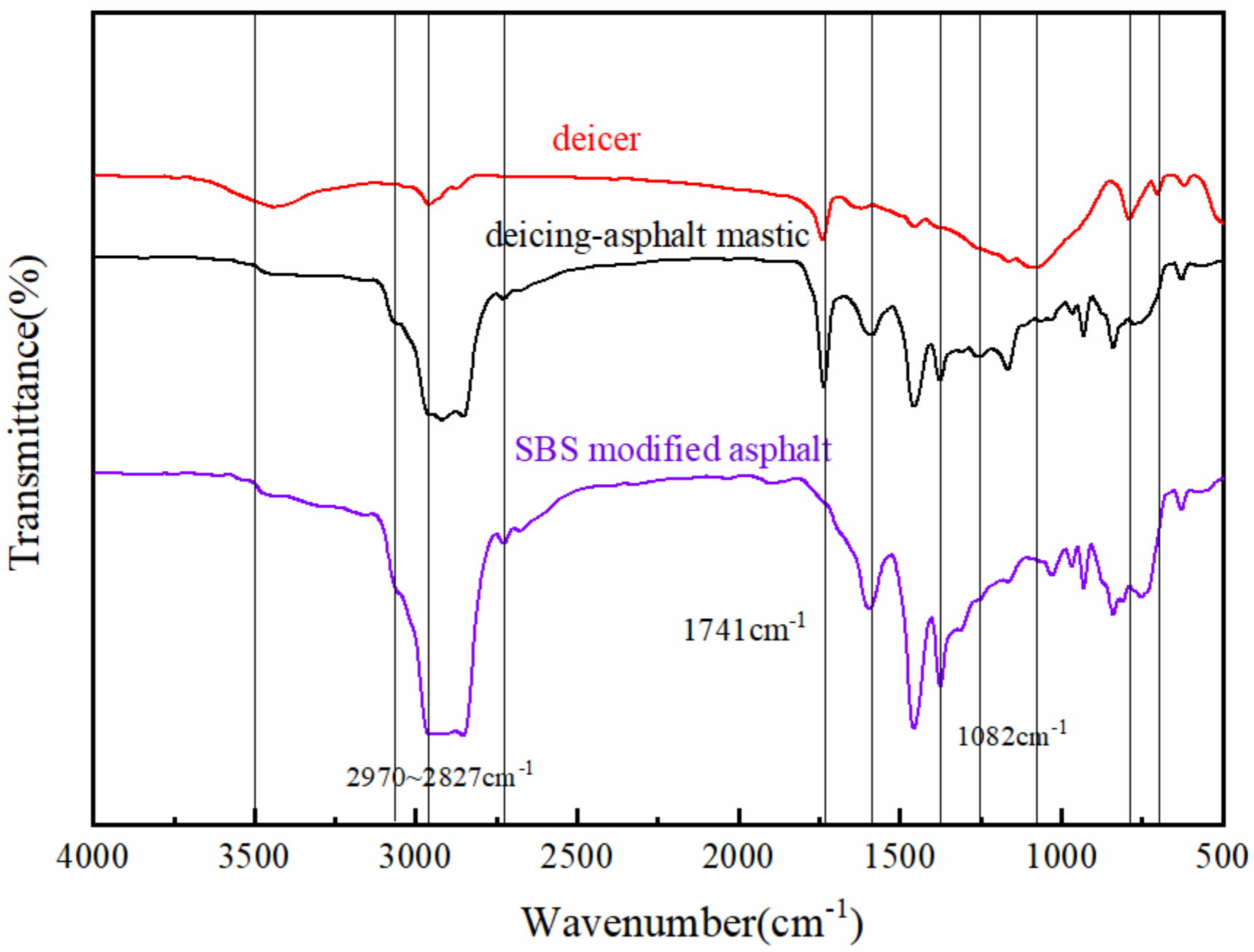
3.2. FTIR Study of Deicing Asphalt Mastics
3.3. Deicing Asphalt Mixture
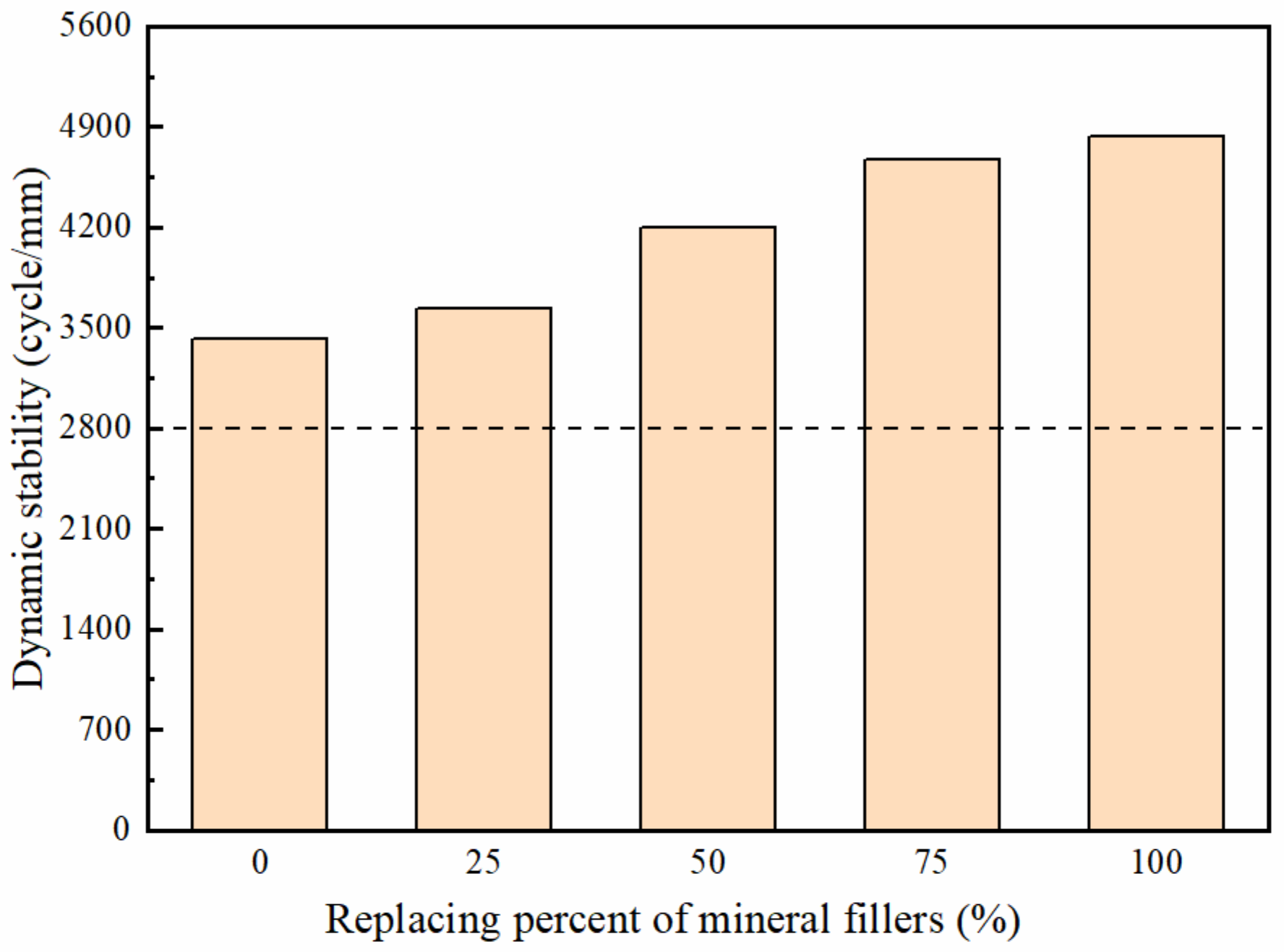
3.3.1. Rutting Resistance
3.3.2. Low-Temperature Performance
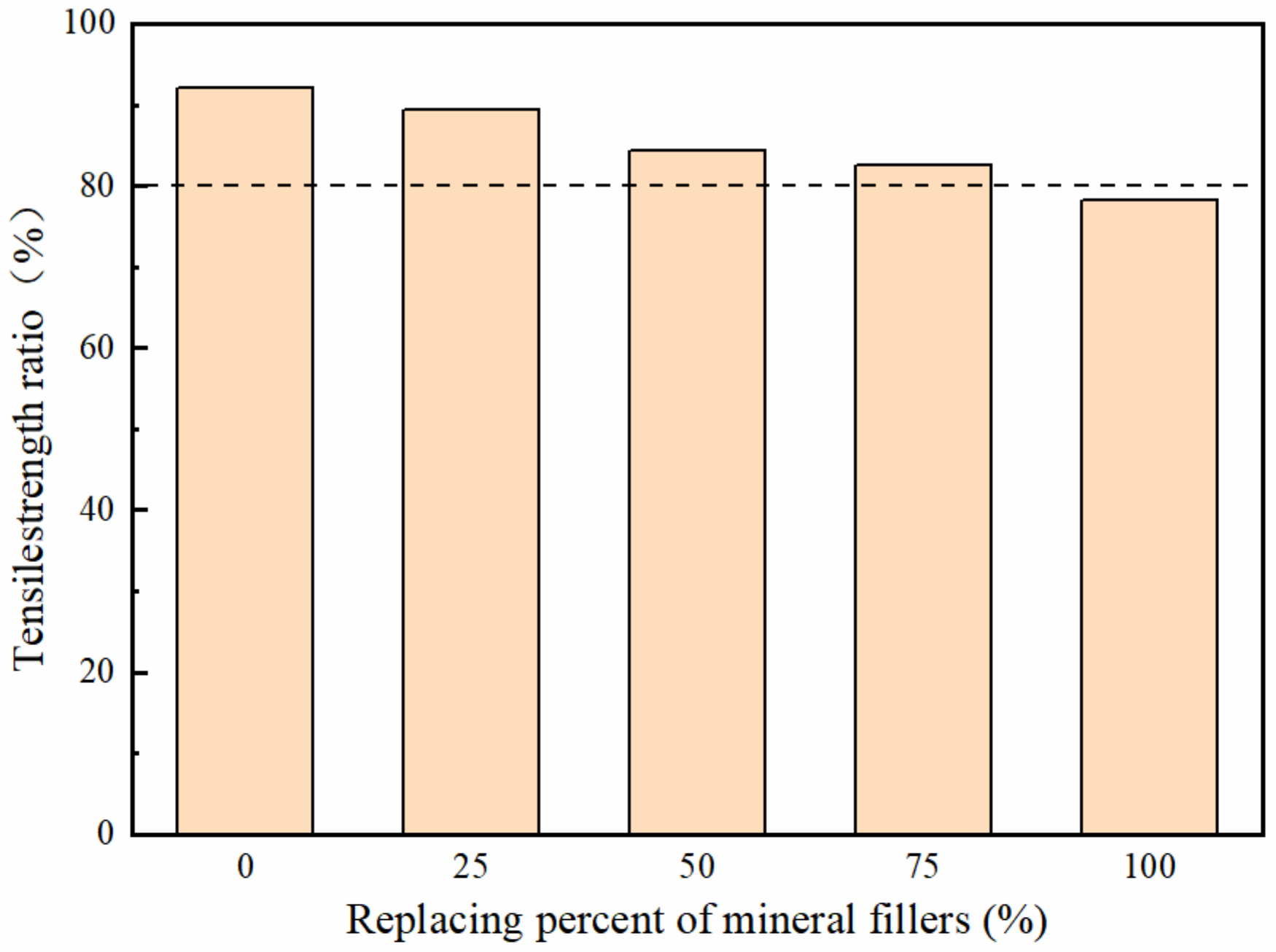
3.3.3. Moisture Damage Resistance
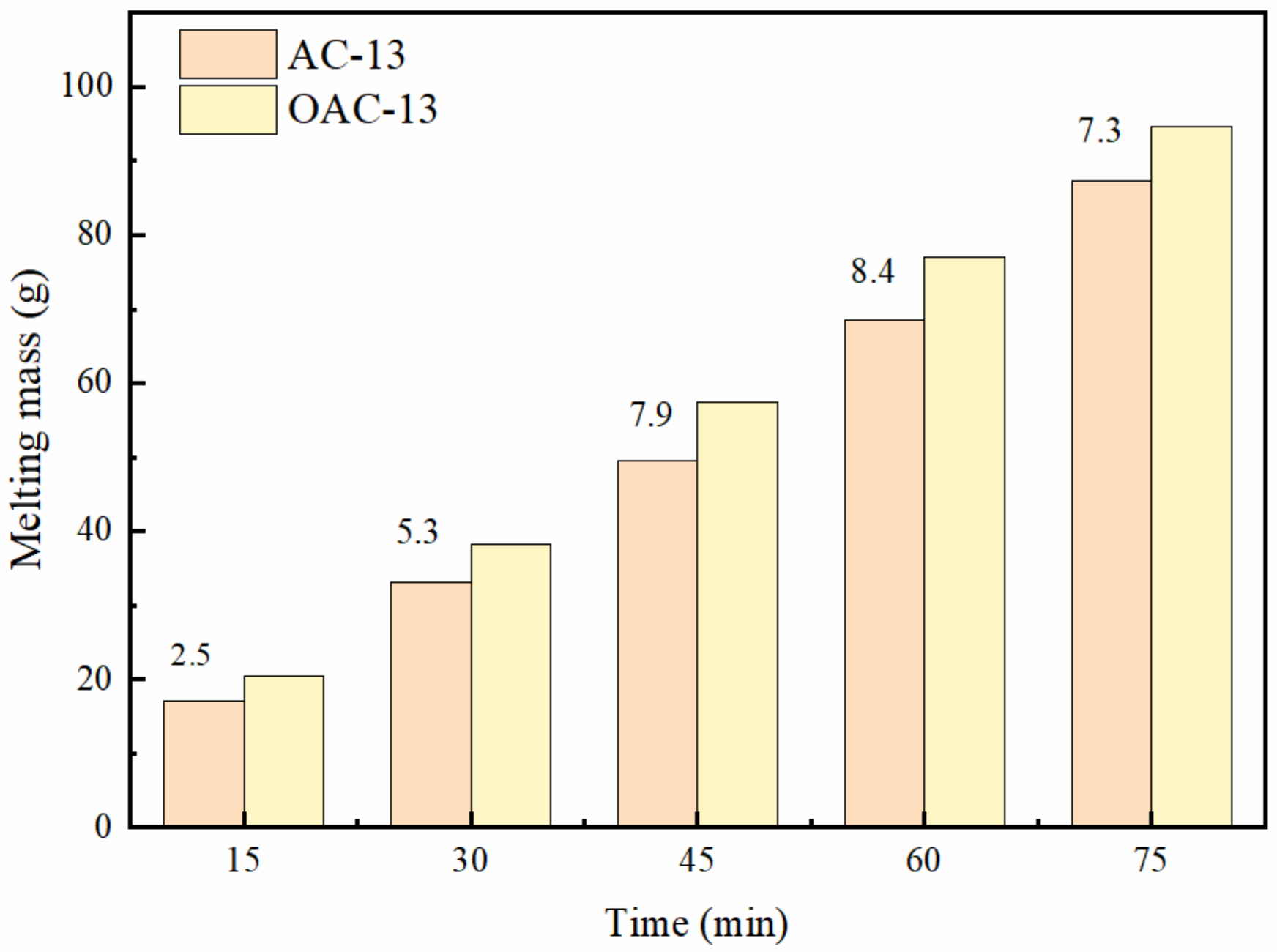
3.3.4. Snow or Ice Melting Capacity
4. Conclusions
- (1)
- The slow-releasing deicers with core-shell were prepared through in-situ polymerization, which have good thermal stability and ice-melting performance and can meet the requirements in winter and effectively reduce the loss of salts in rain and humidity.
- (2)
- The rutting susceptibility got better, while the low temperature cracking resistance and the water stability of mixtures got worse, with the increasing replacement of deicer filler. Considering the road performance, the replacement rate of the deicer is less than 75%.
- (3)
- According to the results of the ice melting test and field verification, the deicing asphalt mixtures would obtain a remarkable improvement in ice melting capacity and slow the bonding strength between mixture and pavement.
- (4)
- Of course, in this study, there are some limitations. Only sodium chloride is considered, while other chlorine salts are not considered from the perspective of deicer material properties. Research on the durability of deicer and deicing asphalt mixture and evaluation of the cost–benefit ration have not been carried out, which will be conducted in the future.
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rafiq, W.; Musarat, M.A.; Altaf, M.; Napiah, M.; Sutanto, M.H.; Alaloul, W.S.; Javed, M.F.; Mosavi, A. Life Cycle Cost Analysis Comparison of Hot Mix Asphalt and Reclaimed Asphalt Pavement: A Case Study. Sustainability 2021, 13, 4411. [Google Scholar] [CrossRef]
- Strong, C.K.; Ye, Z.; Shi, X. Safety effects of winter weather: The state of knowledge and remaining challenges. Transp. Rev. 2010, 30, 677–699. [Google Scholar] [CrossRef]
- Hassan, Y.; Halim, A.A.E. Effects of Runway Deicers on Pavement Materials and Mixes: Comparison with Road Salt. J. Transp. Eng. 2002, 128, 385–391. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, C.; Xu, H.; Tian, D. Snow melting and deicing characteristics and pavement performance of active deicing and snow melting pavement. China J. Highw. Transp. 2019, 32, 1–17. (In Chinese) [Google Scholar]
- Smith, K.H.; Harriott, D.M.; Cox, B.H.; Hibbs, J.O.; Minsk, D.L.; Shah, S.C. Highway Operations: Progress and Products Update; National Research Council: Washington, DC, USA, 1991. [Google Scholar]
- Zhou, C. Study on Granulated Crumb Rubber Asphalt Mixture Technology in Frost Region. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2006. (In Chinese). [Google Scholar]
- Wang, X.; Zhu, Y.; Zhu, M.; Zhu, Y.; Fan, H.; Wang, Y.J.A.T.E. Thermal analysis and optimization of an ice and snow melting system using geothermy by super-long flexible heat pipes. Appl. Therm. Eng. 2017, 112, 1353–1363. [Google Scholar] [CrossRef]
- Pan, P.; Wu, S.; Xiao, Y.; Liu, G.J.R.; Reviews, S.E. A review on hydronic asphalt pavement for energy harvesting and snow melting. Renew. Sustain. Energy Rev. 2015, 48, 624–634. [Google Scholar] [CrossRef]
- Wu, S.; Mo, L.; Shui, Z.; Chen, Z. Investigation of the conductivity of asphalt concrete containing conductive fillers. Carbon 2005, 43, 1358–1363. [Google Scholar] [CrossRef]
- Liu, Q.; Schlangen, E.; García, Á.; van de Ven, M. Induction heating of electrically conductive porous asphalt concrete. Constr. Build. Mater. 2010, 24, 1207–1213. [Google Scholar] [CrossRef]
- Wu, S.; Chen, M.; Wang, H.; Zhang, Y. Laboratory study on solar collector of thermal conductive asphalt concrete. Int. J. Pavement Res. Technol. 2009, 2, 130–136. [Google Scholar] [CrossRef]
- Yu, W.; Yi, X.; Guo, M.; Chen, L. State of the art and practice of pavement anti-icing and de-icing techniques. Sci. Cold Arid. Reg. 2014, 6, 14–21. [Google Scholar] [CrossRef]
- Ma, T.; Geng, L.; Ding, X.; Zhang, D.; Huang, X. Experimental study of deicing asphalt mixture with anti-icing additives. Constr. Build. Mater. 2016, 127, 653–662. [Google Scholar] [CrossRef]
- Gao, J. Analysis and assessment of the risk of snow and freezing disaster in China. Int. J. Disaster Risk Reduct. 2016, 19, 334–340. [Google Scholar] [CrossRef]
- Lee, B.D.; Choi, Y.S.; Kim, Y.G.; Kim, I.S.; Yang, E.I. A comparison study of performance and environmental impacts of chloride-based deicers and eco-label certified deicers in South Korea. Cold Reg. Sci. Technol. 2017, 143, 43–51. [Google Scholar] [CrossRef]
- Murphy, C.; Wallace, S.D.; Knight, R.; Cooper, D.J.; Sellers, T.G.J.E.E. Treatment performance of an aerated constructed wetland treating glycol from de-icing operations at a UK airport. Ecol. Eng. 2015, 80, 117–124. [Google Scholar] [CrossRef]
- Augeri, F. Placement of an Experimental Bituminous Concrete Mixture Utilizing an Asphalt Additive-“Verglimit”; Connecticut Department of Transportation: Washington, DC, USA, 1987. [Google Scholar]
- Turgeon, C.M.J.F. Evaluation of Verglimit (a Deicing Additive in Plant Mixed Bituminous Surface); Final Report; Physical Research Section, Office of Materials, Research and Standards, Minnesota Department of Transportation: Washington, DC, USA, 1989. [Google Scholar]
- Wang, Z.j.; Li, F.; Ma, R.; Yan, E. technology. Experiment on Road Performance of Asphalt Mixture with Automatic Long-term Snowmelt Agent. Int. J. Pavement Res. Technol. 2012, 6, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Wuori, A. Ice-Pavement Bond Disbanding-Surface Modification and Disbanding; National Research Council: Washington, DC, USA, 1993. [Google Scholar]
- Burtwell, M.; Öberg, G. COST Action 344: Improvements to Snow and Ice Control on European Roads and Bridges; World Road Association (PIARC): Paris, France, 2002. [Google Scholar]
- Tan, Y.; Hou, M.; Shan, L.; Sun, R. Development of sustained release complex salt filler for asphalt pavement included salt. J. Build. Mater. 2014, 17, 5. (In Chinese) [Google Scholar]
- Min, Z.; Xia, Y.; Li, X.; Tao, Z. Performances evaluation of epoxy asphalt mixture containing snow-melting agent. Constr. Build. Mater. 2017, 155, 762–769. [Google Scholar] [CrossRef]
- Liu, Z.; Xing, M.; Chen, S.; He, R.; Cong, P. Influence of the chloride-based anti-freeze filler on the properties of asphalt mixtures. Constr. Build. Mater. 2014, 51, 133–140. [Google Scholar] [CrossRef]
- Giuliani, F.; Merusi, F.; Polacco, G.; Filippi, S.; Paci, M. Effectiveness of of sodium chloride-based anti-icing filler in asphalt mixtures. Constr. Build. Mater. 2012, 30, 174–179. [Google Scholar] [CrossRef]
- Kumar, M.; Marks, D.; Dozier, J.; Reba, M.; Winstral, A. Evaluation of distributed hydrologic impacts of temperature-index and energy-based snow models. Adv. Water Resour. 2013, 56, 77–89. [Google Scholar] [CrossRef]
- Trenouth, W.R.; Gharabaghi, B.; Perera, N. Road salt application planning tool for winter de-icing operations. J. Hydrol. 2015, 524, 401–410. [Google Scholar] [CrossRef]
- Wu, S.; Yang, J.; Yang, R.; Zhu, J.; Liu, S.; Wang, C. Investigation of microscopic air void structure of anti-freezing asphalt pavement with X-ray CT and MIP. Constr. Build. Mater. 2018, 178, 473–483. [Google Scholar] [CrossRef]
- Liu, H.H.; Jia, J.; Liu, N.Y.Z.; Zeng, Z.X. Influence of the mixing time on the anti-freezing performance of chloride-based asphalt mixtures. Constr. Build. Mater. 2019, 213, 637–642. [Google Scholar] [CrossRef]
- Liu, Z.; Sha, A.; Xing, M.; Li, Z. Low temperature property and salt releasing characteristics of antifreeze asphalt concrete under static and dynamic conditions. Cold Reg. Sci. Technol. 2015, 114, 9–14. [Google Scholar] [CrossRef]
- Xu, O.; Han, S.; Zhang, C.; Liu, Y.; Xiao, F.; Xu, J. Laboratory investigation of andesite and limestone asphalt mixtures containing sodium chloride-based anti-icing filler. Constr. Build. Mater. 2015, 98, 671–677. [Google Scholar] [CrossRef]
- Xia, H.Y.; Zhao, X.; Wu, Y.C.; Yuan, T.; Song, L.F.; Yan, M.J.; Wang, F.Y.; Chen, H.X. Preparation and performance of antifreeze adhesive materials for asphalt pavement. Constr. Build. Mater. 2020, 258, 119554. [Google Scholar] [CrossRef]
- Goh, S.W.; Akin, M.; You, Z.P.; Shi, X.M. Effect of deicing solutions on the tensile strength of micro- or nano-modified asphalt mixture. Constr. Build. Mater. 2011, 25, 195–200. [Google Scholar] [CrossRef]
- Peng, C.; Yu, J.; Zhao, Z.; Dai, J.; Fu, J.; Wang, W. Effects of a sodium chloride deicing additive on the rheological properties of asphalt mastic. Road Mater. Pavement Des. 2016, 17, 382–395. [Google Scholar] [CrossRef]
- Noii, N.; Khodadadian, A.; Ulloa, J.; Aldakheel, F.; Wick, T.; François, S.; Wriggers, P. Bayesian Inversion with Open-Source Codes for Various One-Dimensional Model Problems in Computational Mechanics. Arch. Comput. Methods Eng. 2022, 7, 1–34. [Google Scholar] [CrossRef]
- Khodadadian, A.; Nima, N.; Maryam, P.; Mostafa, A.; Thomas, W.; Clemens, H. A Bayesian estimation method for variational phase-field fracture problems. Comput. Mech. 2020, 66, 827–849. [Google Scholar] [CrossRef] [PubMed]
- Ketcham, S.; Minsk, L.; Blackburn, R.; Fleege, E. Manual of Practice for an Effective Anti-icing Program: A Guide for Highway Winter Maintenance Personnel; US Army Cold Regions Research and Engineering Laboratory: Hanover, NH, USA, 1995. [Google Scholar]
- Awan, H.H.; Hussain, A.; Javed, M.F.; Qiu, Y.; Alrowais, R.; Mohamed, A.M.; Fathi, D.; Alzahrani, A.M. Predicting Marshall Flow and Marshall Stability of Asphalt Pavements Using Multi Expression Programming. Buildings 2022, 12, 3. [Google Scholar] [CrossRef]
- Ministry of Transport of the People’s Republic of China. JTG E20-2019 Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering; Ministry of Transport of the People’s Republic of China: Beijing, China, 2019. [Google Scholar]







| Chemical Name | Eutectic Temp. (°C) | Effective Temp. (°C) |
|---|---|---|
| sodium chloride (NaCl) | −21 | −9 |
| magnesium chloride (MgCl2) | −33 | −15 |
| calcium chloride (CaCl2) | −51 | −29 |
| Test Items | 25 °C Penetration (0.1 mm) | 5 °C Ductility (cm) | Softening Point (°C) | Penetration Index |
|---|---|---|---|---|
| Test values | 68.5 | 37 | 65 | 0.6 |
| Property | Test Values |
|---|---|
| Crushed value (%) | 16.3 |
| Los Angeles abrasion loss (%) | 13.5 |
| Soundness (%) | 4.0 |
| Flat and elongated particles (%) | 4.2 |
| Apparent specific gravity | 2.712 (limestone) |
| 2.386 (deicer) |
| Passing Percent | Sieving Size (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 13.2 | 9.5 | 4.75 | 2.36 | 1.18 | 0.6 | 0.3 | 0.15 | 0.075 | |
| Upper limit gradation (%) | 100 | 100 | 85 | 68 | 50 | 38 | 28 | 20 | 15 | 8 |
| Design gradation (%) | 100 | 96 | 70.3 | 42.5 | 30.5 | 22.0 | 17.0 | 11.5 | 8.5 | 6 |
| Lower graduation (%) | 100 | 90 | 65 | 38 | 24 | 15 | 10 | 7 | 5 | 4 |
| Deicer Volume | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| Mineral (g) | 60 | 45 | 30 | 15 | 0 |
| Deicer (g) | 0 | 13.2 | 26.4 | 39.6 | 52 |
| Elements K Line | C | O | Na | Si | Cl | K | Al | Mg | Ca | Ti | Fe | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 18.51 | 18.42 | 17.16 | 2.17 | 29.3 | 0.29 | 0.99 | 0.21 | 10.28 | 0.32 | 2.32 | 0.03 |
| wt% Sigma | 0.9 | 0.34 | 0.23 | 0.06 | 0.36 | 0.05 | 0.05 | 0.04 | 0.16 | 0.07 | 0.16 | 0.05 |
| Elements K Line | C | O | Na | Al | Si | Cl | Fe |
|---|---|---|---|---|---|---|---|
| wt% | 30.86 | 7.16 | 20.26 | 0.15 | 2.38 | 38.96 | 0.22 |
| wt% Sigma | 0.36 | 0.10 | 0.12 | 0.02 | 0.03 | 0.22 | 0.06 |
| Category | Core Material | Deicer | ||||
|---|---|---|---|---|---|---|
| Value of test (µS/cm) | 8960 | 8720 | 8890 | 1340 | 1290 | 1320 |
| Average value (µS/cm) | 8857 | 1317 | ||||
| Moisture Absorption Before (g) | Moisture Absorption After (g) | |||||
|---|---|---|---|---|---|---|
| Average (g) | 0.5002 | 0.4974 | 0.5012 | 0.7134 | 0.7118 | 0.7141 |
| 0.4996 | 0.7131 | |||||
| ω (%) | 42.7 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Luo, Y.; Gao, J.; Guo, P.; Jiang, Y.; Liu, F. Preparation and Application of a Novel Slow-Releasing with Core-Shell Deicer in Asphalt Mixtures. Polymers 2022, 14, 2615. https://doi.org/10.3390/polym14132615
Feng Y, Luo Y, Gao J, Guo P, Jiang Y, Liu F. Preparation and Application of a Novel Slow-Releasing with Core-Shell Deicer in Asphalt Mixtures. Polymers. 2022; 14(13):2615. https://doi.org/10.3390/polym14132615
Chicago/Turabian StyleFeng, Yunxia, Yuhong Luo, Junfeng Gao, Peng Guo, Yuntao Jiang, and Fumao Liu. 2022. "Preparation and Application of a Novel Slow-Releasing with Core-Shell Deicer in Asphalt Mixtures" Polymers 14, no. 13: 2615. https://doi.org/10.3390/polym14132615
APA StyleFeng, Y., Luo, Y., Gao, J., Guo, P., Jiang, Y., & Liu, F. (2022). Preparation and Application of a Novel Slow-Releasing with Core-Shell Deicer in Asphalt Mixtures. Polymers, 14(13), 2615. https://doi.org/10.3390/polym14132615







