Impact of Polymers on Magnesium-Based Hydrogen Storage Systems
Abstract
:1. Introduction
- Its abundance and lightweight;
- Clean and green energy system (eco-friendly and clean source of energy);
- The by-product is only water without any greenhouse gas emissions or any harmful emissions during the energy conversion;
- Higher energy conversion rate (the energy of H2 is approximately 3 times higher than petroleum fuels;
- Possibilities to derive from various sources and different methods;
- Reliable and sustainable energy system.
2. Significance of Magnesium Hydride and Benefits of Polymeric Materials for Hydrides
3. Carboxymethyl Cellulose
4. Polystyrene
5. Polyimide
6. Polypyrrole
7. Polyvinylpyrrolidone
8. Polyvinylidene Fluoride
9. Polymethylpentene
10. Poly(Methyl Methacrylate)
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falcone, P.M.; Hiete, M.; Sapio, A. Hydrogen economy and sustainable development goals: Review and policy insights. Curr. Opin. Green Sustain. Chem. 2021, 31, 100506. [Google Scholar] [CrossRef]
- Mahato, N.; Jang, H.; Dhyani, A.; Cho, S. Recent progress in conducting polymers for hydrogen storage and fuel cell applications. Polymers 2020, 12, 2480. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, S.; Chen, Y.; Hua, L. Thickness-prediction method involving tow redistribution for the dome of composite hydrogen storage vessels. Polymers 2022, 14, 902. [Google Scholar] [CrossRef] [PubMed]
- Mah, A.X.Y.; Ho, W.S.; Bong, C.P.C.; Hassim, M.H.; Liew, P.Y.; Asli, U.A.; Kamaruddin, M.J.; Chemmangattuvalappil, N.G. Review of hydrogen economy in Malaysia and its way forward. Int. J. Hydrogen Energy 2019, 44, 5661–5675. [Google Scholar] [CrossRef]
- Cousins, K.; Zhang, R. Highly porous organic polymers for hydrogen fuel storage. Polymers 2019, 11, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.-F.; Sun, Y.-X.; Zhang, S.-T.; Chi, M.-H. Hydrogen storage, magnetism and electrochromism of silver doped fau zeolite: First-principles calculations and molecular simulations. Polymers 2019, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Sim, U.; Kim, J.K. Biopolymer-inspired N-doped nanocarbon using carbonized polydopamine: A high-performance electrocatalyst for hydrogen-evolution reaction. Polymers 2020, 12, 912. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Lee, C.; Lee, J.H.; Kim, S.-K.; Cho, H.-S.; Kim, M.; Cho, W.C.; Joo, J.H.; Kim, C.H. Cerium oxide–polysulfone composite separator for an advanced alkaline electrolyzer. Polymers 2020, 12, 2821. [Google Scholar] [CrossRef]
- Verma, S.; Sinha-Ray, S.; Sinha-Ray, S. Electrospun CNF supported ceramics as electrochemical catalysts for water splitting and fuel cell: A review. Polymers 2020, 12, 238. [Google Scholar] [CrossRef] [Green Version]
- Lo Vecchio, C.; Carbone, A.; Trocino, S.; Gatto, I.; Patti, A.; Baglio, V.; Aricò, A.S. Anionic exchange membrane for photo-electrolysis application. Polymers 2020, 12, 2991. [Google Scholar] [CrossRef]
- Djara, R.; Lacour, M.-A.; Merzouki, A.; Cambedouzou, J.; Cornu, D.; Tingry, S.; Holade, Y. Iridium and ruthenium modified polyaniline polymer leads to nanostructured electrocatalysts with high performance regarding water splitting. Polymers 2021, 13, 190. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Tolba, G.M.K.; Khalil, K.A. Methylene blue dye as photosensitizer for scavenger-less water photo splitting: New insight in green hydrogen technology. Polymers 2022, 14, 523. [Google Scholar] [CrossRef]
- Jilani, A.; Hussain, S.Z.; Melaibari, A.A.; Abu-Hamdeh, N.H. Development and mechanistic studies of ternary nanocomposites for hydrogen production from water splitting to yield sustainable/green energy and environmental remediation. Polymers 2022, 14, 1290. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Thangarasu, S.; Jung, H.-Y.; Wee, J.-H.; Kim, Y.A.; Roh, S.-H. A new strategy of carbon—Pb composite as a bipolar plate material for unitized regenerative fuel cell system. Electrochim. Acta 2021, 391, 138921. [Google Scholar] [CrossRef]
- Kim, M.; Ko, H.; Nam, S.Y.; Kim, K. Study on control of polymeric architecture of sulfonated hydrocarbon-based polymers for high-performance polymer electrolyte membranes in fuel cell applications. Polymers 2021, 13, 3520. [Google Scholar] [CrossRef]
- Raja Sulaiman, R.R.; Walvekar, R.; Khalid, M.; Wong, W.Y.; Priyanka, J. Recent progress in the development of aromatic polymer-based proton exchange membranes for fuel cell applications. Polymers 2020, 12, 1061. [Google Scholar]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.F.; Compañ, V. Recent progress in the development of composite membranes based on polybenzimidazole for high temperature proton exchange membrane (PEM) fuel cell applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef]
- Roh, S.-H.; Sadhasivam, T.; Kim, H.; Park, J.-H.; Jung, H.-Y. Carbon free SiO2–SO3H supported Pt bifunctional electrocatalyst for unitized regenerative fuel cells. Int. J. Hydrogen Energy 2016, 41, 20650–20659. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, S.; Mosquera, M.E.G. Conducting polymer-based nanohybrids for fuel cell application. Polymers 2020, 12, 2993. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton exchange membrane fuel cells (PEMFCs): Advances and challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, T.; Dhanabalan, K.; Roh, S.-H.; Kim, T.-H.; Park, K.-W.; Jung, S.; Kurkuri, M.D.; Jung, H.Y. A comprehensive review on unitized regenerative fuel cells: Crucial challenges and developments. Int. J. Hydrogen Energy 2017, 42, 4415–4433. [Google Scholar] [CrossRef]
- Yarar Kaplan, B.; Haghmoradi, N.; Biçer, E.; Merino, C.; Alkan Gürsel, S. High performance electrocatalysts supported on graphene based hybrids for polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2018, 43, 23221–23230. [Google Scholar] [CrossRef]
- Kiani, M.; Tian, X.Q.; Zhang, W. Non-precious metal electrocatalysts design for oxygen reduction reaction in polymer electrolyte membrane fuel cells: Recent advances, challenges and future perspectives. Coord. Chem. Rev. 2021, 441, 213954. [Google Scholar] [CrossRef]
- Kiani, M.; Zhang, J.; Luo, Y.; Jiang, C.; Fan, J.; Wang, G.; Chen, J.; Wang, R. Recent developments in electrocatalysts and future prospects for oxygen reduction reaction in polymer electrolyte membrane fuel cells. J. Energy Chem. 2018, 27, 1124–1139. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Duan, X.; Wang, S.; Zheng, L.; Li, Y.; Duan, H.; Kuang, Y.; Sun, X. Amorphous ruthenium-sulfide with isolated catalytic sites for Pt-like electrocatalytic hydrogen production over whole pH range. Small 2019, 15, 1904043. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.-B. The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy 2021, 87, 106162. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Yang, G.; Yu, S.; Kang, Z.; Dohrmann, Y.; Bender, G.; Pivovar, B.S.; Green, J.B., Jr.; Retterer, S.T.; Cullen, D.A.; Zhang, F.Y. A novel PEMEC with 3D printed non-conductive bipolar plate for low-cost hydrogen production from water electrolysis. Energy Convers. Manag. 2019, 182, 108–116. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Sterlin Leo Hudson, M.; Pandey, S.K.; Bhatnagar, A.; Singh, M.K.; Gurunathan, K.; Srivastava, O.N. Effects of nano size mischmetal and its oxide on improving the hydrogen sorption behaviour of MgH2. Int. J. Hydrogen Energy 2013, 38, 7353–7362. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Thangarasu, S.; Palanisamy, G.; Im, Y.M.; Oh, T.H. An alternative platform of solid-state hydrides with polymers as composite/encapsulation for hydrogen storage applications: Effects in intermetallic and complex hydrides. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Mao, M.; Luo, C.; Pollard, T.P.; Hou, S.; Gao, T.; Fan, X.; Cui, C.; Yue, J.; Tong, Y.; Yang, G.; et al. A pyrazine-based polymer for fast-charge batteries. Angew. Chem. Int. Ed. 2019, 58, 17820–17826. [Google Scholar] [CrossRef]
- Bitenc, J.; Pirnat, K.; Bančič, T.; Gaberšček, M.; Genorio, B.; Randon-Vitanova, A.; Dominko, R. Anthraquinone-based polymer as cathode in rechargeable magnesium batteries. ChemSusChem 2015, 8, 4128–4132. [Google Scholar] [CrossRef]
- Du, A.; Zhang, H.; Zhang, Z.; Zhao, J.; Cui, Z.; Zhao, Y.; Dong, S.; Wang, L.; Zhou, X.; Cui, G. A crosslinked polytetrahydrofuran-borate-based polymer electrolyte enabling wide-working-temperature-range rechargeable magnesium batteries. Adv. Mater. 2019, 31, 1805930. [Google Scholar] [CrossRef]
- Shuai, H.; Xu, J.; Huang, K. Progress in retrospect of electrolytes for secondary magnesium batteries. Coord. Chem. Rev. 2020, 422, 213478. [Google Scholar] [CrossRef]
- Iwakura, C.; Nohara, S.; Furukawa, N.; Inoue, H. The possible use of polymer gel electrolytes in nickel/metal hydride battery. Solid State Ion. 2002, 148, 487–492. [Google Scholar] [CrossRef]
- Yuan, A.; Zhao, J. Composite alkaline polymer electrolytes and its application to nickel–metal hydride batteries. Electrochim. Acta 2006, 51, 2454–2462. [Google Scholar] [CrossRef]
- Saal, A.; Hagemann, T.; Schubert, U.S. Polymers for battery applications—Active materials, membranes, and binders. Adv. Energy Mater. 2021, 11, 2001984. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Nofal, M.M.; Abdulwahid, R.T.; Hussen, S.A.; Hussein, A.M.; Karim, W.O. A comprehensive review on optical properties of polymer electrolytes and composites. Materials 2020, 13, 3675. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Kadir, M.F.Z.; Brza, M.A.; Karim, W.O. The study of EDLC device fabricated from plasticized magnesium ion conducting chitosan based polymer electrolyte. Polym. Test. 2020, 90, 106714. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Baziz, S.; Nofal, M.M.; Brza, M.A.; Abdulwahid, R.T.; Hadi, J.M.; Karim, W.O.; Kadir, M.F.Z. Characteristics of EDLC device fabricated from plasticized chitosan: MgCl2 based polymer electrolyte. J. Mater. Res. Technol. 2020, 9, 10635–10646. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Kapelewski, M.T.; Runčevski, T.; Tarver, J.D.; Jiang, H.Z.H.; Hurst, K.E.; Parilla, P.A.; Ayala, A.; Gennett, T.; FitzGerald, S.A.; Brown, C.M.; et al. Record high hydrogen storage capacity in the metal–organic framework Ni2(m-dobdc) at near-ambient temperatures. Chem. Mater. 2018, 30, 8179–8189. [Google Scholar] [CrossRef] [PubMed]
- García-Holley, P.; Schweitzer, B.; Islamoglu, T.; Liu, Y.; Lin, L.; Rodriguez, S.; Weston, M.H.; Hupp, J.T.; Gómez-Gualdrón, D.A.; Yildirim, T.; et al. Benchmark study of hydrogen storage in metal–organic frameworks under temperature and pressure swing conditions. ACS Energy Lett. 2018, 3, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P.; et al. Reversible ammonia-based and liquid organic hydrogen carriers for high-density hydrogen storage: Recent progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The prospect of hydrogen storage using liquid organic hydrogen carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Nagrimanov, R.N.; Zaitsau, D.H.; Konnova, M.E.; Pimerzin, A.A. Thermochemical properties of pyrazine derivatives as seminal liquid organic hydrogen carriers for hydrogen storage. J. Chem. Thermodyn. 2021, 158, 106406. [Google Scholar] [CrossRef]
- Goto, K.; Hirata, T.; Yamamoto, I.; Nakao, W. Suitability evaluation of LaNi5 as hydrogen-storage-alloy actuator by in-situ displacement measurement during hydrogen pressure change. Molecules 2019, 24, 2420. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Haraki, T.; Uchida, H. Effect of LaNi5H6 hydride particles size on desorption kinetics. J. Alloys Compd. 2002, 330–332, 488–491. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, X.; Nyahuma, F.M.; Yan, N.; Xiao, J.; Su, S.; Zhang, L. Enhancing hydrogen storage properties of MgH2 by transition metals and carbon materials: A brief review. Front. Chem. 2020, 8, 25. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Hou, Q.; Yang, X.; Zhang, J. Review on hydrogen storage performance of MgH2: Development and trends. ChemistrySelect 2021, 6, 1589–1606. [Google Scholar] [CrossRef]
- Liu, H.; Lu, C.; Wang, X.; Xu, L.; Huang, X.; Wang, X.; Ning, H.; Lan, Z.; Guo, J. Combinations of V2C and Ti3C2 MXenes for boosting the hydrogen storage performances of MgH2. ACS Appl. Mater. Interfaces 2021, 13, 13235–13247. [Google Scholar] [CrossRef]
- Ali, N.A.; Ismail, M. Modification of NaAlH4 properties using catalysts for solid-state hydrogen storage: A review. Int. J. Hydrogen Energy 2021, 46, 766–782. [Google Scholar] [CrossRef]
- Sazelee, N.; Mustafa, N.S.; Yahya, M.S.; Ismail, M. Enhanced dehydrogenation performance of NaAlH4 by the addition of spherical SrTiO3. Int. J. Energy Res. 2021, 45, 8648–8658. [Google Scholar] [CrossRef]
- Chen, W.; You, L.; Xia, G.; Yu, X. A balance between catalysis and nanoconfinement towards enhanced hydrogen storage performance of NaAlH4. J. Mater. Sci. Technol. 2021, 79, 205–211. [Google Scholar] [CrossRef]
- Jin, J.-H.; Shin, S.; Jung, J. Solid-phase hydrogen storage based on NH3BH3-SiO2 nanocomposite for thermolysis. J. Nanomater. 2019, 2019, 6126031. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Chen, W.; Lv, G.; Yang, Z.; Yan, J.; Liu, X.; Dai, Z. Hydrolysis of NH3BH3 and NaBH4 by graphene quantum dots-transition metal nanoparticles for highly effective hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 796–805. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, L.; Xu, F.; Liu, Z. Improved hydrogen storage of LiBH4 and NH3BH3 by catalysts. J. Mater. Chem. A 2018, 6, 7293–7309. [Google Scholar] [CrossRef]
- Rao, P.C.; Yoon, M. Potential liquid-organic hydrogen carrier (LOHC) systems: A review on recent progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Yadav, T.P.; Kumar, A.; Verma, S.K.; Mukhopadhyay, N.K. High-entropy alloys for solid hydrogen storage: Potentials and prospects. Trans. Indian Natl. Acad. Eng. 2022, 7, 147–156. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.; Ashuri, T.; Krohn, J. Hydrogen fuel cell vehicles; Current status and future prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef] [Green Version]
- Valenti, G. 2-Hydrogen liquefaction and liquid hydrogen storage. In Compendium of Hydrogen Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 27–51. [Google Scholar]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Elberry, A.M.; Thakur, J.; Santasalo-Aarnio, A.; Larmi, M. Large-scale compressed hydrogen storage as part of renewable electricity storage systems. Int. J. Hydrogen Energy 2021, 46, 15671–15690. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Gambini, M.; Stilo, T.; Vellini, M. Hydrogen storage systems for fuel cells: Comparison between high and low-temperature metal hydrides. Int. J. Hydrogen Energy 2019, 44, 15118–15134. [Google Scholar] [CrossRef]
- Butova, V.V.; Burachevskaya, O.A.; Podshibyakin, V.A.; Shepelenko, E.N.; Tereshchenko, A.A.; Shapovalova, S.O.; Il’in, O.I.; Bren’, V.A.; Soldatov, A.V. Photoswitchable zirconium MOF for light-driven hydrogen storage. Polymers 2021, 13, 4052. [Google Scholar] [CrossRef]
- Xu, T.; Chen, J.; Yuan, W.; Li, B.; Li, L.; Wu, H.; Zhou, X. Preparation and hydrogen storage characteristics of surfactant-modified graphene. Polymers 2018, 10, 1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.-L.; Zhang, Y.-H. Research progress in Mg-based hydrogen storage alloys. Rare Met. 2014, 33, 499–510. [Google Scholar] [CrossRef]
- Lin, H.-J.; Lu, Y.-S.; Zhang, L.-T.; Liu, H.-Z.; Edalati, K.; Révész, Á. Recent advances in metastable alloys for hydrogen storage: A review. Rare Met. 2022, 41, 1797–1817. [Google Scholar] [CrossRef]
- He, L.; Wang, S.; Li, Z.; Liu, X.; Jiang, L. Synthesis of magnesium alanate by ball milling MgH2 and AlCl3 mixtures. Rare Met. 2011, 30, 55–58. [Google Scholar] [CrossRef]
- Cui, J.; Wang, H.; Sun, D.-L.; Zhang, Q.-A.; Zhu, M. Realizing nano-confinement of magnesium for hydrogen storage using vapour transport deposition. Rare Met. 2016, 35, 401–407. [Google Scholar] [CrossRef]
- Ismail, M.; Yahya, M.S.; Sazelee, N.A.; Ali, N.A.; Yap, F.A.H.; Mustafa, N.S. The effect of K2SiF6 on the MgH2 hydrogen storage properties. J. Magnes. Alloy. 2020, 8, 832–840. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Yu, H.; Lu, Z.; He, J.; Zheng, J.; Wu, F.; Chen, L. Achieving superior hydrogen storage properties of MgH2 by the effect of TiFe and carbon nanotubes. Chem. Eng. J. 2021, 422, 130101. [Google Scholar] [CrossRef]
- Oshchapovsky, I.V.; Zavaliy, I.Y.; Pavlyuk, V.V. The investigation of hydrogen sublattice in Mg2NiHx (x = 0.3) hydride by first-principle calculations. Mater. Today Commun. 2021, 27, 102174. [Google Scholar] [CrossRef]
- Shin, H.-W.; Hwang, J.-H.; Kim, E.-A.; Hong, T.-W. Evaluation of hydrogenation kinetics and life cycle assessment on Mg2NiHx–CaO composites. Materials 2021, 14, 2848. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, Y.; Zhang, X.; Zhou, Y.; Gao, M.; Pan, H. Synthesis and hydrogen storage thermodynamics and kinetics of Mg(AlH4)2 submicron rods. Int. J. Hydrogen Energy 2012, 37, 18148–18154. [Google Scholar] [CrossRef]
- Kang, S.; Karthikeyan, S.; Lee, J.Y. Enhancement of the hydrogen storage capacity of Mg(AlH4)2 by excess electrons: A DFT study. Physical Chem. Chem. Phys. 2013, 15, 1216–1221. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.-T.; Jung, S.; Roh, S.-H.; Park, J.-H.; Jung, H.-Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sustain. Energy Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- Singh, R.K.; Sadhasivam, T.; Sheeja, G.I.; Singh, P.; Srivastava, O.N. Effect of different sized CeO2 nano particles on decomposition and hydrogen absorption kinetics of magnesium hydride. Int. J. Hydrogen Energy 2013, 38, 6221–6225. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, J.; Huang, H.; Lv, Y.; Liu, B.; Li, Z.; Zhang, B.; Lv, W.; Wu, Y. Enhanced hydrogen storage properties of NaBH4–Mg(BH4)2 composites by NdF3 addition. Prog. Nat. Sci. Mater. Int. 2021, 31, 521–526. [Google Scholar] [CrossRef]
- Sulaiman, N.N.; Ismail, M.; Timmiati, S.N.; Lim, K.L. Improved hydrogen storage performances of LiAlH4+ Mg(BH4)2 composite with TiF3 addition. Int. J. Energy Res. 2021, 45, 2882–2898. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Gurunathan, K. The role of nanoparticles, catalytic additives and alternative/advanced techniques on magnesium hydride. Adv. Sci. Eng. Med. 2015, 7, 1–17. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Lu, Z.-Y.; Yu, H.-J.; Lu, X.; Song, M.-C.; Wu, F.-Y.; Zheng, J.-G.; Yuan, Z.F.; Zhang, L.T. Two-dimensional vanadium nanosheets as a remarkably effective catalyst for hydrogen storage in MgH2. Rare Met. 2021, 40, 3195–3204. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Wang, P.; Tian, Z.; Wang, Z.; Xia, C.; Yang, T.; Ou, X. Improved hydrogen storage properties of MgH2 using transition metal sulfides as catalyst. Int. J. Hydrogen Energy 2021, 46, 27107–27118. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Z.; Liu, Z.; Tang, Q.; Zhu, Y.; Lin, H.; Zhang, Y.; Zhang, J.; Liu, Y.; Li, L. Synergistic effect of rGO supported Ni3Fe on hydrogen storage performance of MgH2. Int. J. Hydrogen Energy 2020, 45, 16622–16633. [Google Scholar] [CrossRef]
- Yahya, M.S.; Ismail, M. Improvement of hydrogen storage properties of MgH2 catalyzed by K2NbF7 and multiwall carbon nanotube. J. Phys. Chem. C 2018, 122, 11222–11233. [Google Scholar] [CrossRef]
- Shao, H.; Huang, Y.; Guo, H.; Liu, Y.; Guo, Y.; Wang, Y. Thermally stable Ni MOF catalyzed MgH2 for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 37977–37985. [Google Scholar] [CrossRef]
- Zhang, X.; Leng, Z.; Gao, M.; Hu, J.; Du, F.; Yao, J.; Pan, H.; Liu, Y. Enhanced hydrogen storage properties of MgH2 catalyzed with carbon-supported nanocrystalline TiO2. J. Power Sources 2018, 398, 183–192. [Google Scholar] [CrossRef]
- Yao, P.; Jiang, Y.; Liu, Y.; Wu, C.; Chou, K.-C.; Lyu, T.; Li, Q. Catalytic effect of Ni@rGO on the hydrogen storage properties of MgH2. J. Magnes. Alloy. 2020, 8, 461–471. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Ma, H.; Chen, J. Magnesium nanowires: Enhanced kinetics for hydrogen absorption and desorption. J. Am. Chem. Soc. 2007, 129, 6710–6711. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Wu, Y.; Guo, X.; Ye, J.; Yuan, B.; Wang, S.; Jiang, L. Recent advances on the thermal destabilization of Mg-based hydrogen storage materials. RSC Adv. 2019, 9, 408–428. [Google Scholar] [CrossRef] [Green Version]
- Zahiri, B.; Amirkhiz, B.S.; Mitlin, D. Hydrogen storage cycling of MgH2 thin film nanocomposites catalyzed by bimetallic Cr Ti. Appl. Phys. Lett. 2010, 97, 083106. [Google Scholar] [CrossRef]
- Konarova, M.; Tanksale, A.; Norberto Beltramini, J.; Qing Lu, G. Effects of nano-confinement on the hydrogen desorption properties of MgH2. Nano Energy 2013, 2, 98–104. [Google Scholar] [CrossRef]
- Jia, Y.; Yao, X. Carbon scaffold modified by metal (Ni) or non-metal (N) to enhance hydrogen storage of MgH2 through nanoconfinement. Int. J. Hydrogen Energy 2017, 42, 22933–22941. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Sun, C.; Cheng, L.; Abdul Wahab, M.; Cui, J.; Zou, J.; Zhu, M.; Yao, X. Destabilization of Mg–H bonding through nano-interfacial confinement by unsaturated carbon for hydrogen desorption from MgH2. Phys. Chem. Chem. Phys. 2013, 15, 5814–5820. [Google Scholar] [CrossRef] [Green Version]
- Pedicini, R.; Schiavo, B.; Rispoli, P.; Saccà, A.; Carbone, A.; Gatto, I.; Passalacqua, E. Progress in polymeric material for hydrogen storage application in middle conditions. Energy 2014, 64, 607–614. [Google Scholar] [CrossRef]
- Almeida Neto GRd Gonçalves Beatrice, C.A.; Leiva, D.R.; Pessan, L.A. Polymer-based composite containing nanostructured LaNi5 for hydrogen storage: Improved air stability and processability. Int. J. Hydrogen Energy 2020, 45, 14017–14027. [Google Scholar] [CrossRef]
- Miyake, J.; Ogawa, Y.; Tanaka, T.; Ahn, J.; Oka, K.; Oyaizu, K.; Miyatake, K. Rechargeable proton exchange membrane fuel cell containing an intrinsic hydrogen storage polymer. Commun. Chem. 2020, 3, 138. [Google Scholar] [CrossRef]
- Beatrice, C.A.G.; Moreira, B.R.; Oliveira ADd Passador, F.R.; de Almeida Neto, G.R.; Leiva, D.R.; Pessan, L.A. Development of polymer nanocomposites with sodium alanate for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 5337–5346. [Google Scholar] [CrossRef]
- Zadorozhnyy, M.Y.; Klyamkin, S.N.; Strugova, D.V.; Olifirov, L.K.; Milovzorov, G.S.; Kaloshkin, S.D.; Zadorozhnyy, V.Y. Deposition of polymer coating on metallic powder through ball milling: Application to hydrogen storage intermetallics. Int. J. Energy Res. 2016, 40, 273–279. [Google Scholar] [CrossRef]
- Leela Mohana Reddy, A.; Ramaprabhu, S. Structural and hydrogen absorption kinetics studies of polymer dispersed and boron added Zr-based AB2 alloy. Int. J. Hydrogen Energy 2006, 31, 867–876. [Google Scholar] [CrossRef]
- Uemura, Y.; Yasutsune, R.; Hatate, Y. Encapsulation of hydrogen storage alloy by polymer. J. Chem. Eng. Jpn. 1991, 24, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Checchetto, R.; Bazzanella, N.; Miotello, A.; Carotenuto, G.; Nicolais, L. Hydrogen sorption in metal-polymer composites: The role of interfaces. J. Appl. Phys. 2009, 105, 083513. [Google Scholar] [CrossRef]
- De Almeida Neto, G.R.; Gonçalves Beatrice, C.A.; Leiva, D.R.; Pessan, L.A. Polyetherimide-LaNi5 composite films for hydrogen storage applications. Int. J. Hydrogen Energy 2021, 46, 23767–23778. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, M.; Jia, Y.; Chai, Y.; Hou, D.; Wang, N. Hydrogen storage properties of flexible and porous La0.8Mg0.2Ni3.8/PVDF composite. Int. J. Hydrogen Energy 2013, 38, 10939–10943. [Google Scholar] [CrossRef]
- Pentimalli, M.; Padella, F.; Pilloni, L.; Imperi, E.; Matricardi, P. AB5/ABS composite material for hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 4592–4596. [Google Scholar] [CrossRef]
- Yoshida, A.; Okuyama, T.; Mori, Y.; Saito, N.; Naito, S. Hydrogen storage material composed of polyacetylene and LiH and investigation of its mechanisms. Chem. Mater. 2014, 26, 4076–4081. [Google Scholar] [CrossRef]
- Borodina, T.N.; Grigoriev, D.O.; Andreeva, D.V.; Möhwald, H.; Shchukin, D.G. Polyelectrolyte multilayered nanofilms as a novel approach for the protection of hydrogen storage materials. ACS Appl. Mater. Interfaces 2009, 1, 996–1001. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Wiset, N.; Milanese, C.; Laipple, D.; Marini, A.; Klassen, T.; Dornheim, M.; Gosalawit–Utke, R. Improvement of thermal stability and reduction of LiBH4/polymer host interaction of nanoconfined LiBH4 for reversible hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 392–402. [Google Scholar] [CrossRef]
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of natural, semi-synthetic, and synthetic polymers in cosmetic formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in bacterial cellulose matrices for biotechnological applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Gupta, B.K.; Tripathi, P.; Veziroglu, A.; Hudson, M.S.L.; Shaz, M.A.; Srivastava, O.N. Development and demonstration of air stable rGO-EC@AB5 type hydrogenated intermetallic hybrid for hydrogen fuelled devices. Adv. Sustain. Syst. 2017, 1, 1700087. [Google Scholar] [CrossRef]
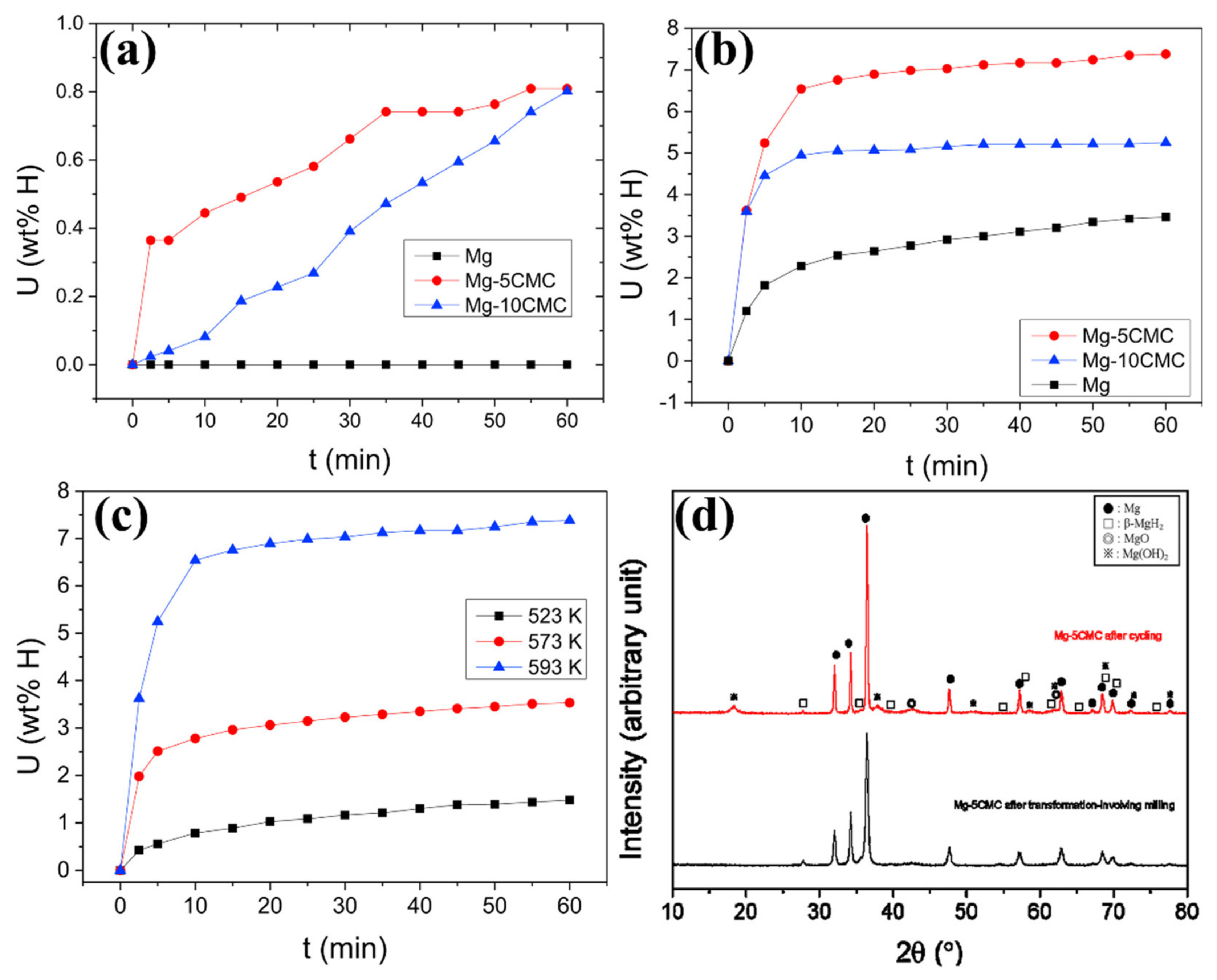
- Song, M.Y.; Choi, E.; Kwak, Y.J. Synthesis of a Mg-based alloy with a hydrogen-storage capacity of over 7 wt% by adding a polymer CMC via transformation-involving milling. Mater. Res. Bull. 2018, 108, 23–31. [Google Scholar] [CrossRef]
- Song, M.Y.; Choi, E.; Kwak, Y.J. Preparation of a Mg-Based alloy with a high hydrogen-storage capacity by adding a polymer CMC via milling in a hydrogen atmosphere. Int. J. Hydrogen Energy 2019, 44, 3779–3789. [Google Scholar] [CrossRef]
- Song, M.Y.; Choi, E.; Kwak, Y.J. Development of a Mg-based alloy with a hydrogen-storage capacity of 7 wt% by adding a polymer CMC via transformation-involving milling. Korean J. Met. Mater. 2018, 56, 392–399. [Google Scholar] [CrossRef]
- Choi, E.; Kwak, Y.J.; Song, M.Y. Increasing the hydrogenation and dehydrogenation rates of magnesium by incorporating CMC(Na) (Carboxymethylcellulose-Sodium Salt) and nickel. J. Nanosci. Nanotechnol. 2019, 19, 6580–6589. [Google Scholar] [CrossRef]
- Song, M.Y.; Choi, E.; Kwak, Y.J. Increase in the dehydrogenation rate of Mg–CMC (Carboxymethylcellulose, Sodium Salt) by adding Ni via hydride-forming milling. Met. Mater. Int. 2019, 25, 516–527. [Google Scholar] [CrossRef]
- Setijadi, E.J.; Boyer, C.; Aguey-Zinsou, K.-F. Switching the thermodynamics of MgH2 nanoparticles through polystyrene stabilisation and oxidation. RSC Adv. 2014, 4, 39934–39940. [Google Scholar] [CrossRef]
- Liu, W.; Aguey-Zinsou, K.-F. Size effects and hydrogen storage properties of Mg nanoparticles synthesised by an electroless reduction method. J. Mater. Chem. A 2014, 2, 9718–9726. [Google Scholar] [CrossRef]
- Orme, C.J.; Stone, M.L.; Benson, M.T.; Peterson, E.S. Testing of polymer membranes for the selective permeability of hydrogen. Sep. Sci. Technol. 2003, 38, 3225–3238. [Google Scholar] [CrossRef]
- Hashimoto, T.; Notomi, M. Hydrogen storage properties of Mg-based multilayer films. Mech. Eng. J. 2016, 3, 16–00228. [Google Scholar] [CrossRef] [Green Version]
- Yokota, R.; Yamamoto, S.; Yano, S.; Sawaguchi, T.; Hasegawa, M.; Yamaguchi, H.; Ozawa, H.; Sato, R. Molecular design of heat resistant polyimides having excellent processability and high glass transition temperature. High Perform. Polym. 2001, 13, S61–S72. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
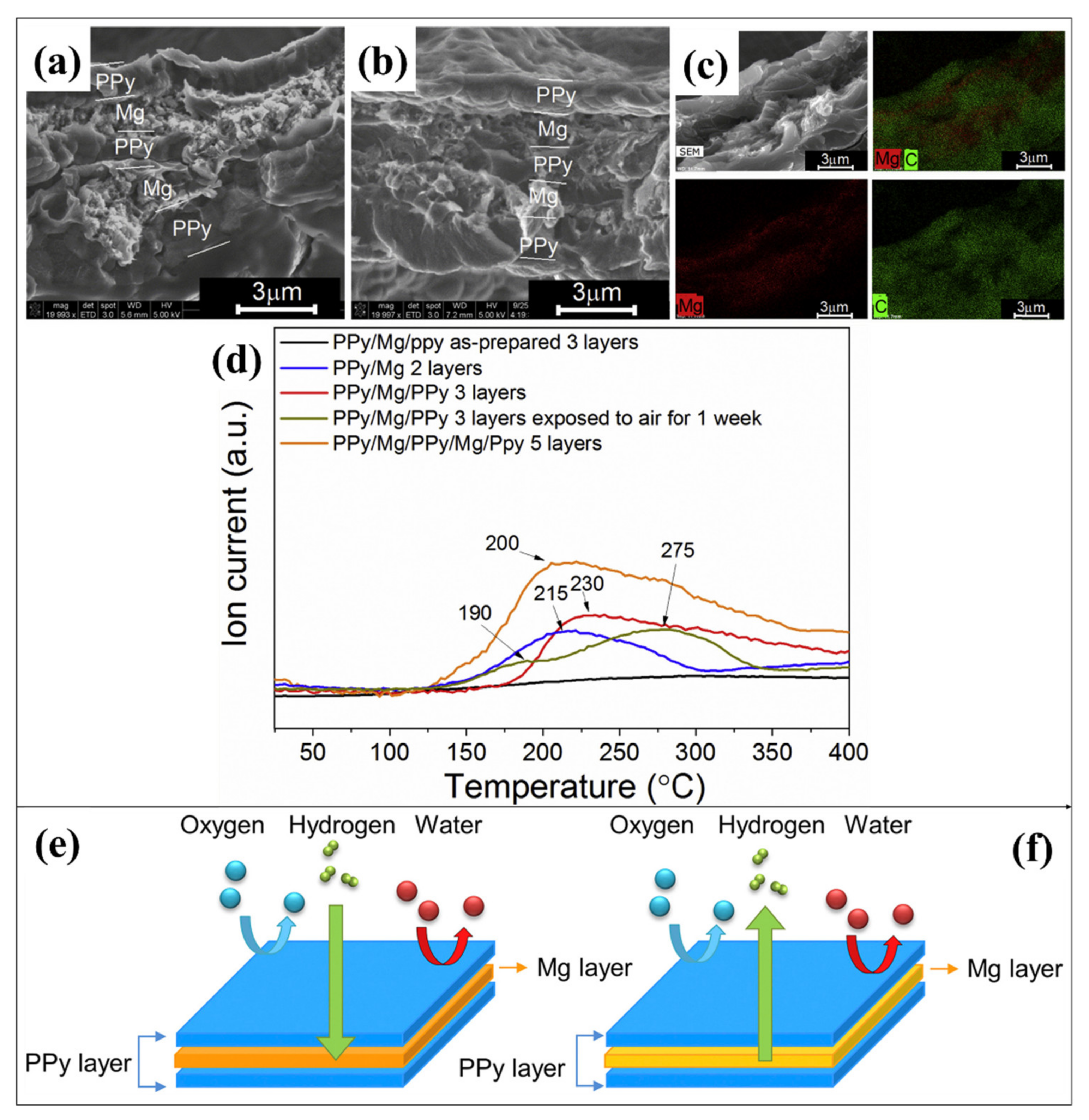
- Shen, C.; Aguey-Zinsou, K.-F. Electrochemical deposited Mg-PPy multilayered film to store hydrogen. Int. J. Hydrogen Energy 2018, 43, 22385–22390. [Google Scholar] [CrossRef]
- Diaz, A.F.; Castillo, J.I.; Logan, J.A.; Lee, W.-Y. Electrochemistry of conducting polypyrrole films. J. Electroanal. Chem. Interfacial Electrochem. 1981, 129, 115–132. [Google Scholar] [CrossRef]
- Yao, L.; Han, H.; Liu, Y.; Zhu, Y.; Zhang, Y.; Li, L. Improved dehydriding property of polyvinylpyrrolidone coated Mg-Ni hydrogen storage nano-composite prepared by hydriding combustion synthesis and wet mechanical milling. Prog. Nat. Sci. Mater. Int. 2018, 28, 7–14. [Google Scholar] [CrossRef]
- Song, M.Y.; Kwak, Y.J. Hydrogen storage properties of Mg alloy prepared by incorporating polyvinylidene fluoride via reactive milling. Korean J. Met. Mater. 2018, 56, 878–884. [Google Scholar] [CrossRef]
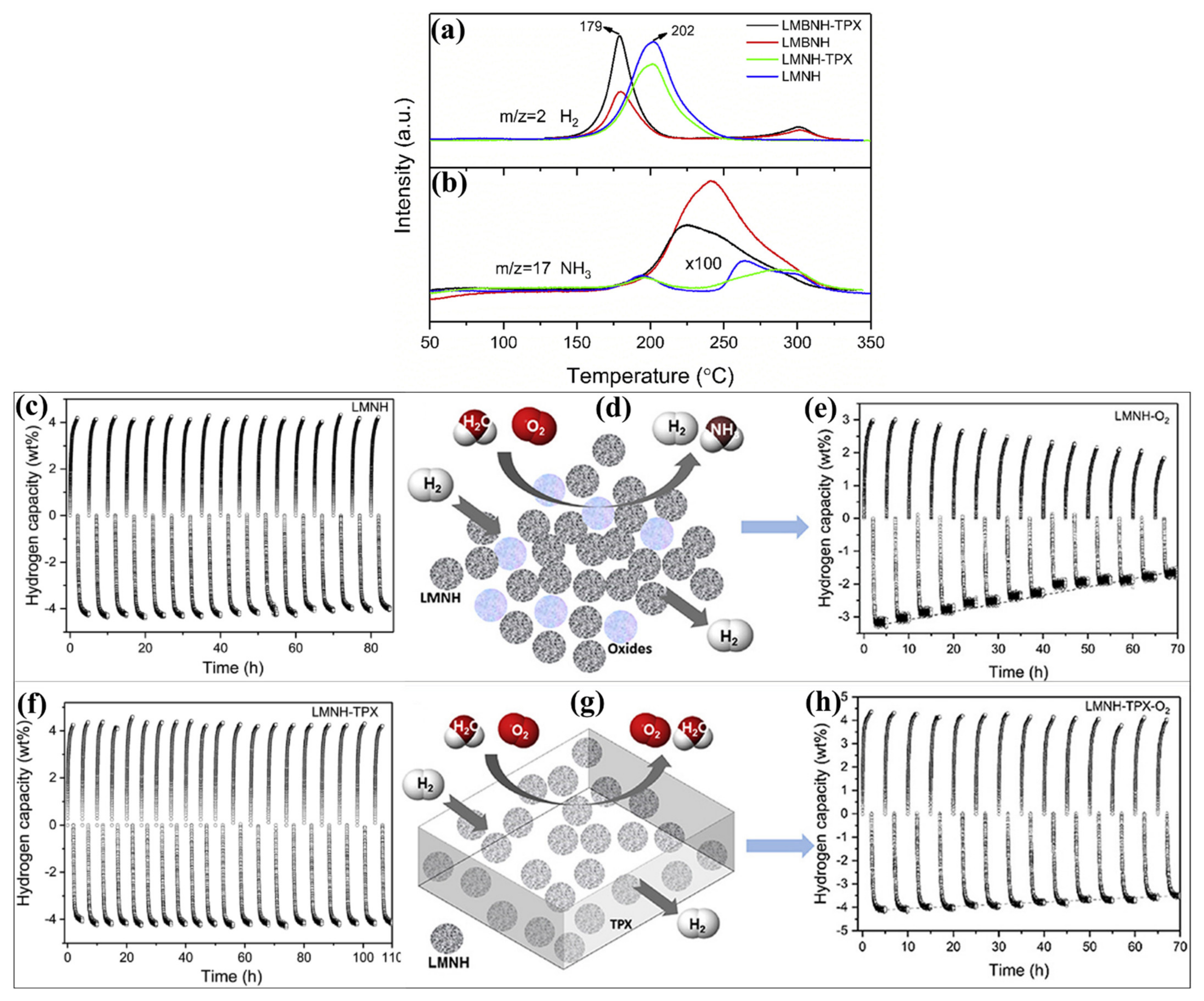
- Cao, H.; Georgopanos, P.; Capurso, G.; Pistidda, C.; Weigelt, F.; Chaudhary, A.-L.; Filiz, V.; Tseng, J.C.; Wharmby, M.T.; Dornheim, M.; et al. Air-stable metal hydride-polymer composites of Mg(NH2)2–LiH and TPX™. Mater. Today Energy 2018, 10, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Michaljaničová, I.; Slepička, P.; Hadravová, J.; Rimpelová, S.; Ruml, T.; Malinský, P.; Veselý, M.; Švorčík, V. High power plasma as an efficient tool for polymethylpentene cytocompatibility enhancement. RSC Adv. 2016, 6, 76000–76010. [Google Scholar] [CrossRef]
- Barmore, L.M.; Knudson, M.D. Mechanical and optical response of polymethylpentene under dynamic compression. J. Appl. Phys. 2019, 126, 185901. [Google Scholar] [CrossRef]
- Xiong, Z.; Hu, J.; Wu, G.; Chen, P.; Luo, W.; Gross, K.; Wang, J. Thermodynamic and kinetic investigations of the hydrogen storage in the Li–Mg–N–H system. J. Alloys Compd. 2005, 398, 235–239. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, D.; Zhao, W.; Cui, K.; Li, P.; Qu, X. Enhanced anti-poisoning performance against carbon monoxide of LaNi4.7Al0.3 alloy encapsulated in polymethyl methacrylate. Mater. Lett. 2021, 302, 130409. [Google Scholar] [CrossRef]
- Gosalawit-Utke, R.; Meethom, S.; Pistidda, C.; Milanese, C.; Laipple, D.; Saisopa, T.; Marini, A.; Klassen, T.; Dornheim, M. Destabilization of LiBH4 by nanoconfinement in PMMA–co–BM polymer matrix for reversible hydrogen storage. Int. J. Hydrogen Energy 2014, 39, 5019–5029. [Google Scholar] [CrossRef]
- Huang, J.; Yan, Y.; Ouyang, L.; Wang, H.; Liu, J.; Zhu, M. Increased air stability and decreased dehydrogenation temperature of LiBH4via modification within poly(methylmethacrylate). Dalton Trans. 2014, 43, 410–413. [Google Scholar] [CrossRef]
- Huang, J.; Yan, Y.; Ouyang, L.; Wang, H.; Zhu, M. Dehydrogenation mechanism of LiBH4 by Poly(methyl methacrylate). J. Alloys Compd. 2015, 645, S100–S102. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, D.; Yuan, Z.; Chen, Q.; Fan, G.; Zhao, D.; Liu, B. Flexible, water-resistant and air-stable LiBH4 nanoparticles loaded melamine foam with improved dehydrogenation. Front. Chem. 2020, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Hosseinabadi, N. The alkaline-earth metal alanate embedded selective gas permeable PMMA matrix-nanocomposite. Int. J. Hydrogen Energy 2021, 46, 2362–2375. [Google Scholar] [CrossRef]
- Jeon, K.-J.; Moon, H.R.; Ruminski, A.M.; Jiang, B.; Kisielowski, C.; Bardhan, R.; Urban, J.J. Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalysts. Nat. Mater. 2011, 10, 286–290. [Google Scholar] [CrossRef] [PubMed]
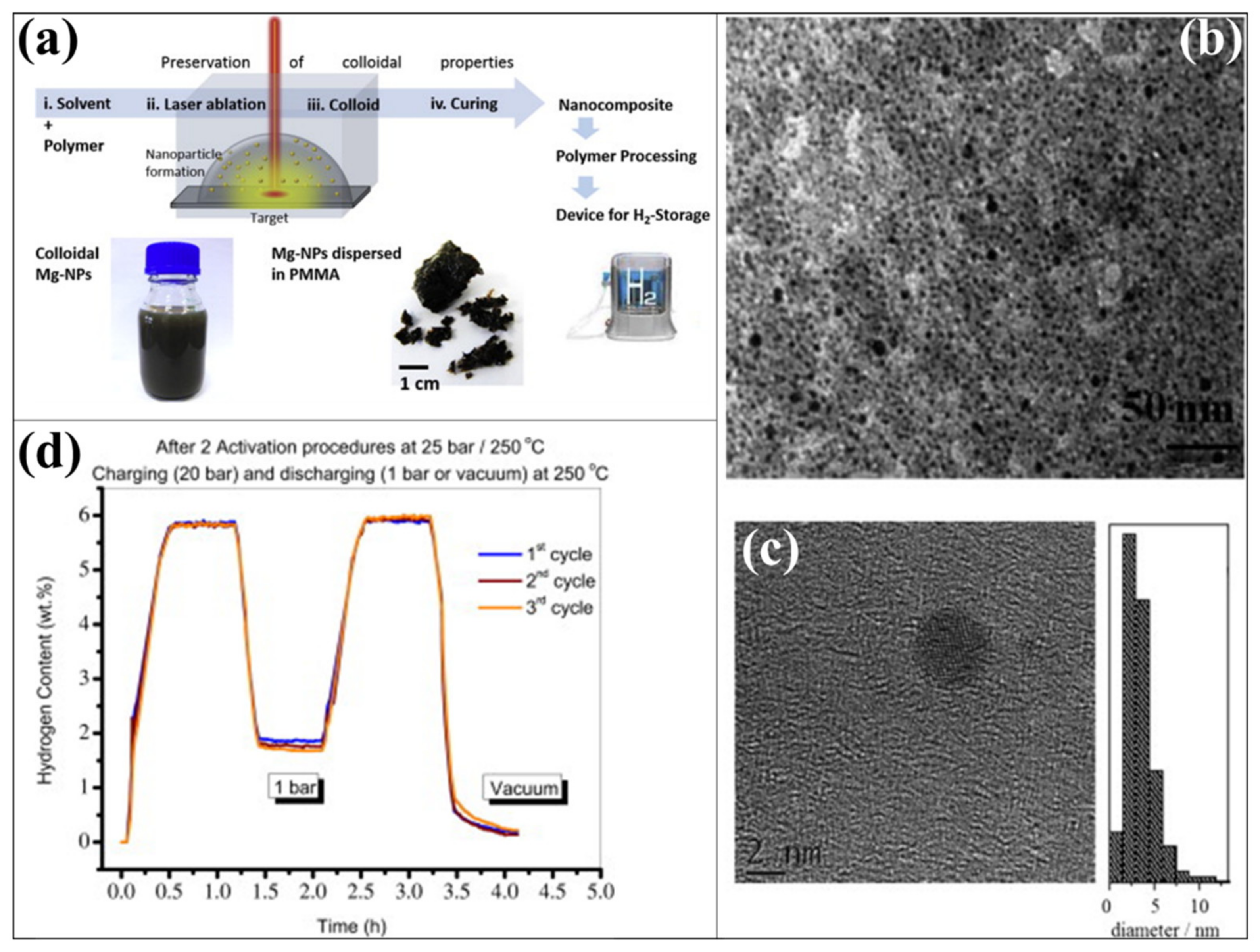
- Makridis, S.S.; Gkanas, E.I.; Panagakos, G.; Kikkinides, E.S.; Stubos, A.K.; Wagener, P.; Barcikowski, S. Polymer-stable magnesium nanocomposites prepared by laser ablation for efficient hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 11530–11535. [Google Scholar] [CrossRef] [Green Version]
- Rafatnejad, M.; Raygan, S.; Sefidmooy Azar, M. Investigation of dehydrogenation performance and air stability of MgH2–PMMA nanostructured composite prepared by direct high-energy ball-milling. Mater. Renew. Sustain. Energy 2020, 9, 14. [Google Scholar] [CrossRef]
- Xie, S.; Wang, D.; Zhang, S.; Xu, J.; Fu, J. High performance poly(methyl methacrylate) via hindered urea bond crosslinking. J. Mater. Chem. A 2022, 10, 9457–9467. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H.; Picchioni, F. Material encapsulation in poly(methyl methacrylate) shell: A review. J. Appl. Polym. Sci. 2019, 136, 48039. [Google Scholar] [CrossRef] [Green Version]
- Goseki, R.; Ishizone, T. Poly(methyl methacrylate) (PMMA). In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–11. [Google Scholar]
- Zaremba, D.; Evert, R. 5–Materials, chemical properties and analysis. In Polymer Optical Fibres; Bunge, C.-A., Gries, T., Beckers, M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 153–186. [Google Scholar]
- Grubbs, R.B. Roles of polymer ligands in nanoparticle stabilization. Polym. Rev. 2007, 47, 197–215. [Google Scholar] [CrossRef]
- Ruminski, A.M.; Bardhan, R.; Brand, A.; Aloni, S.; Urban, J.J. Synergistic enhancement of hydrogen storage and air stability via Mg nanocrystal–polymer interfacial interactions. Energy Environ. Sci. 2013, 6, 3267–3271. [Google Scholar] [CrossRef]
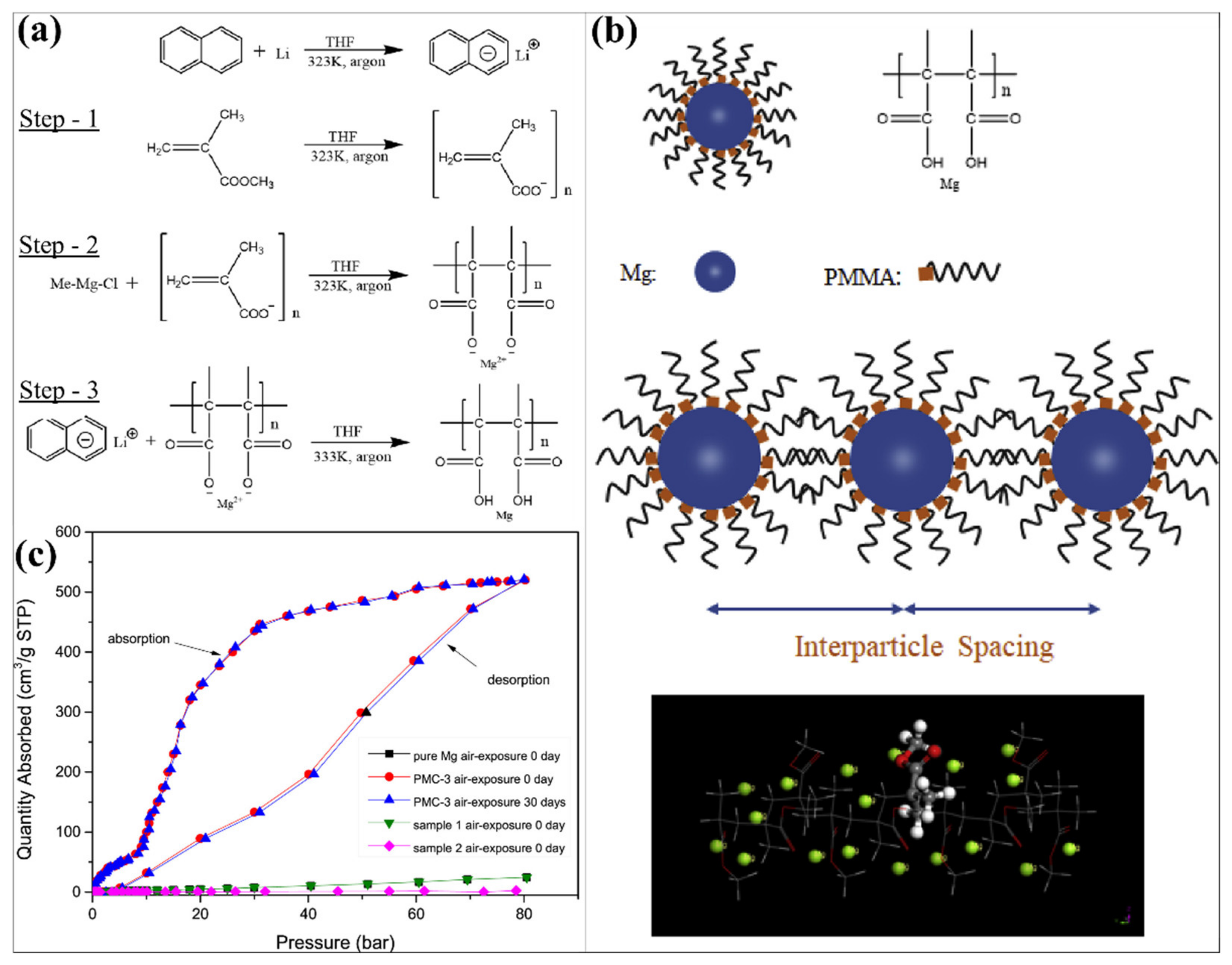
- Liang, H.; Chen, D.; Chen, M.; Li, W.; Snyders, R. Study of the synthesis of PMMA-Mg nanocomposite for hydrogen storage application. Int. J. Hydrogen Energy 2020, 45, 4743–4753. [Google Scholar] [CrossRef]
- Liang, H.; Chen, D.; Thiry, D.; Li, W.; Chen, M.; Snyders, R. Efficient hydrogen storage with the combination of metal Mg and porous nanostructured material. Int. J. Hydrogen Energy 2019, 44, 16824–16832. [Google Scholar] [CrossRef]
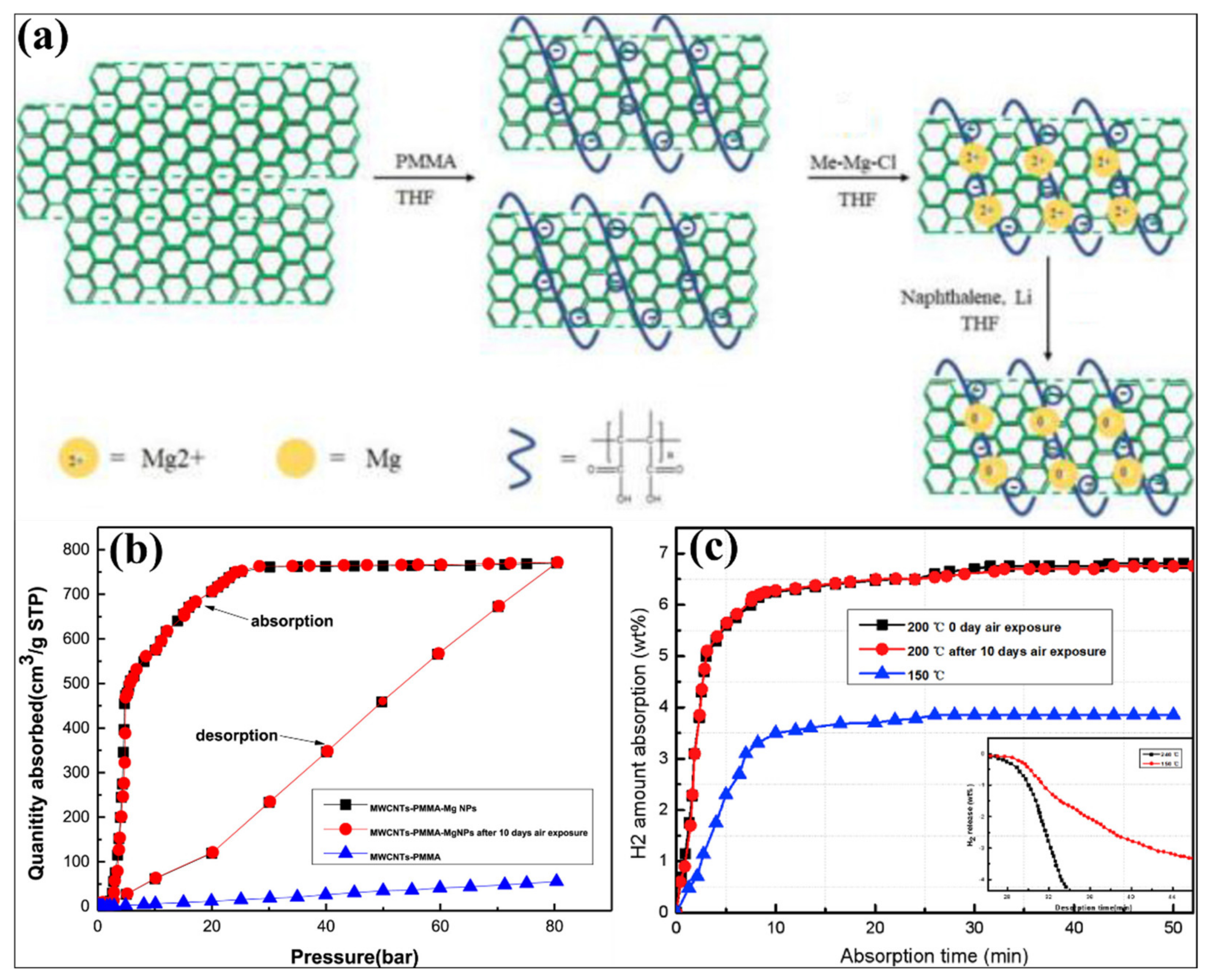
- Chen, Q.; Zhu, Y.; Zhang, Y.; Liu, Y.; Zhang, J.; Liu, Z.; Chen, W.; Li, L. Electrochemical properties of Mg3MnNi2-x% polymethyl methacrylate-multiwalled carbon nanotubes (PMMA-MWCNTs) (x = 25, 50, 75, 100). J. Mater. Sci. 2018, 53, 6033–6041. [Google Scholar] [CrossRef]
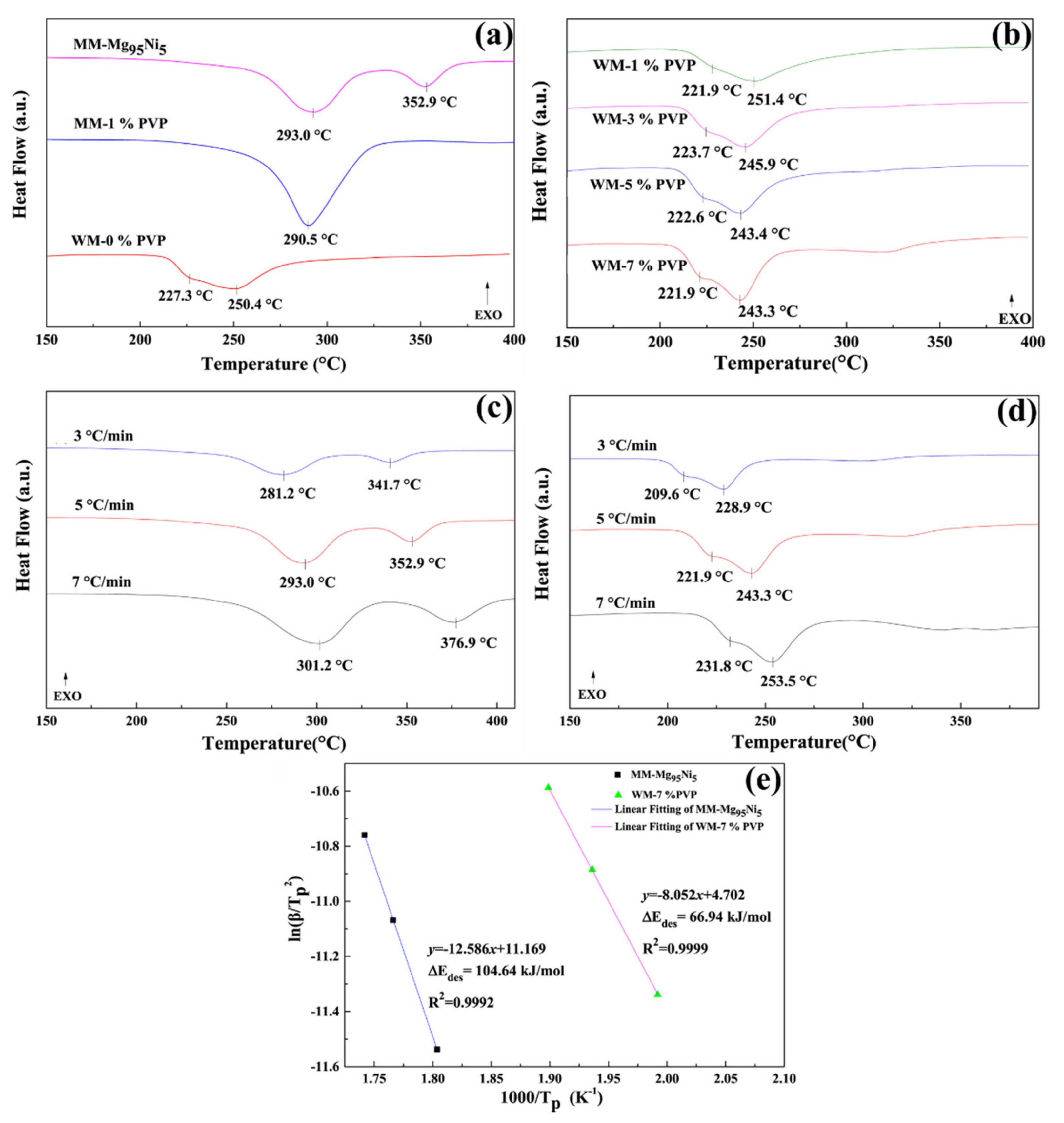
- Yuan, J.G.; Zhu, Y.F.; Li, L.Q.; Wu, Y.; Zhou, S.X. Preparation and hydrogen storage property of Mg-based hydrogen storage composite embedded by polymethyl methacrylate. Int. J. Hydrogen Energy 2017, 42, 22366–22372. [Google Scholar] [CrossRef]
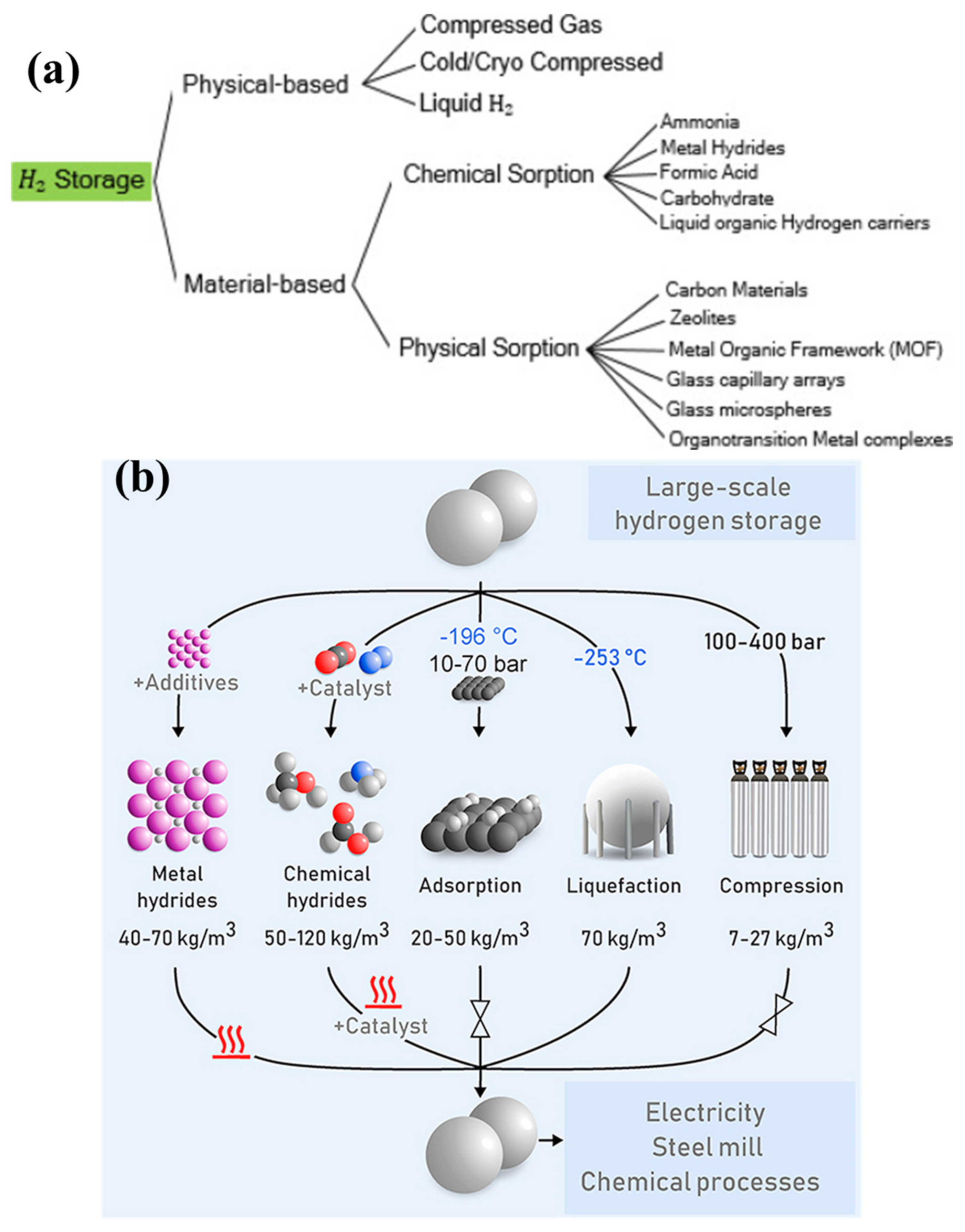

| Target | Year | ||
|---|---|---|---|
| 2020 | 2025 | Ultimate | |
| Gravimetric capacity (wt %) | 4.5% | 5.5% | 6.5% |
| Volumetric capacity (g/L) | 30 | 40 | 50 |
| Cost ($/kg H2) | 333 | 300 | 266 |
| Durability/Operability: | |||
| • Operating temperature (°C) | −40/60 | −40/60 | −40/60 |
| • Min/max delivery temperature (°C) | −40/85 | −40/85 | −40/85 |
| • Operational cycles | 1500 | 1500 | 1500 |
| • Min/max delivery pressure (bar) | 5/12 | 5/12 | 5/12 |
| • Onboard efficiency | 90% | 90% | 90% |
| Charging/Discharging rate: | |||
| • System fill time (min) | 3–5 | 3–5 | 3–5 |
| • Min full flow rate ((g/s)/kW) | 0.02 | 0.02 | 0.02 |
| • Average flow rate ((g/s)/kW) | 0.004 | 0.004 | 0.004 |
| • Start time to full flow @ 20 °C (s) | 5 | 5 | 5 |
| • Start time to full flow @ −20 °C (s) | 15 | 15 | 15 |
| • Transient response at operating temperature 10–90% and 90–0% (based on full flow rate) (s) | 0.75 | 0.75 | 0.75 |
| Hydrogen Storage Materials/Hydrides with PMMA Polymer | Hydrogen Sorption Information | Ref. |
|---|---|---|
| Mg NCs/PMMA composites |
| [153] |
| Laser ablated nano Mg/PMMA composite |
| [154] |
| Mg–PMMA nanocomposites |
| [161] |
| Mg–polyethylene nanocomposites |
| |
| Mg–polystyrene nanocomposites |
| |
| Mg–polylactic acid nanocomposite |
| |
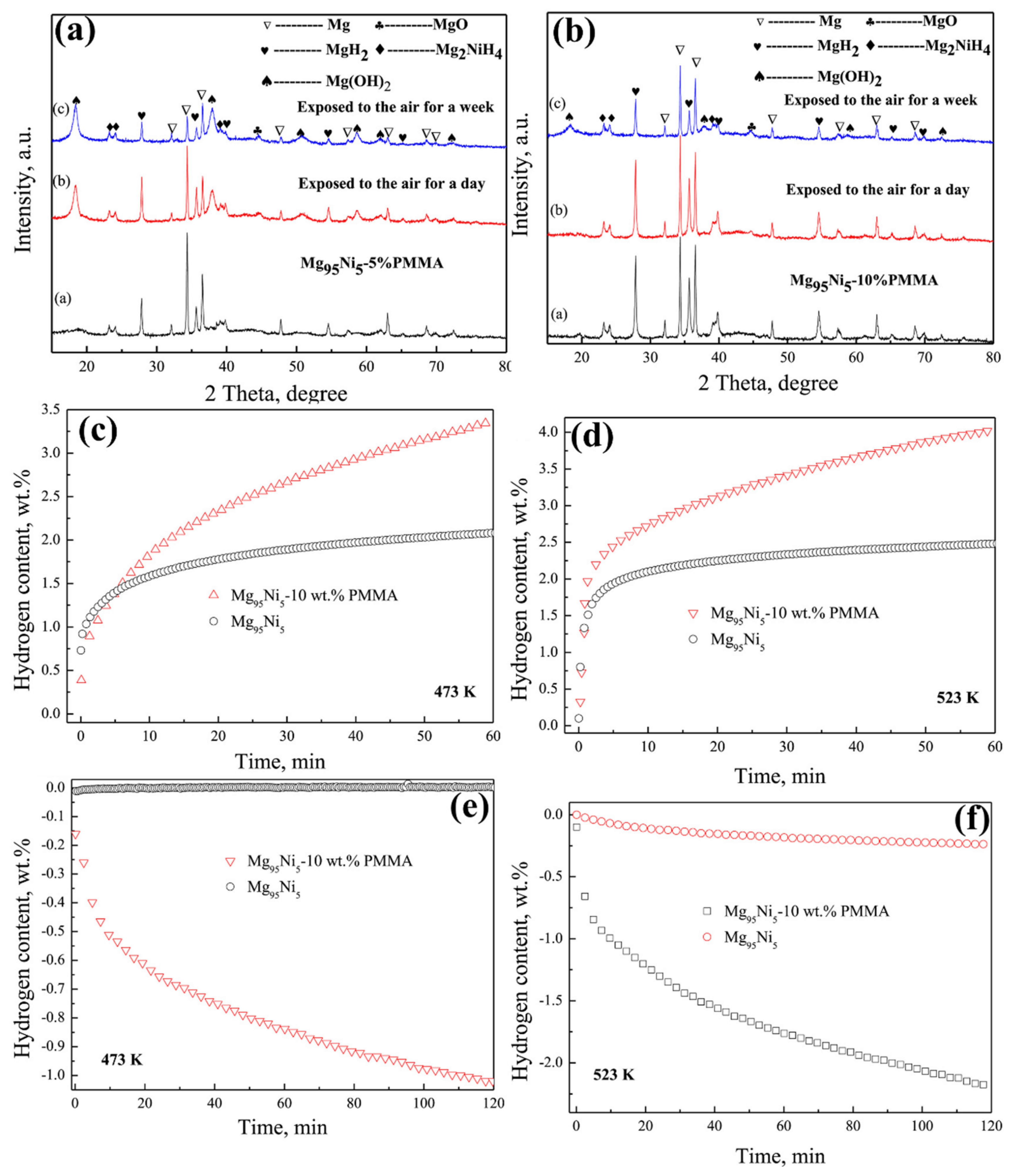
| Mg95Ni5-PMMA |
| [165] |
| MWCNTs–PMMA–Mg NPs composites |
| [163] |
| Mg NPs in porous PMMA |
| [162] |
| MgH2–PMMA nanostructured composite |
| [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangarasu, S.; Oh, T.H. Impact of Polymers on Magnesium-Based Hydrogen Storage Systems. Polymers 2022, 14, 2608. https://doi.org/10.3390/polym14132608
Thangarasu S, Oh TH. Impact of Polymers on Magnesium-Based Hydrogen Storage Systems. Polymers. 2022; 14(13):2608. https://doi.org/10.3390/polym14132608
Chicago/Turabian StyleThangarasu, Sadhasivam, and Tae Hwan Oh. 2022. "Impact of Polymers on Magnesium-Based Hydrogen Storage Systems" Polymers 14, no. 13: 2608. https://doi.org/10.3390/polym14132608
APA StyleThangarasu, S., & Oh, T. H. (2022). Impact of Polymers on Magnesium-Based Hydrogen Storage Systems. Polymers, 14(13), 2608. https://doi.org/10.3390/polym14132608






