Cytocompatibility and Antibacterial Properties of Coaxial Electrospun Nanofibers Containing Ciprofloxacin and Indomethacin Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Coaxial Nanofibers
2.3. Characterizations of the Scaffold
2.3.1. The Morphological Study
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. The Tensile Property Evaluation
2.3.4. The Physical Properties Evaluation
2.4. Drug Release Study
2.5. Biocompatibility Evaluation
2.6. Antibacterial Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Co-Electrospinning Conditions
3.2. Morphology of the Scaffolds after Crosslinking
3.3. The Core–Shell Structure of the Prepared Scaffold
3.4. The Chemical Structure of the Core–Shell Scaffold
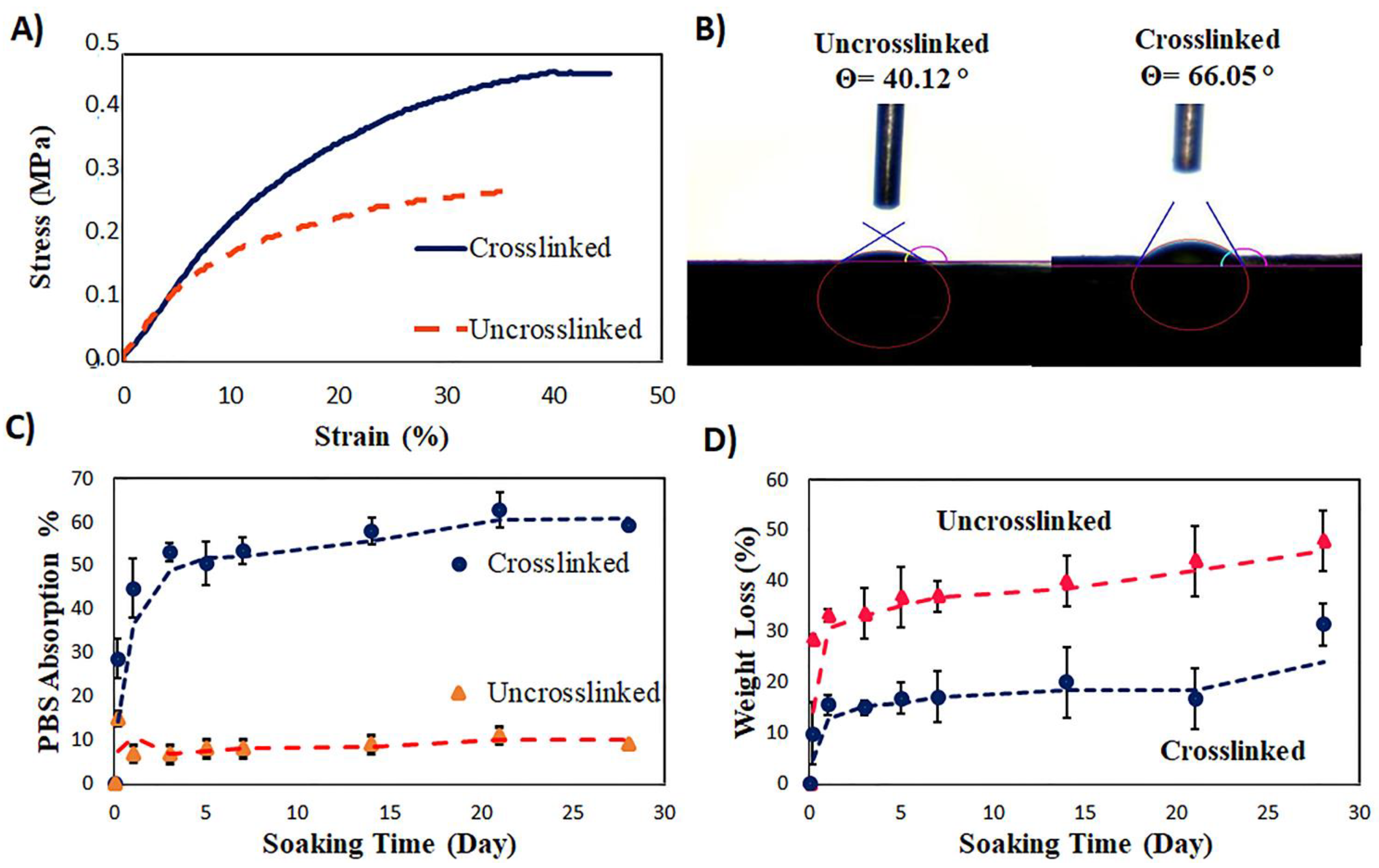
3.5. The Tensile Properties of the Core–Shell Scaffold
3.6. The Surface Hydrophilicity of the Coaxial Scaffold
3.7. The Swelling Ratio and Biodegradation of the Coaxial Scaffolds before and after Crosslinking
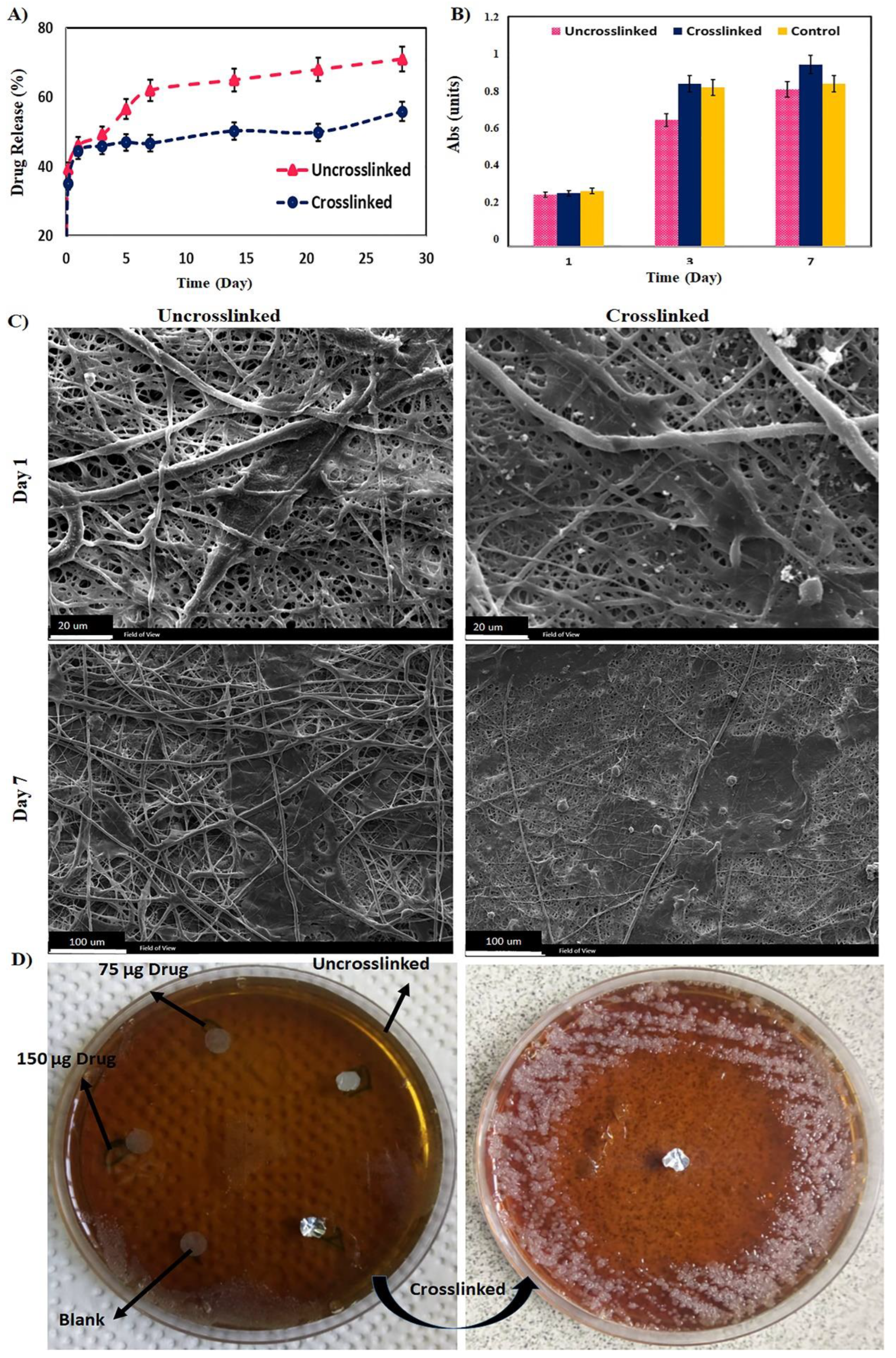
3.8. The Drug Release Study
- -
- Kinetics and Mechanism of the Drug Release
3.9. The Biological Study
3.10. The Antibacterial Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanmugam, K.S.V.; Renganathan, S. Type 1 collagen scaffold functionalized with ciprofloxacin loaded gelatin microspheres—Fabrication, In vitro & In vivo evaluation, histological and biochemical analysis. MOJ Drug Des. Dev. Ther. 2019, 3, 1–10. [Google Scholar]
- Iga, C.; Agata, T.; Marcin, Ł.; Natalia, F.; Justyna, K.-L. Ciprofloxacin-Modified Degradable Hybrid Polyurethane-Polylactide Porous Scaffolds Developed for Potential Use as an Antibacterial Scaffold for Regeneration of Skin. Polymers 2020, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Kaviannasab, E.; Semnani, D.; Nouri Khorasani, S.; Varshosaz, J.; Khalili, S.; Ghahreman, F. Core-Shell nanofibers of Poly (ε–caprolactone) and Polyvinylpyrrolidone for drug delivery system. Mater. Res. Express 2019, 6, 115015. [Google Scholar] [CrossRef]
- Ghahreman, F.; Semnani, D.; Khorasani, S.N.; Varshosaz, J.; Khalili, S.; Mohammadi, S.; Kaviannasab, E. Polycaprolactone–Gelatin Membranes in Controlled Drug Delivery of 5-Fluorouracil. Polym. Sci. Ser. A 2020, 62, 636–647. [Google Scholar] [CrossRef]
- Milkova, V.; Kamburova, K.; Radeva, T. Nanocolloids of indomethacin prepared using sonication and subsequent encapsulation with polysaccharide films. Colloids Surf. B Biointerfaces 2013, 108, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Suresh, N.; Nagesh, H.N.; Sekhar, K.V.; Kumar, A.; Shirazi, A.N.; Parang, K. Synthesis of novel ciprofloxacin analogues and evaluation of their anti-proliferative effect on human cancer cell lines. Bioorganic Med. Chem. Lett. 2013, 23, 6292–6295. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Abasian, P.; Ghanavati, S.; Rahebi, S.; Nouri Khorasani, S.; Khalili, S. Polymeric nanocarriers in targeted drug delivery systems: A review. Polym. Adv. Technol. 2020, 31, 2939–2954. [Google Scholar] [CrossRef]
- Abasian, P.; Shakibi, S.; Maniati, M.S.; Nouri Khorasani, S.; Khalili, S. Targeted delivery, drug release strategies, and toxicity study of polymeric drug nanocarriers. Polym. Adv. Technol. 2021, 32, 931–944. [Google Scholar] [CrossRef]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef]
- Jung, J.-W.; Lee, C.-L.; Yu, S.; Kim, I.-D. Electrospun nanofibers as a platform for advanced secondary batteries: A comprehensive review. J. Mater. Chem. A 2016, 4, 703–750. [Google Scholar] [CrossRef]
- Qu, H.; Wei, S.; Guo, Z. Coaxial electrospun nanostructures and their applications. J. Mater. Chem. A 2013, 1, 11513–11528. [Google Scholar] [CrossRef]
- Yoon, J.; Yang, H.-S.; Lee, B.-S.; Yu, W.-R. Recent Progress in Coaxial Electrospinning: New Parameters, Various Structures, and Wide Applications. Adv. Mater. 2018, 30, 1704765. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Malrautu, P.; Laha, A.; Ramakrishna, S. Gelatin Nanofibers in Drug Delivery Systems and Tissue Engineering. Eng. Sci. 2021, 16, 71–81. [Google Scholar] [CrossRef]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef]
- Nosar, M.N.; Salehi, M.; Ghorbani, S.; Pour Beiranvand, S.; Goodarzi, A.; Azami, M. Characterization of wet-electrospun cellulose acetate based 3-dimensional scaffolds for skin tissue engineering applications: Influence of cellulose acetate concentration. Cellulose 2016, 23, 3239–3248. [Google Scholar] [CrossRef]
- Abedini, F.; Ebrahimi, M.; Roozbehani, A.H.; Domb, A.J.; Hosseinkhani, H. Overview on natural hydrophilic polysaccharide polymers in drug delivery. Polym. Adv. Technol. 2018, 29, 2564–2573. [Google Scholar] [CrossRef]
- Khalili, S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous Scaffolds with Biomimetic Composition for Skin Regeneration. Appl. Biochem. Biotechnol. 2019, 187, 1193–1203. [Google Scholar] [CrossRef]
- Khalili, S.; Nouri Khorasani, S.; Razavi, M.; Hashemi Beni, B.; Heydari, F.; Tamayol, A. Nanofibrous scaffolds with biomimetic structure. J. Biomed. Mater. Res. Part A 2018, 106, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Nadim, A.; Khorasani, S.N.; Kharaziha, M.; Davoodi, S.M. Design and characterization of dexamethasone-loaded poly (glycerol sebacate)-poly caprolactone/gelatin scaffold by coaxial electro spinning for soft tissue engineering. Mater. Sci. Eng. C 2017, 78, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Khorasani, S.N.; Saadatkish, N.; Khoshakhlagh, K. Characterization of gelatin/cellulose acetate nanofibrous scaffolds: Prediction and optimization by response surface methodology and artificial neural networks. Polym. Sci. Ser. A 2016, 58, 399–408. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Zhang, C.; Ahmad, Z.; Huang, J.; Li, J.-S.; Chang, M.-W. Designer fibers from 2D to 3D–Simultaneous and controlled engineering of morphology, shape and size. Chem. Eng. J. 2018, 334, 89–98. [Google Scholar] [CrossRef]
- Thevenot, P.; Nair, A.; Dey, J.; Yang, J.; Tang, L. Method to analyze three-dimensional cell distribution and infiltration in degradable scaffolds. Tissue Eng. Part C Methods 2008, 14, 319–331. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.G.; Jayaram, L.; Biswas, R.; Nair, M. Evaluation of antibacterial activity and cytocompatibility of ciprofloxacin loaded gelatin-hydroxyapatite scaffolds as a local drug delivery system for osteomyelitis treatment. Tissue Eng. Part A 2015, 21, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Shao, J.; Ma, J.; Zou, Q.; Li, J.; Zuo, Y.; Yang, F.; Li, Y. Development of ciprofloxacin and nano-hydroxyapatite dual-loaded polyurethane scaffolds for simultaneous treatment of bone defects and osteomyelitis. Mater. Lett. 2019, 253, 86–89. [Google Scholar] [CrossRef]
- Redenti, S.; Neeley, W.L.; Rompani, S.; Saigal, S.; Yang, J.; Klassen, H.; Langer, R.; Young, M.J. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials 2009, 30, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Nivison-Smith, L.; Rnjak, J.; Weiss, A.S. Synthetic human elastin microfibers: Stable cross-linked tropoelastin and cell interactive constructs for tissue engineering applications. Acta Biomater. 2010, 6, 354–359. [Google Scholar] [CrossRef]
- Jaramillo, P.; Pérez, P.; Contreras, R.; Tiznado, W.; Fuentealba, P. Definition of a Nucleophilicity Scale. J. Phys. Chem. A 2006, 110, 8181–8187. [Google Scholar] [CrossRef]
- Lavik, E.B.; Klassen, H.; Warfvinge, K.; Langer, R.; Young, M.J. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials 2005, 26, 3187–3196. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef]
- Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Theoretical cross-link density of the nanofibrous scaffolds. Mater. Des. Processing Commun. 2019, 1, e22. [Google Scholar] [CrossRef][Green Version]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. Part B Rev. 2011, 17, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef]
- Hosseinkhani, H.; Domb, A.J. Biodegradable polymers in gene-silencing technology. Polym. Adv. Technol. 2019, 30, 2647–2655. [Google Scholar] [CrossRef]
- Santos, L.F.; Correia, I.J.; Silva, A.S.; Mano, J.F. Biomaterials for drug delivery patches. Eur. J. Pharm. Sci. 2018, 118, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Marin, Ș.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pîrvu, C.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enachescu, M. Collagen-Polyvinyl Alcohol-Indomethacin Biohybrid Matrices as Wound Dressings. Pharmaceutics 2018, 10, 224. [Google Scholar] [CrossRef]
- Basar, A.O.; Castro, S.; Torres-Giner, S.; Lagaron, J.M.; Turkoglu Sasmazel, H. Novel poly(epsilon-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Romo, I.L.; Leija Gutiérrez, H.M.; Barrera, E.V.; Elías-Zúñiga, A. Development of forcespun fiber-aligned scaffolds from gelatin-zein composites for potential use in tissue engineering and drug release. MRS Commun. 2018, 8, 885–892. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Investigation of the swelling behavior of crosslinked hyaluronic acid films and hydrogels produced using homogeneous reactions. J. Appl. Polym. Sci. 2008, 109, 923–931. [Google Scholar] [CrossRef]
- Kim, G.-M.; Le, K.H.T.; Giannitelli, S.M.; Lee, Y.J.; Rainer, A.; Trombetta, M. Electrospinning of PCL/PVP blends for tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Badri, W.; Miladi, K.; Robin, S.; Viennet, C.; Nazari, Q.A.; Agusti, G.; Fessi, H.; Elaissari, A. Polycaprolactone Based Nanoparticles Loaded with Indomethacin for Anti-Inflammatory Therapy: From Preparation to Ex Vivo Study. Pharm. Res. 2017, 34, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Letha, S.S.; Kumar, A.S.; Nisha, U.; Rosemary, M.J. Electrospun polyurethane-gelatin artificial skin scaffold for wound healing. J. Text. Inst. 2022, 113, 378–387. [Google Scholar] [CrossRef]
- Salehi, S.; Fathi, M.; Javanmard, S.H.; Bahners, T.; Gutmann, J.S.; Ergün, S.; Steuhl, K.P.; Fuchsluger, T.A. Generation of PGS/PCL Blend Nanofibrous Scaffolds Mimicking Corneal Stroma Structure. Macromol. Mater. Eng. 2014, 299, 455–469. [Google Scholar] [CrossRef]
- Geng, Y.; Li, K.; Simonsen, J. Further investigation of polyaminoamide-epichlorohydrin/stearic anhydride compatibilizer system for wood-polyethylene composites. J. Appl. Polym. Sci. 2006, 99, 712–718. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Luong-Van, E.; Grondahl, L.; Chua, K.N.; Leong, K.W.; Nurcombe, V.; Cool, S.M. Controlled release of heparin from poly(epsilon-caprolactone) electrospun fibers. Biomaterials 2006, 27, 2042–2050. [Google Scholar] [CrossRef]
- Sukul, A.; Das, S.; Saha, S.; Rahman, S.M. Screening of Analgesic, Antimicrobial, Cytotoxic and Antioxidant Activities of Metal Complexes of Indomethacin. Dhaka Univ. J. Pharm. Sci. 2014, 13, 175–180. [Google Scholar] [CrossRef]
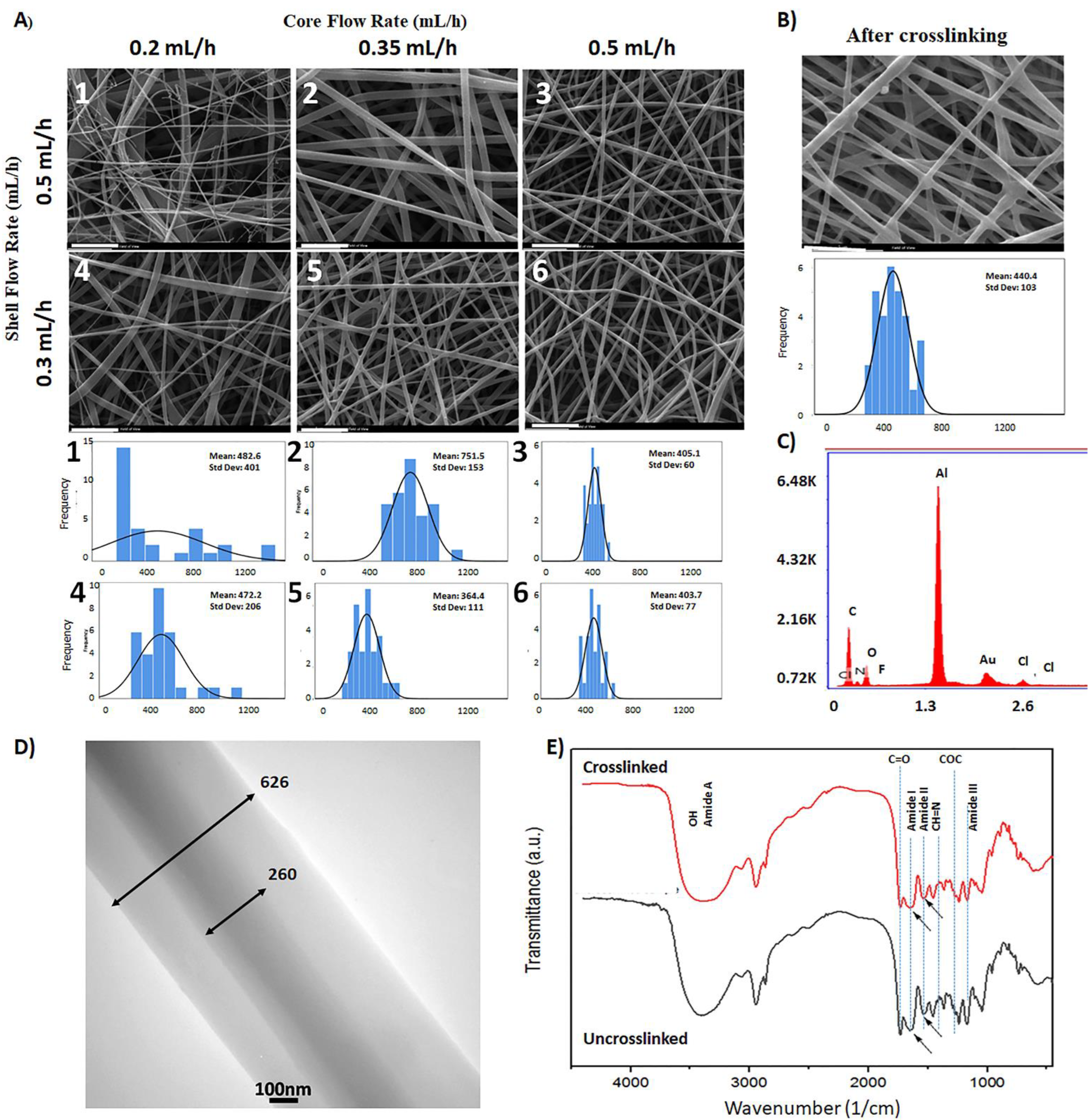

| Sample Code | Shell Flow Rate (mL·h−1) | Core Flow Rate (mL·h−1) | Fiber Diameter (nm) | Pore Diameter (µm) | Surface Porosity (%) |
|---|---|---|---|---|---|
| 1 | 0.5 | 0.2 | 482 ± 401 | 3.0 ± 0.1 | 87 |
| 2 | 0.5 | 0.35 | 752 ± 153 | 4.5 ± 0.2 | 87 |
| 3 | 0.5 | 0.5 | 405 ± 60 | 2.6 ± 0.1 | 88 |
| 4 | 0.3 | 0.2 | 472 ± 206 | 2.3 ± 0.1 | 91 |
| 5 | 0.3 | 0.35 | 364 ± 111 | 2.9 ± 0.1 | 89 |
| 6 | 0.3 | 0.5 | 404 ± 77 | 2.8 ± 0.1 | 87 |
| Property | Tensile Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | |
|---|---|---|---|---|
| Sample | ||||
| Before crosslinking | 1.85 ± 0.2 | 0.26 ± 0.02 | 36 ± 4 | |
| After crosslinking | 2.4 ± 0.3 | 0.47 ± 0.04 | 44 ± 1 | |
| Scaffold | Peppas Eq. | Zero-Order Eq. Ct = C0 + K0t | First-Order Eq. dC/dt = −K1t | Higuchi Eq. Q = KH t 1/2 |
|---|---|---|---|---|
| Uncrosslinked | y = 0.6117x0.0746 R2 = 0.9708 | y = 0.0095x + 26.989 R2 = 0.8222 | y = −0.0001x + 1.8917 R2 = 0.3084 | y = 0.2905x + 25.385 R2 = 0.9272 |
| Crosslinked | y = 0.6x0.073 R2 = 0.8673 | y = 0.0202x + 34.461 R2 = 0.4569 | y = −0.0002x + 1.8717 R2 = 0.4582 | y = 0.7114x + 29.845 R2 = 0.6739 |
| Control Disc | Disc with 75 µg Drug | Disc with 150 µg Drug | Uncrosslinked Scaffold | Crosslinked Scaffold |
|---|---|---|---|---|
| 0 | 29.5 ± 0.03 | 30.3 ± 0.02 | 27.4 ± 0.01 | 27.9 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalili, S.; Ghane, N.; Nouri Khorasani, S.; Heydari, F.; Atwal, A.; Davoodi, P. Cytocompatibility and Antibacterial Properties of Coaxial Electrospun Nanofibers Containing Ciprofloxacin and Indomethacin Drugs. Polymers 2022, 14, 2565. https://doi.org/10.3390/polym14132565
Khalili S, Ghane N, Nouri Khorasani S, Heydari F, Atwal A, Davoodi P. Cytocompatibility and Antibacterial Properties of Coaxial Electrospun Nanofibers Containing Ciprofloxacin and Indomethacin Drugs. Polymers. 2022; 14(13):2565. https://doi.org/10.3390/polym14132565
Chicago/Turabian StyleKhalili, Shahla, Nazanin Ghane, Saied Nouri Khorasani, Fariba Heydari, Arjan Atwal, and Pooya Davoodi. 2022. "Cytocompatibility and Antibacterial Properties of Coaxial Electrospun Nanofibers Containing Ciprofloxacin and Indomethacin Drugs" Polymers 14, no. 13: 2565. https://doi.org/10.3390/polym14132565
APA StyleKhalili, S., Ghane, N., Nouri Khorasani, S., Heydari, F., Atwal, A., & Davoodi, P. (2022). Cytocompatibility and Antibacterial Properties of Coaxial Electrospun Nanofibers Containing Ciprofloxacin and Indomethacin Drugs. Polymers, 14(13), 2565. https://doi.org/10.3390/polym14132565








