Polymer-Magnetic Semiconductor Nanocomposites for Industrial Electronic Applications
Abstract
1. Introduction
2. Fundamentals
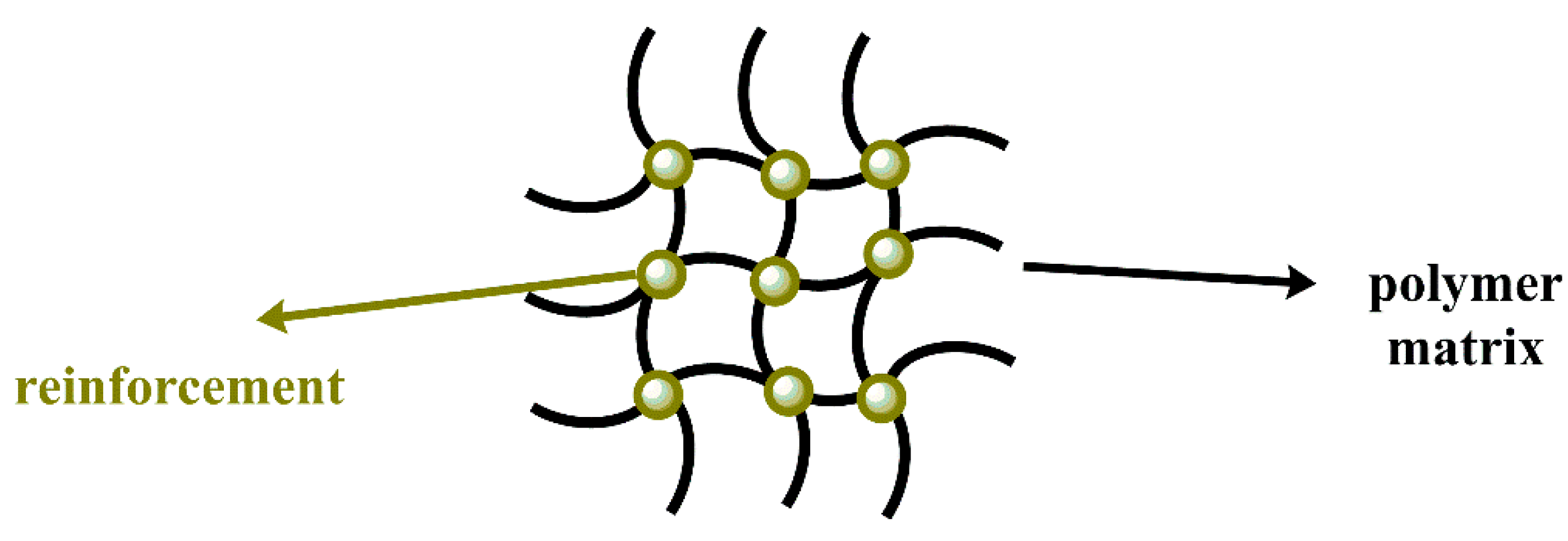
2.1. Composites
2.1.1. Polymer Matrix
Hydrogels
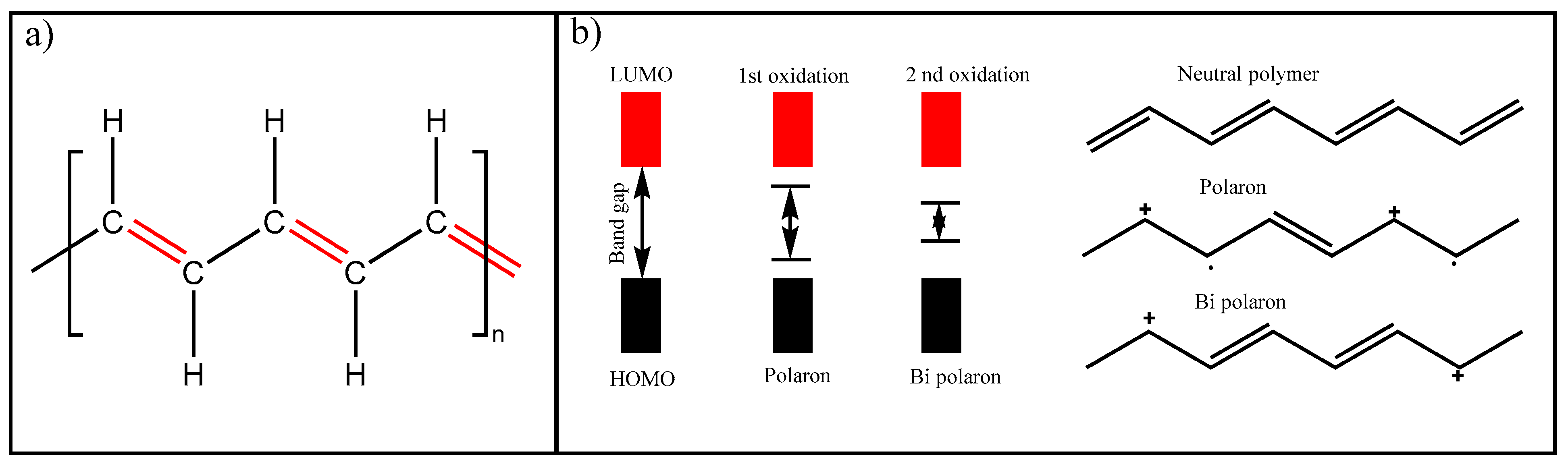
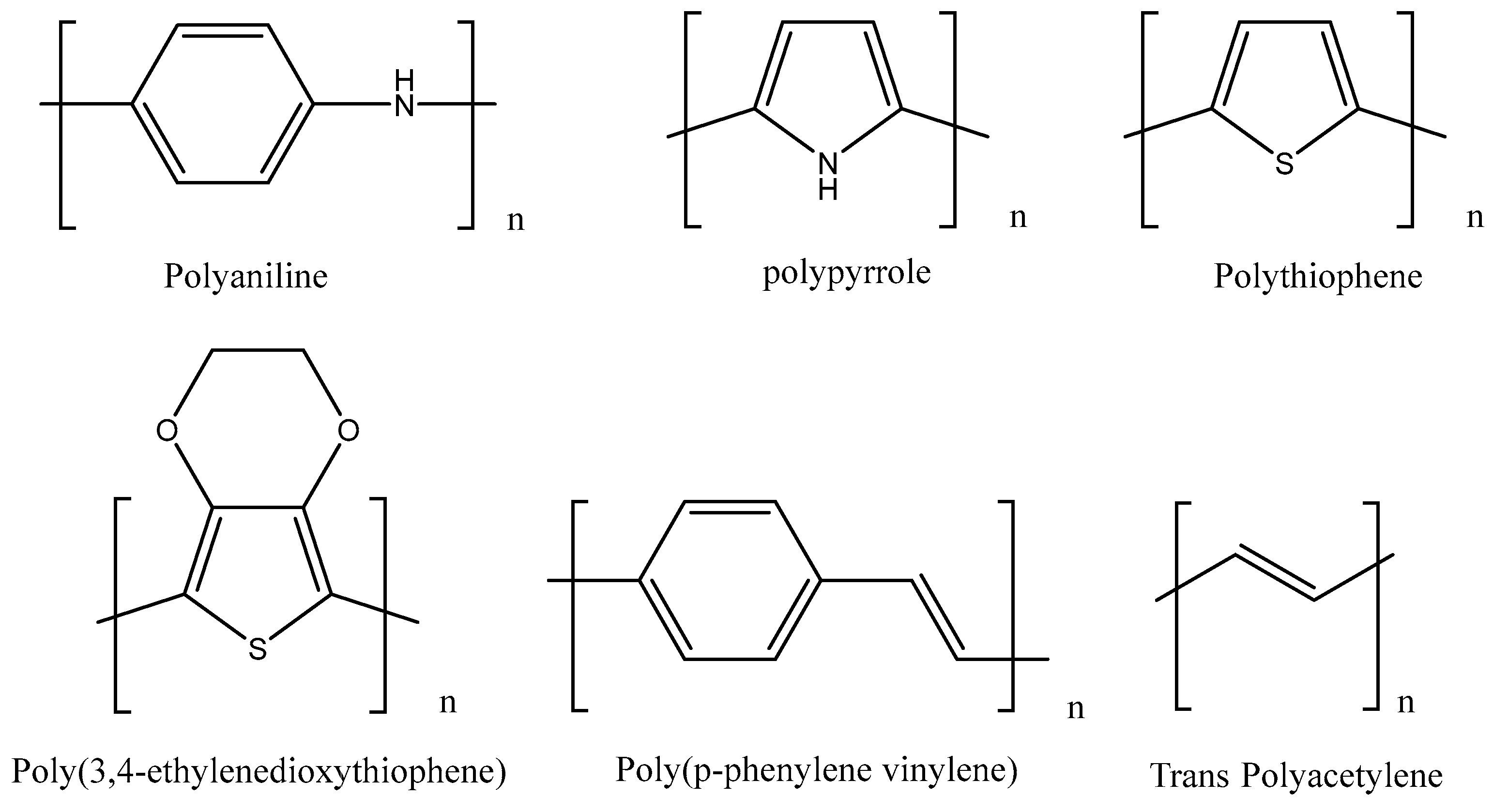
Conducting and Semiconducting Polymers
2.1.2. Reinforcement
Nanoparticles as Reinforcement
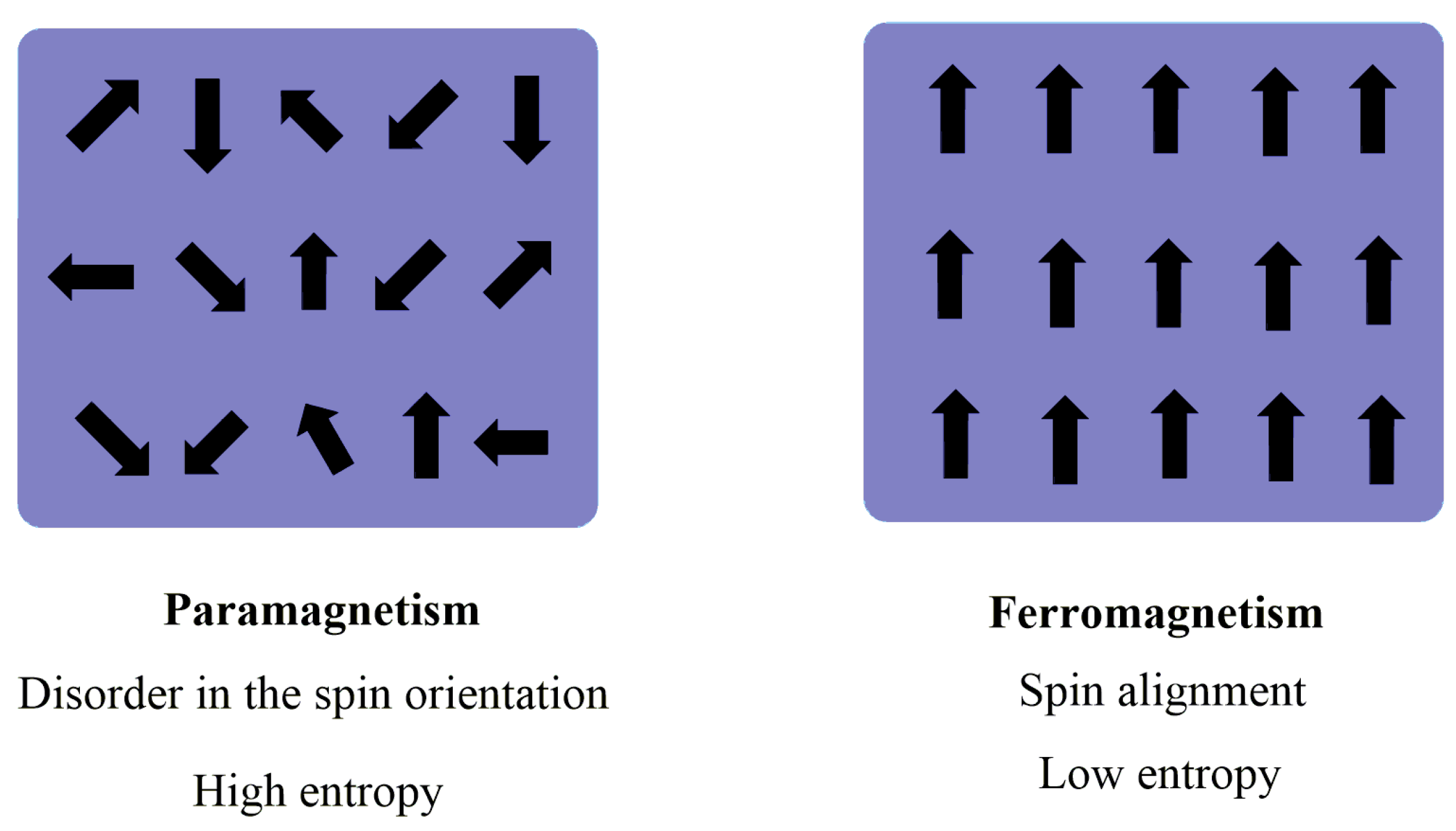
2.2. Magnetic Nanoparticles
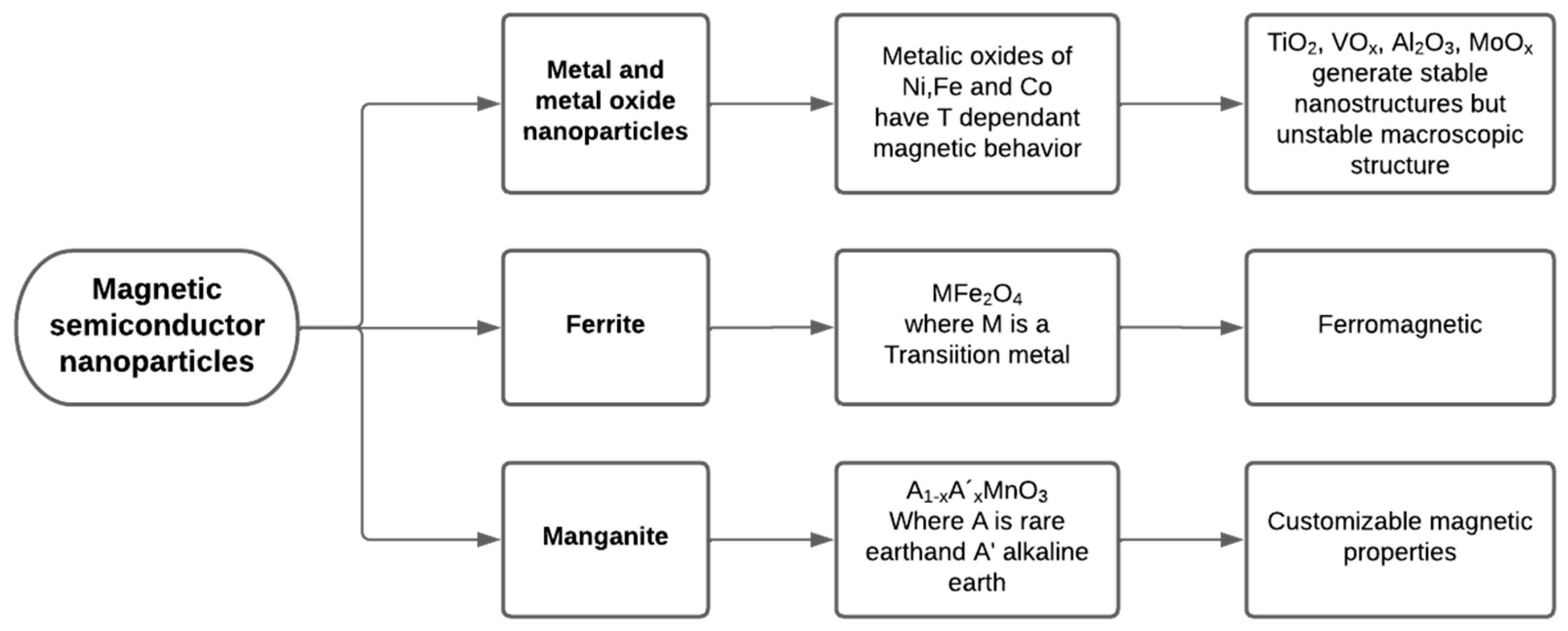
2.2.1. Types of Magnetic Semiconductor Nanoparticles
Metal and Metal Oxide Nanoparticles
Ferrite
Manganite
2.3. Polymer Nanocomposites
Magnetic Polymer Nanocomposites
3. Synthesis of MNCs
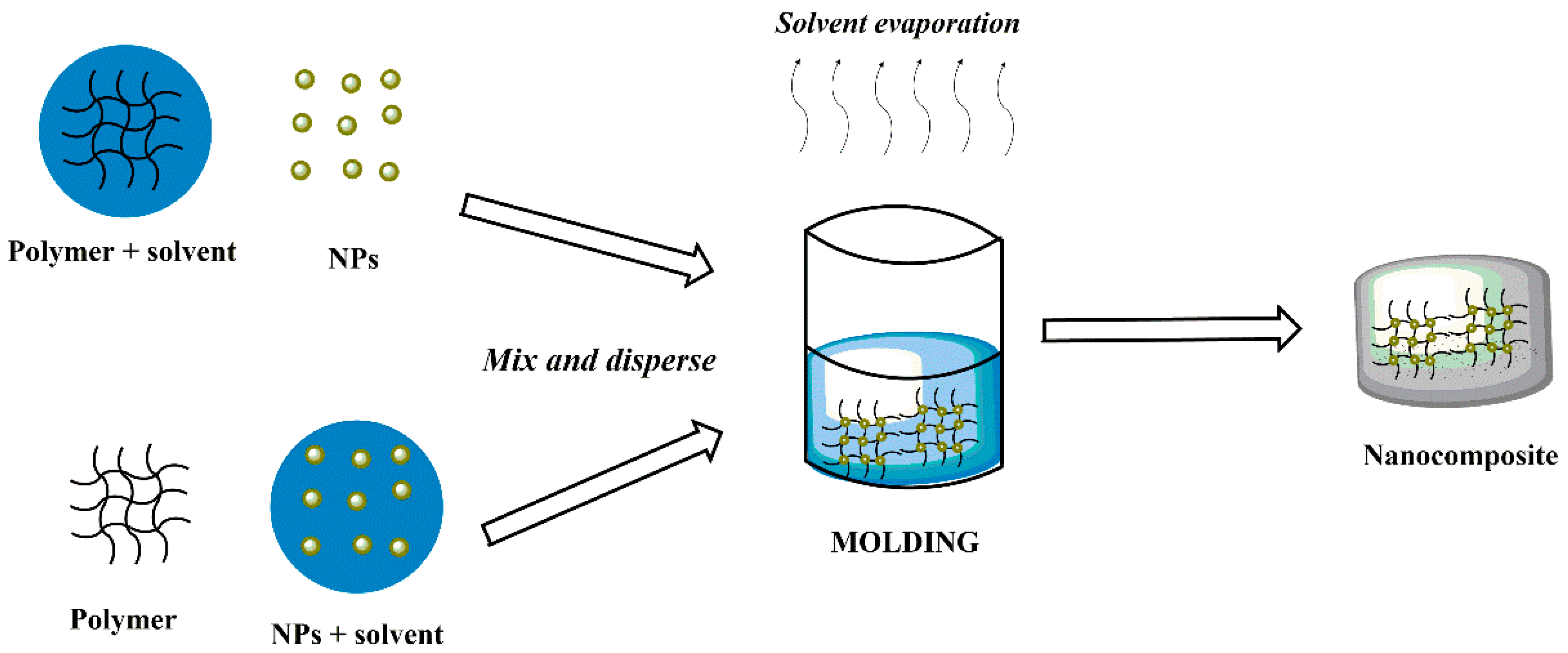
3.1. Molding
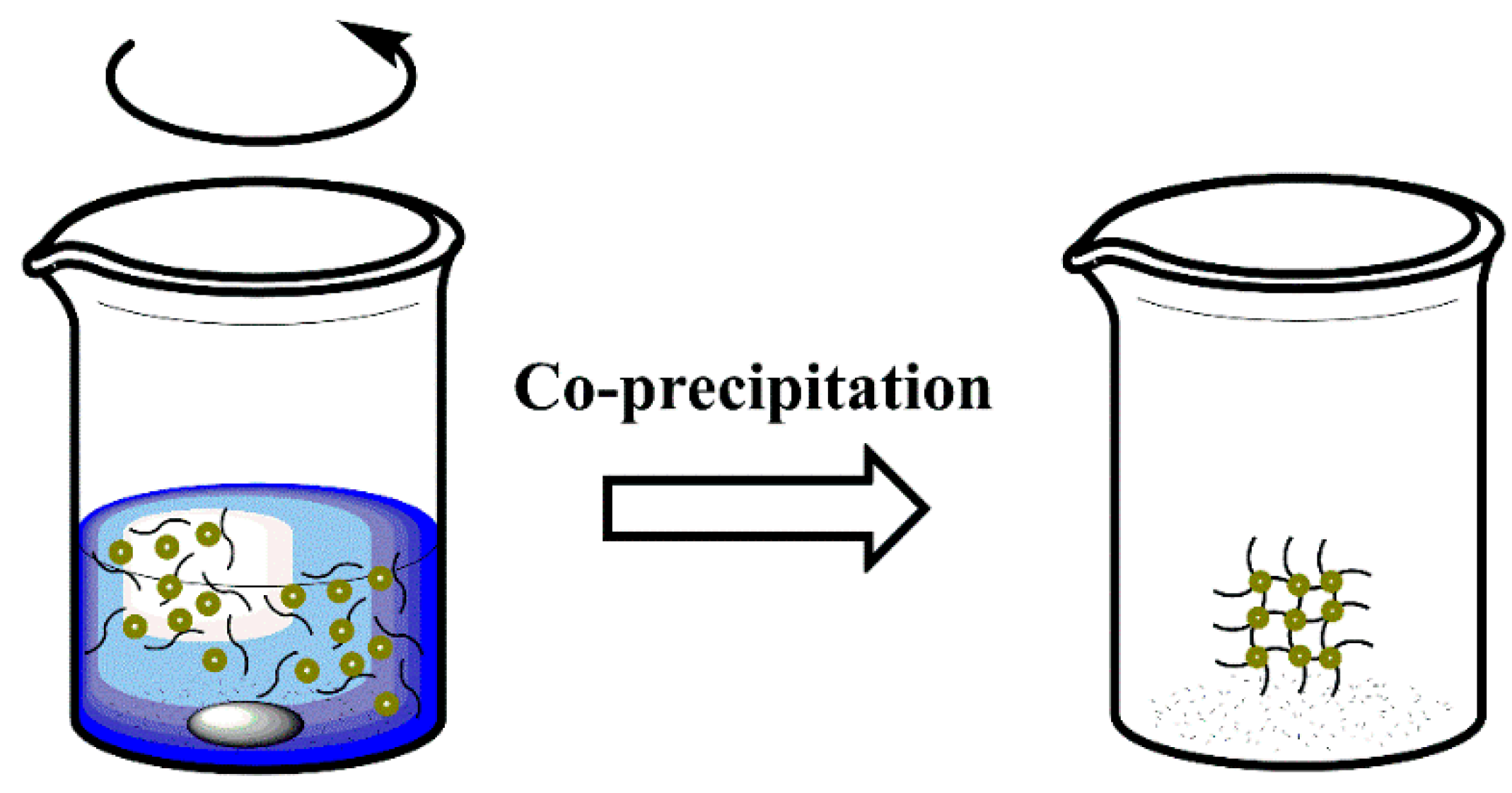
3.2. Coprecipitation
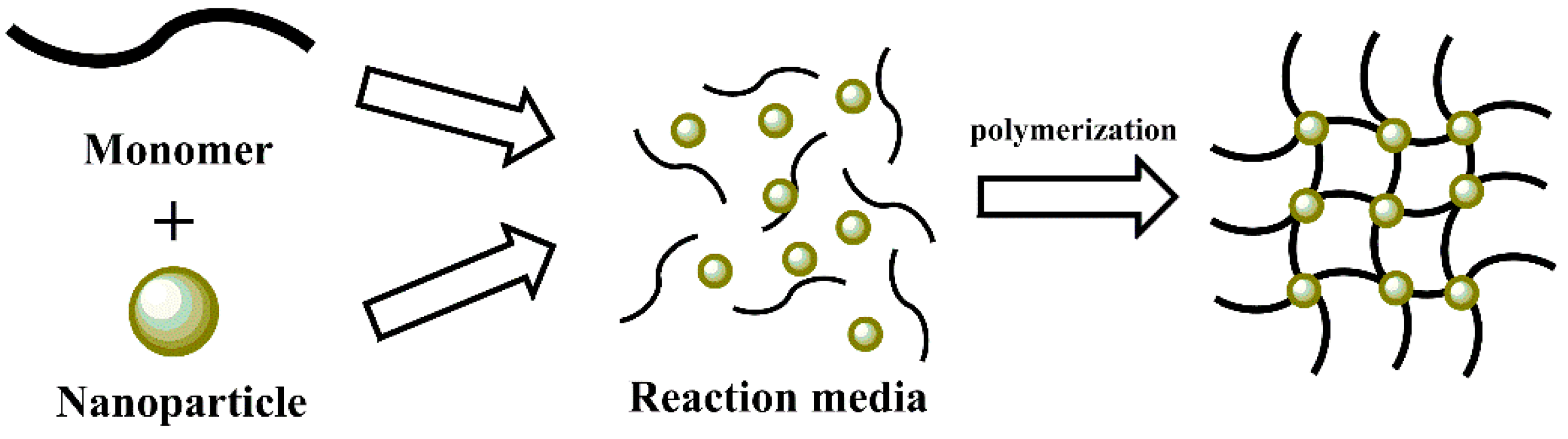
3.3. In Situ Polymerization
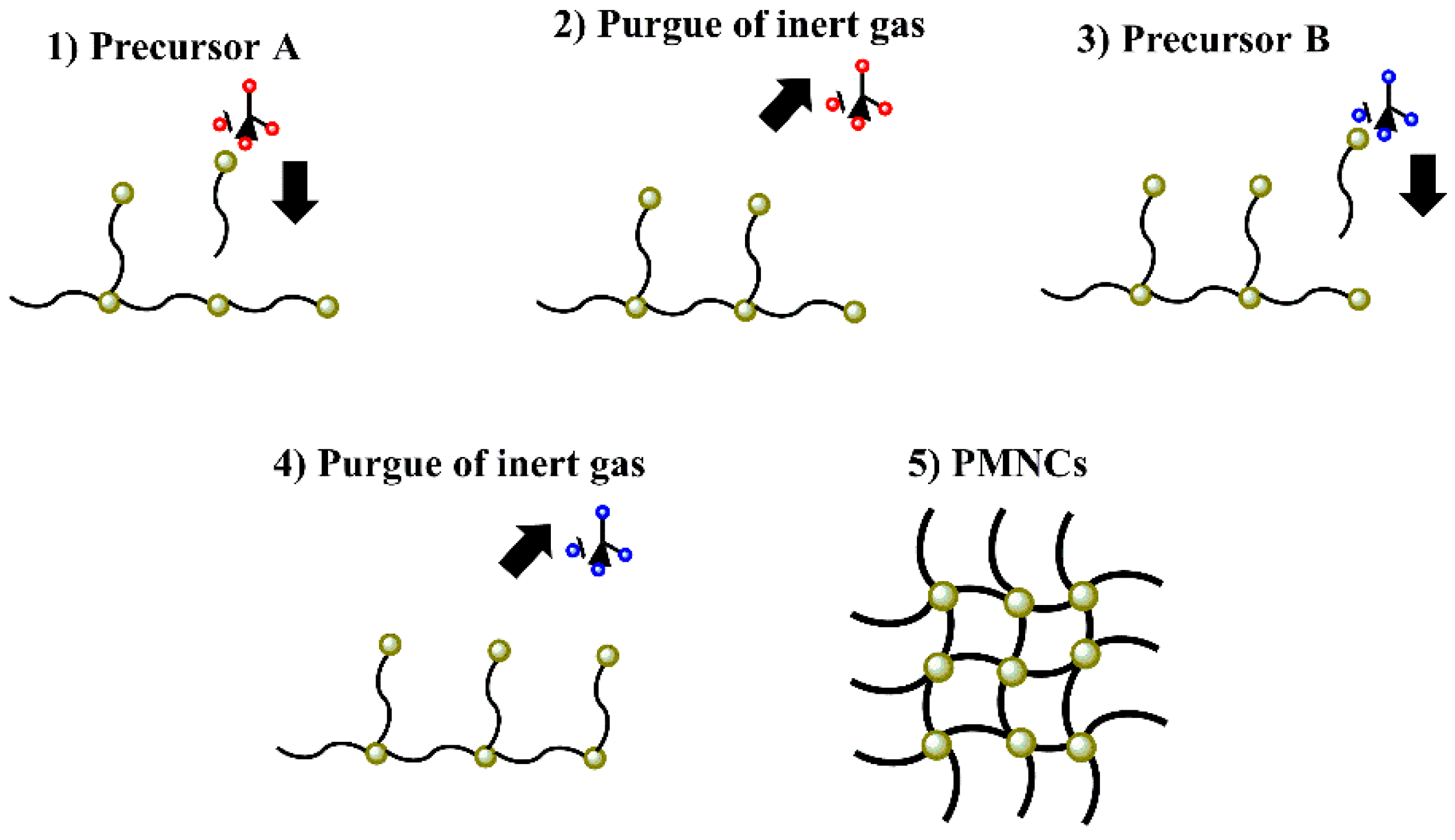
3.4. Chemical Vapor Deposition (CVD)
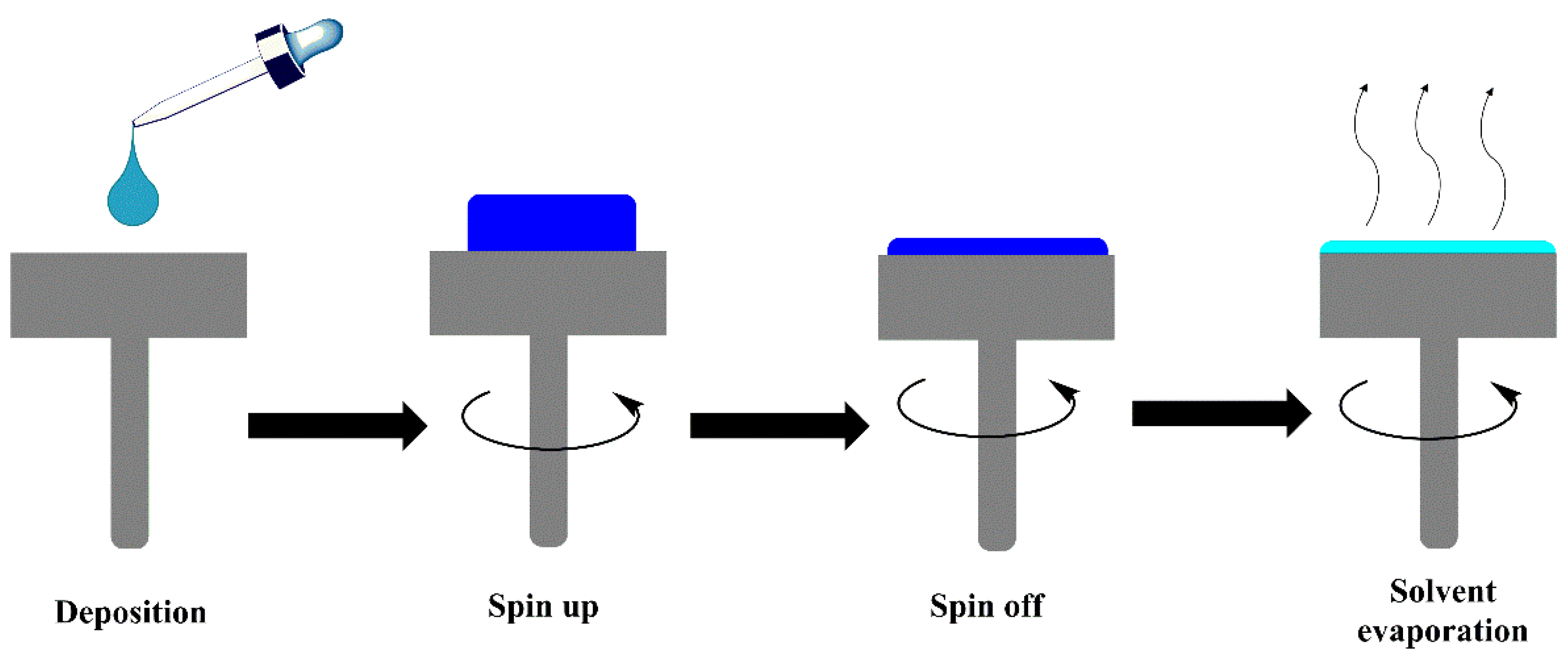
3.5. Spin Coating
4. Electronic Applications
4.1. Supercapacitors
4.2. Sensors
4.3. Light-Emitting Diodes (LED)
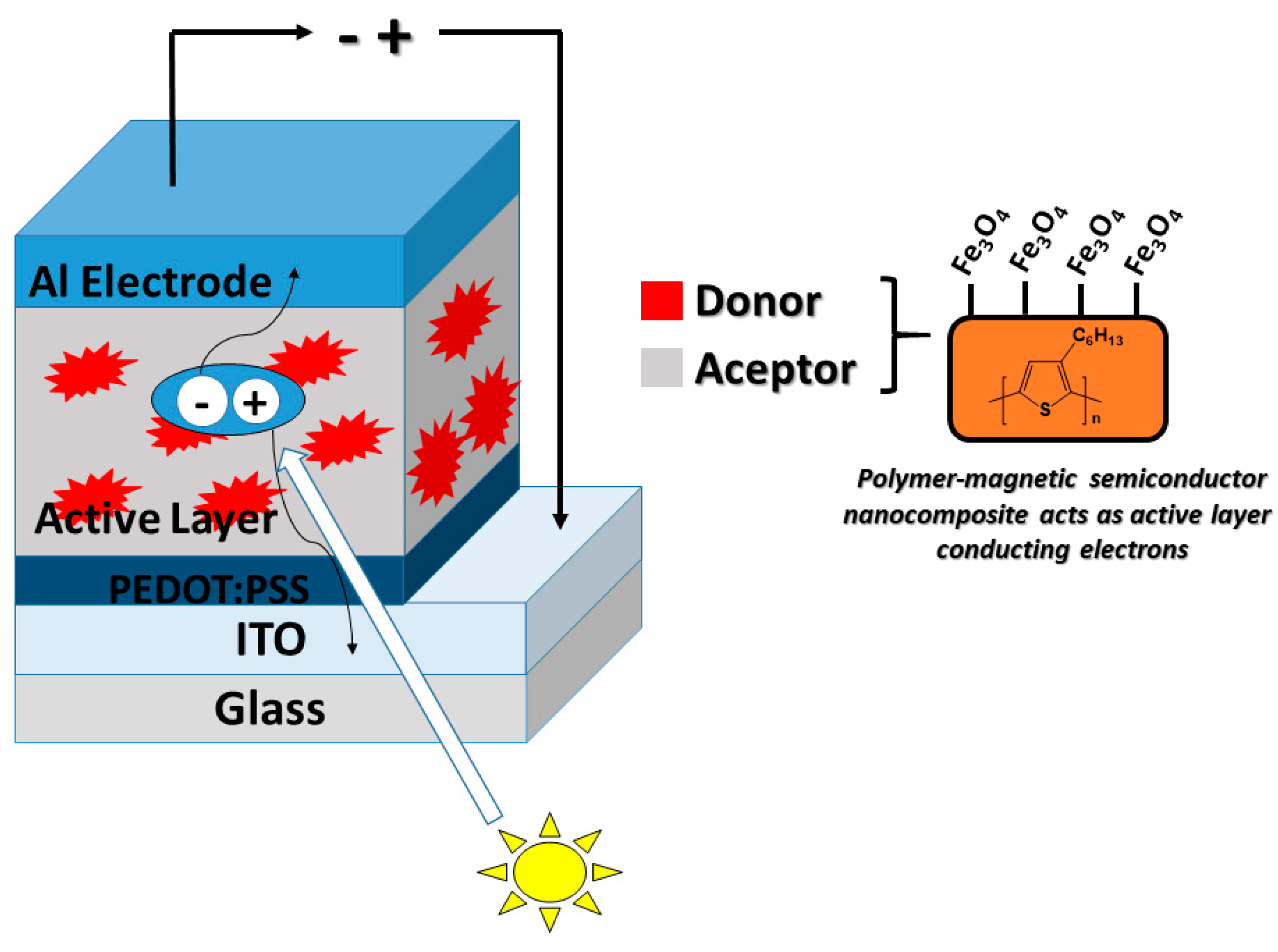
4.4. Solar Cells
5. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghouse, H.; Slewa, L.; Mahmood, M.; Rehmat, S.; Musharrat, S.; Dahman, Y. Importance of Nanotechnology, Various Applications in Electronic Field. In Nanotechnology for Electronic Applications; Springer Nature Singapore: Singapore, 2022; pp. 1–28. [Google Scholar]
- Coey, J.M.D. Applications of soft magnets. In Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2001; pp. 439–463. [Google Scholar]
- Coey, J.M.D. Applications of hard magnets. In Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2001; pp. 464–493. [Google Scholar]
- Zverev, V.I.; Pyatakov, A.P.; Shtil, A.A.; Tishin, A.M. Novel applications of magnetic materials and technologies for medicine. J. Magn. Magn. Mater. 2018, 459, 182–186. [Google Scholar] [CrossRef]
- Lewis, L.H.; Jiménez-Villacorta, F. Perspectives on Permanent Magnetic Materials for Energy Conversion and Power Generation. Metall. Mater. Trans. A 2013, 44, 2–20. [Google Scholar] [CrossRef]
- Sadiku, M.N.O.; Ashaolu, T.J.; Ajayi-Majebi, A.; Musa, S.M. Future of Nanotechnology. Int. J. Sci. Adv. 2021, 2. [Google Scholar] [CrossRef]
- Gubin, S.P.; Koksharov, Y.A.; Khomutov, G.B.; Yurkov, G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev. 2005, 74, 489–520. [Google Scholar] [CrossRef]
- Payal; Pandey, P. Role of Nanotechnology in Electronics: A Review of Recent Developments and Patents. Recent Pat. Nanotechnol. 2022, 16, 45–66. [Google Scholar] [CrossRef]
- Pandey, G.; Jain, P. Assessing the nanotechnology on the grounds of costs, benefits, and risks. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 63. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. An Introduction to Nanotechnology. In An Introduction to Green Nanotechnology; Academic Press: Delhi, India, 2019; pp. 1–27. [Google Scholar]
- Vatta, L.L.; Sanderson, R.D.; Koch, K.R. Magnetic nanoparticles: Properties and potential applications. Pure Appl. Chem. 2006, 78, 1793–1801. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010, 7, 1–37. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Yeap, S.P.; Lim, J.; Ooi, B.S.; Ahmad, A.L. Agglomeration, colloidal stability, and magnetic separation of magnetic nanoparticles: Collective influences on environmental engineering applications. J. Nanopart. Res. 2017, 19, 368. [Google Scholar] [CrossRef]
- Kalia, S.; Kango, S.; Kumar, A.; Haldorai, Y.; Kumari, B.; Kumar, R. Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid Polym. Sci. 2014, 292, 2025–2052. [Google Scholar] [CrossRef]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Vasiliev, V.V.; Morozov, E.V. Introduction. In Advanced Mechanics of Composite Materials and Structures; Elsevier: Canberra, Australia, 2018; pp. xvii–xxv. [Google Scholar]
- Amir, S.M.M.; Sultan, M.T.H.; Jawaid, M.; Ariffin, A.H.; Mohd, S.; Salleh, K.A.M.; Ishak, M.R.; Shah, A.U.M. Nondestructive testing method for Kevlar and natural fiber and their hybrid composites. In Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–388. [Google Scholar]
- Sapuan, S.M. Composite Materials. In Composite Materials; Sapuan, S.M., Ed.; Elsevier: Boston, MA, USA, 2017; pp. 57–93. [Google Scholar]
- Abbas, Q. Advances of Electrode Materials. In Reference Module in Materials Science and Materials Engineering; Elsevier: Durhan, NC, USA, 2020; ISBN 978-0-12-803581-8. [Google Scholar]
- Ngo, T.-D. Introduction to Composite Materials. In Composite and Nanocomposite Materials—From Knowledge to Industrial Applications; IntechOpen: Edmonton, AB, Canada, 2020. [Google Scholar]
- Rahman, M.R.; Hamdan, S. bin Study on physical, mechanical, morphological and thermal properties of styrene- co -glycidyl methacrylate/fumed silica/clay nanocomposites. In Silica and Clay Dispersed Polymer Nanocomposites; Elsevier: New Delhi, India, 2018; pp. 71–85. [Google Scholar]
- Saba, N.; Jawaid, M.; Sultan, M.T.H. Thermal properties of oil palm biomass based composites. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Elsevier: New Delhi, India, 2017; pp. 95–122. [Google Scholar]
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B 2021, 9, 2561–2583. [Google Scholar] [CrossRef]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Frontispiece: Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem. A Eur. J. 2018, 24, chem.201886461. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2, 843–865. [Google Scholar] [CrossRef]
- Rawat, N.K.; Ahmad, S. Unveiling nanoconducting polymers and composites for corrosion protection. In Nanomaterials-Based Coatings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 373–395. [Google Scholar]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- Mishra, A.K. Conducting Polymers: Concepts and Applications. J. At. Mol. Condens. Nano Phys. 2018, 5, 159–193. [Google Scholar] [CrossRef]
- Roth, S. Conductive polymers. Phys. B+C 1984, 127, 151–157. [Google Scholar] [CrossRef]
- Pham Truong, T.N.; Banet, P.; Aubert, P. Conducting Polymers Nanowires with Carbon Nanotubes or Graphene-Based Nanocomposites for Supercapacitors Applications. In Conjugated Polymer Nanostructures for Energy Conversion and Storage Applications; Wiley: Weinheim, Germany, 2021; pp. 445–497. [Google Scholar]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; N. Abdullah, S.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef]
- Phan, S.; Luscombe, C.K. Recent Advances in the Green, Sustainable Synthesis of Semiconducting Polymers. Trends Chem. 2019, 1, 670–681. [Google Scholar] [CrossRef]
- Han, Y.; Dai, L. Conducting Polymers for Flexible Supercapacitors. Macromol. Chem. Phys. 2019, 220, 1800355. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent developments in conducting polymers: Applications for electrochemistry. RSC Adv. 2020, 10, 37834–37856. [Google Scholar] [CrossRef]
- Arumugaprabu, V.; Ko, T.J.; Uthayakumar, M.; Joel Johnson, R.D. Failure analysis in hybrid composites prepared using industrial wastes. In Failure Analysis in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 229–244. [Google Scholar]
- Hasan, Z. Composite materials. In Tooling for Composite Aerospace Structures; Elsevier: Oxford, UK, 2020; pp. 21–48. [Google Scholar]
- Abhilash, M.; Thomas, D. Biopolymers for Biocomposites and Chemical Sensor Applications. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 405–435. [Google Scholar]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Díez-Pascual, A. Nanoparticle Reinforced Polymers. Polymers 2019, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, G.Y.; Baranov, D.A.; Dotsenko, I.P.; Gubin, S.P. New magnetic materials based on cobalt and iron-containing nanoparicles. Compos. Part B Eng. 2006, 37, 413–417. [Google Scholar] [CrossRef]
- Kapranos, P.; Brabazon, D.; Midson, S.P.; Naher, S.; Haga, T. Advanced Casting Methodologies. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–37. [Google Scholar]
- Keçili, R.; Büyüktiryaki, S.; Dolak, İ.; Hussain, C.M. The use of magnetic nanoparticles in sample preparation devices and tools. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–95. [Google Scholar]
- Jeong, J.-R.; Shin, S.-C.; Lee, S.-J.; Kim, J.-D. Magnetic properties of superparamagnetic γ-Fe2O3 nanoparticles prepared by coprecipitation technique. J. Magn. Magn. Mater. 2005, 286, 5–9. [Google Scholar] [CrossRef]
- Acar, H.Y.C.; Garaas, R.S.; Syud, F.; Bonitatebus, P.; Kulkarni, A.M. Superparamagnetic nanoparticles stabilized by polymerized PEGylated coatings. J. Magn. Magn. Mater. 2005, 293, 1–7. [Google Scholar] [CrossRef]
- Mikhaylova, M.; Kim, D.K.; Bobrysheva, N.P.; Osmolowsky, M.; Semenov, V.; Tsakalakos, T.; Muhammed, M. Superparamagnetism of Magnetite Nanoparticles: Dependence on Surface Modification. Langmuir 2004, 20, 2472–2477. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Arcentales-Vera, B.; Chichande-Proaño, E.; Bucio, E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers 2022, 14, 752. [Google Scholar] [CrossRef]
- Sommertune, J.; Sugunan, A.; Ahniyaz, A.; Bejhed, R.S.; Sarwe, A.; Johansson, C.; Balceris, C.; Ludwig, F.; Posth, O.; Fornara, A. Polymer/iron oxide nanoparticle composites—A straight forward and scalable synthesis approach. Int. J. Mol. Sci. 2015, 16, 19752–19768. [Google Scholar] [CrossRef]
- Ansari, S.A.M.K.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials 2019, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Maicas, M.; Sanz, M.; Cui, H.; Aroca, C.; Sánchez, P. Magnetic properties and morphology of Ni nanoparticles synthesized in gas phase. J. Magn. Magn. Mater. 2010, 322, 3485–3489. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, Y.; Wang, Y.; Yuan, G.; Liu, J.-M. A review of flexible perovskite oxide ferroelectric films and their application. J. Mater. 2020, 6, 1–16. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, K.; Wang, J. Perovskites for photovoltaics: A combined review of organic–inorganic halide perovskites and ferroelectric oxide perovskites. J. Mater. Chem. A 2015, 3, 18809–18828. [Google Scholar] [CrossRef]
- Tian, M.; Xu, L.; Yang, Y. Perovskite Oxide Ferroelectric Thin Films. Adv. Electron. Mater. 2022, 2101409. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Tran, V.V.; Moon, J.-Y.; Park, D.; Lee, Y.-C. Titanium Dioxide Microscale and Macroscale Structures: A Mini-Review. Nanomaterials 2020, 10, 1190. [Google Scholar] [CrossRef]
- Kryszewski, M.; Jeszka, J.K. Nanostructured conducting polymer composites—Superparamagnetic particles in conducting polymers. Synth. Met. 1998, 94, 99–104. [Google Scholar] [CrossRef]
- Koo, J.H. Polymer Nanocomposites: Processing, Characterization, and Applications; McGraw-Hill Education: New York, NY, USA, 2006; ISBN 9780071458214. [Google Scholar]
- Petkov, P.; Tsiulyanu, D.; Popov, C.; Kulisch, W. Advanced Nanotechnologies for Detection and Defence against CBRN Agents; Springer: New York, NY, USA, 2018; ISBN 978-94-024-1297-0. [Google Scholar]
- Mazrouaa, A.M.; Mohamed, M.G.; Fekry, M. Physical and magnetic properties of iron oxide nanoparticles with a different molar ratio of ferrous and ferric. Egypt. J. Pet. 2019, 28, 165–171. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Yang, G. Synthesis and magnetic properties of Mn-Zn ferrite nanoparticles. J. Magn. Magn. Mater. 2007, 312, 464–469. [Google Scholar] [CrossRef]
- Ichiyanagi, Y.; Kubota, M.; Moritake, S.; Kanazawa, Y.; Yamada, T.; Uehashi, T. Magnetic properties of Mg-ferrite nanoparticles. J. Magn. Magn. Mater. 2007, 310, 2378–2380. [Google Scholar] [CrossRef]
- Brommer, P.E.; Duc, N.H. Magnetoelasticity in Nanoscale Heterogeneous Materials; Elsevier: Oxford, UK, 2004; pp. 1–6. ISBN 978-0-08-043152-9. [Google Scholar]
- Fiebig, M.; Lottermoser, T.; Kneip, M.K.; Bayer, M. Correlations between magnetic and electrical orderings in multiferroic manganites. J. Appl. Phys. 2006, 99, 08E302. [Google Scholar] [CrossRef]
- Roy, S.B. Materials in a High Magnetic Field. In Materials Under Extreme Conditions; Elsevier: Amsterdam, The Netherlands, 2017; pp. 755–789. ISBN 978-0-12-801300-7. [Google Scholar]
- Joshi, R.S.; Kumar, P.S.A. Magnetic Solid-State Materials. In Comprehensive Inorganic Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; pp. 271–316. ISBN 978-0-08-096529-1. [Google Scholar]
- Pirzada, B.M.; Sabir, S. Polymer-Based Nanocomposites for Significantly Enhanced Dielectric Properties and Energy Storage Capability; Elsevier Ltd.: Duxford, UK, 2018; ISBN 9780081019115. [Google Scholar]
- Mishra, R.; Militky, J. Nanocomposites. In Nanotechnology in Textiles; Elsevier: Duxford, UK, 2019; pp. 263–310. [Google Scholar]
- Parhi, R. Nanocomposite for transdermal drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Duxford, UK, 2018; pp. 353–389. [Google Scholar]
- Nathani, H.; Gubbala, S.; Misra, R.D.K. Magnetic behavior of nickel ferrite-polyethylene nanocomposites synthesized by mechanical milling process. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004, 111, 95–100. [Google Scholar] [CrossRef]
- Passaglia, E.; Bertoldo, M.; Coiai, S.; Augier, S.; Savi, S.; Ciardelli, F. Nanostructured polyolefins/clay composites: Role of the molecular interaction at the interface. Polym. Adv. Technol. 2008, 19, 560–568. [Google Scholar] [CrossRef]
- Jiles, D.C.; Lo, C.C.H. The role of new materials in the development of magnetic sensors and actuators. Sens. Actuators A Phys. 2003, 106, 3–7. [Google Scholar] [CrossRef]
- Varga, Z.; Filipcsei, G.; Zrínyi, M. Magnetic field sensitive functional elastomers with tuneable elastic modulus. Polymer 2006, 47, 227–233. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Sánchez-Marcos, J.; Herrasti, P. Magnetic Nanoparticles-Based Conducting Polymer Nanocomposites; Springer: Dehradun, India, 2017; ISBN 978-3-319-46456-5. [Google Scholar]
- Fulco, A.P.P.; Melo, J.D.D.; Paskocimas, C.A.; de Medeiros, S.N.; de Araujo Machado, F.L.; Rodrigues, A.R. Magnetic properties of polymer matrix composites with embedded ferrite particles. NDT E Int. 2016, 77, 42–48. [Google Scholar] [CrossRef]
- Bellucci, F.S.; Lobato de Almeida, F.C.; Lima Nobre, M.A.; Rodríguez-Pérez, M.A.; Paschoalini, A.T.; Job, A.E. Magnetic properties of vulcanized natural rubber nanocomposites as a function of the concentration, size and shape of the magnetic fillers. Compos. Part B Eng. 2016, 85, 196–206. [Google Scholar] [CrossRef]
- Makled, M.H.; Matsui, T.; Tsuda, H.; Mabuchi, H.; El-Mansy, M.K.; Morii, K. Magnetic and dynamic mechanical properties of barium ferrite–natural rubber composites. J. Mater. Process. Technol. 2005, 160, 229–233. [Google Scholar] [CrossRef]
- Junliang, L.; Liu, P.; Zhang, X.; Lu, P.; Jivio, Z.; Zhang, M. Fabrication of Magnetic Rubber Composites by Recycling Waste Rubber Powders via a Microwave-assisted In Situ Surface Modification and Semi-devulcanization Process. Chem. Eng. J. 2016, 295, 79. [Google Scholar] [CrossRef]
- Sunny, V.; Kurian, P.; Mohanan, P.; Joy, P.A.; Anantharaman, M.R. A flexible microwave absorber based on nickel ferrite nanocomposite. J. Alloys Compd. 2010, 489, 297–303. [Google Scholar] [CrossRef]
- Kong, I.; Ahmad, S.; Abdullah, M.; Hui, D.; Yusoff, A.; Puryanti, D. Magnetic and microwave absorbing properties of magnetite–thermoplastic natural rubber nanocomposites. J. Magn. Magn. Mater. 2010, 322, 3401–3409. [Google Scholar] [CrossRef]
- Ramajo, L.A.; Cristóbal, A.A.; Botta, P.M.; Porto López, J.M.; Reboredo, M.M.; Castro, M.S. Dielectric and magnetic response of Fe3O4/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 388–393. [Google Scholar] [CrossRef]
- Weidenfeller, B.; Höfer, M.; Schilling, F. Thermal and electrical properties of magnetite filled polymers. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1041–1053. [Google Scholar] [CrossRef]
- Garzón, A.O.; Landínez, D.A.; Roa-Rojas, J.; Fajardo-Tolosa, F.E.; Peña-Rodríguez, G.; Parra-Vargas, C.A. Production and structural, electrical and magnetic characterization of a composite material based on powdered magnetite and high density polyethylene. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2017, 41, 154. [Google Scholar] [CrossRef][Green Version]
- Palmero, E.M.; Bollero, A. 3D and 4D Printing of Functional and Smart Composite Materials. In Encyclopedia of Materials: Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 402–419. [Google Scholar]
- Wu, S.; Hu, W.; Ze, Q.; Sitti, M.; Zhao, R. Multifunctional magnetic soft composites: A review. Multifunct. Mater. 2020, 3, 042003. [Google Scholar] [CrossRef] [PubMed]
- Trochu, F.; Gauvin, R.; Gao, D.-M. Numerical analysis of the resin transfer molding process by the finite element method. Adv. Polym. Technol. 1993, 12, 329–342. [Google Scholar] [CrossRef]
- Mallick, P.K. Failure of polymer matrix composites (PMCs) in automotive and transportation applications. In Failure Mechanisms in Polymer Matrix Composites; Elsevier: Cambridge, UK, 2012; pp. 368–392. [Google Scholar]
- Marques, A.T. Fibrous materials reinforced composites production techniques. In Fibrous and Composite Materials for Civil Engineering Applications; Fangueiro, R., Ed.; Elsevier: Cambridge, UK, 2011; pp. 191–215. [Google Scholar]
- Chen, A.; Wang, Q.; Li, M.; Peng, Z.; Lai, J.; Zhang, J.; Xu, J.; Huang, H.; Lei, C. Combined Approach of Compression Molding and Magnetic Attraction to Micropatterning of Magnetic Polydimethylsiloxane Composite Surfaces with Excellent Anti-Icing/Deicing Performance. ACS Appl. Mater. Interfaces 2021, 13, 48153–48162. [Google Scholar] [CrossRef]
- Hui, B.H.; Salimi, M.N. Production of Iron Oxide Nanoparticles by Co-Precipitation method with Optimization Studies of Processing Temperature, pH and Stirring Rate. IOP Conf. Ser. Mater. Sci. Eng. 2020, 743, 012036. [Google Scholar] [CrossRef]
- Gautam, R.K.; Chattopadhyaya, M.C. Functionalized Magnetic Nanoparticles: Adsorbents and Applications. In Nanomaterials for Wastewater Remediation; Elsevier: Oxford, UK, 2016; pp. 139–159. [Google Scholar]
- Fatimah, I.; Fadillah, G.; Yudha, S.P. Synthesis of iron-based magnetic nanocomposites: A review. Arab. J. Chem. 2021, 14, 103301. [Google Scholar] [CrossRef]
- Mehra, N.; Jeske, M.; Yang, X.; Gu, J.; Kashfipour, M.A.; Li, Y.; Baughman, J.A.; Zhu, J. Hydrogen-Bond Driven Self-Assembly of Two-Dimensional Supramolecular Melamine-Cyanuric Acid Crystals and Its Self-Alignment in Polymer Composites for Enhanced Thermal Conduction. ACS Appl. Polym. Mater. 2019, 1, 1291–1300. [Google Scholar] [CrossRef]
- Adnan, M.; Dalod, A.; Balci, M.; Glaum, J.; Einarsrud, M.-A. In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites. Polymers 2018, 10, 1129. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials; Elsevier: Duxford, UK, 2018; pp. 121–139. [Google Scholar]
- Harito, C.; Bavykin, D.V.; Yuliarto, B.; Dipojono, H.K.; Walsh, F.C. Polymer nanocomposites having a high filler content: Synthesis, structures, properties, and applications. Nanoscale 2019, 11, 4653–4682. [Google Scholar] [CrossRef]
- Yadav, R.; Tirumali, M.; Wang, X.; Naebe, M.; Kandasubramanian, B. Polymer composite for antistatic application in aerospace. Def. Technol. 2020, 16, 107–118. [Google Scholar] [CrossRef]
- Pu, S.; Xue, S.; Yang, Z.; Hou, Y.; Zhu, R.; Chu, W. In situ co-precipitation preparation of a superparamagnetic graphene oxide/Fe3O4 nanocomposite as an adsorbent for wastewater purification: Synthesis, characterization, kinetics, and isotherm studies. Environ. Sci. Pollut. Res. 2018, 25, 17310–17320. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Xue, Y.; Wang, J.; Li, X.; Wu, X.; Qin, Y.; Chen, W. Sequential in-situ route to synthesize novel composite hydrogels with excellent mechanical, conductive, and magnetic responsive properties. Mater. Des. 2020, 193, 108759. [Google Scholar] [CrossRef]
- Hermán, V.; Takacs, H.; Duclairoir, F.; Renault, O.; Tortai, J.H.; Viala, B. Core double–shell cobalt/graphene/polystyrene magnetic nanocomposites synthesized by in situ sonochemical polymerization. RSC Adv. 2015, 5, 51371–51381. [Google Scholar] [CrossRef]
- Shabzendedar, S.; Modarresi-Alam, A.R.; Noroozifar, M.; Kerman, K. Core-shell nanocomposite of superparamagnetic Fe3O4 nanoparticles with poly(m-aminobenzenesulfonic acid) for polymer solar cells. Org. Electron. 2020, 77, 105462. [Google Scholar] [CrossRef]
- Tahir, M.B.; Rafique, M.; Rafique, M.S.; Nawaz, T.; Rizwan, M.; Tanveer, M. Photocatalytic nanomaterials for degradation of organic pollutants and heavy metals. In Nanotechnology and Photocatalysis for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–138. [Google Scholar]
- Yamaguchi, A.; Hirohata, A.; Stadler, B.J.H. Synthesis and processing. In Nanomagnetic Materials: Fabrication, Characterization and Application; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–118. [Google Scholar]
- Pottathara, Y.B.; Grohens, Y.; Kokol, V.; Kalarikkal, N.; Thomas, S. Synthesis and Processing of Emerging Two-Dimensional Nanomaterials. In Nanomaterials Synthesis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–25. [Google Scholar]
- Madhuri, K.V. Thermal protection coatings of metal oxide powders. In Metal Oxide Powder Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 209–231. [Google Scholar]
- Augello, C.; Liu, H. Surface modification of magnesium by functional polymer coatings for neural applications. In Surface Modification of Magnesium and its Alloys for Biomedical Applications; Elsevier: Cambridge, UK, 2015; pp. 335–353. [Google Scholar]
- Mammeri, F. Nanostructured flexible PVDF and fluoropolymer-based hybrid films. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–101. [Google Scholar]
- Yilbas, B.S.; Al-Sharafi, A.; Ali, H. Surfaces for Self-Cleaning. In Self-Cleaning of Surfaces and Water Droplet Mobility; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–98. [Google Scholar]
- Sasikumar, M.; Subiramaniyam, N.P. Microstructure, electrical and humidity sensing properties of TiO2/polyaniline nanocomposite films prepared by sol–gel spin coating technique. J. Mater. Sci. Mater. Electron. 2018, 29, 7099–7106. [Google Scholar] [CrossRef]
- Chou, W.-Y.; Fang, P.-H.; Chiang, W.-C.; Cheng, H.-L. Room temperature ferromagnetism in Fe3O4 nanoparticle-embedded polymer semiconductors. J. Phys. Chem. Solids 2022, 167, 110750. [Google Scholar] [CrossRef]
- Sharif, A.; Mustaqeem, M.; Saleh, T.A.; ur Rehman, A.; Ahmad, M.; Warsi, M.F. Synthesis, structural and dielectric properties of Mg/Zn ferrites -PVA nanocomposites. Mater. Sci. Eng. B 2022, 280, 115689. [Google Scholar] [CrossRef]
- Bogue, R. Nanocomposites: A review of technology and applications. Assem. Autom. 2011, 31, 106–112. [Google Scholar] [CrossRef]
- Talaat, A.; Suraj, M.V.; Byerly, K.; Wang, A.; Wang, Y.; Lee, J.K.; Ohodnicki, P.R. Review on soft magnetic metal and inorganic oxide nanocomposites for power applications. J. Alloys Compd. 2021, 870, 159500. [Google Scholar] [CrossRef]
- Peng, S.; Yu, Y.; Wu, S.; Wang, C.-H. Conductive Polymer Nanocomposites for Stretchable Electronics: Material Selection, Design, and Applications. ACS Appl. Mater. Interfaces 2021, 13, 43831–43854. [Google Scholar] [CrossRef]
- Hossain, S.K.S.; Hoque, M.E. Polymer nanocomposite materials in energy storage: Properties and applications. In Polymer-Based Nanocomposites for Energy and Environmental Applications; Elsevier: Duxford, UK, 2018; pp. 239–282. [Google Scholar]
- Arunachalam, P. Polymer-based nanocomposites for energy and environmental applications. In Polymer-Based Nanocomposites for Energy and Environmental Applications; Elsevier: Duxford, UK, 2018; pp. 185–203. [Google Scholar]
- Alatai, S.; Salem, M.; Ishak, D.; Das, H.S.; Alhuyi Nazari, M.; Bughneda, A.; Kamarol, M. A Review on State-of-the-Art Power Converters: Bidirectional, Resonant, Multilevel Converters and Their Derivatives. Appl. Sci. 2021, 11, 10172. [Google Scholar] [CrossRef]
- Magu, T.; Agobi, A.; Hitler, L.; Dass, P. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. Capacitance enhancement of polyaniline coated curved-graphene supercapacitors in a redox-active electrolyte. Nanoscale 2013, 5, 4134. [Google Scholar] [CrossRef]
- Prasankumar, T.; Wiston, B.R.; Gautam, C.R.; Ilangovan, R.; Jose, S.P. Synthesis and enhanced electrochemical performance of PANI/Fe3O4 nanocomposite as supercapacitor electrode. J. Alloys Compd. 2018, 757, 466–475. [Google Scholar] [CrossRef]
- Sadeghinia, M.; Shayeh, J.S.; Fatemi, F.; Rahmandoust, M.; Ehsani, A.; Rezaei, M. Electrochemical study of perlite-barium ferrite/conductive polymer nano composite for super capacitor applications. Int. J. Hydrogen Energy 2019, 44, 28088–28095. [Google Scholar] [CrossRef]
- Asen, P.; Shahrokhian, S.; Zad, A.I. Ternary nanostructures of Cr2O3/graphene oxide/conducting polymers for supercapacitor application. J. Electroanal. Chem. 2018, 823, 505–516. [Google Scholar] [CrossRef]
- Sun, L.; Shi, Y.; He, Z.; Li, B.; Liu, J. Synthesis and characterization of SnO2/polyaniline nanocomposites by sol–gel technique and microemulsion polymerization. Synth. Met. 2012, 162, 2183–2187. [Google Scholar] [CrossRef]
- Prasanna, B.P.; Avadhani, D.N.; Raj, V.; Kumar, K.Y.; Raghu, M.S. Fabrication of PANI/SnO2 Hybrid Nanocomposites via Interfacial Polymerization for High Performance Supercapacitors Applications. Surf. Eng. Appl. Electrochem. 2019, 55, 463–471. [Google Scholar] [CrossRef]
- Ghebache, Z.; Hamidouche, F.; Safidine, Z.; Trari, M.; Bellal, B. Synthesis and Electrical Conducting Properties of Poly(aniline) Doped With Zeolite HY Nanocomposites Containing SnO2 for High-Performance Supercapacitor Electrode. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1548–1558. [Google Scholar] [CrossRef]
- Kandasamy, M.; Seetharaman, A.; Babu, I.M.; William, J.J.; Muralidharan, G.; Sivasubramanian, D.; Jothivenkatachalam, K.; Imran, M.; Chakraborty, B. Experimental and theoretical investigations of a multiwalled carbon nanotubes/SnO2/polyaniline ternary nanohybrid electrode for energy storage. Surf. Interfaces 2022, 30, 101978. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef]
- Jin, L.; Wang, T.; Feng, Z.-Q.; Leach, M.K.; Wu, J.; Mo, S.; Jiang, Q. A facile approach for the fabrication of core–shell PEDOT nanofiber mats with superior mechanical properties and biocompatibility. J. Mater. Chem. B 2013, 1, 1818. [Google Scholar] [CrossRef]
- Ates, M.; Bayrak, Y.; Ozkan, H.; Yoruk, O.; Yildirim, M.; Kuzgun, O. Synthesis of rGO/TiO2/PEDOT nanocomposites, supercapacitor device performances and equivalent electrical circuit models. J. Polym. Res. 2019, 26, 49. [Google Scholar] [CrossRef]
- Gupta, M.; Tyagi, S.; Kumari, N. Electrochemical evaluation of highly stable Mn ferrite/PEDOT/rGO ternary nanocomposite for supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2022, 33, 7838–7852. [Google Scholar] [CrossRef]
- Megha, R.; Ali, F.A.; Ravikiran, Y.T.; Ramana, C.H.V.V.; Kiran Kumar, A.B.V.; Mishra, D.K.; Vijayakumari, S.C.; Kim, D. Conducting polymer nanocomposite based temperature sensors: A review. Inorg. Chem. Commun. 2018, 98, 11–28. [Google Scholar] [CrossRef]
- Chow, W.S.; Mohd Ishak, Z.A. Smart polymer nanocomposites: A review. Express Polym. Lett. 2020, 14, 416–435. [Google Scholar] [CrossRef]
- Muhammed Shameem, M.; Sasikanth, S.M.; Annamalai, R.; Ganapathi Raman, R. A brief review on polymer nanocomposites and its applications. Mater. Today Proc. 2021, 45, 2536–2539. [Google Scholar] [CrossRef]
- Péres, L.O.; Li, R.W.C.; Yamauchi, E.Y.; Lippi, R.; Gruber, J. Conductive polymer gas sensor for quantitative detection of methanol in Brazilian sugar-cane spirit. Food Chem. 2012, 130, 1105–1107. [Google Scholar] [CrossRef]
- Sáaedi, A.; Shabani, P.; Yousefi, R. High performance of methanol gas sensing of ZnO/PAni nanocomposites synthesized under different magnetic field. J. Alloys Compd. 2019, 802, 335–344. [Google Scholar] [CrossRef]
- Wang, X.; Gong, L.; Zhang, D.; Fan, X.; Jin, Y.; Guo, L. Room temperature ammonia gas sensor based on polyaniline/copper ferrite binary nanocomposites. Sens. Actuators B Chem. 2020, 322, 128615. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Thermally stable and highly sensitive ethene gas sensor based on polythiophene/zirconium oxide nanocomposites. Mater. Today Commun. 2019, 20, 100574. [Google Scholar] [CrossRef]
- Miao, J.; Liu, A.; Wu, L.; Yu, M.; Wei, W.; Liu, S. Magnetic ferroferric oxide and polydopamine molecularly imprinted polymer nanocomposites based electrochemical impedance sensor for the selective separation and sensitive determination of dichlorodiphenyltrichloroethane (DDT). Anal. Chim. Acta 2020, 1095, 82–92. [Google Scholar] [CrossRef]
- Sohrabi, N.; Mohammadi, R.; Ghassemzadeh, H.R.; Heris, S.S.S. Design and synthesis of a new magnetic molecularly imprinted polymer nanocomposite for specific adsorption and separation of diazinon insecticides from aqueous media. Microchem. J. 2022, 175, 107087. [Google Scholar] [CrossRef]
- Song, J.; Lee, H.; Jeong, E.G.; Choi, K.C.; Yoo, S. Organic Light-Emitting Diodes: Pushing Toward the Limits and Beyond. Adv. Mater. 2020, 32, 1907539. [Google Scholar] [CrossRef]
- Kandulna, R.; Das, U.; Rimpi, M.; Kachhap, B.; Prasad, N. Hybrid Polymeric Nanocomposites Based High Performance Oleds: A Review. Shodh Sankalp J. 2021, 1, 16–34. [Google Scholar] [CrossRef]
- Alarifi, I.; Prasad, B.; Uddin, M.K. Conducting Polymer Membranes and Their Applications. In Self-Standing Substrates; Springer: Cham, Switzerland, 2020; pp. 147–176. [Google Scholar]
- Skoda, D.; Urbanek, P.; Sevcik, J.; Munster, L.; Nadazdy, V.; Cullen, D.A.; Bazant, P.; Antos, J.; Kuritka, I. Colloidal cobalt-doped ZnO nanoparticles by microwave-assisted synthesis and their utilization in thin composite layers with MEH-PPV as an electroluminescent material for polymer light emitting diodes. Org. Electron. 2018, 59, 337–348. [Google Scholar] [CrossRef]
- Jamatia, T.; Skoda, D.; Urbanek, P.; Sevcik, J.; Maslik, J.; Munster, L.; Kalina, L.; Kuritka, I. Microwave-assisted synthesis of FexZn1−xO nanoparticles for use in MEH-PPV nanocomposites and their application in polymer light-emitting diodes. J. Mater. Sci. Mater. Electron. 2019, 30, 11269–11281. [Google Scholar] [CrossRef]
- Kumar, S.; Baruah, S.; Puzari, A. Poly(p-phenylenediamine)-based nanocomposites with metal oxide nanoparticle for optoelectronic and magneto-optic application. Polym. Bull. 2020, 77, 441–457. [Google Scholar] [CrossRef]
- Hadavand, M.; Jafari, M.R.; Pakpour, F.; Ghanbari, D. CdS/ZnS nanocomposite: Synthesis and utilization in organic light-emitting diodes for a lower turn-on voltage. J. Nanopart. Res. 2021, 23, 61. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, X.; Liu, J.; Chen, S.; Xiang, W. Environmentally Friendly and Ultrastable Narrow-Band Emitter: CsPbX 3 @SEBS Flexible Film for Light-Emitting Diode Displays. ACS Sustain. Chem. Eng. 2021, 9, 10291–10298. [Google Scholar] [CrossRef]
- Xue, L.; Liu, Y.; Li, F.; Sun, K.; Chen, W.; Yang, K.; Hu, H.; Lin, J.; Chen, H.; Yang, Z.; et al. Highly flexible light emitting diodes based on a quantum dots-polymer composite emitting layer. Vacuum 2019, 163, 282–286. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, M.; Li, J.; Yang, S.; Tao, X.; Yu, Y.; Jiang, Y. Independent dispersed and highly water-oxygen environment stable FAPbBr3 QDs-polymer composite for down-conversion display films. Chem. Eng. J. 2022, 428, 130974. [Google Scholar] [CrossRef]
- Wang, G.; Melkonyan, F.S.; Facchetti, A.; Marks, T.J. All-Polymer Solar Cells: Recent Progress, Challenges, and Prospects. Angew. Chem. Int. Ed. 2019, 58, 4129–4142. [Google Scholar] [CrossRef]
- Tessema Mola, G.; Mbuyise, X.G.; Oseni, S.O.; Dlamini, W.M.; Tonui, P.; Arbab, E.A.A.; Kaviyarasu, K.; Maaza, M. Nanocomposite for Solar Energy Application. Nano Hybrids Compos. 2018, 20, 90–107. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The Applications of Polymers in Solar Cells: A Review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef]
- Meng, L.; Watson, B.W.; Qin, Y. Hybrid conjugated polymer/magnetic nanoparticle composite nanofibers through cooperative non-covalent interactions. Nanoscale Adv. 2020, 2, 2462–2470. [Google Scholar] [CrossRef]
| Application | Polymer Matrix | Magnetic Filler | Properties | References |
|---|---|---|---|---|
| Supercapacitors | PANI | Fe3O4 | High specific capacitance Cycling stability Excellent capacitance retention | [130] |
| BaFe12O19 | Specific capacitance (225–330 F/g) | [131] | ||
| Cr2O3-graphene oxide | A high specific capacitance value of 525 F/g | [132] | ||
| SnO2 | A specific capacitance of 337 F/g | [134] | ||
| HY zeolite/SnO2 | A maximum capacitance of 1085 F/g | [135] | ||
| CN/SiO2 | A specific capacitance value of 221 F/g | [136] | ||
| PPy | Cr2O3-graphene oxide | A high specific capacitance value of 495 F/g | [132] | |
| PEDOT | rGO/TiO2 | Improved specific capacitance depending on the ratio content | [139] | |
| rGO/MnFe2O4 | A good specific capacitance of 298.97 F/g | [140] | ||
| Sensors | PANI | ZnO | A magnetic flux of 0.5 T Resistance of 865 k Response time of 18.2 s Recovery time of 5.1 s | [145] |
| CuFe2O4 | Fast response time | [146] | ||
| PTh | ZrO2 | High thermal stability Highest electrical conductivity | [147] | |
| Polydopamine | Fe3O4 | Enhanced limit of detection of DDT | [148] | |
| PDA | Clay/Fe3O4 | Improved magnetic saturation Highest detection of diazinon Enhanced adsorption capacity | [149] | |
| LED | MEH-PPV | CoxZn1−xO | Band gap narrowing Luminescence quenching | [153] |
| FexZn1−xO | Enhanced electroluminescence | [154] | ||
| Poly(p-phenylenediamine | ZnO, Fe3O4, or TiO2 | Improved soft magnetic properties Semiconducting material | [155] | |
| PEDOT: PSS | CDs/ZnS | Decreased turn-on voltage | [156] | |
| PSEBS | CsPbX3 (X = Cl/Br, Br, Br/I) | Flexible films Wide color gamut | [157] | |
| PMMA | FAPbBr3 QDs | Enhanced Lumen Efficiency Improved Color Rendering Index | [159] | |
| Solar cells | π-conjugated polymer donor | Nanoscale coating | Improved stability and efficiency | [160,162] |
| P3HT | Fe3O4 | Improved magnetic responsiveness Controlled and stable morphologies | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Fierro, D.; Bustamante-Torres, M.; Bravo-Plascencia, F.; Magaña, H.; Bucio, E. Polymer-Magnetic Semiconductor Nanocomposites for Industrial Electronic Applications. Polymers 2022, 14, 2467. https://doi.org/10.3390/polym14122467
Romero-Fierro D, Bustamante-Torres M, Bravo-Plascencia F, Magaña H, Bucio E. Polymer-Magnetic Semiconductor Nanocomposites for Industrial Electronic Applications. Polymers. 2022; 14(12):2467. https://doi.org/10.3390/polym14122467
Chicago/Turabian StyleRomero-Fierro, David, Moises Bustamante-Torres, Francisco Bravo-Plascencia, Héctor Magaña, and Emilio Bucio. 2022. "Polymer-Magnetic Semiconductor Nanocomposites for Industrial Electronic Applications" Polymers 14, no. 12: 2467. https://doi.org/10.3390/polym14122467
APA StyleRomero-Fierro, D., Bustamante-Torres, M., Bravo-Plascencia, F., Magaña, H., & Bucio, E. (2022). Polymer-Magnetic Semiconductor Nanocomposites for Industrial Electronic Applications. Polymers, 14(12), 2467. https://doi.org/10.3390/polym14122467








