One-Pot Synthesis of UPy-Functionalized Nanocellulose under Mechanochemical Synergy for High-Performance Epoxy Nanocomposites
Abstract
:1. Introduction
2. Experimental Model
2.1. Materials
2.2. Preparation of NCC-UPy
2.3. Construction of Epoxy Nanocomposite Membrane
2.4. Performance Characterization
3. Results and Discussion
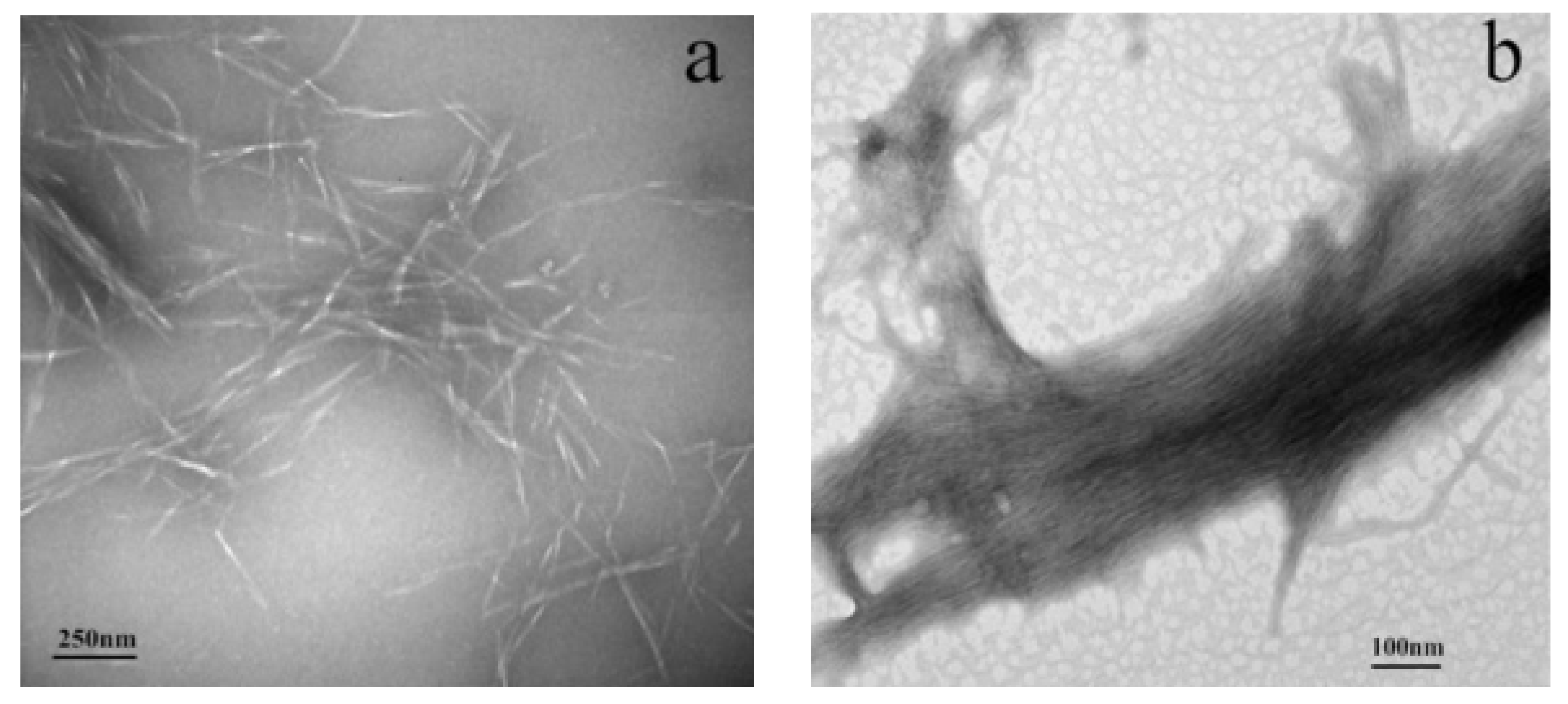
3.1. Morphology and Dispersion Stability
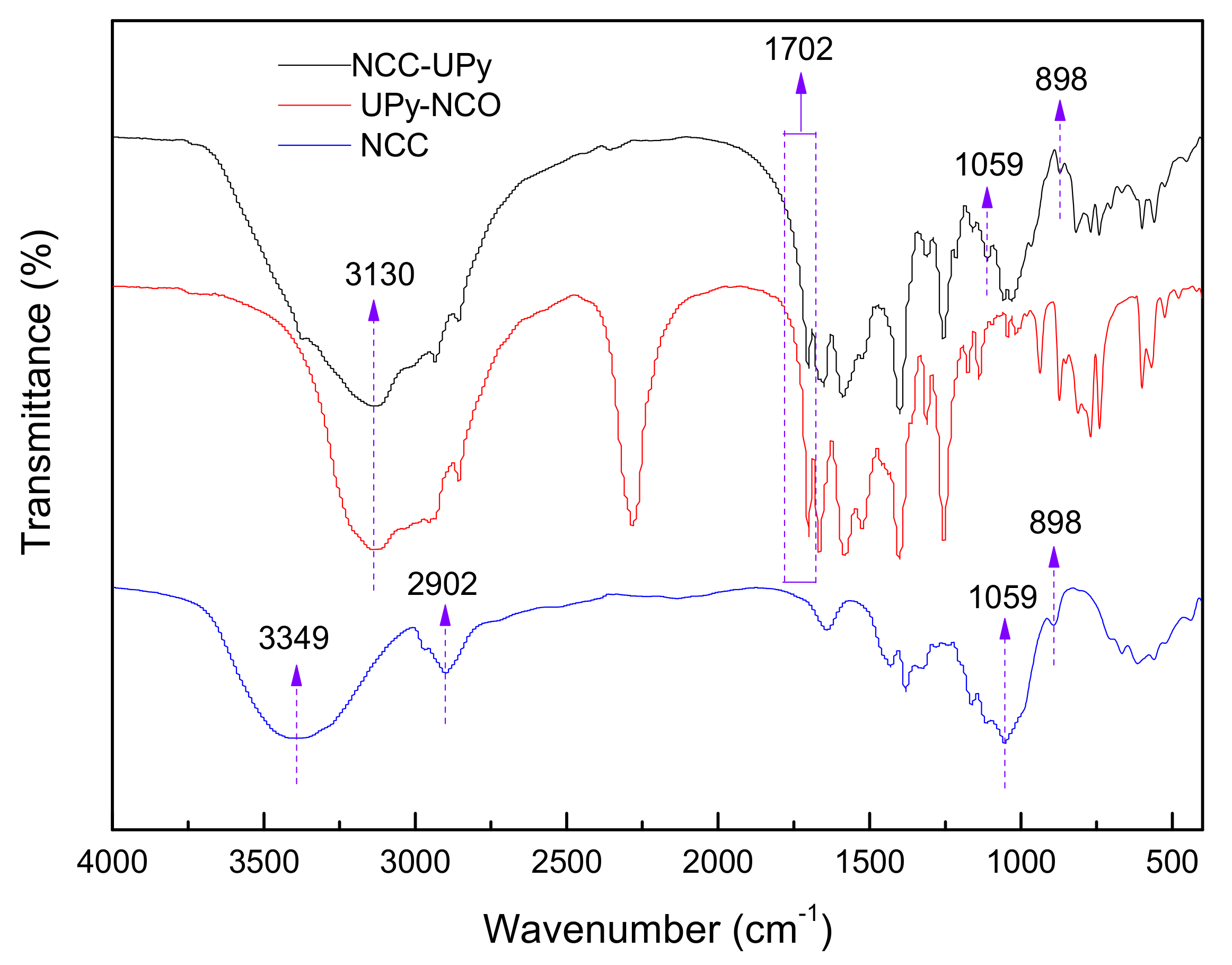
3.2. FTIR Analysis
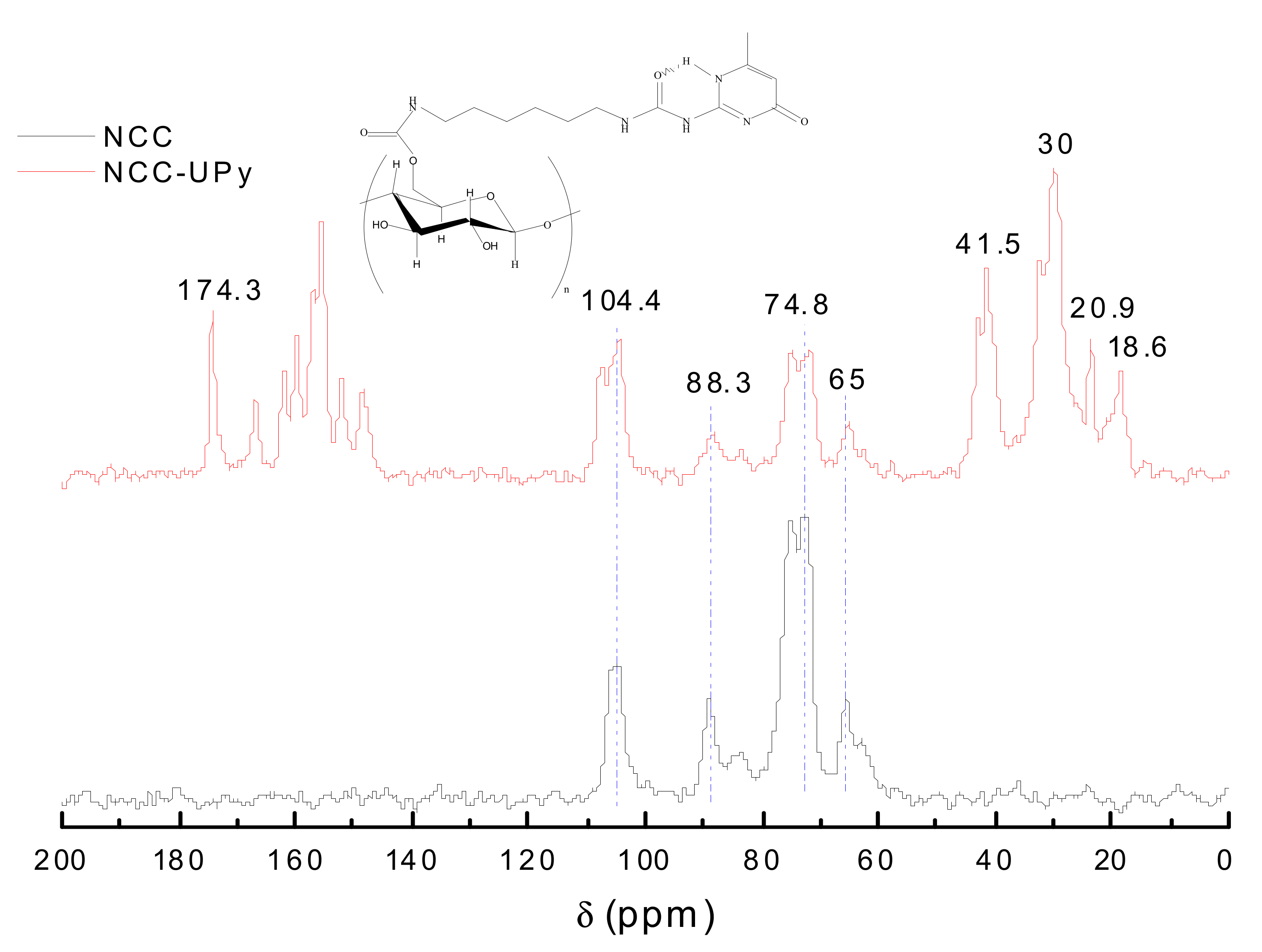
3.3. Solid-State 13C NMR Analysis
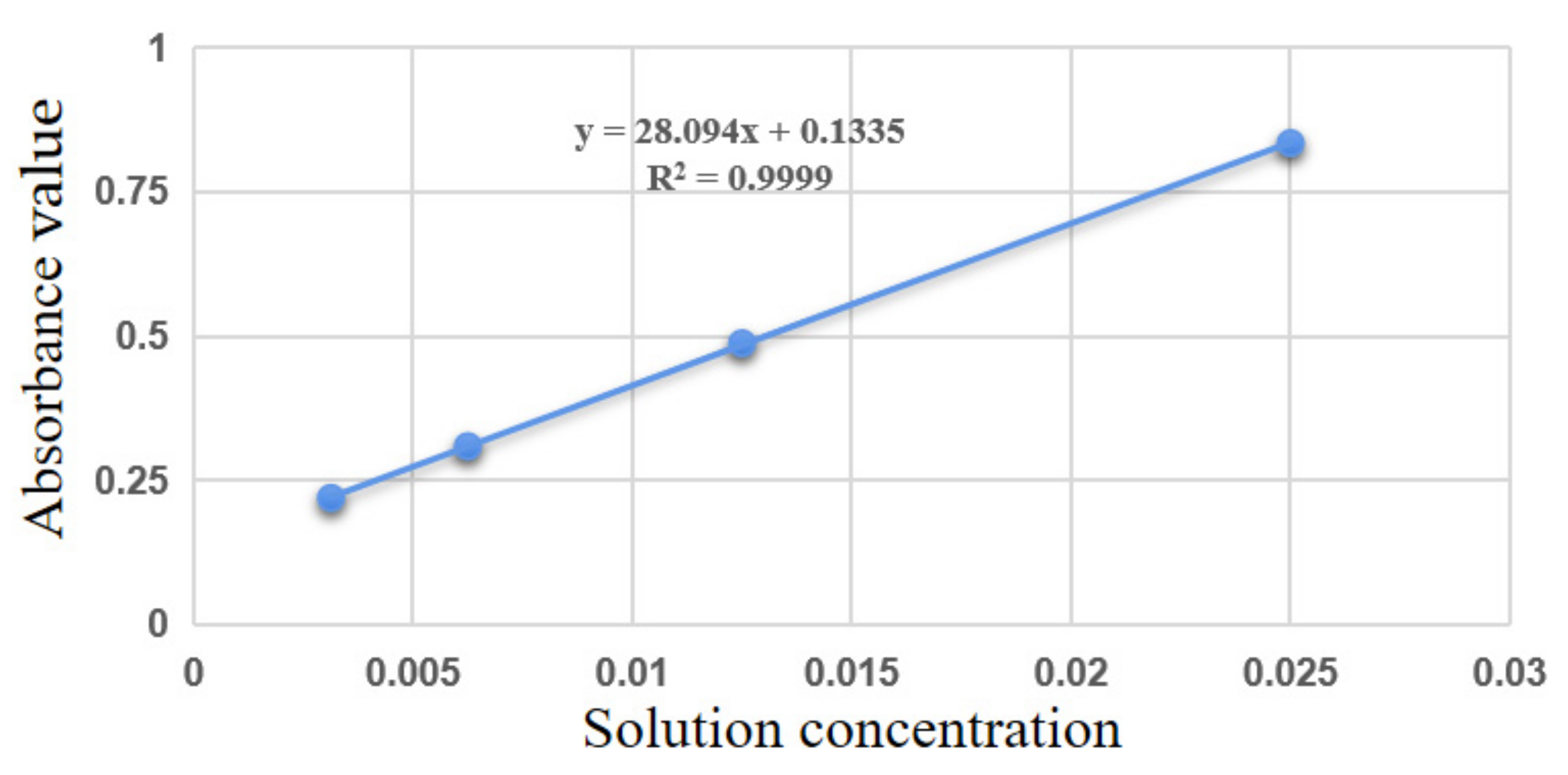
3.4. Degree of Substitution Measurement
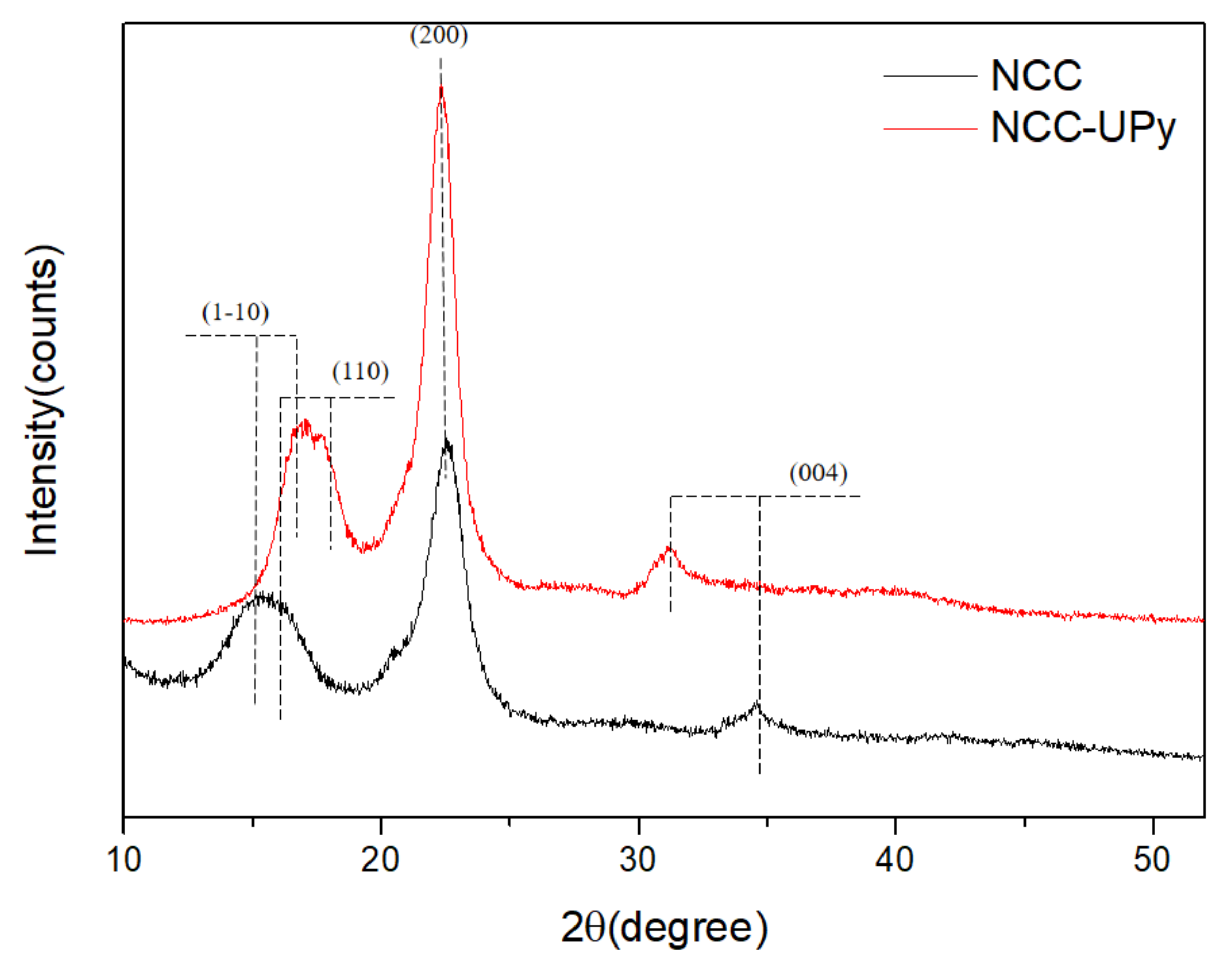
3.5. Crystal Structure
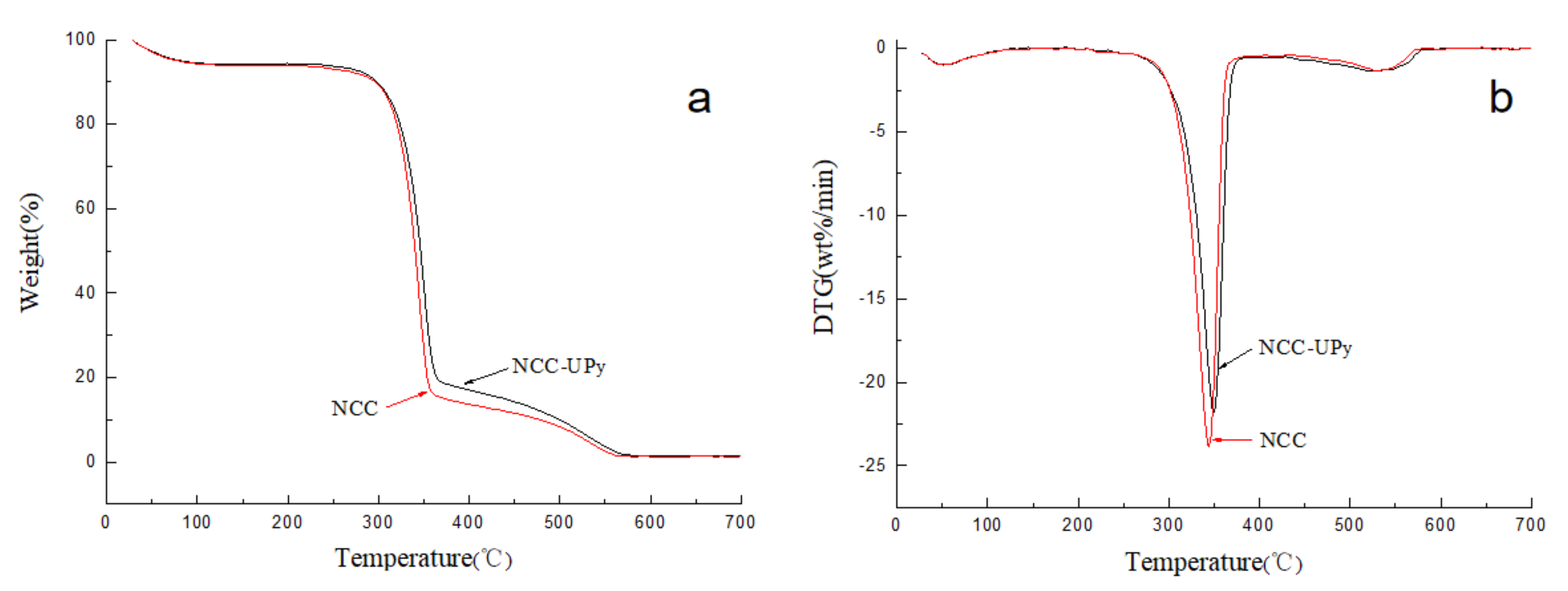
3.6. Thermostability of NCC-UPy
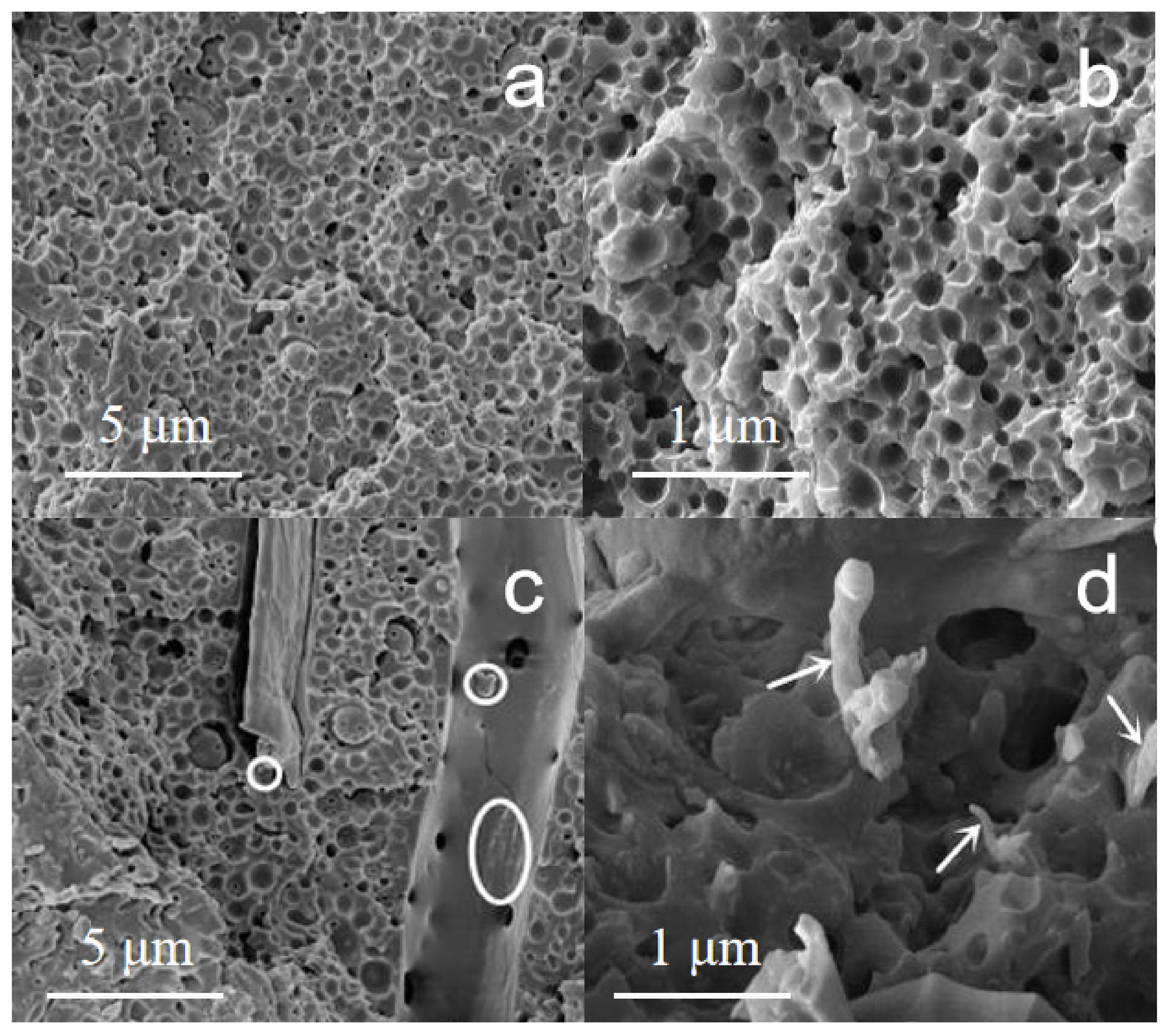
3.7. Morphology of Epoxy Nanocomposites
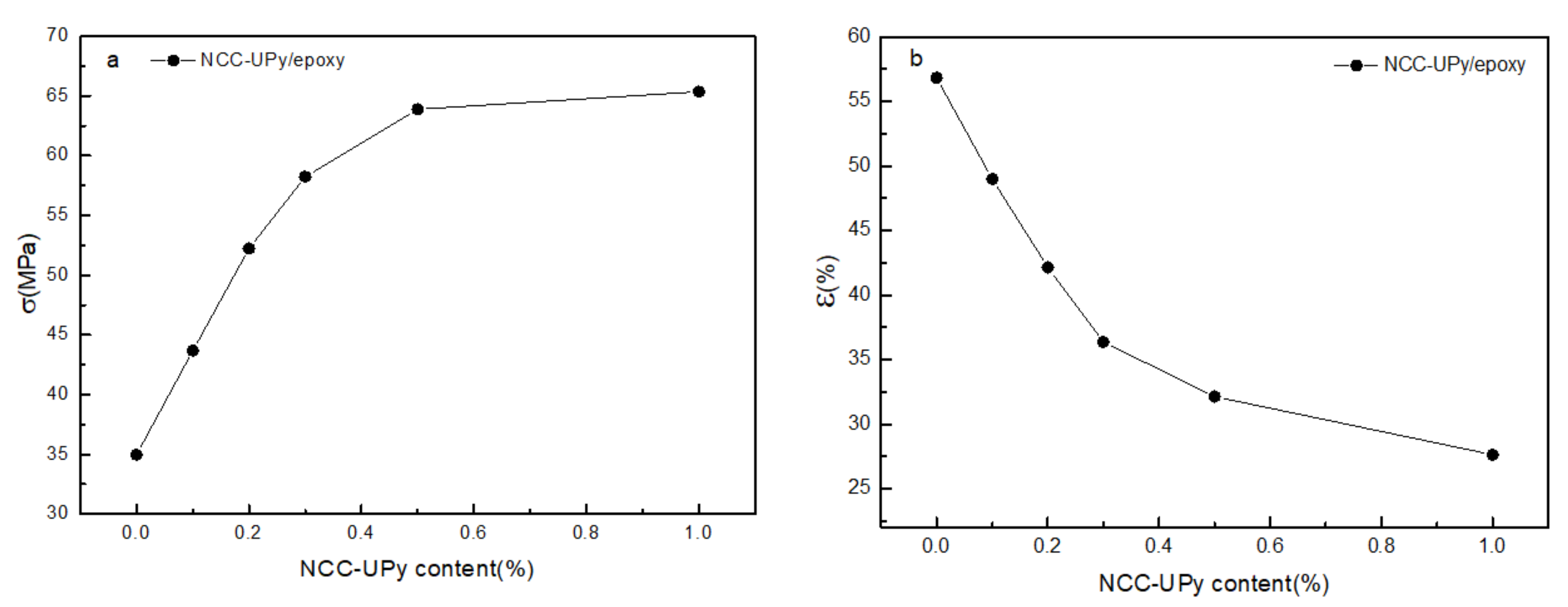
3.8. Mechanical Properties of Epoxy Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Ikkala, O. Nanocellulose: Recent fundamental advances and emerging biological and biomimicking applications. Adv. Mater. 2021, 33, 2004349. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Ruiz, M.M.; Cavaillé, J.Y.; Dufresne, A.; Graillat, C.; Gérard, J.F. New waterborne epoxy coatings based on cellulose nanofillers. In Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2001; Volume 169, pp. 211–222. [Google Scholar]
- Lu, J.; Askeland, P.; Drzal, L.T. Surface modification of microfibrillated cellulose for epoxy composite applications. Polymer 2008, 49, 1285–1296. [Google Scholar] [CrossRef]
- Maleki, S.E.; Shokrollahi, P.; Barzin, J. Impact of supramolecular interactions on swelling and release behavior of UPy functionalized HEMA-based hydrogels. Polym. Adv. Technol. 2018, 29, 1670–1683. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Y.; Luo, Y.; Wang, Y. Poly (dl-Lactic Acid)-Based Linear Polyurethanes Capable of Forming Physical Crosslinking via UPy Quadruple Hydrogen Bonding Assembly. Macromol. Mater. Eng. 2020, 305, 2000042. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, Z.; Lin, F.; Tang, L.; Wang, S.; Huang, B. Extraction of cellulose nanocrystals with a high yield of 88% by simultaneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef]
- Monemian, S.; Korley, L.S.T.J. Exploring the role of supramolecular associations in mechanical toughening of interpenetrating polymer networks. Macromolecules 2015, 48, 7146–7155. [Google Scholar] [CrossRef]
- Anthamatten, M. Hydrogen bonding in supramolecular polymer networks: Glasses, melts, and elastomers. Supramol. Polym. Netw. Gels 2015, 47–99. [Google Scholar]
- Abd Hamid, S.B.; Zain, S.K.; Das, R.; Centi, G. Synergic effect of tungstophosphoric acid and sonication for rapid synthesis of crystalline nanocellulose. Carbohydr. Polym. 2016, 138, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Effect of Arabic gum, xanthan gum and orange oil contents on ζ-potential, conductivity, stability, size index and pH of orange beverage emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 47–56. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Zawawy, W.K. Extraction of cellulose nanofibers from cotton linter and their composites. In Handbook of Polymer Nanocomposites: Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2015; pp. 145–164. [Google Scholar]
- Rambabu, N.; Panthapulakkal, S.; Sain, M.; Dalai, A.K. Production of nanocellulose fibers from pinecone biomass: Evaluation and optimization of chemical and mechanical treatment conditions on mechanical properties of nanocellulose films. Ind. Crops Prod. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Aydin, M.; Uyar, T.; Tasdelen, M.A.; Yagci, Y. Polymer/clay nanocomposites through multiple hydrogen-bonding interactions. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 650–658. [Google Scholar] [CrossRef]
- Bobade, S.L.; Malmgren, T.; Baskaran, D. Micellar-cluster association of ureidopyrimidone functionalized monochelic polybutadiene. Polym. Chem. 2014, 5, 910–920. [Google Scholar] [CrossRef]
- Biyani, M.V.; Foster, E.J.; Weder, C. Light-healable supramolecular nanocomposites based on modified cellulose nanocrystals. ACS Macro Lett. 2013, 2, 236–240. [Google Scholar] [CrossRef]
- Delgado, P.A.; Hillmyer, M.A. Combining block copolymers and hydrogen bonding for poly (lactide) toughening. Rsc Adv. 2014, 4, 13266–13273. [Google Scholar] [CrossRef]
- Kono, H.; Yunoki, S.; Shikano, T.; Fujiwara, M.; Erata, T.; Takai, M. CP/MAS 13C NMR study of cellulose and cellulose derivatives. 1. Complete assignment of the CP/MAS 13C NMR spectrum of the native cellulose. J. Am. Chem. Soc. 2002, 124, 7506–7511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; Deng, Q.; Wu, X. Synthesis and characterization of cellulose derivatives prepared in NaOH/urea aqueous solutions. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5911–5920. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Shebani, A.N.; Van Reenen, A.J.; Meincken, M. The effect of wood extractives on the thermal stability of different wood species. Thermochim. Acta 2008, 471, 43–50. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, S.; Xiong, M.; Lin, F.; Tang, L.; Huang, B.; Chen, Y. One-pot construction of cellulose-gelatin supramolecular hydrogels with high strength and pH-responsive properties. Carbohydr. Polym. 2018, 196, 225–232. [Google Scholar] [CrossRef]
- Abraham, E.; Kam, D.; Nevo, Y.; Slattegard, R.; Rivkin, A.; Lapidot, S.; Shoseyov, O. Highly modified cellulose nanocrystals and formation of epoxy-nanocrystalline cellulose (CNC) nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 28086–28095. [Google Scholar] [CrossRef]
- Rahmanian, S.; Suraya, A.R.; Shazed, M.A.; Zahari, R.; Zainudin, E.S. Mechanical characterization of epoxy composite with multiscale reinforcements: Carbon nanotubes and short carbon fibers. Mater. Des. 2014, 60, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Khelifa, F.; Habibi, Y.; Bonnaud, L.; Dubois, P. Epoxy monomers cured by high cellulosic nanocrystal loading. ACS Appl. Mater. Interfaces 2016, 8, 10535–10544. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Y.; Feng, J. Polydopamine as an efficient and robust platform to functionalize carbon fiber for high-performance polymer composites. ACS Appl. Mater. Interfaces 2014, 6, 349–356. [Google Scholar] [CrossRef]
- Qin, X.; Ge, W.; Mei, H.; Li, L.; Zheng, S. Toughness improvement of epoxy thermosets with cellulose nanocrystals. Polym. Int. 2021, 70, 1640–1648. [Google Scholar] [CrossRef]
- Ansari, F.; Galland, S.; Johansson, M.; Plummer, C.J.; Berglund, L.A. Cellulose nanofiber network for moisture stable, strong and ductile biocomposites and increased epoxy curing rate. Compos. Part A Appl. Sci. Manuf. 2014, 63, 35–44. [Google Scholar] [CrossRef] [Green Version]




| Sample | C% | H% | N% | O% |
|---|---|---|---|---|
| NCC | 41.29 | 6.88 | 0 | 51.83 |
| NCC-UPy | 47.89 | 7.59 | 13.96 | 30.56 |
| Sample | Onset Temperature (°C) | Maximum Temperature (°C) | Char Residue (wt.%) |
|---|---|---|---|
| NCC-UPy | 326.6 | 350.2 | 1.52 |
| NCC | 322.4 | 343.6 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, J.; Huang, B.; Lu, Q.-L. One-Pot Synthesis of UPy-Functionalized Nanocellulose under Mechanochemical Synergy for High-Performance Epoxy Nanocomposites. Polymers 2022, 14, 2428. https://doi.org/10.3390/polym14122428
Wang H, Wu J, Huang B, Lu Q-L. One-Pot Synthesis of UPy-Functionalized Nanocellulose under Mechanochemical Synergy for High-Performance Epoxy Nanocomposites. Polymers. 2022; 14(12):2428. https://doi.org/10.3390/polym14122428
Chicago/Turabian StyleWang, Hanchen, Jiayin Wu, Biao Huang, and Qi-Lin Lu. 2022. "One-Pot Synthesis of UPy-Functionalized Nanocellulose under Mechanochemical Synergy for High-Performance Epoxy Nanocomposites" Polymers 14, no. 12: 2428. https://doi.org/10.3390/polym14122428
APA StyleWang, H., Wu, J., Huang, B., & Lu, Q.-L. (2022). One-Pot Synthesis of UPy-Functionalized Nanocellulose under Mechanochemical Synergy for High-Performance Epoxy Nanocomposites. Polymers, 14(12), 2428. https://doi.org/10.3390/polym14122428








