Cell Immobilization Using Alginate-Based Beads as a Protective Technique against Stressful Conditions of Hydrolysates for 2G Ethanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Inoculum
2.3. Preparation of Chitosan Gel
2.4. Yeast Encapsulation in Different Alginate-Based Beads
2.5. Acetic Acid Tolerance Experiments
2.6. Repeated Batch Experiments
2.7. Analytical Methods
2.7.1. Substrate and Product Quantification
2.7.2. Cell Concentration and Viability
2.8. Calculations
2.9. Scanning Electron Microscopy (SEM) of Alginate-Based Beads
3. Results
3.1. Acetic Acid Tolerance of T18 Yeast Encapsulated in Different Alginate-Based Beads
3.2. Repeated Batch Experiments in Crude Sugarcane Bagasse Hemicellulose Hydrolysate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vries, L.; Guevara-Rozo, S.; Cho, M.; Liu, L.Y.; Renneckar, S.; Mansfield, S.D. Tailoring renewable materials via plant biotechnology. Biotechnol. Biofuels 2021, 14, 167. [Google Scholar] [CrossRef]
- Aguiar, A.; Milessi, T.S.; Mulinari, D.R.; Lopes, M.S.; Costa, S.M.; Candido, R.G. Sugarcane straw as a potential second generation feedstock for biorefinery and white biotechnology applications. Biomass Bioenerg. 2021, 144, 105896. [Google Scholar] [CrossRef]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [CrossRef] [Green Version]
- Robak, K.; Balcerek, M. Review of Second Generation Bioethanol Production from Residual Biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Perez, C.L.; Pereira, L.P.R.; Milessi, T.S.; Sandri, J.P.; Demeke, M.; Foulquié-Moreno, M.R.; Thevelein, J.M.; Zangirolami, T.C. Towards a practical industrial 2G ethanol production process based on immobilized recombinant S. cerevisiae: Medium and strain selection for robust integrated fixed-bed reactor operation. Renew. Energy 2022, 185, 363–375. [Google Scholar] [CrossRef]
- Narisetty, V.; Cox, R.; Bommareddy, R.; Agrawal, D.; Ahmad, E.; Pant, K.K.; Chandel, A.K.; Bhatia, S.K.; Kumar, D.; Binod, P.; et al. Valorisation of xylose to renewable fuels and chemicals, an essential step in augmenting the commercial viability of lignocellulosic biorefineries. Sustain. Energy Fuels 2022, 6, 29. [Google Scholar] [CrossRef]
- Chandel, A.K.; Forte, M.B.S.; Gonçalves, I.S.; Milessi, T.S.; Arruda, T.S.; Carvalho, W.; Mussatto, S.I. Brazilian biorefineries from second generation biomass: Critical insights from industry and future perspectives. Biofuels Bioprod. Bioref. 2021, 15, 1190–1208. [Google Scholar] [CrossRef]
- Bonan, C.I.D.G.; Biazi, L.E.; Dionísio, S.R.; Soares, L.B.; Tramontina, R.; Sousa, A.S.; de Oliveira Filho, C.A.; Costa, A.C.; Ienczak, J.L. Redox potential as a key parameter for monitoring and optimization of xylose fermentation with yeast Spathaspora passalidarum under limited-oxygen conditions. Bioprocess Biosyst. Eng. 2020, 43, 1509–1519. [Google Scholar] [CrossRef]
- Singla, A.; Paroda, S.; Dhamija, S.S.; Goyal, S.; Shekhawat, K.; Amachi, S.; Inubushi, K. Bioethanol Production from Xylose: Problems and Possibilities. J. Biofuels 2012, 3, 39–49. [Google Scholar] [CrossRef]
- Belissimi, E.; Dijken, J.P.; Pronk, J.T.; Maris, A.J.A. Effects of acetic acid on the kinetics of xylose fermentation by an engineered, xylose-isomerase-based Saccharomyces cerevisiae strain. FEMS Yeast Res. 2009, 9, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Demeke, M.M.; Dietz, H.; Li, Y.; Foulquié-Moreno, M.R.; Mutturi, S.; Deprez, S.; Abt, T.D.; Bonini, B.M.; Liden, G.; Dumortier, F.; et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulosic hydrolysates using metabolic and evolutionary engineering. Biotechnol. Biofuels 2013, 6, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanmarcke, G.; Demeke, M.M.; Foulquié-Moreno, M.R.; Thevelein, J.M. Identification of the major fermentation inhibitors of recombinant 2G yeasts in diverse lignocellulose hydrolysates. Biotechnol. Biofuels 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour. Technol. 2020, 299, 122633. [Google Scholar] [CrossRef] [PubMed]
- Betancur, G.J.V.; Pereira, N., Jr. Sugar cane bagasse as feedstock for second generation ethanol production. Part I: Diluted acid pretreatment optimization. Eletron. J. Biotechnol. 2010, 13, 3. [Google Scholar]
- Milessi, T.S.; Perez, C.L.; Zangirolami, T.C.; Corradini, F.A.S.; Sandri, J.P.; Foulquié-Moreno, M.R.; Giordano, R.C.; Thevelein, J.M.; Giordano, R.L.C. Repeated batches as a strategy for high 2G ethanol production from undetoxified hemicellulose hydrolysate using immobilized cells of recombinant Saccharomyces cerevisiae in a fixed-bed reactor. Biotechnol. Biofuels 2020, 13, 85. [Google Scholar] [CrossRef]
- Milessi, T.S.; Zangirolami, T.C.; Perez, C.L.; Sandri, J.P.; Corradini, F.A.S.; Foulquié-Moreno, M.R.; Thevelein, J.M.; Giordano, R.C.; Giordano, R.L.C. Bioethanol Production from Xylose-Rich Hydrolysate by Immobilized Recombinant Saccharomyces cerevisiae in Fixed-Bed Reactor. Ind. Biotechnol. 2020, 16, 75–80. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Ramos, M.D.; Milessi, T.S.; Candido, R.G.; Mendes, A.A.; Aguiar, A. Enzymatic catalysis as a tool in biofuels production in Brazil: Current status and perspectives. Energy Sustain. Dev. 2022, 68, 103–119. [Google Scholar] [CrossRef]
- Guo, X.; Deng, G.; Xu, J.; Wang, M. Immobilization of Rhodococcus sp. AJ270 in alginate capsules and its application in enantioselective biotransformation of trans-2-methyl-3-phenyl-oxiranecarbonitrile and amide. Enzyme Microb. Technol. 2006, 39, 1–5. [Google Scholar] [CrossRef]
- Guidoni, I.; Chlapanidas, T.; Bucco, M.; Crovato, F.; Marazzi, M.; Vigo, D.; Torre, M.L.; Faustini, M. Alginate cell encapsulation: New advances in reproduction and cartilage regenerative medicine. Cytotechnology 2008, 58, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Covizzi, L.G.; Giese, E.C.; Gomes, E.; Dekker, R.F.H.; Silva, R. Imobilização de células microbianas e suas aplicações biotecnológicas. Semin. Ciênc. Exatas Tecnol. 2007, 28, 143–160. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, T.B.; Silva, M.G.C.; Vieira, M.G.A. Equilibrium, Thermodynamic, Reuse, and Selectivity Studies for the Bioadsorption of Lanthanum onto Sericin/Alginate/Poly(vinyl alcohol) Particles. Polymers 2021, 13, 623. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biomol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Surtirman, Z.A.; Sanagi, M.M.; Aini, W.I.W. Alginate-based adsorbents for removal of metal ions and radionuclides from aqueous solutions: A review. Int. J. Biol. Macromol. 2021, 174, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérome, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Polyelectrolye Complex Formation Between Alginate and Chitosan as a Function of pH. J. Appl. Polym. Sci. 2003, 88, 346–351. [Google Scholar] [CrossRef]
- Silva, C.R.; Zangirolami, T.C.; Rodrigues, J.P.; Matugi, K.; Giordano, R.C.; Giordano, R.L.C. An innovative biocatalyst for production of ethanol from xylose in a continuous bioreactor. Enzyme Microb. Technol. 2012, 50, 35–42. [Google Scholar] [CrossRef]
- Trovati, J.; Giordano, R.C.; Giordano, R.L.C. Improving the Performance of a Continuous Process for the Production of Ethanol from Starch. Appl. Biochem. Biotechnol. 2009, 156, 506–520. [Google Scholar] [CrossRef]
- Bonfiglio, F.; Cagno, M.; Yamakawa, C.K.; Mussatto, S.I. Production of xylitol and carotenoids from switchgrass and Eucalyptus globulus hydrolysates obtained by intensified steam explosion pretreatment. Ind. Crops Prod. 2021, 170, 113800. [Google Scholar] [CrossRef]
- Mesquita, T.J.B.; Sandri, J.P.; Giordano, R.C.; Horta, A.C.L.; Zangirolami, T.C. A high-throughput approach for modeling and simulation of homofermentative microorganisms applied to ethanol fermentation by S. cerevisiae. Ind. Biotechnol. 2021, 17, 13–26. [Google Scholar] [CrossRef]
- Wang, F.Z.; Xie, T.; Hui, M. Increase of ethanol productivity by cell-recycle fermentation of flocculating yeast. Appl. Biochem. Microbiol. 2010, 47, 527–531. [Google Scholar] [CrossRef]
- Enari, T.M. EBC Analytica Microbiology, Method 2.2.2.3 Methylene blue staining. J. Inst. Brew. 1977, 83, 109–118. [Google Scholar]
- Shuler, M.L.; Kargi, F. Bioprocess Engineering: Basic Concepts, 2nd ed.; Prentice Hall Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Meijnen, J.; Randazzo, P.; Foulquié-Moreno, M.R.; van den Brink, J.; Vandecruys, P.; Stojiljkovic, M.; Dumortier, F.; Zalar, P.; Boekhout, T.; Gunde-Cimerman, N.; et al. Polygenic analysis and targeted improvement of the complex trait of high acetic acid tolerance in the yeast Saccharomyces cerevisiae. Biotechnol. Biofuels 2016, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/chitosan polyelectrolyte complexes: A comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 2017, 172, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Sohail, R.; Abbas, S.R. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45. [Google Scholar] [CrossRef]
- Kopplin, G.; Lervik, A.; Draget, K.I.; Aachmann, F.L. Alginate gels crosslinked with chitosan oligomers—A systematic investigation into alginate block structure and chitosan oligomer interaction. RSC Adv. 2021, 11, 13780. [Google Scholar] [CrossRef]
- Saheed, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Mi, F.L.; Sung, H.W.; Shyu, S.S. Drug release from chitosan-alginate complex beads reinforced by a naturally occurring cross-link agent. Carbohydr. Polym. 2002, 48, 61–72. [Google Scholar] [CrossRef]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Caetano, L.A.; Almeida, A.j.; Gonçalves, L.M.D. Effect of Experimental Parameters on Alginate/Chitosan Microparticles for BCG Encapsulation. Mar. Drugs 2016, 14, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Granobles, M.; Saeys, M. Dynamic pressure-swing chemical looping process for the recovery of CO from blast furnace gas. Energy Convers. Manag. 2022, 258, 115515. [Google Scholar] [CrossRef]

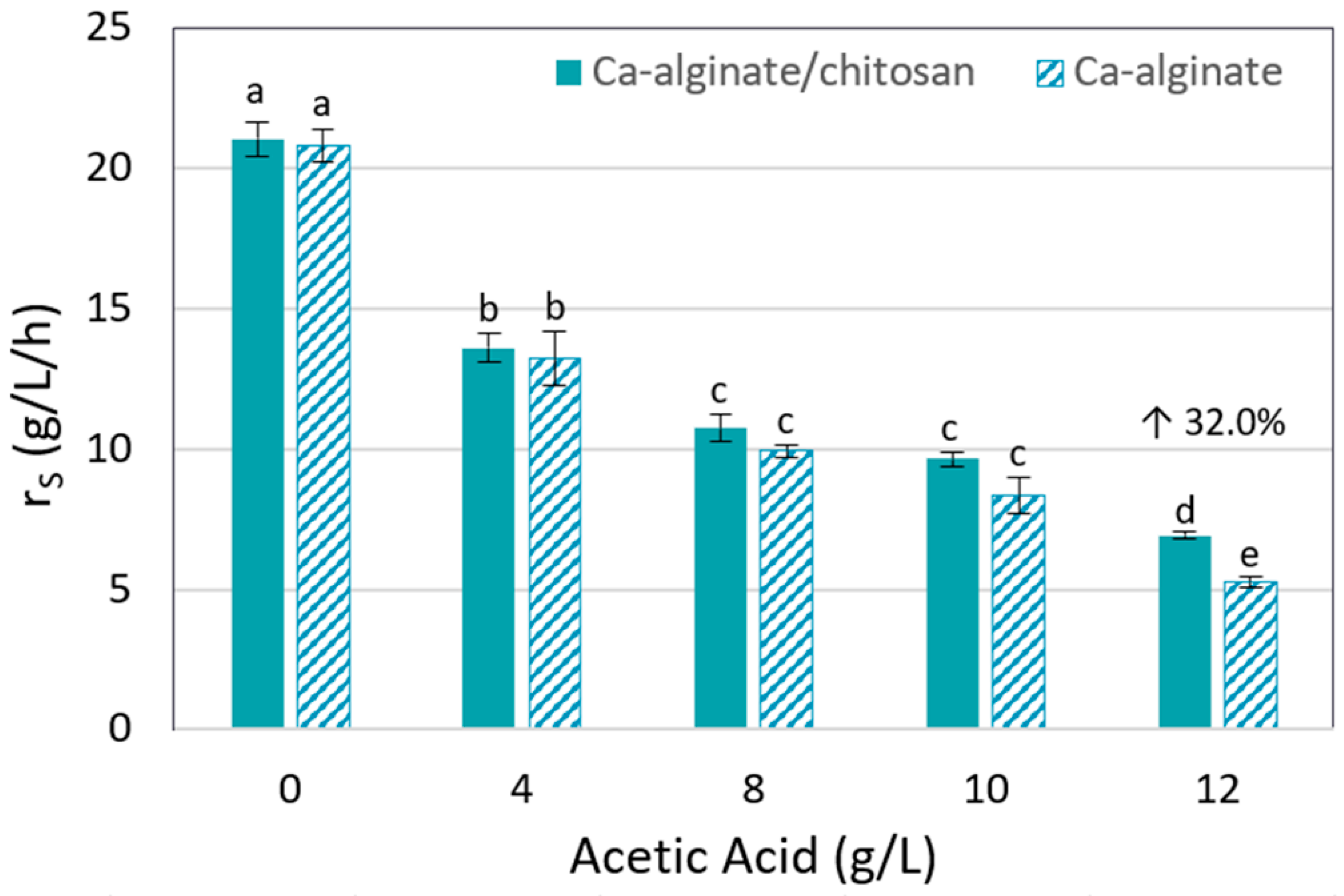
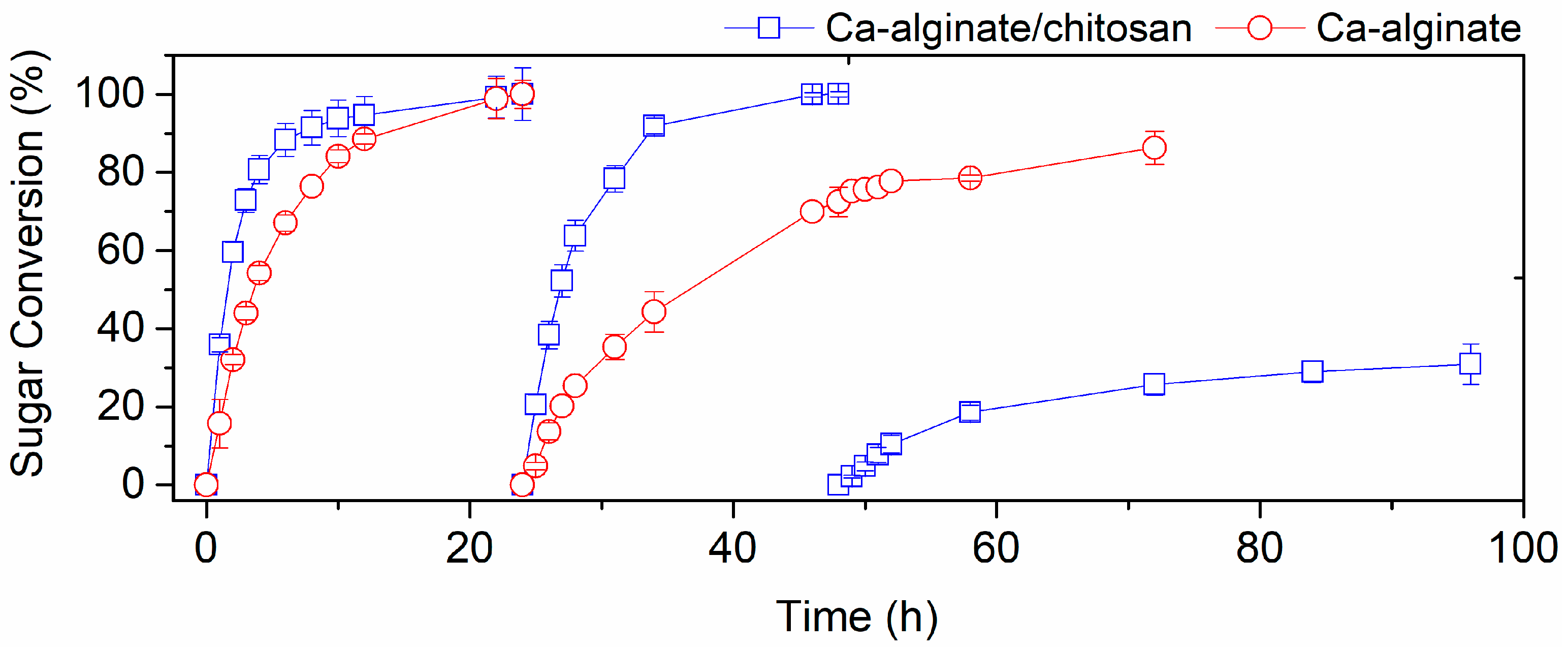

| Immobilization Gel | Acetic Acid (g/L) | Ethanol (g/L) | QP (g/L/h) | YP/S (g/g) | Residual Xylose (g/L) |
|---|---|---|---|---|---|
| Ca-alginate beads | 0.0 | 14.4 ± 0.0 a | 3.60 ± 0.01 c | 0.427 ± 0.066 g | 0.0 ± 0.0 h |
| 4.0 | 15.4 ± 0.0 a | 1.93 ± 0.00 d | 0.388 ± 0.000 g | 0.0 ± 0.0 h | |
| 8.0 | 15.6 ± 0.5 a | 1.30 ± 0.01 e | 0.391 ± 0.012 g | 0.0 ± 0.0 h | |
| 10.0 | 14.2 ± 0.6 a | 1.19 ± 0.01 e | 0.356 ± 0.016 g | 0.0 ± 0.0 h | |
| 12.0 | 11.8 ± 0.8 b | 0.99 ± 0.07 f | 0.353 ± 0.023 g | 6.4 ± 0.1 i | |
| Ca-alginate/chitosan beads | 0.0 | 14.5 ± 0.1 a | 3.61 ± 0.02 c | 0.424 ± 0.065 g | 0.0 ± 0.0 h |
| 4.0 | 14.9 ± 0.7 a | 1.80 ± 0.09 d | 0.397 ± 0.004 g | 0.0 ± 0.0 h | |
| 8.0 | 14.9 ± 0.1 a | 1.24 ± 0.01 e | 0.405 ± 0.012 g | 0.0 ± 0.0 h | |
| 10.0 | 14.3 ± 0.3 a | 1.19 ± 0.02 e | 0.388 ± 0.007 g | 0.0 ± 0.0 h | |
| 12.0 | 13.6 ± 0.8 a | 1.13 ± 0.06 ef | 0.387 ± 0.015 g | 0.0 ± 0.0 h |
| Immobilization Gel | Recycle | Ethanol (g/L) | QP (g/L/h) | YP/S (g/g) | rS (g/L/h) | Cell Viability (%) | Residual Sugars (g/L) |
|---|---|---|---|---|---|---|---|
| Ca-alginate beads | 1 | 24.8 ± 0.2 a | 1.03 ± 0.01 d | 0.436 ± 0.003 g | 8.2 ± 0.3 h | 76.8 ± 1.6 l | 0.0 ± 0.0 o |
| 2 | 21.8 ± 0.2 b | 0.45 ± 0.00 e | 0.444 ± 0.004 g | 4.2 ± 0.0 i | 0.0 ± 0.0 m | 7.8 ± 0.5 p | |
| Ca-alginate/chitosan beads | 1 | 25.0 ± 0.3 a | 1.04 ± 0.01 d | 0.439 ± 0.005 g | 17.0 ± 0.4 j | 95.6 ± 3.2 n | 0.0 ± 0.0 o |
| 2 | 25.3 ± 0.1 a | 1.06 ± 0.00 d | 0.445 ± 0.002 g | 9.3 ± 0.3 h | 87.8 ± 4.9 n | 0.0 ± 0.0 o | |
| 3 | 7.8 ± 0.7 c | 0.16 ± 0.01 f | 0.443 ± 0.038 g | 1.6 ± 0.1 k | 0.0 ± 0.0 m | 39.3 ± 1.5 q |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, R.C.; Zangirolami, T.C.; Giordano, R.L.C.; Demeke, M.M.; Thevelein, J.M.; Milessi, T.S. Cell Immobilization Using Alginate-Based Beads as a Protective Technique against Stressful Conditions of Hydrolysates for 2G Ethanol Production. Polymers 2022, 14, 2400. https://doi.org/10.3390/polym14122400
Soares RC, Zangirolami TC, Giordano RLC, Demeke MM, Thevelein JM, Milessi TS. Cell Immobilization Using Alginate-Based Beads as a Protective Technique against Stressful Conditions of Hydrolysates for 2G Ethanol Production. Polymers. 2022; 14(12):2400. https://doi.org/10.3390/polym14122400
Chicago/Turabian StyleSoares, Raiane C., Teresa C. Zangirolami, Raquel L. C. Giordano, Mekonnen M. Demeke, Johan M. Thevelein, and Thais S. Milessi. 2022. "Cell Immobilization Using Alginate-Based Beads as a Protective Technique against Stressful Conditions of Hydrolysates for 2G Ethanol Production" Polymers 14, no. 12: 2400. https://doi.org/10.3390/polym14122400
APA StyleSoares, R. C., Zangirolami, T. C., Giordano, R. L. C., Demeke, M. M., Thevelein, J. M., & Milessi, T. S. (2022). Cell Immobilization Using Alginate-Based Beads as a Protective Technique against Stressful Conditions of Hydrolysates for 2G Ethanol Production. Polymers, 14(12), 2400. https://doi.org/10.3390/polym14122400






