One-Step Lignin Refining Process: The Influence of the Solvent Nature on the Properties and Quality of Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
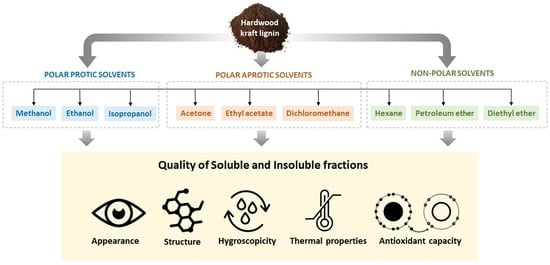
2.2. One-Step Fractionation of Hardwood Kraft Lignin
2.3. Physical Appearance of Lignin Fractions
2.4. Chemical Structure Characterization
2.5. Hygroscopic Properties
2.6. Total Phenolic Content (TPC) and DPPH Assay
2.7. Thermal Characterization
3. Results and Discussion
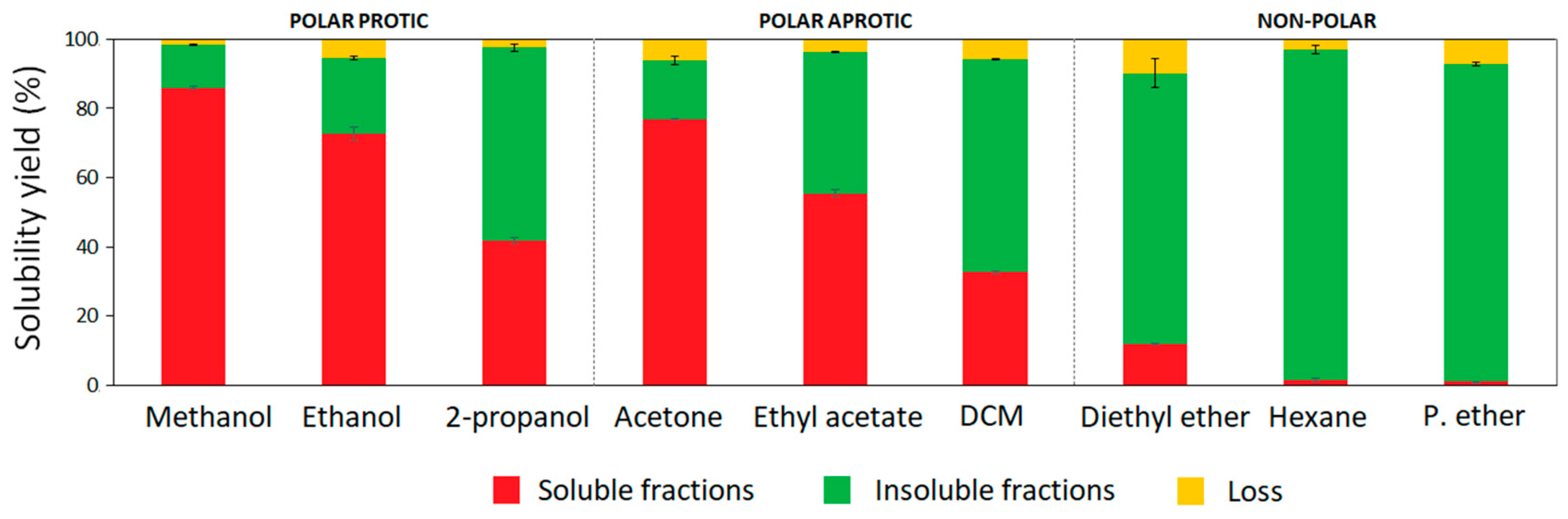
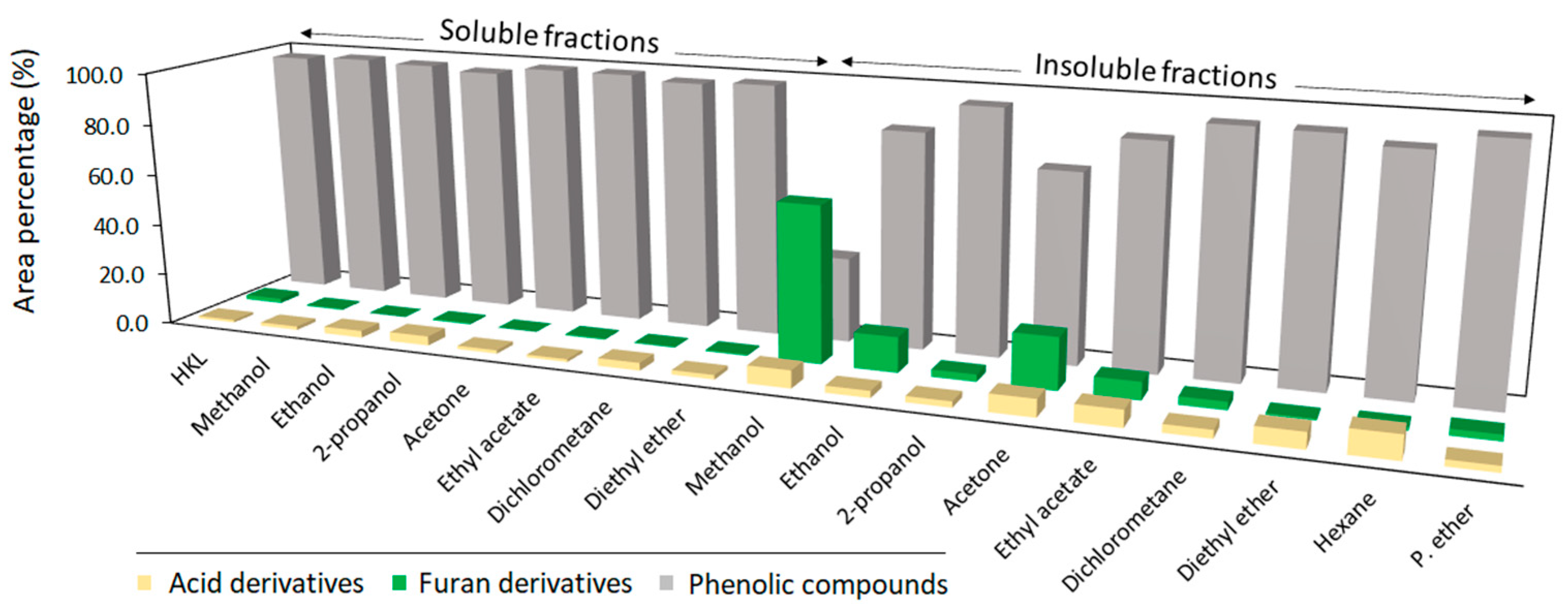
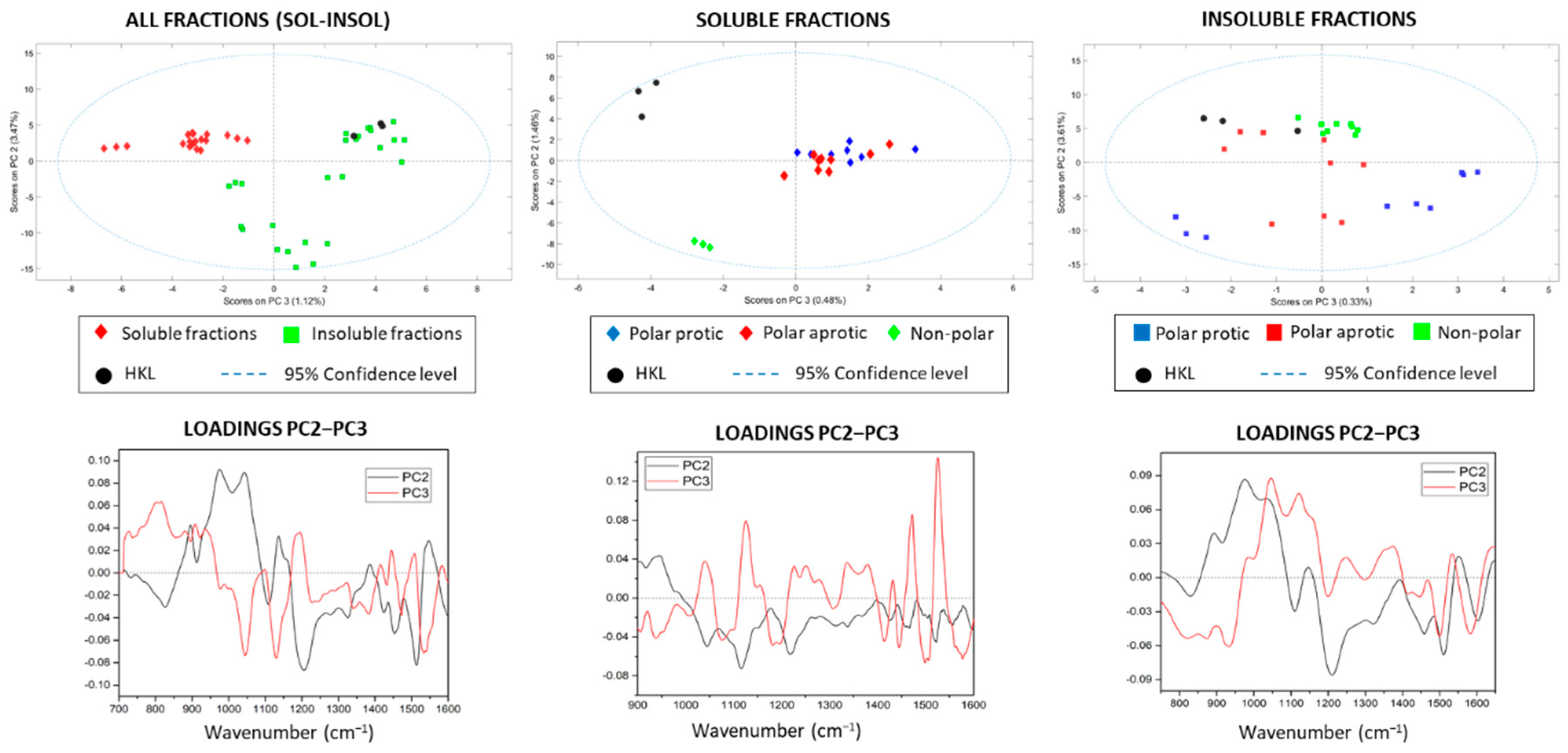
3.1. Solubility Yields, Appearance, and Molecular Features of Lignin Fractions
3.2. Hygroscopic Properties of Lignin Fractions
3.3. Total Phenolic Content and Antioxidant Capacity of Lignin Fractions
3.4. Thermal Properties of Lignin Fractions
4. Valorization Perspectives of Lignin Fractions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Haq, I.; Mazumder, P.; Kalamdhad, A. Recent advances in removal of lignin from paper industry wastewater and its industrial applications—A review. Bioresour. Technol. 2020, 312, 123636. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, S.; Zhang, S.; Ok, Y.S.; Matsagar, B.M.; Wu, K.C.-W.; Tsang, D.C. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery. Bioresour. Technol. 2019, 291, 121878. [Google Scholar] [CrossRef] [PubMed]
- Gil-Chávez, J.; Gurikov, P.; Hu, X.; Meyer, R.; Reynolds, W.; Smirnova, I. Application of novel and technical lignins in food and pharmaceutical industries: Structure-function relationship and current challenges. Biomass Convers. Biorefinery 2019, 11, 2387–2403. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in Industrial Applications of Technical Lignins. BioResources 2011. [Google Scholar] [CrossRef]
- Lourençon, T.V.; de Lima, G.G.; Ribeiro, C.S.; Hansel, F.A.; Maciel, G.M.; da Silva, K.; Winnischofer, S.M.B.; de Muniz, G.I.B.; Magalhães, W.L.E. Antioxidant, antibacterial and antitumoural activities of kraft lignin from hardwood fractionated by acid precipitation. Int. J. Biol. Macromol. 2021, 166, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Weinwurm, F.; Drljo, A.; Waldmüller, W.; Fiala, B.; Niedermayer, J.; Friedl, A. Lignin concentration and fractionation from ethanol organosolv liquors by ultra- and nanofiltration. J. Clean. Prod. 2016, 136, 62–71. [Google Scholar] [CrossRef]
- Allegretti, C.; Fontanay, S.; Rischka, K.; Strini, A.; Troquet, J.; Turri, S.; Griffini, G.; D’Arrigo, P. Two-Step Fractionation of a Model Technical Lignin by Combined Organic Solvent Extraction and Membrane Ultrafiltration. ACS Omega 2019, 4, 4615–4626. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kim, J.-Y.; Youn, H.J.; Choi, J.W. Fractionation of lignin macromolecules by sequential organic solvents systems and their characterization for further valuable applications. Int. J. Biol. Macromol. 2018, 106, 793–802. [Google Scholar] [CrossRef]
- Passoni, V.; Scarica, C.; Levi, M.; Turri, S.; Griffini, G. Fractionation of Industrial Softwood Kraft Lignin: Solvent Selection as a Tool for Tailored Material Properties. ACS Sustain. Chem. Eng. 2016, 4, 2232–2242. [Google Scholar] [CrossRef]
- Jiang, X.; Savithri, D.; Du, X.; Pawar, S.; Jameel, H.; Chang, H.-M.; Zhou, X. Fractionation and Characterization of Kraft Lignin by Sequential Precipitation with Various Organic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 835–842. [Google Scholar] [CrossRef]
- Duval, A.; Vilaplana, F.; Crestini, C.; Lawoko, M. Solvent screening for the fractionation of industrial kraft lignin. Holzforschung 2016, 70, 11–20. [Google Scholar] [CrossRef]
- Jääskeläinen, A.-S.; Liitiä, T.; Mikkelson, A.; Tamminen, T. Aqueous organic solvent fractionation as means to improve lignin homogeneity and purity. Ind. Crop. Prod. 2017, 103, 51–58. [Google Scholar] [CrossRef]
- Ajao, O.; Jeaidi, J.; Benali, M.; Abdelaziz, O.Y.; Hulteberg, C.P. Green solvents-based fractionation process for kraft lignin with controlled dispersity and molecular weight. Bioresour. Technol. 2019, 291, 121799. [Google Scholar] [CrossRef]
- Jiang, X.; de Assis, C.A.; Kollman, M.; Sun, R.; Jameel, H.; Chang, H.-M.; Gonzalez, R. Lignin fractionation from laboratory to commercialization: Chemistry, scalability and techno-economic analysis. Green Chem. 2020, 22, 7448–7459. [Google Scholar] [CrossRef]
- Wang, S.; Ru, B.; Lin, H.; Sun, W.; Luo, Z. Pyrolysis behaviors of four lignin polymers isolated from the same pine wood. Bioresour. Technol. 2015, 182, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.; Gordobil, O.; Robles, E.; González-Alriols, M.; Labidi, J. Lignin valorization from side-streams produced during agricultural waste pulping and total chlorine free bleaching. J. Clean. Prod. 2017, 142, 2609–2617. [Google Scholar] [CrossRef]
- Liapis, A.I.; Bruttini, R. Freeze Drying. Handb. Ind. Dry. 2020, 309–343. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Vebber, G.C.; Pranke, P.; Pereira, C.N. Calculating hansen solubility parameters of polymers with genetic algorithms. J. Appl. Polym. Sci. 2014, 131, 1–12. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Schuerch, C. The Solvent Properties of Liquids and Their Relation to the Solubility, Swelling, Isolation and Fractionation of Lignin. J. Am. Chem. Soc. 1952, 74, 5061–5067. [Google Scholar] [CrossRef]
- Wang, K.; Xu, F.; Sun, R. Molecular Characteristics of Kraft-AQ Pulping Lignin Fractionated by Sequential Organic Solvent Extraction. Int. J. Mol. Sci. 2010, 11, 2988–3001. [Google Scholar] [CrossRef] [PubMed]
- Melro, E.; Alves, L.; Antunes, F.E.; Medronho, B. A brief overview on lignin dissolution. J. Mol. Liq. 2018, 265, 578–584. [Google Scholar] [CrossRef]
- Wen, J.-L.; Xue, B.-L.; Xu, F.; Sun, R.-C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crop. Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Tagami, A.; Gioia, C.; Lauberts, M.; Budnyak, T.; Moriana, R.; Lindström, M.E.; Sevastyanova, O. Solvent fractionation of softwood and hardwood kraft lignins for more efficient uses: Compositional, structural, thermal, antioxidant and adsorption properties. Ind. Crop. Prod. 2019, 129, 123–134. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. Effect of the Temperature on the Composition of Lignin Pyrolysis Products. Energy Fuels 2010, 24, 4470–4475. [Google Scholar] [CrossRef]
- Lin, X.; Sui, S.; Tan, S.; Pittman, J.C.U.; Sun, J.; Zhang, Z. Fast Pyrolysis of Four Lignins from Different Isolation Processes Using Py-GC/MS. Energies 2015, 8, 5107–5121. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Resende, F.; Moutsoglou, A.; Raynie, D.E. Pyrolysis of lignin extracted from prairie cordgrass, aspen, and Kraft lignin by Py-GC/MS and TGA/FTIR. J. Anal. Appl. Pyrolysis 2012, 98, 65–71. [Google Scholar] [CrossRef]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef] [Green Version]
- Dignac, M.-F.; Pechot, N.; Thevenot, M.; Lapierre, C.; Bahri, H.; Bardoux, G.; Rumpel, C. Isolation of soil lignins by combination of ball-milling and cellulolysis: Evaluation of purity and isolation efficiency with pyrolysis/GC/MS. J. Anal. Appl. Pyrolysis 2009, 85, 426–430. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin-Carbohydrate Complexes: Properties, Applications, Analyses, and Methods of Extraction: A Review. Biotechnol. Biofuels 2018, 11, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-Robles, J.; Tamminen, T.; Liitiä, T.; Peresin, M.S.; Rodríguez, A.; Jääskeläinen, A.-S. Aqueous acetone fractionation of kraft, organosolv and soda lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; Van Dam, J.E.G.; de Jong, E.; Scott, E.L.; Sanders, J.P.M.; Li, J.; Gellerstedt, G. Fractionation, analysis, and PCA modeling of properties of four technical lignins for prediction of their application potential in binders. Holzforschung 2010, 64, 193–200. [Google Scholar] [CrossRef]
- Sevastyanova, O.; Helander, M.; Chowdhury, S.; Lange, H.; Wedin, H.; Zhang, L.; Ek, M.; Kadla, J.F.; Crestini, C.; Lindström, M.E. Tailoring the molecular and thermo-mechanical properties of kraft lignin by ultrafiltration. J. Appl. Polym. Sci. 2014, 131, 9505–9515. [Google Scholar] [CrossRef] [Green Version]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Viksna, A.; Dobele, G.; Bikovens, O.; Telysheva, G. Characterization of Softwood and Hardwood LignoBoost Kraft Lignins with Emphasis on their Antioxidant Activity. BioResources 2014, 9, 2051–2068. [Google Scholar] [CrossRef]
- Thring, R.W.; Vanderlaan, M.N.; Griffin, S.L. Fractionation Of Alcell® Lignin By Sequential Solvent Extraction. J. Wood Chem. Technol. 1996, 16, 139–154. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Gordobil, O.; Diaz, R.H.; Sandak, A.; Sandak, J. Fractionation of lignin using organic solvents: A combined experimental and theoretical study. Int. J. Biol. Macromol. 2021, 168, 792–805. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Volperts, A.; Dobele, G.; Telysheva, G. Analytical pyrolysis—A tool for revealing of lignin structure-antioxidant activity relationship. J. Anal. Appl. Pyrolysis 2015, 113, 360–369. [Google Scholar] [CrossRef]
- Almeida, G.; Rémond, R.; Perré, P. Hygroscopic behaviour of lignocellulosic materials: Dataset at oscillating relative humidity variations. J. Build. Eng. 2018, 19, 320–333. [Google Scholar] [CrossRef]
- Xiao, T.; Yuan, H.; Ma, Q.; Guo, X.; Wu, Y. An approach for in situ qualitative and quantitative analysis of moisture adsorption in nanogram-scaled lignin by using micro-FTIR spectroscopy and partial least squares regression. Int. J. Biol. Macromol. 2019, 132, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Yuan, H.; Xiao, T.; Wu, Y. Application of micro-FTIR spectroscopy to study molecular association of adsorbed water with lignin. Int. J. Biol. Macromol. 2019, 131, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Gordobil, O.; Herrera, R.; Poohphajai, F.; Sandak, J.; Sandak, A. Impact of drying process on kraft lignin: Lignin-water interaction mechanism study by 2D NIR correlation spectroscopy. J. Mater. Res. Technol. 2021, 12, 159–169. [Google Scholar] [CrossRef]
- Cui, C.; Sun, R.; Argyropoulos, D.S. Fractional Precipitation of Softwood Kraft Lignin: Isolation of Narrow Fractions Common to a Variety of Lignins. ACS Sustain. Chem. Eng. 2014, 2, 959–968. [Google Scholar] [CrossRef]
- Dodd, A.P.; Kadla, J.F.; Straus, S.K. Characterization of Fractions Obtained from Two Industrial Softwood Kraft Lignins. ACS Sustain. Chem. Eng. 2014, 3, 103–110. [Google Scholar] [CrossRef]
- El Moustaqim, M.; El Kaihal, A.; El Marouani, M.; Men-La-Yakhaf, S.; Taibi, M.; Sebbahi, S.; El Hajjaji, S.; Kifani-Sahban, F. Thermal and thermomechanical analyses of lignin. Sustain. Chem. Pharm. 2018, 9, 63–68. [Google Scholar] [CrossRef]
- Gordobil, O.; Moriana, R.; Zhang, L.; Labidi, J.; Sevastyanova, O. Assesment of technical lignins for uses in biofuels and biomaterials: Structure-related properties, proximate analysis and chemical modification. Ind. Crop. Prod. 2016, 83, 155–165. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Perspective on Technical Lignin Fractionation. ACS Sustain. Chem. Eng. 2020, 8, 8086–8101. [Google Scholar] [CrossRef]
- Yuan, T.-Q.; He, J.; Xu, F.; Sun, R.-C. Fractionation and physico-chemical analysis of degraded lignins from the black liquor of Eucalyptus pellita KP-AQ pulping. Polym. Degrad. Stab. 2009, 94, 1142–1150. [Google Scholar] [CrossRef]
- Araújo, L.C.P.; Yamaji, F.M.; Lima, V.H.; Botaro, V.R. Kraft lignin fractionation by organic solvents: Correlation between molar mass and higher heating value. Bioresour. Technol. 2020, 314, 123757. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; McDonald, A.G. Fractionation and characterization of industrial lignins. Ind. Crop. Prod. 2014, 62, 67–76. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New Opportunities in the Valorization of Technical Lignins. ChemSusChem 2021, 14, 1016–1036. [Google Scholar] [CrossRef] [PubMed]
- Gigli, M.; Crestini, C. Fractionation of industrial lignins: Opportunities and challenges. Green Chem. 2020, 22, 4722–4746. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Avérous, L.; Boquillon, N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Alexy, P.; Košíková, B.; Podstránska, G. The effect of blending lignin with polyethylene and polypropylene on physical properties. Polymer 2000, 41, 4901–4908. [Google Scholar] [CrossRef]
- Bai, J.; Wang, S.; Li, Y.; Wang, Z.; Tang, J. Effect of chemical structure and molecular weight on the properties of lignin-based ultrafine carbon fibers. Int. J. Biol. Macromol. 2021, 187, 594–602. [Google Scholar] [CrossRef]


| Soluble Fractions | Insoluble Fractions | |||||
|---|---|---|---|---|---|---|
| Mn (g/mol) | Mw (g/mol) | PDI (Mw/Mn) | Mn (g/mol) | Mw (g/mol) | PDI (Mw/Mn) | |
| Crude HKL | 669 | 2477 | 3.7 | - | - | - |
| Methanol | 651 | 2272 | 3.5 | 3110 | 1,4370 | 4.6 |
| Ethanol | 633 | 1982 | 3.1 | 1762 | 8492 | 4.8 |
| 2-propanol | 587 | 1245 | 2.1 | 1144 | 4302 | 3.8 |
| Acetone | 661 | 2251 | 3.4 | 1670 | 1,0384 | 6.2 |
| Ethyl acetate | 561 | 1430 | 2.6 | 1272 | 5568 | 4.4 |
| Dichloromethane | 562 | 1195 | 2.1 | 1158 | 4453 | 3.8 |
| Diethyl ether | 412 | 563 | 1.4 | 759 | 2894 | 3.8 |
| Hexane | - | - | - | 676 | 2569 | 3.8 |
| Petroleum ether | - | - | - | 675 | 2614 | 3.9 |
| Sample | Phenolic Compounds: Structure of the Side Chain | S/G | ||||||
|---|---|---|---|---|---|---|---|---|
| Non-Substituted Saturated Chains (%) a | Unsaturated Side Chains (%) b | Oxygenated Groups in the Side Chains (%) c | Short Side Chain (%) d | Long Side Chain (%) e | (ArC1 + ArC2)/ArC3 f | |||
| HKL | 29.9 | 6.7 | 1.4 | 36.1 | 2.1 | 17.1 | 4.8 | |
| Methanol | S | 31.2 | 4.5 | 3.0 | 37.0 | 1.6 | 22.9 | 2.8 |
| I | 20.4 | 9.3 | 35.1 | 46.4 | 3.9 | 11.9 | 3.5 | |
| Ethanol | S | 30.8 | 5.7 | 6.5 | 40.0 | 3.3 | 12.0 | 3.4 |
| I | 25.6 | 10.9 | 2.3 | 35.5 | 5.5 | 6.5 | 2.7 | |
| Isopropanol | S | 36.7 | 6.4 | 1.9 | 41.6 | 3.0 | 14.1 | 3.3 |
| I | 27.9 | 7.5 | 3.5 | 35.4 | 3.6 | 9.9 | 3.1 | |
| Acetone | S | 34.5 | 6.6 | 2.0 | 40.7 | 3.4 | 11.8 | 3.0 |
| I | 16.5 | 9.0 | 5.1 | 27.2 | 3.7 | 7.4 | 3.1 | |
| Ethyl Acetate | S | 45.8 | 4.8 | 1.9 | 49.6 | 2.9 | 17.3 | 2.6 |
| I | 22.8 | 6.7 | 4.0 | 28.2 | 5.3 | 5.3 | 2.8 | |
| Dichloromethane | S | 37.1 | 5.4 | 7.2 | 46.0 | 3.9 | 11.9 | 3.1 |
| I | 23.0 | 7.4 | 3.7 | 30.3 | 3.5 | 8.8 | 3.2 | |
| Diethyl ether | S | 40.1 | 3.5 | 3.6 | 45.7 | 2.3 | 20.2 | 5.2 |
| I | 21.7 | 7.4 | 18.6 | 42.8 | 4.7 | 9.0 | 4.3 | |
| Hexane | S | - | - | - | - | - | - | - |
| I | 25.0 | 6.6 | 9.6 | 37.2 | 4.9 | 7.5 | 3.7 | |
| Petroleum Ether | S | - | - | - | - | - | - | - |
| I | 27.8 | 8.6 | 3.7 | 35.5 | 4.1 | 8.7 | 3.0 | |
| Included Data | Data Preprocessing | PCs | % Cumulative Variance | RMSEC | RMSECV |
|---|---|---|---|---|---|
| All fractions | Baseline–EMSC–SVN | 5 | 99.82 | 0.0424 | 0.0527 |
| Analysis of individual class groups | |||||
| Insoluble fractions | Baseline–EMSC–SVN | 4 | 99.90 | 0.0310 | 0.0414 |
| Soluble fractions | 4 | 99.79 | 0.0457 | 0.0703 | |
| PCs = principal components | |||||
| Soluble Fractions | Insoluble Fractions | |||
|---|---|---|---|---|
| TPC (µg GA/mg Lignin) | IC50 (µg/mL) | TPC (µg GA/mg Lignin) | IC50 (µg/mL) | |
| Crude HKL | 355.5 ± 3.4 | 11.4 ± 0.4 | ||
| Methanol | 377.3 ± 1.4 | 9.9 ± 0.4 | 61.9 ± 3.0 | 46.4 ± 4.2 |
| Ethanol | 389.4 ± 2.1 | 9.9 ± 0.6 | 182.8 ± 1.9 | 18.7 ± 0.1 |
| 2-propanol | 411.9 ± 8.6 | 9.5 ± 1.0 | 299.5 ± 0.8 | 13.1 ± 0.8 |
| Acetone | 388.1 ± 0.1 | 9.7 ± 0.0 | 163.2 ± 2.6 | 19.8 ± 0.0 |
| Ethyl acetate | 388.6 ± 3.4 | 8.4 ± 0.0 | 291.7 ± 1.4 | 9.7 ± 0.4 |
| Dichloromethane | 449.0 ± 7.4 | 8.1 ± 0.3 | 325.5 ± 3.8 | 11.9 ± 0.9 |
| Diethyl ether | 407.6 ± 5.4 | 8.5 ± 0.8 | 365.1 ± 4.4 | 9.5 ± 0.5 |
| Hexane | - | - | 361.3 ± 0.2 | 9.9 ± 0.5 |
| Petroleum ether | - | - | 345.2 ± 1.3 | 10.1 ± 0.5 |
| Soluble Fractions | Insoluble Fractions | |
|---|---|---|
| Tg (°C) | ||
| Crude HKL | 133.2 | |
| Methanol | 122.6 | 96.9 |
| Ethanol | 113.8 | 99.4 |
| 2-propanol | 84.8 | 86.6 |
| Acetone | 114.9 | 93.5 |
| Ethyl acetate | 89.2 | 138.3 |
| Dichloromethane | 79.2 | 173.1 |
| Diethyl ether | 52.9 | 151.8 |
| Hexane | - | 137.0 |
| Petroleum ether | - | 127.0 |
| Homogeneity a | Hygroscopic Stability | Antioxidant Capacity | Thermal Stability b | Ash Content | |
|---|---|---|---|---|---|
| Soluble fractions | |||||
| Methanol | + | + | + | = | + |
| Ethanol | + | + | + | + | ++ |
| 2-propanol | ++ | + | + | + | ++ |
| Acetone | + | + | + | = | ++ |
| Ethyl acetate | + | + | ++ | + | ++ |
| Dicloromethane | + | + | ++ | = | ++ |
| Diethyl ether | ++ | + | ++ | = | ++ |
| Insoluble fractions | |||||
| Methanol | − | − | − | − | − |
| Ethanol | − | − | − | − | − |
| 2-propanol | − | = | − | − | − |
| Acetone | − | − | − | − | − |
| Ethyl acetate | − | = | + | − | − |
| Dicloromethane | − | = | = | − | − |
| Diethyl ether | = | = | + | = | = |
| Hexane | = | = | + | = | = |
| Petroleum ether | = | = | + | = | = |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordobil, O.; Diaz, R.H.; Sandak, J.; Sandak, A. One-Step Lignin Refining Process: The Influence of the Solvent Nature on the Properties and Quality of Fractions. Polymers 2022, 14, 2363. https://doi.org/10.3390/polym14122363
Gordobil O, Diaz RH, Sandak J, Sandak A. One-Step Lignin Refining Process: The Influence of the Solvent Nature on the Properties and Quality of Fractions. Polymers. 2022; 14(12):2363. https://doi.org/10.3390/polym14122363
Chicago/Turabian StyleGordobil, Oihana, René Herrera Diaz, Jakub Sandak, and Anna Sandak. 2022. "One-Step Lignin Refining Process: The Influence of the Solvent Nature on the Properties and Quality of Fractions" Polymers 14, no. 12: 2363. https://doi.org/10.3390/polym14122363
APA StyleGordobil, O., Diaz, R. H., Sandak, J., & Sandak, A. (2022). One-Step Lignin Refining Process: The Influence of the Solvent Nature on the Properties and Quality of Fractions. Polymers, 14(12), 2363. https://doi.org/10.3390/polym14122363