Effect of Different Pre-Treatment on the Microstructure and Intumescent Properties of Rice Husk Ash-Based Geopolymer Hybrid Coating
Abstract
:1. Introduction
2. Materials and Methods
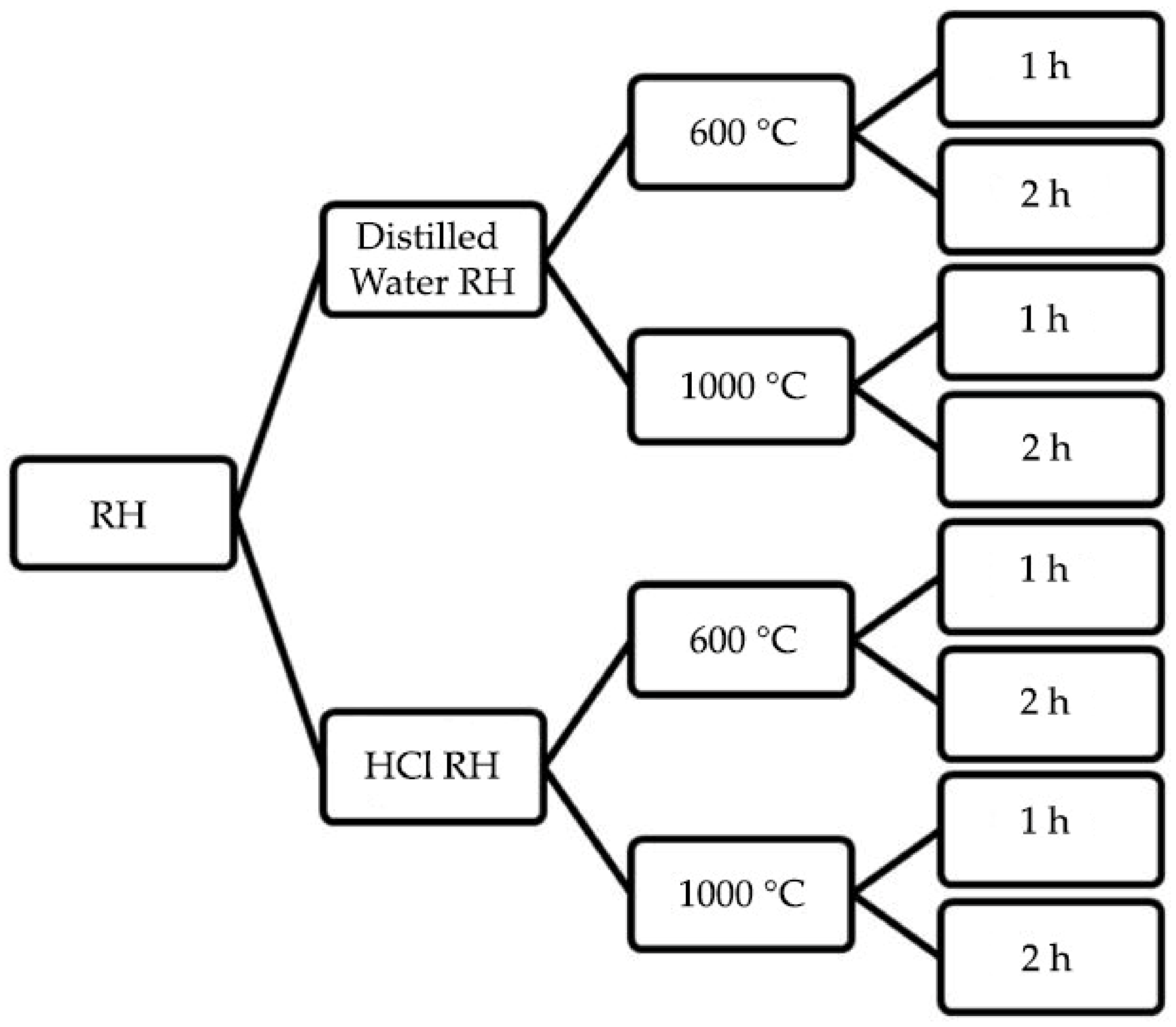
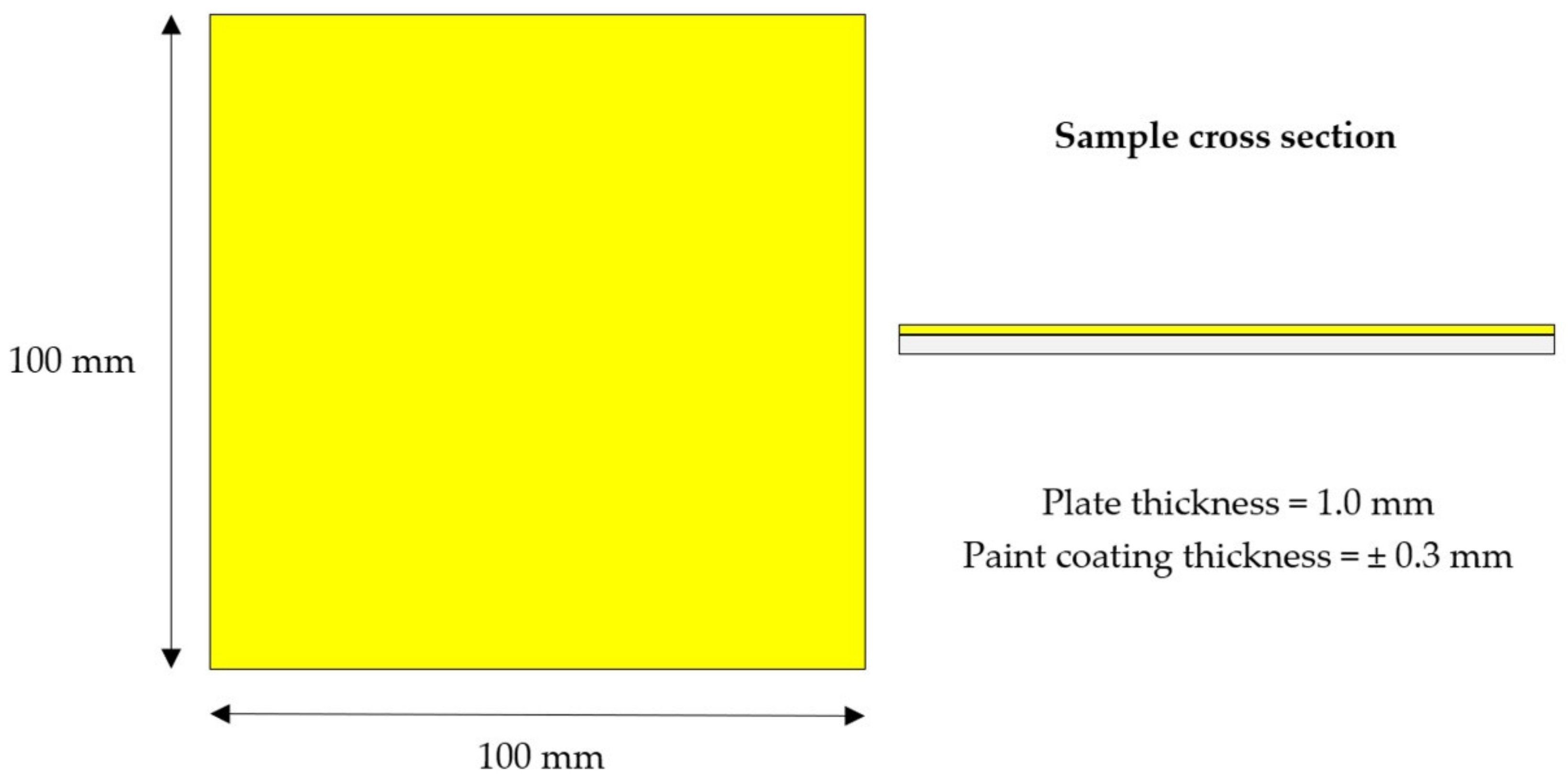
2.1. Sample Preparation
2.2. Rice Husk Pre-Treated Using Distilled Water
2.3. Rice Husk Acid Leaching Pre-Treatment
2.4. Preparation of Geopolymer Hybrid Alkyd Coating
2.5. Material Characterization and Microstructural Analysis
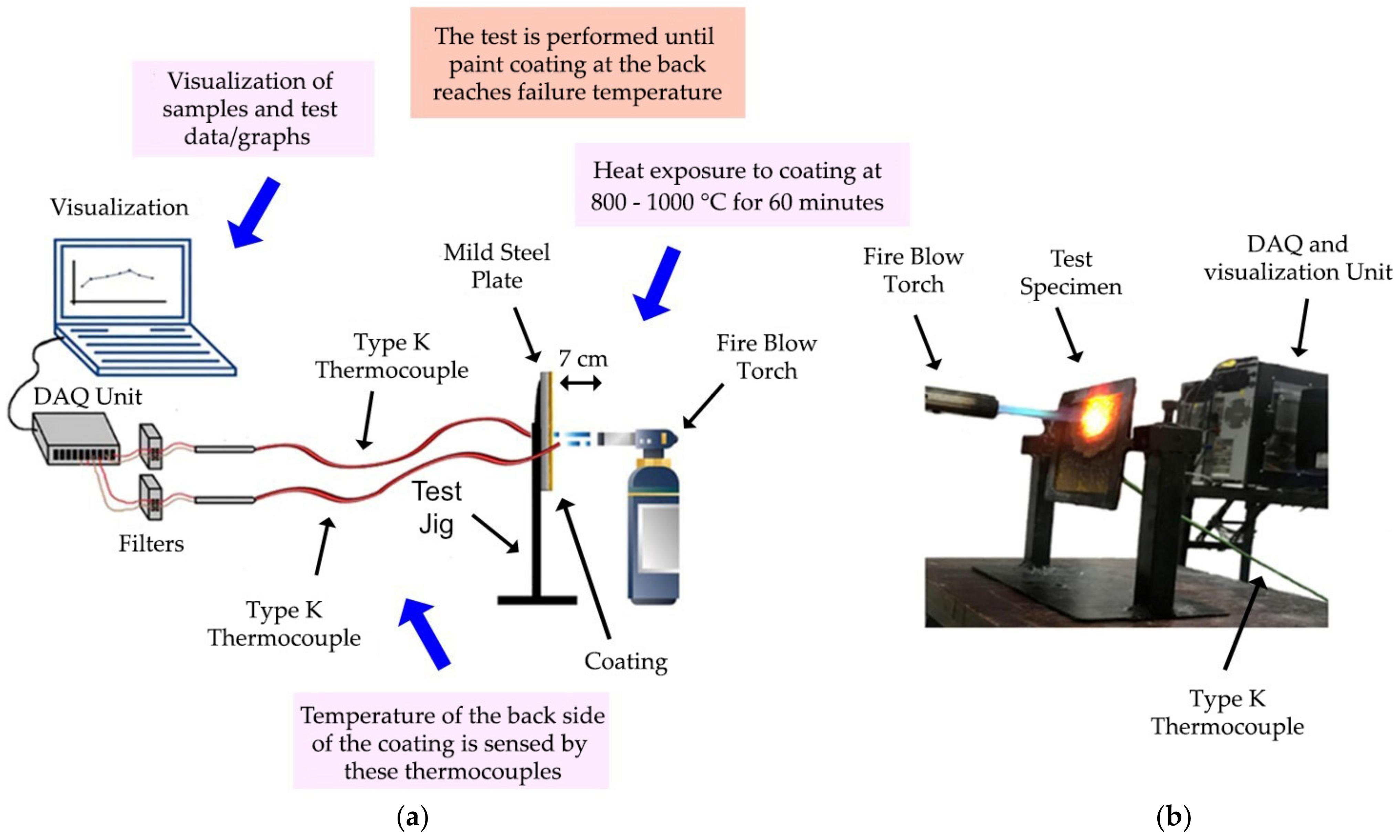
2.6. Fire Resistance and Furnace Test
3. Results and Discussion
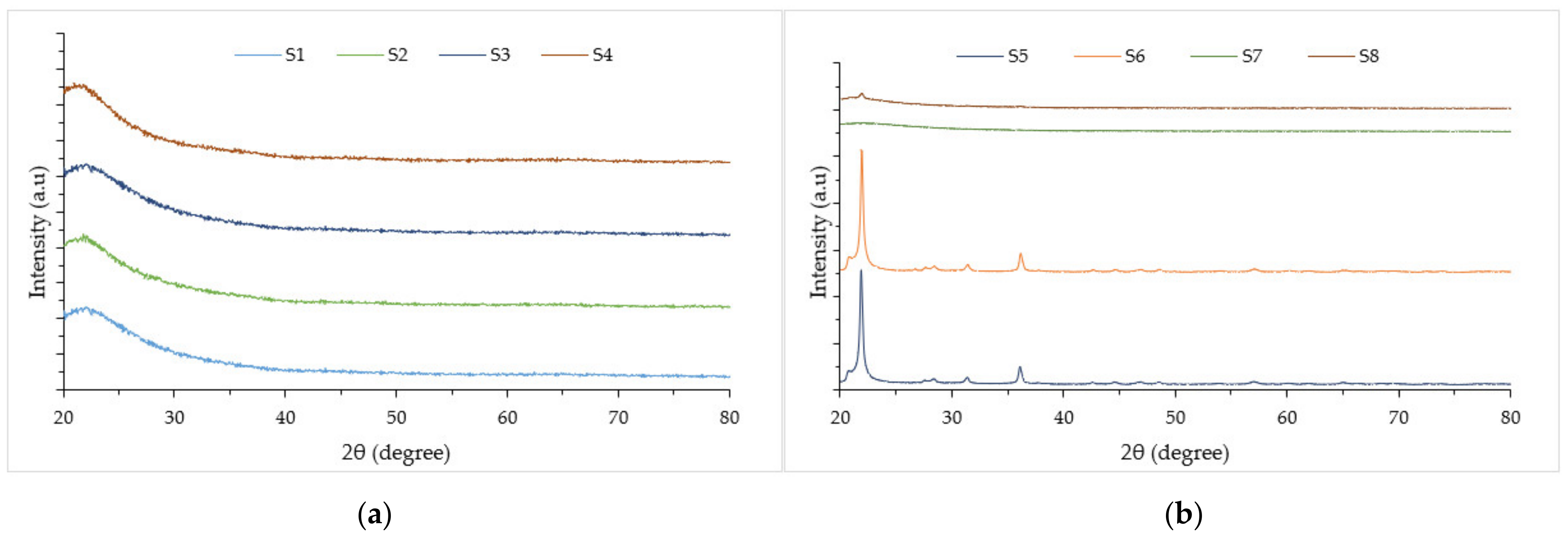
3.1. RHA X-ray Fluorescent Characterization
3.2. RHA X-ray Diffraction Characterization
3.3. Coatings Fire Retardant Performance Comparison
3.4. Coatings Characterization Comparison
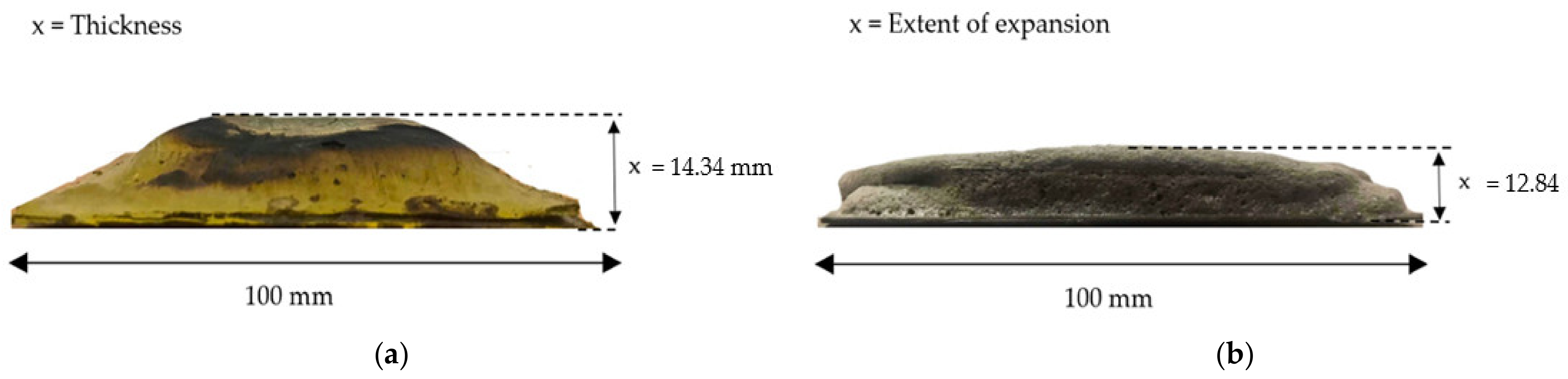
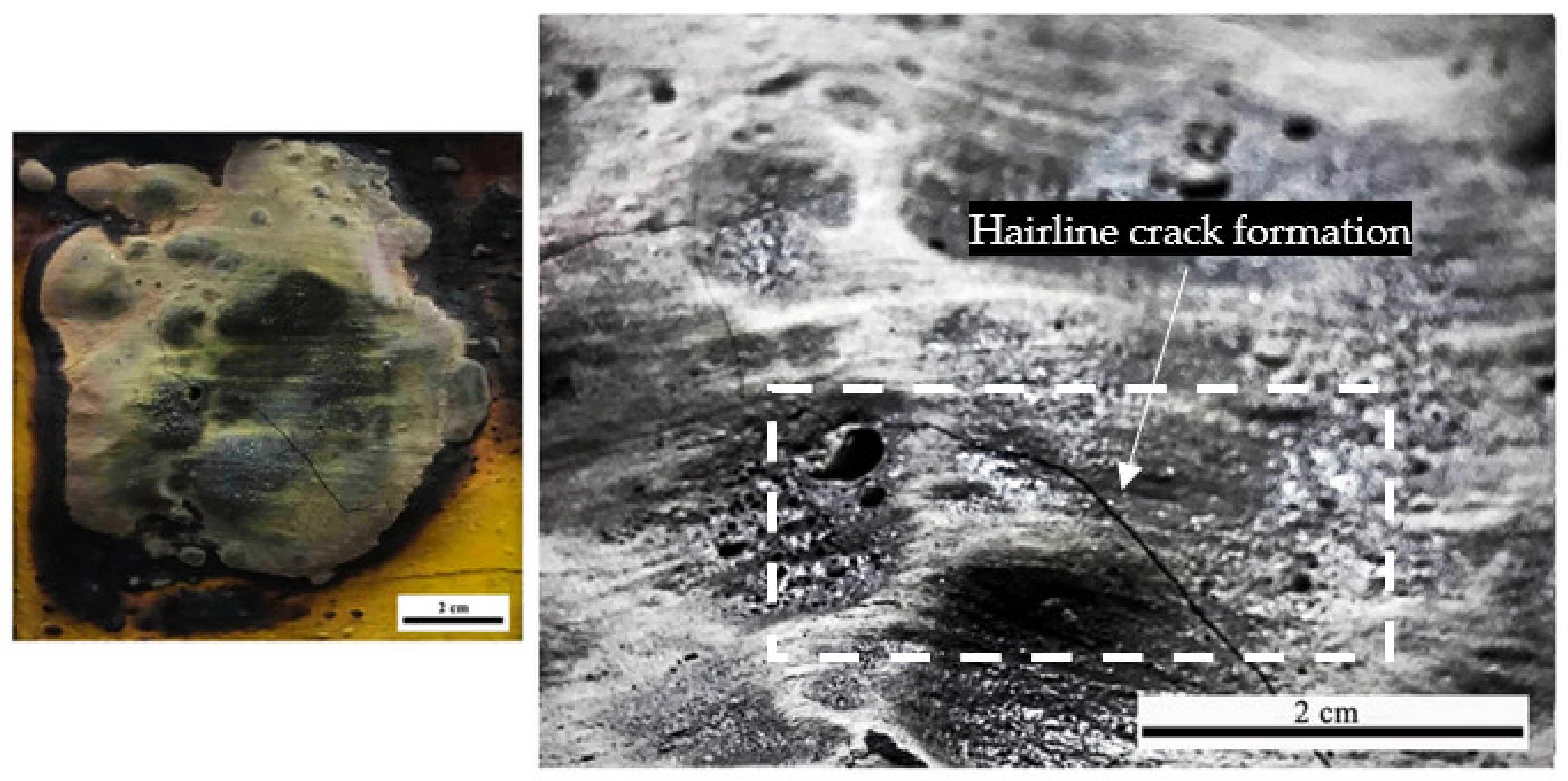
3.4.1. Char Thickness of the Coating Char Layer
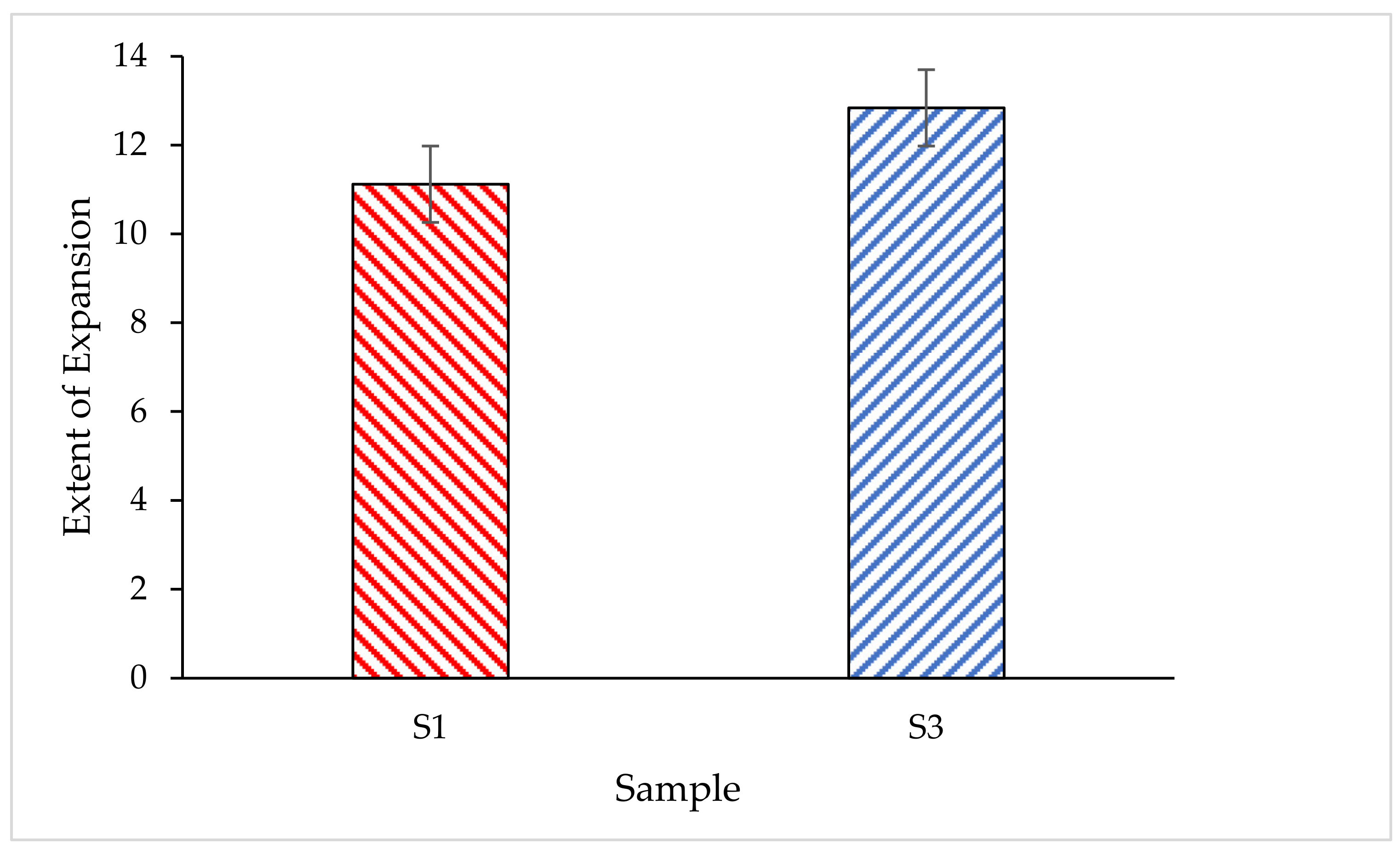
3.4.2. Expansion Rate of the Coating Char Layer
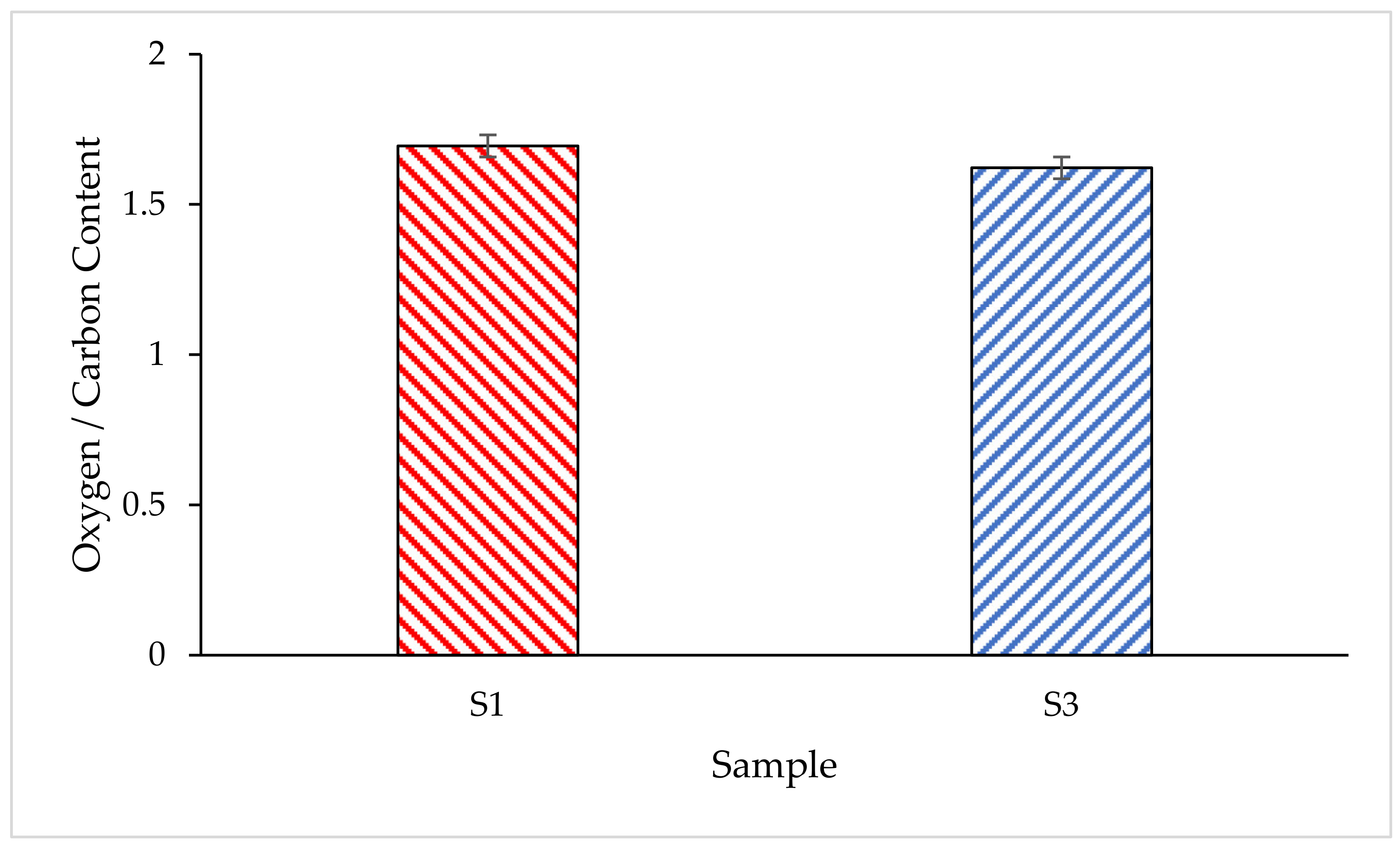
3.4.3. Anti-Oxidant Properties of the Samples
4. Conclusions
- It was observed that RH that underwent the HCl pre-treatment at 600 °C for 1 h (Sample S3) produced the best amount of silica content in RHA (93.92%) and the least amount of carbon impurities. An incineration time of 1 h was sufficient to yield high SiO2 content in RHA.
- From XRD analysis, at 600 °C incineration temperature, unleached and leached samples have shown the amorphous phase, while unleached RH for samples S5, S6 and leached RH for sample S8 showed the crystalline formed at 1000 °C incineration temperature. Raising the incineration temperature or extending the incineration duration can reduce the silica concentration and lead to accelerated formation of crystalline compounds.
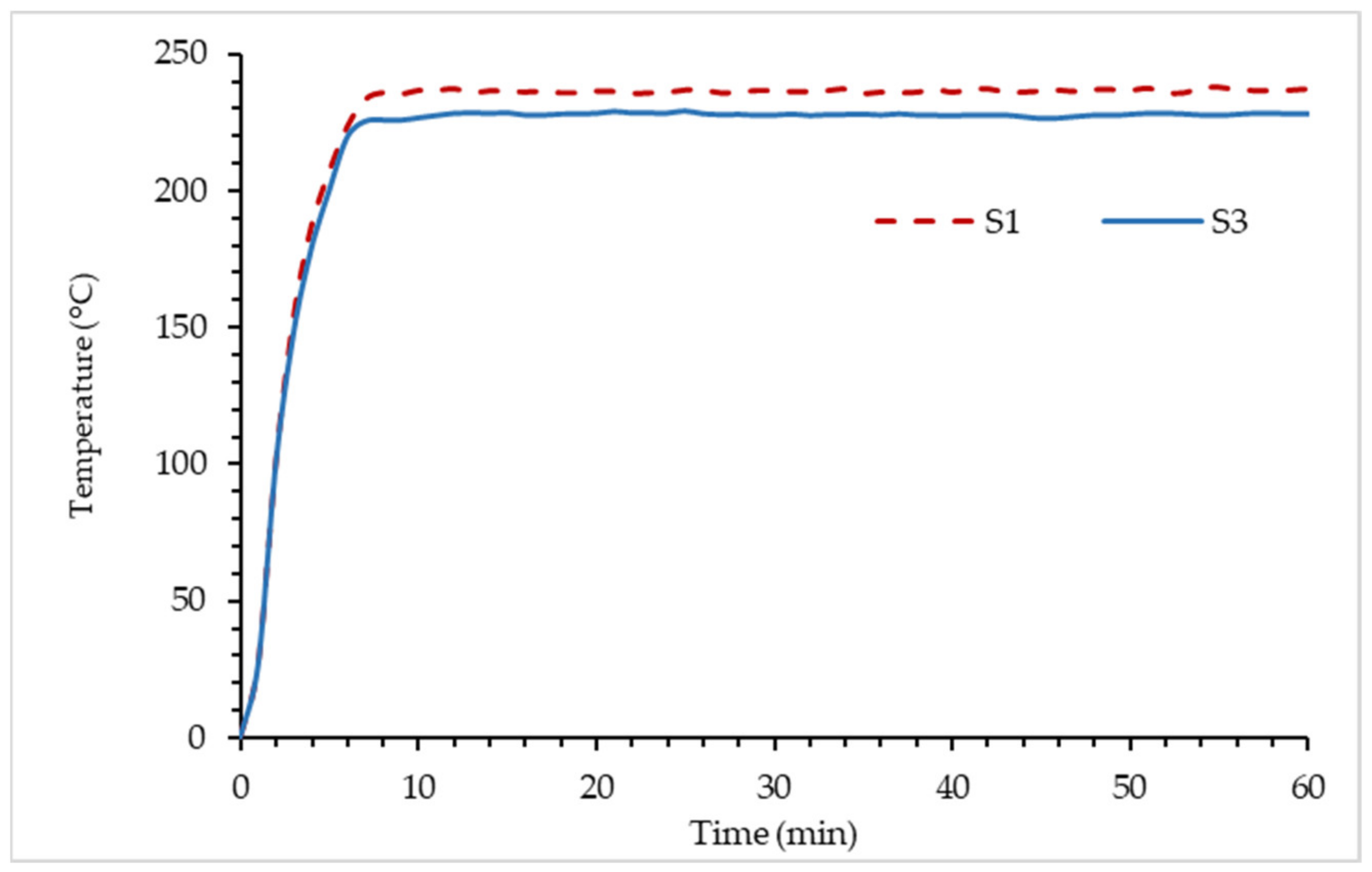
- The comparison in thermal properties between RHA-based GB hybrid coating using optimum RHA and the lowest silica content RHA was conducted. Based on fire resistance results, TAE and TT200 responses for sample S3 showed 5.83% and 3.48% improvement compared to sample S1.
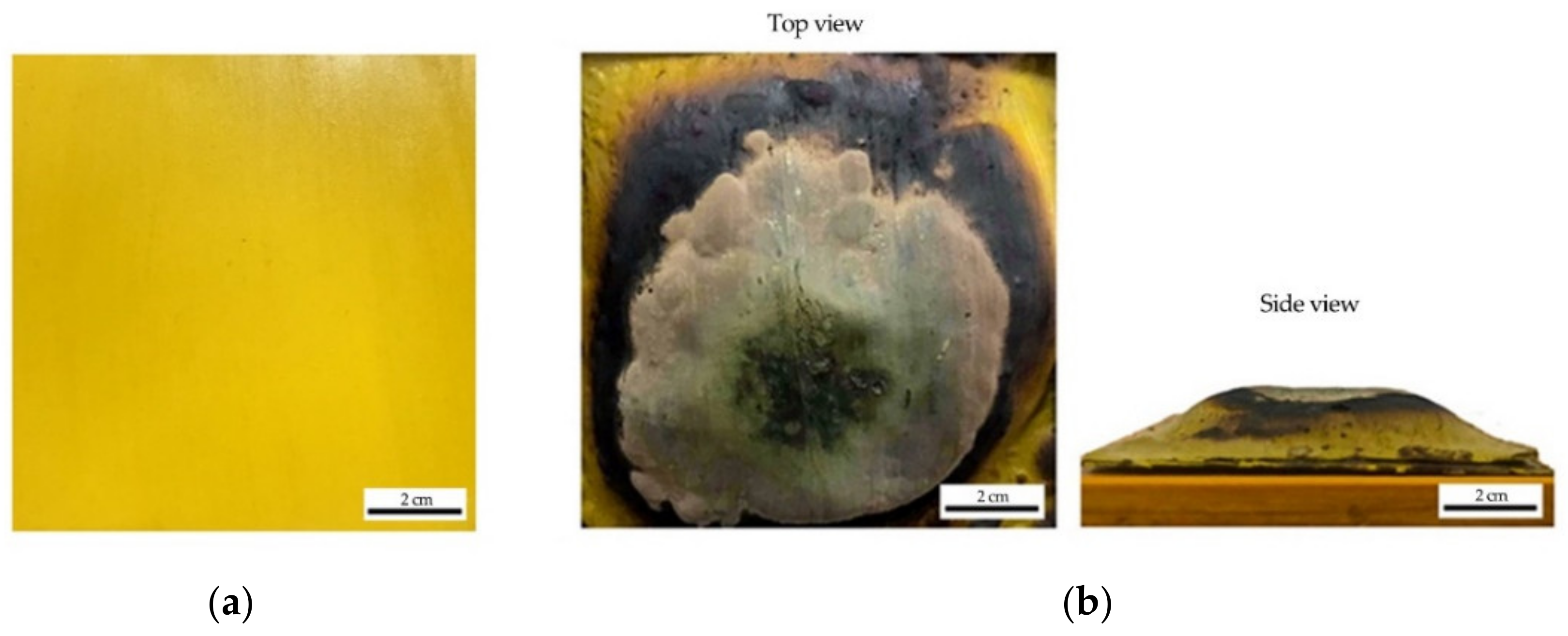
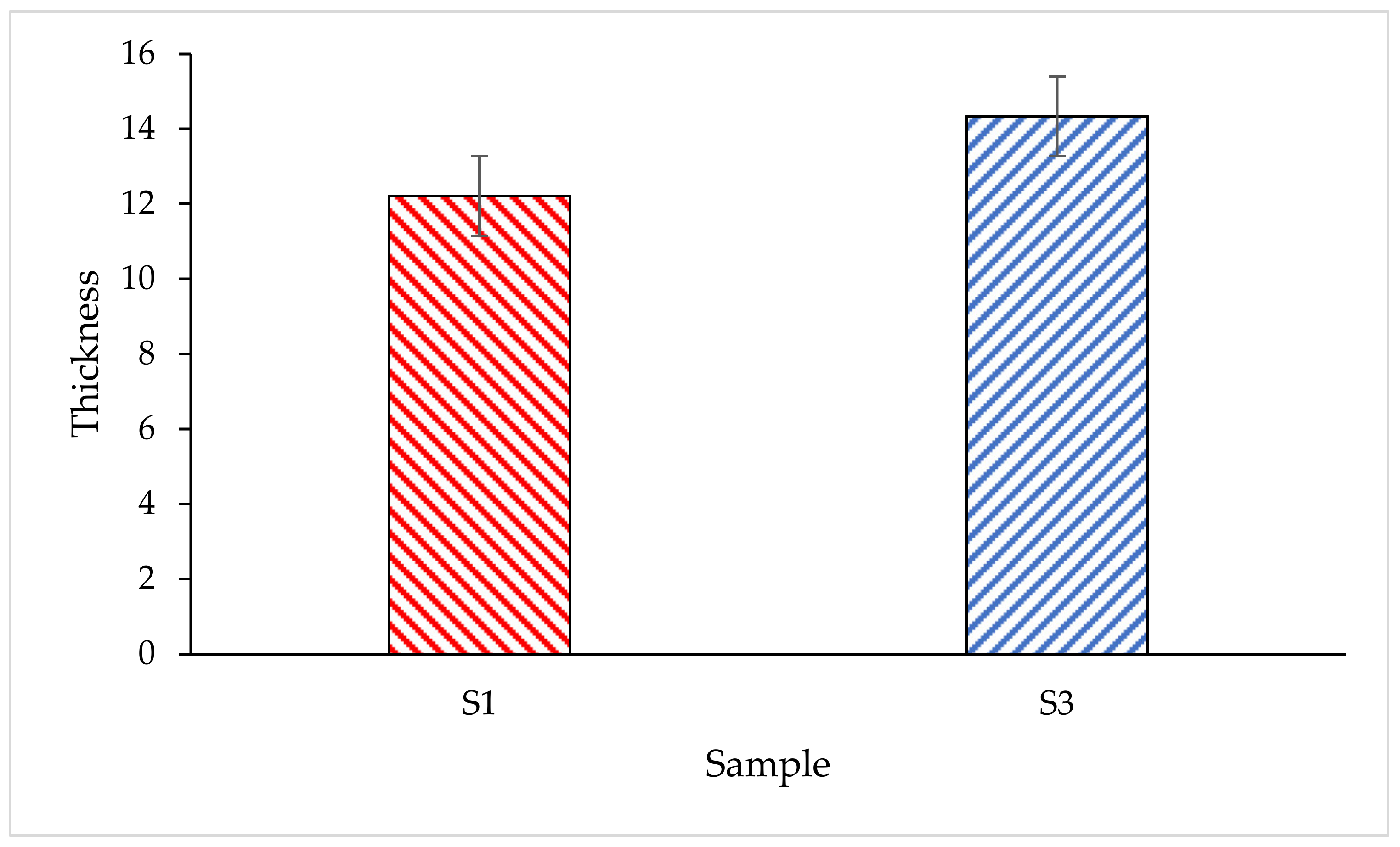
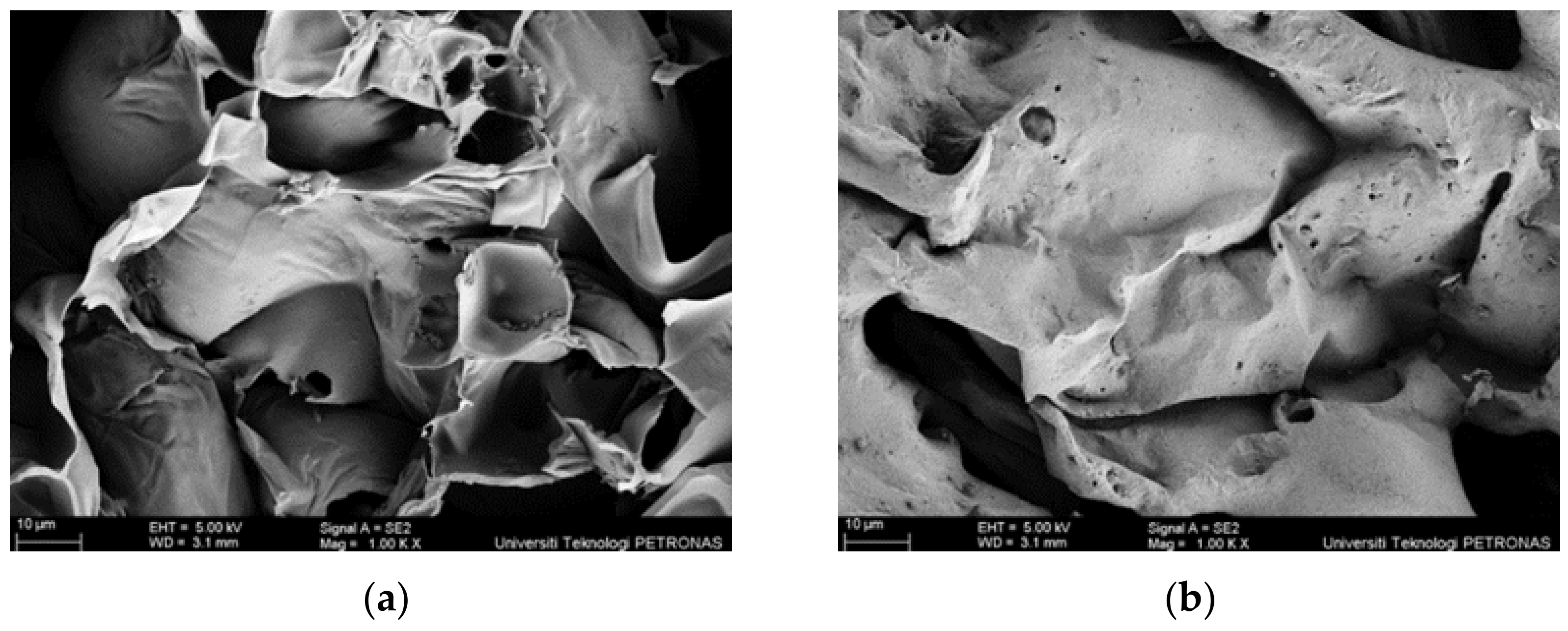
- SEM analysis revealed that the surface structure for sample S1 is more porous and less dense compared to sample S3. Furthermore, another major difference between S1 and S3 coating samples was the char layer compactness. Sample S1 was fragile and contained big voids while the char layer of sample S3 was more compact and smoother.
- It can be summarized that SiO2 efficiently improved the strength and compactness of the char layer, which resulted in a relatively higher fire-retardant efficiency. RHA has proven to be an effective alternative aluminosilicate source for geopolymer due to its intumescent formation and char layer properties.
- The RHA-based geopolymer hybrid coating has the potential to potentially improve building fire safety through passive fire protection and as thermal insulation material. Furthermore, utilizing silica content in RH is an innovative solution to transform “waste to wealth”.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Alkaline Activator |
| Al2O3 | Aluminum Oxide |
| ASTM | American Society for Testing and Materials |
| CaO | Calcium Oxide |
| DAQ | Data Acquisition |
| EDX | Energy Dispersive X-ray Spectroscopy |
| Fe2O3 | Ferric Oxide |
| GB | Geopolymer Binder |
| HCL | Hydrochloric Acid |
| ISO | International Organization for Standardization |
| K2O | Potassium Oxide |
| LOI | Loss of Ignition |
| K | Potassium |
| MgO | Magnesium oxide |
| Na | Sodium |
| Na2SiO3 | Sodium Silicate |
| NaOH | Sodium Hydroxide |
| OH | Hydroxide |
| RH | Rice Husk |
| RHA | Rice Husk Ash |
| SEM | Scanning Electron Microscopy |
| Si | Silica |
| TAE | Temperature at Equilibrium |
| TGA | Thermogravimetry Analysis |
| TiO2 | Titanium Dioxide |
| TT200 | Time Taken to Reach 200 °C |
| Wt. | Weight (g) |
| XRD | X-ray Diffraction |
References
- Mazela, B.; Batista, A.; Grzeskowiak, W. Expandable Graphite as a Fire Retardant for Cellulosic Materials—A Review. Forests 2020, 11, 755. [Google Scholar] [CrossRef]
- Kruger, H.J.; Focke, W.W.; Mhike, W.; Taute, A.; Roberson, A. Thermal properties of polyethylene flame retarded with expandable graphite and intumescent fire-retardant additives. Fire Mater. 2017, 41, 573–586. [Google Scholar] [CrossRef] [Green Version]
- Lahoti, M.; Tan, K.H.; Yang, E.H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Abdullah, M.N.; Mustapha, M.; Sallih, N.; Ahmad, A.; Mustapha, F.; Dahliyanti, A. Study and Use of Rice Husk Ash as a Source of Aluminosilicate in Refractory Coating. Materials 2021, 14, 3440. [Google Scholar] [CrossRef]
- Neupane, K.; Chalmers, D.; Kidd, P. High-strength geopolymer concrete-properties, advantages and challenges. J. Adv. Mater. 2018, 7, 15–25. [Google Scholar] [CrossRef]
- Mohseni, E. Assessment of Na₂SiO₃ to NaOH ratio impact on the performance of polypropylene fiber-reinforced geopolymer composites. Constr. Build Mater. 2018, 186, 904–911. [Google Scholar] [CrossRef]
- Sitarz, M.; Figiela, B.; Łach, M.; Korniejenko, K.; Mróz, K.; Castro-Gomes, J.; Hager, I. Mechanical Response of Geopolymer Foams to Heating—Managing Coal Gangue in Fire-Resistant Materials Technology. Energies 2022, 15, 3363. [Google Scholar] [CrossRef]
- Singh, N.B. Fly ash-based geopolymer binder: A future construction material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental impact assessment of fly ash and silica fume based geopolymer concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Hamdi, N.; Messaoud, I.B.; Srasra, E. Production of geopolymer binders using clay minerals and industrial wastes. Comptes Rendus Chim. 2019, 22, 220–226. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Bonet-Martínez, E.; Pérez-Villarejo, L.; Castro, E.; Sánchez-Soto, P.J. Effects of an Illite Clay Substitution on Geopolymer Synthesis as an Alternative to Metakaolin. J. Mater. Civ. Eng. 2021, 33, 04021072. [Google Scholar] [CrossRef]
- Wang, Y.S.; Alrefaei, Y.; Dai, J.G. Silico-aluminophosphate and alkali-aluminosilicate geopolymers: A comparative review. Front. Mater. 2019, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, B.A.; Lăzărescu, A.V.; Hegyi, A. The possibility of using slag for the production of geopolymer materials and its influence on mechanical performances—A review. Proceedings 2020, 63, 30. [Google Scholar]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- MacKenzie, K.J.; Bradley, S.; Hanna, J.V.; Smith, M.E. Magnesium analogues of aluminosilicate inorganic polymers (geopolymers) from magnesium minerals. J. Mater. Sci. 2013, 48, 1787–1793. [Google Scholar] [CrossRef]
- Kaze, R.C.; à Moungam, L.M.B.; Cannio, M.; Rosa, R.; Kamseu, E.; Melo, U.C.; Leonelli, C. Microstructure and engineering properties of Fe2O3 (FeO)-Al2O3-SiO2 based geopolymer composites. J. Clean. Prod. 2018, 199, 849–859. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Ming, L.Y.; Yong, H.C.; Tahir, M.F. Clay-based materials in geopolymer technology. In Cement Based Materials; Books on Demand: Norderstedt, Germany, 2018; Volume 239. [Google Scholar]
- Rasaki, S.A.; Bingxue, Z.; Guarecuco, R.; Thomas, T.; Minghui, Y. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Clean. Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Abdul Rashid, M.K.; Ramli Sulong, N.H.; Alengaram, U.J. Fire resistance performance of composite coating with geopolymer-based bio-fillers for lightweight panel application. J. Appl. Polym. Sci. 2020, 137, 49558. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Yahya, Z.; Abdul Razak, R. Fly ash-based geopolymer lightweight concrete using foaming agent. Int. J. Mol. Sci. 2012, 13, 7186–7198. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Fire Retardant performance of rice husk ash-based geopolymer coated mild steel-A factorial design and microstructure analysis. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2016; pp. 48–54. [Google Scholar]
- Sharma, A.; Ahmad, J. Factors affecting compressive strength of geopolymer concrete-a review. Int. Res. J. Eng. Technol. 2017, 4, 2026–2031. [Google Scholar]
- Bai, C.; Colombo, P. Processing, properties and applications of highly porous geopolymers: A review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Liang, G.; Liu, T.; Li, H.; Dong, B.; Shi, T. A novel synthesis of lightweight and high-strength green geopolymer foamed material by rice husk ash and ground-granulated blast-furnace slag. Resour. Conserv. Recycl. 2022, 176, 105922. [Google Scholar] [CrossRef]
- Steven, S.; Restiawaty, E.; Bindar, Y. Routes for energy and bio-silica production from rice husk: A comprehensive review and emerging prospect. Renew. Sust. Energ. Rev. 2021, 149, 111329. [Google Scholar] [CrossRef]
- Xu, W.; Wei, J.; Chen, J.; Zhang, B.; Xu, P.; Ren, J.; Yu, Q. Comparative study of water-leaching and acid-leaching pretreatment on the thermal stability and reactivity of biomass silica for viability as a pozzolanic additive in cement. Materials 2018, 11, 1697. [Google Scholar] [CrossRef] [Green Version]
- Bakar, R.A.; Yahya, R.; Gan, S.N. Production of high purity amorphous silica from rice husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Rice-Husk-Ash-Based Geopolymer Coating: Fire-Retardant, Optimize Composition, Microstructural, Thermal and Element Characteristics Analysis. Polymers 2021, 13, 3747. [Google Scholar] [CrossRef]
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio (nano) materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Calheiro, D.; Sánchez, F.A.; Camacho, A.L.D.; Rocha, T.L.A.D.C.; Moraes, C.A.M.; Sousa, V.C.D. Characterization of silica produced from rice husk ash: Comparison of purification and processing methods. Mater. Res. 2017, 20, 512–518. [Google Scholar] [CrossRef]
- Borouni, M.; Niroumand, B.; Maleki, A. A study on crystallization of amorphous nano silica particles by mechanical activation at the presence of pure aluminum. J. Solid State Chem. 2018, 263, 208–215. [Google Scholar] [CrossRef]
- Prasetyoko, D.; Ramli, Z.; Endud, S.; Hamdan, H.; Sulikowski, B. Conversion of rice husk ash to zeolite beta. J. Waste Manag. 2006, 26, 1173–1179. [Google Scholar] [CrossRef]
- Kwan, W.H.; Wong, Y.S. Acid leached rice husk ash (ARHA) in concrete: A review. J Mater Sci. Technol. 2020, 3, 501–507. [Google Scholar] [CrossRef]
- Shinde, P.S.; Suryawanshi, P.S.; Patil, K.K.; Belekar, V.M.; Sankpal, S.A.; Delekar, S.D.; Jadhav, S.A. A brief overview of recent progress in porous silica as catalyst supports. J. Compos. Sci. 2021, 5, 75. [Google Scholar] [CrossRef]
- Zaffar, S.; Kumar, A.; Memon, N.A.; Kumar, R.; Saand, A. Investigating Optimum Conditions for Developing Pozzolanic Ashes from Organic Wastes as Cement Replacing Materials. Materials 2022, 15, 2320. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xu, Z.; Wang, X. Influence of nano-silica on the flame retardancy and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog. Org. Coat. 2017, 112, 319–329. [Google Scholar] [CrossRef]
- Wu, Z.H.; Qu, J.P.; Zhao, Y.Q.; Tang, H.L.; Wen, J.S. Flammable and mechanical effects of silica on intumescent flame retardant/ethylene–octene copolymer/polypropylene composites. J. Thermoplast. Compos. Mater. 2015, 28, 981–994. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Rice Husk Ash-Based Geopolymer Binder: Compressive Strength, Optimize Composition, FTIR Spectroscopy, Microstructural, and Potential as Fire-Retardant Material. Polymers 2021, 13, 4373. [Google Scholar] [CrossRef]
- Beh, J.H.; Yew, M.C.; Yew, M.K.; Saw, L.H. Fire Protection Performance and Thermal Behavior of Thin Film Intumescent Coating. Coatings 2019, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, J.T.; Gonçalves, C.; Hiliou, L.; Coelho, J.F.; Magalhaes, F.D. Effect of binder on performance of intumescent coatings. J. Coat. Technol. Res. 2016, 13, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Liu, H.; Wang, C.; Huang, W.; Tian, Q.; Fu, Q.; Yan, W. Flame-retardant performance of epoxy resin composites with SiO2 nanoparticles and phenethyl-bridged DOPO derivative. ACS Omega 2020, 6, 666–674. [Google Scholar] [CrossRef]
- Yew, M.C.; Sulong, N.R.; Yew, M.K.; Amalina, M.A.; Johan, M.R. Influences of flame-retardant fillers on fire protection and mechanical properties of intumescent coatings. Prog. Org. Coat. 2015, 78, 59–66. [Google Scholar] [CrossRef]
- Aziz, H.; Ahmad, F. Effects from nano-titanium oxide on the thermal resistance of an intumescent fire-retardant coating for structural applications. Prog. Org. Coat. 2016, 101, 431–439. [Google Scholar] [CrossRef]


| Element | SiO2 | PdO | Al2O3 | Fe2O3 | CaO | K2O | Cr2O3 | MnO | LOI |
|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | 84.40 | 5.15 | 2.89 | 1.42 | 0.40 | 0.33 | 0.25 | 0.14 | 5.02 |
| Time | Temperature 600 °C | Temperature 1000 °C | ||
|---|---|---|---|---|
| Unleached | Leached | Unleached | Leached | |
| 1 h | S1 | S3 | S5 | S7 |
| 2 h | S2 | S4 | S6 | S8 |
| Element | Sample | |||
|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |
| (wt.%) | ||||
| SiO2 | 87.49 | 90.58 | 93.92 | 92.10 |
| K2O | 0.80 | 0.82 | 0.07 | 0.07 |
| Al2O3 | 0.05 | 0.06 | 0.37 | 0.36 |
| Fe2O3 | 0.04 | 0.03 | 0.03 | 0.03 |
| CaO | 0.37 | 0.43 | 0.15 | 0.12 |
| TiO2 | 0.01 | 0.01 | 0.01 | 0.01 |
| MgO | 0.01 | 0.01 | 0.01 | 0.00 |
| Na2O | 0.01 | 0.01 | 0.01 | 0.00 |
| MnO | 0.01 | 0.01 | 0.01 | 0.00 |
| LOI | 11.21 | 8.04 | 5.42 | 7.31 |
| Element | Sample | |||
|---|---|---|---|---|
| S5 | S6 | S7 | S8 | |
| (wt.%) | ||||
| SiO2 | 89.26 | 85.54 | 91.59 | 92.10 |
| K2O | 0.23 | 0.27 | 0.08 | 0.10 |
| Al2O3 | 0.10 | 0.04 | 0.39 | 0.31 |
| Fe2O3 | 0.04 | 0.04 | 0.03 | 0.02 |
| CaO | 0.25 | 0.23 | 0.08 | 0.08 |
| TiO2 | 0.01 | 0.01 | 0.00 | 0.00 |
| MgO | 0.01 | 0.01 | 0.00 | 0.00 |
| LOI | 10.10 | 13.86 | 7.83 | 7.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, M.N.; Mustapha, F.; Ahmad, K.A.; Mustapha, M.; Khan, T.; Singh, B.; Sebaey, T.A. Effect of Different Pre-Treatment on the Microstructure and Intumescent Properties of Rice Husk Ash-Based Geopolymer Hybrid Coating. Polymers 2022, 14, 2252. https://doi.org/10.3390/polym14112252
Abdullah MN, Mustapha F, Ahmad KA, Mustapha M, Khan T, Singh B, Sebaey TA. Effect of Different Pre-Treatment on the Microstructure and Intumescent Properties of Rice Husk Ash-Based Geopolymer Hybrid Coating. Polymers. 2022; 14(11):2252. https://doi.org/10.3390/polym14112252
Chicago/Turabian StyleAbdullah, Mohd Na’im, Faizal Mustapha, Kamarul Arifin Ahmad, Mazli Mustapha, Tabrej Khan, Balbir Singh, and Tamer A. Sebaey. 2022. "Effect of Different Pre-Treatment on the Microstructure and Intumescent Properties of Rice Husk Ash-Based Geopolymer Hybrid Coating" Polymers 14, no. 11: 2252. https://doi.org/10.3390/polym14112252
APA StyleAbdullah, M. N., Mustapha, F., Ahmad, K. A., Mustapha, M., Khan, T., Singh, B., & Sebaey, T. A. (2022). Effect of Different Pre-Treatment on the Microstructure and Intumescent Properties of Rice Husk Ash-Based Geopolymer Hybrid Coating. Polymers, 14(11), 2252. https://doi.org/10.3390/polym14112252










