An Efficient Technique for Ammonia Capture in the Haber–Bosch Process Loop—Membrane-Assisted Gas Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Absorbents Screening
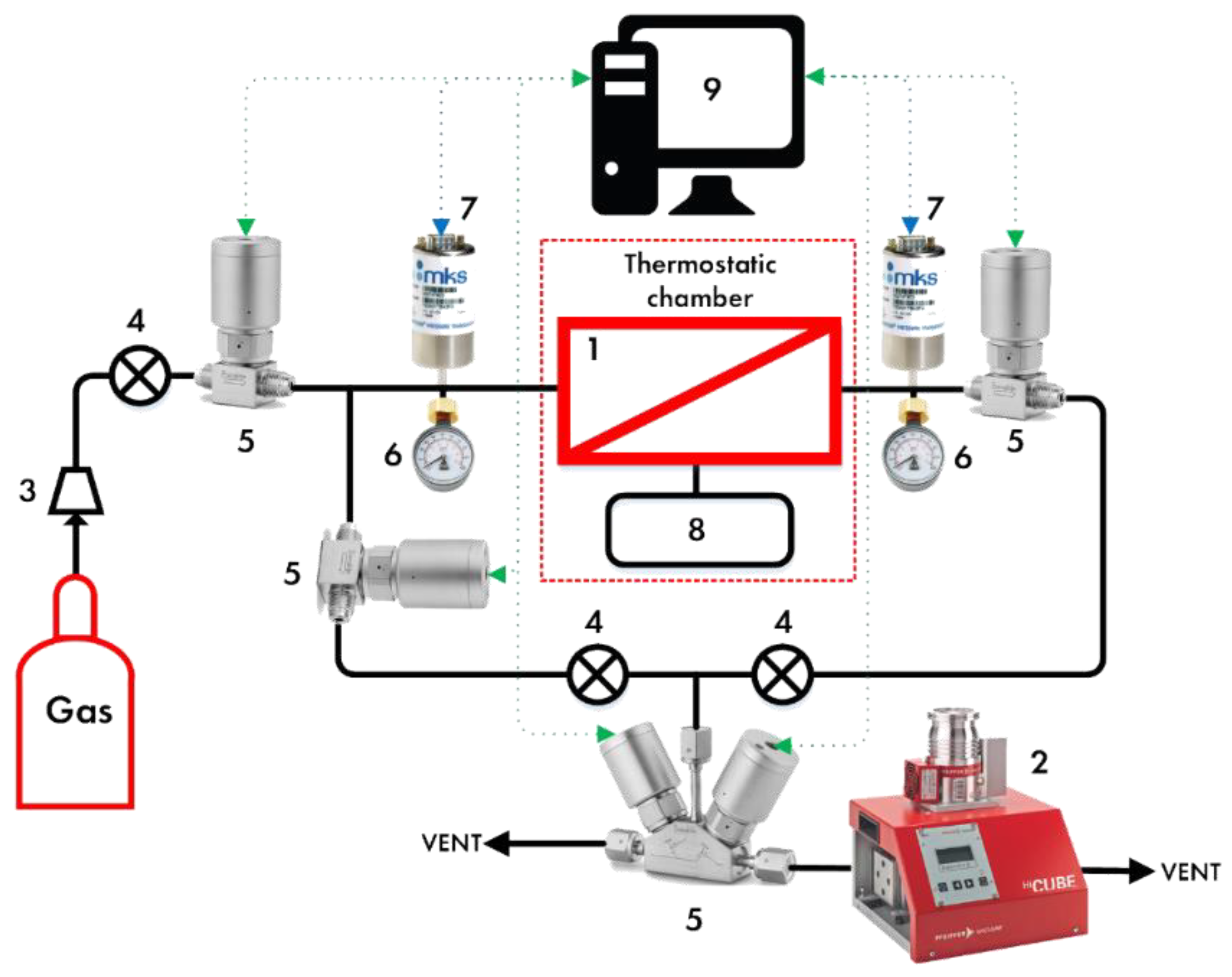
2.3. Experimental Setup
2.4. Membrane-Assisted Gas Absorption Cell Design
2.5. Membrane Permeance Test
3. Results and Discussion
3.1. Membrane Permeance
3.2. Membrane-Assisted Gas Separation Cell Performance: Helium Sweep Mode
3.3. Membrane-Assisted Gas Separation Cell Performance: H2/N2 Mix Sweep Mode
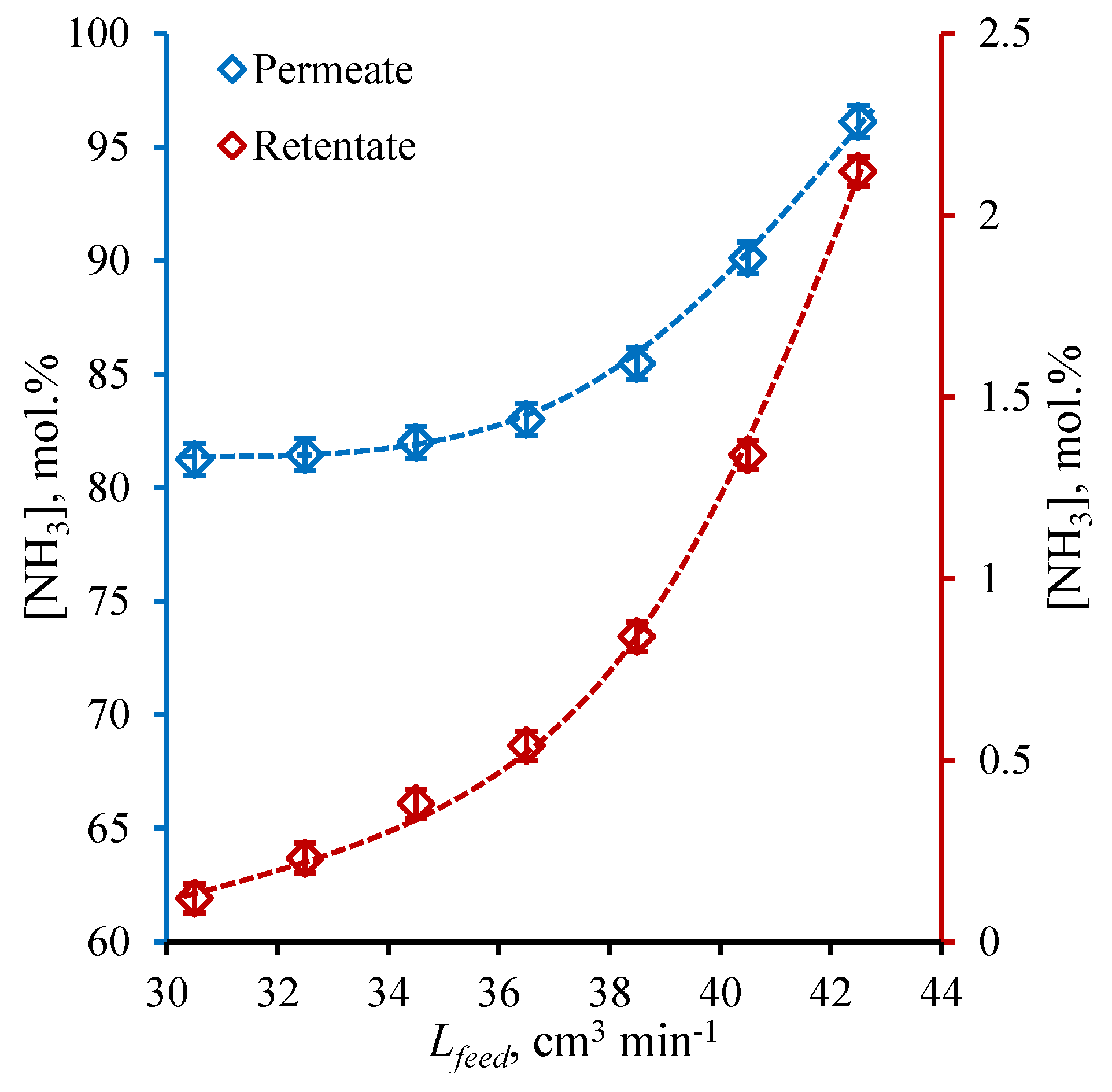
3.4. The Steady-State Establishing Dynamic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Ammonia Annual Production Capacity | Statista. Available online: https://www.statista.com/statistics/1065865/ammonia-production-capacity-globally/ (accessed on 14 April 2022).
- US990191A—Process of Producing Ammonia—Google Patents. Available online: https://patents.google.com/patent/US990191 (accessed on 22 January 2020).
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Chen, P. Recent Progress towards Mild-Condition Ammonia Synthesis. J. Energy Chem. 2019, 36, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Smart, K. Review of Recent Progress in Green Ammonia Synthesis. Johns. Matthey Technol. Rev. 2021, 66, 230. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 34. [Google Scholar] [CrossRef]
- Petukhov, A.N.; Atlaskin, A.A.; Kryuchkov, S.S.; Smorodin, K.A.; Zarubin, D.M.; Petukhova, A.N.; Atlaskina, M.E.; Nyuchev, A.V.; Vorotyntsev, A.V.; Trubyanov, M.M.; et al. A Highly-Efficient Hybrid Technique—Membrane-Assisted Gas Absorption for Ammonia Recovery after the Haber–Bosch Process. Chem. Eng. J. 2021, 421, 127726. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Kryuchkov, S.S.; Smorodin, K.A.; Markov, A.N.; Kazarina, O.V.; Zarubin, D.M.; Atlaskina, M.E.; Vorotyntsev, A.V.; Nyuchev, A.V.; Petukhov, A.N.; et al. Towards the Potential of Trihexyltetradecylphosphonium Indazolide with Aprotic Heterocyclic Ionic Liquid as an Efficient Absorbent for Membrane-Assisted Gas Absorption Technique for Acid Gas Removal Applications. Sep. Purif. Technol. 2021, 257, 117835. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Kryuchkov, S.S.; Yanbikov, N.R.; Smorodin, K.A.; Petukhov, A.N.; Trubyanov, M.M.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Comprehensive Experimental Study of Acid Gases Removal Process by Membrane-Assisted Gas Absorption Using Imidazolium Ionic Liquids Solutions Absorbent. Sep. Purif. Technol. 2020, 239, 11578. [Google Scholar] [CrossRef]
- Vorotyntsev, I.V.; Atlaskin, A.A.; Trubyanov, M.M.; Petukhov, A.N.; Gumerova, O.R.; Akhmetshina, A.I.; Vorotyntsev, V.M. Towards the Potential of Absorbing Pervaporation Based on Ionic Liquids for Gas Mixture Separation. Desalin. Water Treat. 2017, 75, 305–313. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Petukhov, A.N.; Yanbikov, N.R.; Salnikova, M.E.; Sergeeva, M.S.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Evaluation of the Absorbing Pervaporation Technique for Ammonia Recovery after the Haber Process. Chem. Process. Eng.—Inz. Chem. I Proces. 2018, 39, 323–333. [Google Scholar] [CrossRef]
- Deng, D.; Gao, B.; Zhang, C.; Duan, X.; Cui, Y.; Ning, J. Investigation of Protic NH4SCN-Based Deep Eutectic Solvents as Highly Efficient and Reversible NH3 Absorbents. Chem. Eng. J. 2019, 358, 936–943. [Google Scholar] [CrossRef]
- Kazarina, O.V.; Agieienko, V.N.; Petukhov, A.N.; Vorotyntsev, A.V.; Atlaskina, M.E.; Atlaskin, A.A.; Kryuchkov, S.S.; Markov, A.N.; Nyuchev, A.V.; Vorotyntsev, I.V. Deep Eutectic Solvents Composed of Urea and New Salts of a Choline Family for Efficient Ammonia Absorption. J. Chem. Eng. Data 2022, 67, 138–150. [Google Scholar] [CrossRef]
- Atlaskina, M.E.; Atlaskin, A.A.; Kazarina, O.V.; Petukhov, A.N.; Zarubin, D.M.; Nyuchev, A.V.; Vorotyntsev, A.V.; Vorotyntsev, I.V. Synthesis and Comprehensive Study of Quaternary-Ammonium-Based Sorbents for Natural Gas Sweetening. Environments 2021, 8, 134. [Google Scholar] [CrossRef]
- Vekariya, R.L. A Review of Ionic Liquids: Applications towards Catalytic Organic Transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; Macfarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [Green Version]
- Kazarina, O.V.; Agieienko, V.N.; Nagrimanov, R.N.; Atlaskina, M.E.; Petukhov, A.N.; Moskvichev, A.A.; Nyuchev, A.V.; Barykin, A.V.; Vorotyntsev, I.V. A Rational Synthetic Approach for Producing Quaternary Ammonium Halides and Physical Properties of the Room Temperature Ionic Liquids Obtained by This Way. J. Mol. Liq. 2021, 344, 117925. [Google Scholar] [CrossRef]
- Zhong, F.Y.; Huang, K.; Peng, H.L. Solubilities of Ammonia in Choline Chloride plus Urea at (298.2–353.2) K and (0–300) KPa. J. Chem. Thermodyn. 2019, 129, 5–11. [Google Scholar] [CrossRef]
- Zhong, F.Y.; Peng, H.L.; Tao, D.J.; Wu, P.K.; Fan, J.P.; Huang, K. Phenol-Based Ternary Deep Eutectic Solvents for Highly Efficient and Reversible Absorption of NH3. ACS Sustain. Chem. Eng. 2019, 7, 3258–3266. [Google Scholar] [CrossRef]
- Duan, X.; Gao, B.; Zhang, C.; Deng, D. Solubility and Thermodynamic Properties of NH3 in Choline Chloride-Based Deep Eutectic Solvents. J. Chem. Thermodyn. 2019, 133, 79–84. [Google Scholar] [CrossRef]
- Deng, X.; Duan, X.; Gong, L.; Deng, D. Ammonia Solubility, Density, and Viscosity of Choline Chloride-Dihydric Alcohol Deep Eutectic Solvents. J. Chem. Eng. Data 2020, 65, 4845–4854. [Google Scholar] [CrossRef]
- Zhong, F.Y.; Zhou, L.; Shen, J.; Liu, Y.; Fan, J.P.; Huang, K. Rational Design of Azole-Based Deep Eutectic Solvents for Highly Efficient and Reversible Capture of Ammonia. ACS Sustain. Chem. Eng. 2019, 7, 14170–14179. [Google Scholar] [CrossRef]
- Li, Y.; Ali, M.C.; Yang, Q.; Zhang, Z.; Bao, Z.; Su, B.; Xing, H.; Ren, Q. Hybrid Deep Eutectic Solvents with Flexible Hydrogen-Bonded Supramolecular Networks for Highly Efficient Uptake of NH3. ChemSusChem 2017, 10, 3368–3377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Kong, L.Y.; Huang, K. NH3 Solubilities and Physical Properties of Ethylamine Hydrochloride plus Urea Deep Eutectic Solvents. J. Chem. Eng. Data 2019, 64, 3821–3830. [Google Scholar] [CrossRef]
- Jiang, W.J.; Zhong, F.Y.; Liu, Y.; Huang, K. Effective and Reversible Capture of NH3 by Ethylamine Hydrochloride Plus Glycerol Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 10552–10560. [Google Scholar] [CrossRef]
- Luo, Q.; Hao, J.; Wei, L.; Zhai, S.; Xiao, Z.; An, Q. Protic Ethanolamine Hydrochloride-Based Deep Eutectic Solvents for Highly Efficient and Reversible Absorption of NH3. Sep. Purif. Technol. 2021, 260, 118240. [Google Scholar] [CrossRef]
- Jiang, W.J.; Zhong, F.Y.; Zhou, L.S.; Peng, H.L.; Fan, J.P.; Huang, K. Chemical Dual-Site Capture of NH3 by Unprecedentedly Low-Viscosity Deep Eutectic Solvents. Chem. Commun. 2020, 56, 2399–2402. [Google Scholar] [CrossRef]
- Li, K.; Fang, H.; Duan, X.; Deng, D. Efficient Uptake of NH3 by Dual Active Sites NH4SCN-Imidazole Deep Eutectic Solvents with Low Viscosity. J. Mol. Liq. 2021, 339, 116724. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhong, F.Y.; Zhou, L.S.; Tian, Z.Q.; Huang, K. Deep Eutectic Solvents Formed by N-Methylacetamide and Heterocyclic Weak Acids for Highly Efficient and Reversible Chemical Absorption of Ammonia. Ind. Eng. Chem. Res. 2020, 59, 2060–2067. [Google Scholar] [CrossRef]
- Deng, D.; Duan, X.; Gao, B.; Zhang, C.; Deng, X.; Gong, L. Efficient and Reversible Absorption of NH3 by Functional Azole-Glycerol Deep Eutectic Solvents. New J. Chem. 2019, 43, 11636–11642. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Petukhov, A.N.; Mechergui, A.; Vorotyntsev, A.V.; Nyuchev, A.V.; Moskvichev, A.A.; Vorotyntsev, I.V. Evaluation of Methanesulfonate-Based Deep Eutectic Solvent for Ammonia Sorption. J. Chem. Eng. Data 2018, 63, 1896–1904. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.; Zeng, S.; Liu, Y.; Dong, H.; Deng, C. Protic Ionic Liquid-Based Deep Eutectic Solvents with Multiple Hydrogen Bonding Sites for Efficient Absorption of NH3. AIChE J. 2020, 66, e16253. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Q.; Sun, X.; Wu, H.; Hao, J.; Wei, L.; Zhai, S.; Xiao, Z.; An, Q. Dual-Active-Sites Deep Eutectic Solvents Based on Imidazole and Resorcinol for Efficient Capture of NH3. Chem. Eng. J. 2021, 416, 129114. [Google Scholar] [CrossRef]
- Shang, D.; Bai, L.; Zeng, S.; Dong, H.; Gao, H.; Zhang, X.; Zhang, S. Enhanced NH3 Capture by Imidazolium-Based Protic Ionic Liquids with Different Anions and Cation Substituents. J. Chem. Technol. Biotechnol. 2018, 93, 1228–1236. [Google Scholar] [CrossRef]
- Yokozeki, A.; Shiflett, M.B. Ammonia Solubilities in Room-Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2007, 46, 1605–1610. [Google Scholar] [CrossRef]
- Li, G.; Zhou, Q.; Zhang, X.; Lei, W.; Zhang, S.; Li, J. Solubilities of Ammonia in Basic Imidazolium Ionic Liquids. Fluid Phase Equilibria 2010, 297, 34–39. [Google Scholar] [CrossRef]
- Huang, J.; Riisager, A.; Berg, R.W.; Fehrmann, R. Tuning Ionic Liquids for High Gas Solubility and Reversible Gas Sorption. J. Mol. Catal. A Chem. 2008, 279, 170–176. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Dong, H.; Zhang, X.; Gao, H.; Zhang, S.; Li, J.; Wang, C. Efficient Absorption of Ammonia with Hydroxyl-Functionalized Ionic Liquids. RSC Adv. 2015, 5, 81362–81370. [Google Scholar] [CrossRef]
- Yokozeki, A.; Shiflett, M.B. Vapor-Liquid Equilibria of Ammonia + Ionic Liquid Mixtures. Appl. Energy 2007, 84, 1258–1273. [Google Scholar] [CrossRef]
- Arce, A.; Soto, A.; Ortega, J.; Sabater, G. Viscosities and Volumetric Properties of Binary and Ternary Mixtures of Tris(2-Hydroxyethyl) Methylammonium Methylsulfate + Water + Ethanol at 298.15 K. J. Chem. Eng. Data 2008, 53, 770–775. [Google Scholar] [CrossRef]
- Yang, Y.; Faheem, M.; Wang, L.; Meng, Q.; Sha, H.; Yang, N.; Yuan, Y.; Zhu, G. Surface Pore Engineering of Covalent Organic Frameworks for Ammonia Capture through Synergistic Multivariate and Open Metal Site Approaches. ACS Cent. Sci. 2018, 4, 748–754. [Google Scholar] [CrossRef]
- Shang, D.; Zhang, X.; Zeng, S.; Jiang, K.; Gao, H.; Dong, H.; Yang, Q.; Zhang, S. Protic Ionic Liquid [Bim][NTf2] with Strong Hydrogen Bond Donating Ability for Highly Efficient Ammonia Absorption. Green Chem. 2017, 19, 937–945. [Google Scholar] [CrossRef]
- Barrer, R.M. Barrer (1939) XII. Gas Flow in Solids. Lond. Edinb. Dublin Philos. Mag. J. Sci. 2009, 28, 148–162. [Google Scholar] [CrossRef]
- Barrer, R.M.; F. R. S. Diffusion et Solution Dans Quelques Polymères. J. Chim. Phys. 1958, 55, 139–148. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer International Publishing: New York, NY, USA, 2015; ISBN 9783319010953. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; Wiley: New York, NY, USA, 1973; p. 771. [Google Scholar]







| Solvents | Mol W g mol−1 | Density g sm−3 | Viscosity, mPa s | Pressure kPa | T, K | Solubility NH3 g g−1 | Refs |
|---|---|---|---|---|---|---|---|
| Choline-Chloride-based DESs | |||||||
| ChCl + Urea (2:3) | 91.89 | 10.8 | 313.2 | 0.002623 | [18] | ||
| 104.8 | 0.024455 | ||||||
| 297.9 | 0.065991 | ||||||
| ChCl + Urea (1:2) | 86.59 | 17.2 | 298.2 | 0.007561 | |||
| 95 | 0.037687 | ||||||
| 296.6 | 0.117149 | ||||||
| 11.3 | 313.2 | 0.003372 | |||||
| 108.2 | 0.027231 | ||||||
| 302.1 | 0.073927 | ||||||
| ChCl + Urea (2:5) | 82.8 | 19.8 | 313.2 | 0.004785 | |||
| 98.1 | 0.023076 | ||||||
| 299.1 | 0.068597 | ||||||
| ChCl + PhOH + EG (1:5:4) | 142.92 | 1.09 | 29 | 101.3 | 298.2 | 0.16391 | [19] |
| 1.085 | 12.5 | 313.2 | 0.11908 | ||||
| ChCl + PhOH + EG (1:7:4) | 153.8 | 1.08 | 12 | 101.3 | 313.2 | 0.13039 | |
| ChCl + EG (1:2) | 87.9 | 100.5 | 313.15 | 0.046 | [20] | ||
| ChCl + Gly (1:2) | 107.96 | 132.3 | 0.051 | ||||
| ChCl + MU (1:2) | 95.93 | 147.2 | 0.032 | ||||
| ChCl + TA (1:2) | 121.9 | 137.4 | 0.045 | ||||
| ChCl + 1,4-BD (1:4) | 100.02 | 1.0403 | 54.75 | 23.2 | 303.15 | 0.01276 | [21] |
| 115.8 | 0.03408 | ||||||
| 396.9 | 0.15225 | ||||||
| 1.0348 | 36.66 | 33.3 | 313.15 | 0.0118 | |||
| 121.8 | 0.02498 | ||||||
| 389.2 | 0.11117 | ||||||
| ChCl + 1,4-BD (1:3) | 102.5 | 1.0471 | 60.64 | 31.2 | 303.15 | 0.0163 | |
| 113.7 | 0.04961 | ||||||
| 396.9 | 0.15075 | ||||||
| 1.0416 | 40.55 | 47.2 | 313.15 | 0.01557 | |||
| 131.7 | 0.04 | ||||||
| 416.6 | 0.10763 | ||||||
| ChCl + 2,3-BD (1:4) | 100.02 | 1.0307 | 71.79 | 37.6 | 303.15 | 0.01335 | |
| 120.9 | 0.04382 | ||||||
| 395.8 | 0.13483 | ||||||
| 1.0239 | 40.72 | 37 | 313.15 | 0.00908 | |||
| 124.1 | 0.03241 | ||||||
| 384 | 0.09424 | ||||||
| ChCl + 2,3-BD (1:3) | 102.5 | 1.039 | 84.88 | 32.4 | 303.15 | 0.01337 | |
| 121 | 0.04596 | ||||||
| 384.8 | 0.12796 | ||||||
| 1.0325 | 48.73 | 49 | 313.15 | 0.01402 | |||
| 130.4 | 0.0353 | ||||||
| 390.4 | 0.0978 | ||||||
| ChCl + 1,3-PD (1:4) | 88.8 | 1.0705 | 34.45 | 32.7 | 303.15 | 0.02166 | |
| 132.8 | 0.06788 | ||||||
| 391.5 | 0.16814 | ||||||
| 1.0648 | 24.21 | 31.6 | 313.15 | 0.01197 | |||
| 121.8 | 0.04286 | ||||||
| 390.7 | 0.11846 | ||||||
| ChCl + 1,3-PD (1:3) | 91.98 | 1.0753 | 40.05 | 34 | 303.15 | 0.01448 | |
| 131.7 | 0.05489 | ||||||
| 396.2 | 0.14728 | ||||||
| 1.0697 | 27.84 | 31.4 | 313.15 | 0.01083 | |||
| 128.5 | 0.0428 | ||||||
| 401.4 | 0.11376 | ||||||
| ChCl + ImZ + EG (3:7:14) | 73.52 | 1.105 | 15 | 11 | 313.2 | 0.00848 | [22] |
| 101.6 | 0.0836 | ||||||
| ChCl + TrZ + EG (3:7:14) | 73.79 | 1.126 | 15 | 9.1 | 0.02176 | ||
| 104.3 | 0.11061 | ||||||
| ChCl + TetrZ + EG (3:7:14) | 74.1 | 1.158 | 12 | 7.7 | 0.07313 | ||
| 104.9 | 0.16948 | ||||||
| ChCl + Res (1:3) | 117.48 | 101.3 | 313.2 | 0.053 | [23] | ||
| ChCl + Phe (1:3) | 105.49 | 0.081 | |||||
| ChCl + EG (1:2) | 87.9 | 0.041 | |||||
| ChCl + PhA (1:2) | 137.31 | 0.043 | |||||
| ChCl + Gly (1:2) | 107.96 | 0.053 | |||||
| ChCl + Phe + EG (1:3:5) | 81.367 | 0.091 | |||||
| ChCl + Phe + Gly (1:3:5) | 98.046 | 0.095 | |||||
| ChCl + Res + Gly (1:3:5) | 103.375 | 101.3 | 293.15 | 0.18 | |||
| 298.15 | 0.17009 | ||||||
| 1.21 | 313.15 | 0.1303 | |||||
| ChCl + D-fructose + Gly (1:3:5) | 126.73 | 101.3 | 313.15 | 0.11027 | |||
| ChCl + DL-malic Acid + Gly (1:3:5) | 111.37 | 0.045 | |||||
| ChCl + Levulinic Acid + Gly (1:3:5) | 105.38 | 0.055 | |||||
| ChCl + Oxalic Acid + Gly (1:3:5) | 96.69 | 0.074 | |||||
| ChCl + Malonic Acid + Gly (1:3:5) | 101.36 | 0.081 | |||||
| ChCl + Phenylacetic Acid + Gly (1:3:5) | 112.06 | 0.097 | |||||
| Ethylamine-hydrochloride-based DESs | |||||||
| EaCl + Urea (2:1) | 74.38 | 1.103 | 97.8 | 8.5 | 313.2 | 0.00882 | [24] |
| 98.5 | 0.07486 | ||||||
| 301.7 | 0.1877 | ||||||
| EaCl + Urea (1:1) | 70.81 | 1.142 | 197.7 | 9.5 | 0.01102 | ||
| 99.1 | 0.07788 | ||||||
| 300.6 | 0.179 | ||||||
| EaCl + Urea (1:2) | 67.23 | 1.179 | 105.5 | 8.4 | 0.00897 | ||
| 96.3 | 0.07117 | ||||||
| 296.2 | 0.17134 | ||||||
| EaCh + Gly (1:2) | 88.6 | 101.3 | 313.15 | 0.114 | [25] | ||
| EaCl + Gly (1:5) | 90.3 | 101.3 | 313.2 | 0.129 | [26] | ||
| EaCl + Res + Gly (1:1:5) | 93.15 | 0.149 | |||||
| EaCl + Res + Gly (1:2:5) | 95.3 | 0.163 | |||||
| EaCl + Res + Gly (1:3:5) | 96.9 | 0.174 | |||||
| EaCl + Res + Gly (1:4:5) | 98.2 | 1.244 | 0.181 | ||||
| EaCh + PhOH (1:2) | 166.0 | 101.3 | 313.15 | 0.119 | [27] | ||
| EaCl + PhOH (1:5) | 187.1 | 313.2 | 0.138 | ||||
| EaCl + PhOh (1:7) | 192.4 | 298.2 | 0.167 | ||||
| Ammonium-thiocyanate-based DESs | |||||||
| NH4SCN + Gly (2:3) | 85.7 | 1.239 | 71.18 | 101.3 | 313.2 | 0.17642 | [12] |
| NH4SCN + EG (1:3) | 65.58 | 1.138 | 11.46 | 0.1684 | |||
| NH4SCN + Urea (2:3) | 91.3 | 1.256 | 41.04 | 0.1463 | |||
| NH4SCN + AT (2:3) | 65.89 | 0.0918 | |||||
| NH4SCN + CL (2:3) | 98.34 | 0.0295 | |||||
| NH4SCN + Im (1:2) | 70.76 | 1.115 | 17.47 | 100 | 303.15 | 0.164 | [28] |
| 1.106 | 13 | 100 | 313.15 | 0.117 | |||
| NH4SCN + Im (1:3) | 70.09 | 1.125 | 18.22 | 100 | 303.15 | 0.122 | |
| Other DESs | |||||||
| MAA + TetrZ (2:1) | 72.08 | 1.05 | 33 | 9 | 313.2 | 0.07834 | [29] |
| 102.9 | 0.13624 | ||||||
| Tri + Gly (1:3) | 86.32 | 1.249 | 163 | 101.3 | 303.15 | 0.147 | [30] |
| Im + Gly (1:3) | 86.09 | 0.126 | |||||
| Tz + Gly (1:3) | 86.58 | 0.179 | |||||
| [bmim][MeSO3] + Urea (1:1) | 147.19 | 1.189 | 295.72 | 172.6 | 313.2 | 0.01787 | [31] |
| [Im][NO3] + EG (1:3) | 1.213 | 9.8 | 101.3 | 313.15 | 0.172 | [32] | |
| [Mim][NO3] + EG (1:3) | 1.186 | 8.7 | 0.152 | ||||
| [Mmim][NO3] + EG (1:3) | 1.175 | 9 | 0.141 | ||||
| Im + Res (1:1) | 89.09 | 1.2017 | 101.3 | 313.15 | 0.154 | [33] | |
| Im + Res (1.5:1) | 84.89 | 101.3 | 313.15 | 0.128 | |||
| Im + Res (2:1) | 82.08 | 101.3 | 313.15 | 0.107 | |||
| Im + Res (2.5:1) | 80.08 | 101.3 | 313.15 | 0.101 | |||
| [Me2COH2N]Cl + U (1:1) | 114.86 | 1.211 | 723.38 | 101.3 | 313.2 | 0.035 | [13] |
| [MeCOH3N]Cl + U (1:1) | 129.96 | 1.250 | 1026.3 | 0.045 | |||
| Ionic Liquids | |||||||
| [mim][Tf2N] | 362.24 | 6.496 | 313 | 0.04983 | [34] | ||
| 102.71 | 0.12599 | ||||||
| 610.14 | 0.27785 | ||||||
| [eim][Tf2N] | 376.27 | 1.559711 | 33.165 | 8.179 | 0.04979 | ||
| 105.12 | 0.12356 | ||||||
| 622.99 | 0.28106 | ||||||
| [mmim][Tf2N] | 376.27 | 1.569724 | 52.96 | 14.488 | 0.043 | ||
| 98.631 | 0.10862 | ||||||
| 610.92 | 0.25934 | ||||||
| [bim][SCN] | 182.26 | 1.084584 | 152.28 | 51.718 | 303 | 0.16445 | |
| 98.817 | 0.20369 | ||||||
| 546.09 | 0.51297 | ||||||
| 1.078656 | 84.302 | 46.817 | 313 | 0.14483 | |||
| 96.588 | 0.18314 | ||||||
| 563.18 | 0.42981 | ||||||
| [bim][NO3] | 186.18 | 1.172231 | 248 | 100.09 | 303 | 0.13721 | |
| 553.89 | 0.43814 | ||||||
| 1.165499 | 136.95 | 86.01 | 313 | 0.10611 | |||
| 141.53 | 0.15733 | ||||||
| 515.83 | 0.34301 | ||||||
| [bmim][SCN] | 197.3 | 1.068149 | 45.263 | 72.441 | 303 | 0.01381 | |
| 144.51 | 0.02762 | ||||||
| 559.47 | 0.13897 | ||||||
| 1.062258 | 30.461 | 82.918 | 313 | 0.01295 | |||
| 203.46 | 0.03107 | ||||||
| 556.94 | 0.09667 | ||||||
| [bmim][DCA] | 223.3 | 1.058303 | 25.093 | 61.162 | 303 | 0.0122 | |
| 114.28 | 0.02288 | ||||||
| 567.88 | 0.15329 | ||||||
| 1.05196 | 18.133 | 62.376 | 313 | 0.00763 | |||
| 128.32 | 0.01678 | ||||||
| 565.56 | 0.1022 | ||||||
| [bmmim][Tf2N] | 433.39 | 1.414188 | 77.731 | 50.322 | 303 | 0.00393 | |
| 119.43 | 0.00982 | ||||||
| 536.8 | 0.06287 | ||||||
| 1.404927 | 49.703 | 100.49 | 313 | 0.00786 | |||
| 216.34 | 0.01729 | ||||||
| 607.72 | 0.06012 | ||||||
| [bmmim][SCN] | 211.33 | 1.068578 | 192.26 | 95.668 | 303 | 0.01451 | |
| 184.62 | 0.0282 | ||||||
| 544 | 0.10395 | ||||||
| 1.062887 | 108.5 | 40.706 | 313 | 0.00484 | |||
| 149.89 | 0.01692 | ||||||
| 560.58 | 0.07656 | ||||||
| [bmmim][DCA] | 237.33 | 1.053082 | 47.66 | 115.38 | 303 | 0.01794 | |
| 199.86 | 0.03157 | ||||||
| 560.58 | 0.11553 | ||||||
| 1.04702 | 31.616 | 103.42 | 313 | 0.00861 | |||
| 174.97 | 0.02081 | ||||||
| 569.34 | 0.08252 | ||||||
| [bmim][BF4] | 226.02 | 1.203 | 123.41 | 101.3 | 298.2 | 0.01701 | [35] |
| 130 | 0.01543 | ||||||
| 200 | 323 | 0.01027 | |||||
| 220 | 298.15 | 0.03505 | [36] | ||||
| 630 | 0.14856 | ||||||
| 80 | 313.15 | 0.00827 | |||||
| 610 | 0.09648 | ||||||
| 500 | 293 | 0.07535 | [37] | ||||
| [bmim][PF6] | 284.18 | 101.3 | 298.2 | 0.02101 | [35] | ||
| 170 | 0.03227 | ||||||
| 270 | 323 | 0.02448 | |||||
| [bmim][Tf2N] | 419.36 | 60.7 | 101.3 | 299.4 | 0.0053 | ||
| [emim][Tf2N] | 391.31 | 140 | 323 | 0.0065 | |||
| 170 | 0.0043 | ||||||
| [hmim][Cl] | 202.72 | 100 | 323 | 0.00536 | |||
| 130 | 0.02509 | ||||||
| [emim][BF4] | 101.3 | 333.15 | 0.0095 | [38] | |||
| [emim][Tf2N] | 419.36 | 0.0065 | |||||
| [emim][NO3] | 0.0097 | ||||||
| [EtOHmim][SCN] | 1.2 | 55 | 313.15 | 0.031 | |||
| [EtOHmim][NO3] | 1.32 | 90 | 0.02 | ||||
| [EtOHmim][PF6] | 1.54 | 92 | 0.038 | ||||
| [EtOHmim][BF4] | 213.97 | 1.35 | 54.09 | 0.04502 | |||
| [EtOHmim][Tf2N] | 1.57 | 40 | 0.036 | ||||
| [EtOHmim][DCA] | 193.21 | 1.18 | 38.83 | 0.018 | |||
| [emim][Ac] | 170.11 | 101.3 | 298.3 | 0.03202 | [39] | ||
| 590 | 298 | 0.14406 | |||||
| 540 | 323 | 0.11752 | |||||
| [emim][SCN] | 169.24 | 101.3 | 298.1 | 0.04502 | |||
| 440 | 298 | 0.07906 | |||||
| 420 | 323 | 0.07287 | |||||
| [emim][EtOSO3] | 236.29 | 520 | 298 | 0.07808 | |||
| 480 | 323 | 0.06653 | |||||
| 470 | 323 | 0.10123 | |||||
| [DMEA][AC] | 149.19 | 101.3 | 298.1 | 0.10006 | |||
| 470 | 0.10123 | ||||||
| [emim][BF4] | 197.97 | 110 | 298.15 | 0.01487 | [36] | ||
| 550 | 0.13893 | ||||||
| 140 | 313.15 | 0.01235 | |||||
| 620 | 0.09535 | ||||||
| [hmim][BF4] | 254.08 | 220 | 298.15 | 0.03891 | |||
| 600 | 0.15447 | ||||||
| 230 | 313.15 | 0.02507 | |||||
| 600 | 0.09139 | ||||||
| [omim][BF4] | 282.17 | 120 | 298.15 | 0.02333 | |||
| 610 | 0.17877 | ||||||
| 180 | 313.15 | 0.02385 | |||||
| 600 | 0.10908 | ||||||
| [TMGH][BF4] | 201.98 | 101.3 | 293.2 | 0.09006 | [37] | ||
| 520 | 293 | 0.09134 | |||||
| [TMGH][NTf2] | 395.32 | 540 | 293 | 0.05057 | |||
| [TMGHPO2][BF4] | 264.95 | 101.3 | 293.2 | 0.038 | |||
| 420 | 293 | 0.04654 | |||||
| [MTEOA][MeOSO3] | 275.32 | - | 440.01 | 313 | 0.082 | [40] | |
| IL-4 | 1.184 | 5389.8 | 101.3 | 313.15 | 0.012 | [17] | |
| IL-5 | 1.218 | 3366.5 | 0.028 | ||||
| [HOOC]17-COFs | 100 | 298 | 0.15915 | [41] | |||
| [HOOC]33-COFs | 100 | 298 | 0.1399 | ||||
| [HOOC]0-COFs | 100 | 283 | 0.15728 | ||||
| [HOOC]0-COFs | 100 | 298 | 0.11672 | ||||
| [HOOC(CH2)3mim][Tf2N] | 449.23 | 1592 | 313 | 0.058 | [42] | ||
| 9 | Characteristics |
|---|---|
| Detectors | TCD №1, 393.15K TCD №2, 463.15 K TCD №3, 463.15 K |
| Columns | 15% PEG-600 on PTFE, 333.15 K 60/80 mesh 3 m × 2 mm i.d. stainless steel tube (TCD №1) 333.15 K CaA 5A, 333.15 K 60/80 mesh 2 m × 2 mm i.d. stainless steel tube (TCD №1) 333.15 K Hayesep B, 333.15 K 60/80 mesh 3 m × 2 mm i.d. stainless steel tube (TCD №2) 333.15 K Hayesep Q, 333.15 K 60/80 mesh 2 m × 2 mm i.d. stainless steel tube (TCD №3) 333.15 K |
| Sample loop | 0.25 cm3, 383.15 K (TCD №1) 1 cm3, 453.15 K (TCD №2, 3) |
| Carrier gas | He ≥ 99.995 vol.% (TCD №1, 2, 3) 20 cm3 min−1 |
| Parameter | Value |
|---|---|
| Feed pressure | 0.4 MPa |
| Permeate pressure | 0.132–0.135 MPa |
| Temperature | 298.15 K |
| Feed flow rate | 30.5–42.5 cm3 min−1 |
| Retentate flow rate | 30 cm3 min−1 |
| Helium sweep flow rate | 30 cm3 min−1 |
| H2/N2 mix sweep flow rate | 30 cm3 min−1 |
| Permeance, GPU a | α(NH3/x) | |||
|---|---|---|---|---|
| PSF | PEI + PI | PSF | PEI + PI | |
| NH3 | 1691 | 400.9 | - | - |
| H2 | 563 | 135.6 | 3 | 3 |
| N2 | 21.7 | 1.6 | 77.9 | 250.6 |
| Ar | 31.3 | 2.7 | 54 | 148.5 |
| CH4 | 23 | 2.8 | 73.5 | 143.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petukhov, A.N.; Atlaskin, A.A.; Smorodin, K.A.; Kryuchkov, S.S.; Zarubin, D.M.; Atlaskina, M.E.; Petukhova, A.N.; Stepakova, A.N.; Golovacheva, A.A.; Markov, A.N.; et al. An Efficient Technique for Ammonia Capture in the Haber–Bosch Process Loop—Membrane-Assisted Gas Absorption. Polymers 2022, 14, 2214. https://doi.org/10.3390/polym14112214
Petukhov AN, Atlaskin AA, Smorodin KA, Kryuchkov SS, Zarubin DM, Atlaskina ME, Petukhova AN, Stepakova AN, Golovacheva AA, Markov AN, et al. An Efficient Technique for Ammonia Capture in the Haber–Bosch Process Loop—Membrane-Assisted Gas Absorption. Polymers. 2022; 14(11):2214. https://doi.org/10.3390/polym14112214
Chicago/Turabian StylePetukhov, Anton N., Artem A. Atlaskin, Kirill A. Smorodin, Sergey S. Kryuchkov, Dmitriy M. Zarubin, Maria E. Atlaskina, Anastasia N. Petukhova, Anna N. Stepakova, Anna A. Golovacheva, Artem N. Markov, and et al. 2022. "An Efficient Technique for Ammonia Capture in the Haber–Bosch Process Loop—Membrane-Assisted Gas Absorption" Polymers 14, no. 11: 2214. https://doi.org/10.3390/polym14112214
APA StylePetukhov, A. N., Atlaskin, A. A., Smorodin, K. A., Kryuchkov, S. S., Zarubin, D. M., Atlaskina, M. E., Petukhova, A. N., Stepakova, A. N., Golovacheva, A. A., Markov, A. N., Stepanova, E. A., Vorotyntsev, A. V., & Vorotyntsev, I. V. (2022). An Efficient Technique for Ammonia Capture in the Haber–Bosch Process Loop—Membrane-Assisted Gas Absorption. Polymers, 14(11), 2214. https://doi.org/10.3390/polym14112214








