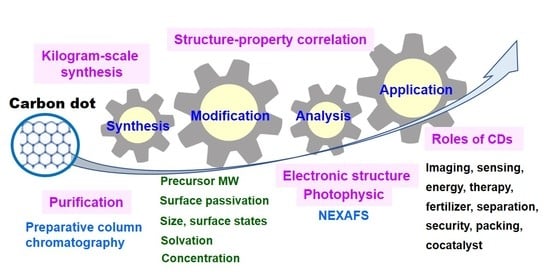
Recent Advances in Synthesis, Modification, Characterization, and Applications of Carbon Dots
Abstract
:1. Introduction
2. Preparation and Modification of CDs
2.1. Synthesis Methods
2.1.1. Top-Down Approach
Laser Ablation
Electrochemical Method
2.1.2. Bottom-Up Approach
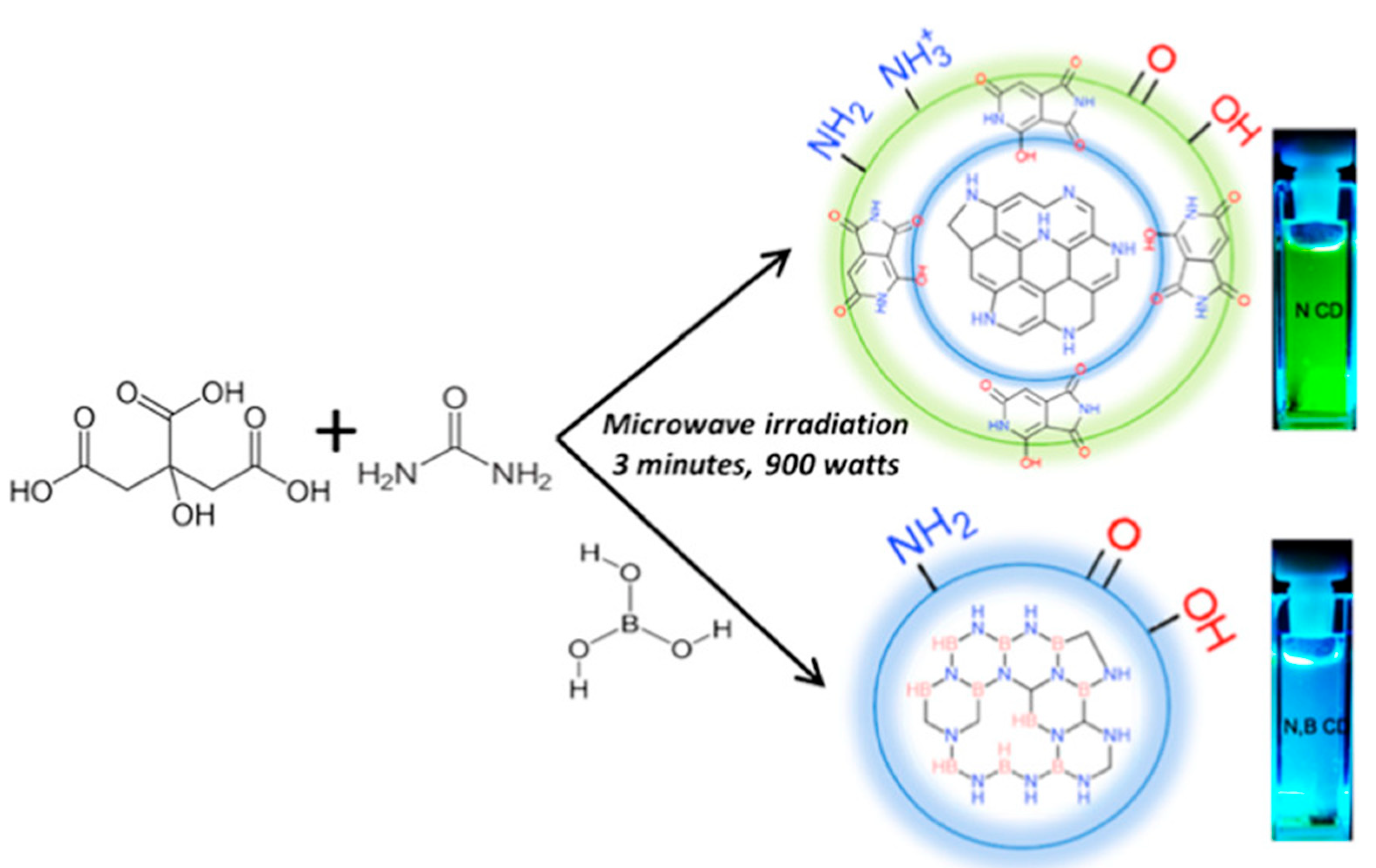
Hydrothermal, Solvothermal Method
Microwave-Assisted Synthesis
Ultrasonic Methods
2.1.3. Fabrication of Kilogram-Scale CDs
2.2. Purification of Carbon Dots
2.3. Doping of CDs
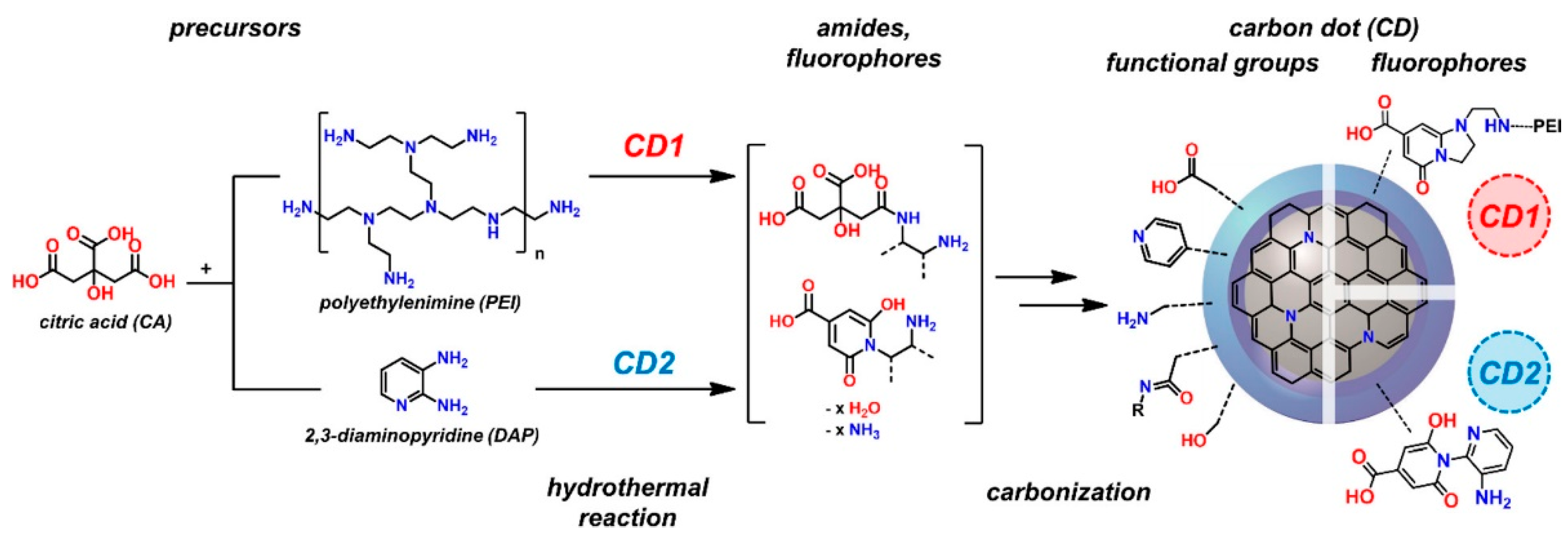
2.4. Surface Modification
3. Key Factors
3.1. Synthesis Parameters Affecting the Properties/Performance of CDs
3.1.1. Process Parameters
- (1)
- Microwave-assisted process: incubation time and precursor ratio:
- (2)
- Laser ablation synthesis: laser-pulse width
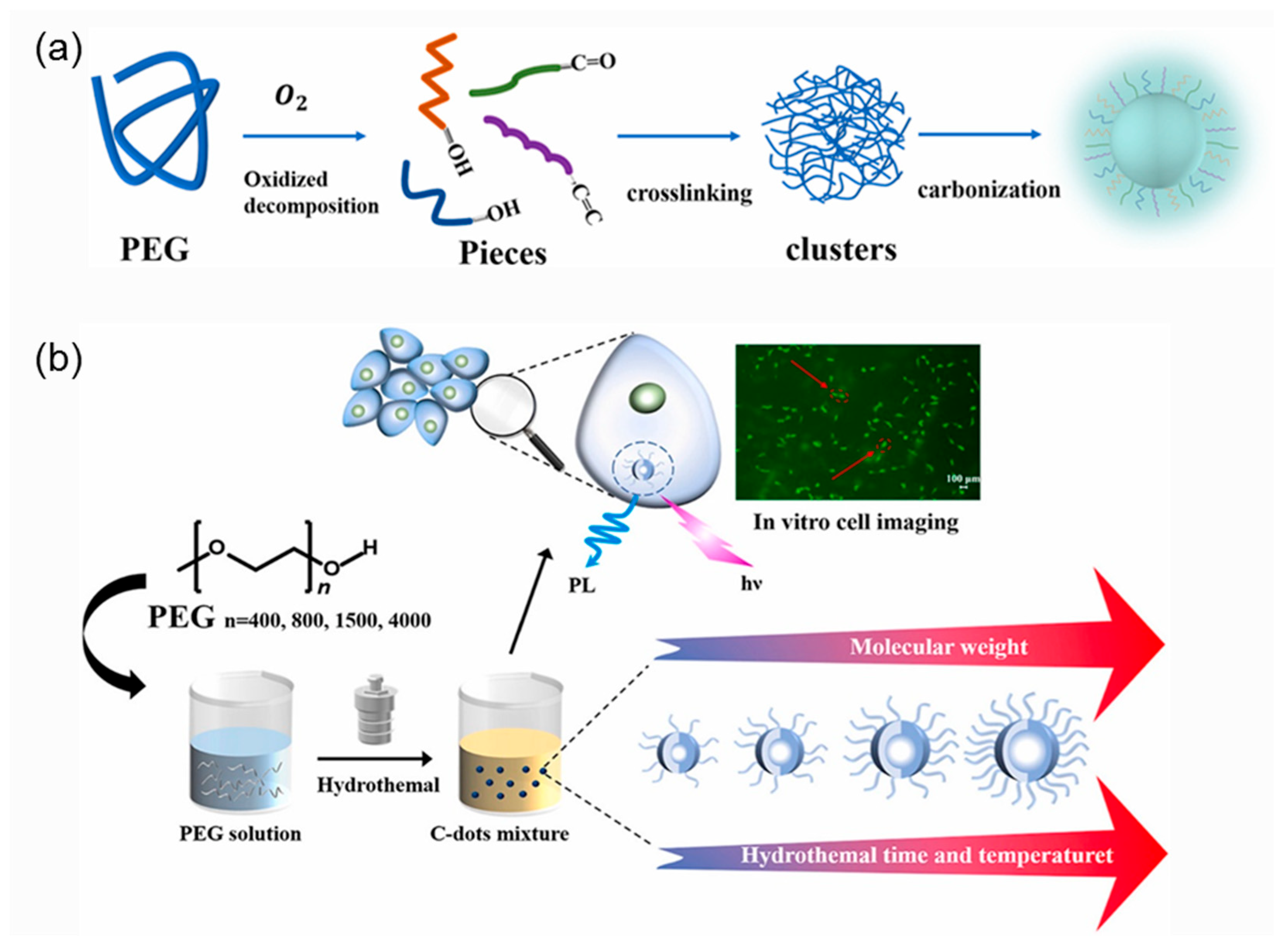
3.1.2. Average Molar Mass of Polymeric Precursor
3.1.3. Surface Passivation Effect
3.1.4. Purification by Preparative Column Chromatography
3.2. Factors Affecting the Properties/Performance of CDs
3.2.1. Concentration-Dependent Multicolor Luminescence
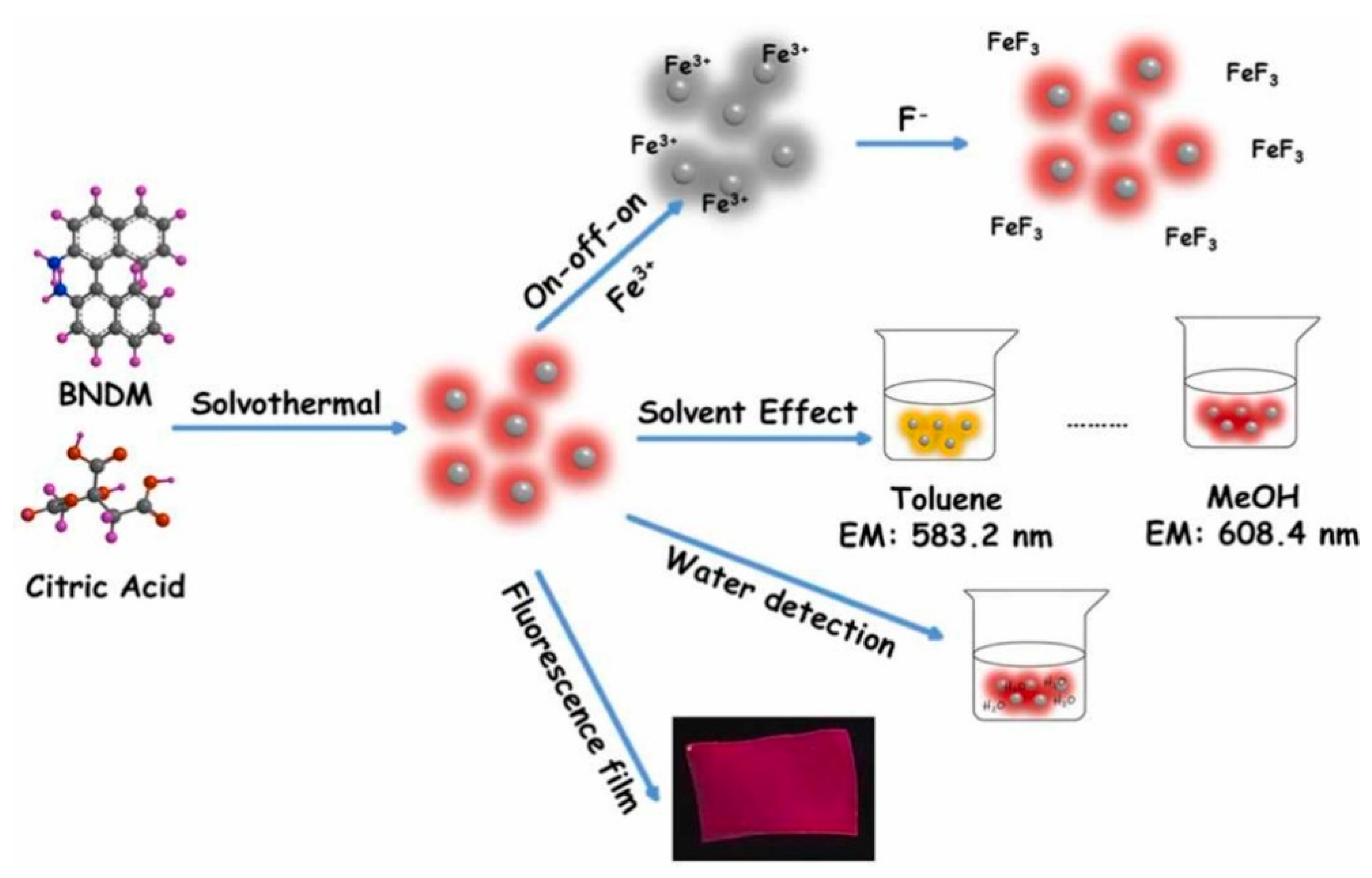
3.2.2. Solvation Effects (Polarity-Sensitive Fluorescence Effect)
3.2.3. Size and Surface States
3.2.4. Electronic Structures and Photophysics Analysis of CDs
4. Applications of CDs
4.1. Degradation of Organic Toxicants
4.1.1. Organic Dyes
4.1.2. Possible Mechanism
4.1.3. Pharmaceutical Pollutant Removal
4.2. Treatment of Inorganic Toxicant
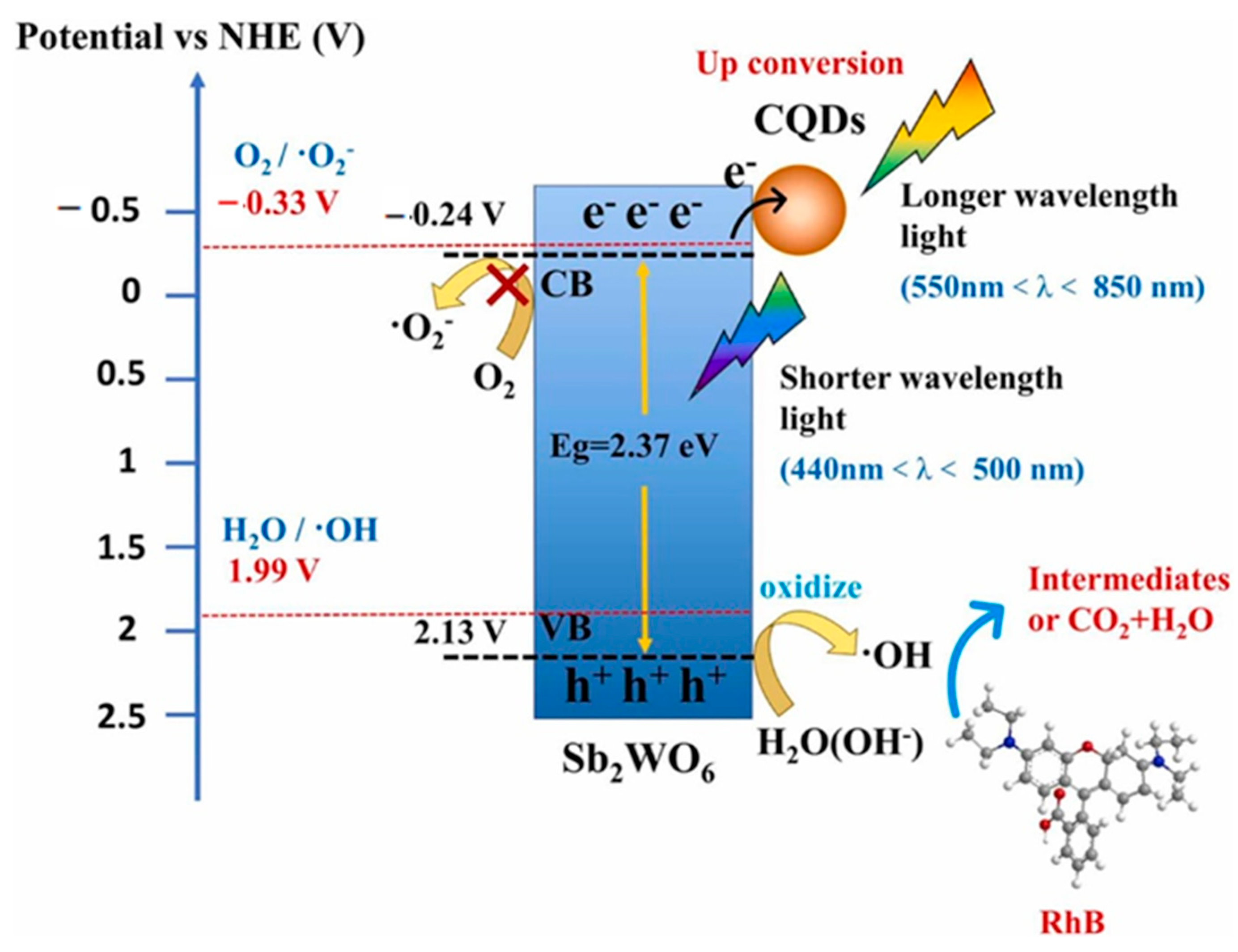
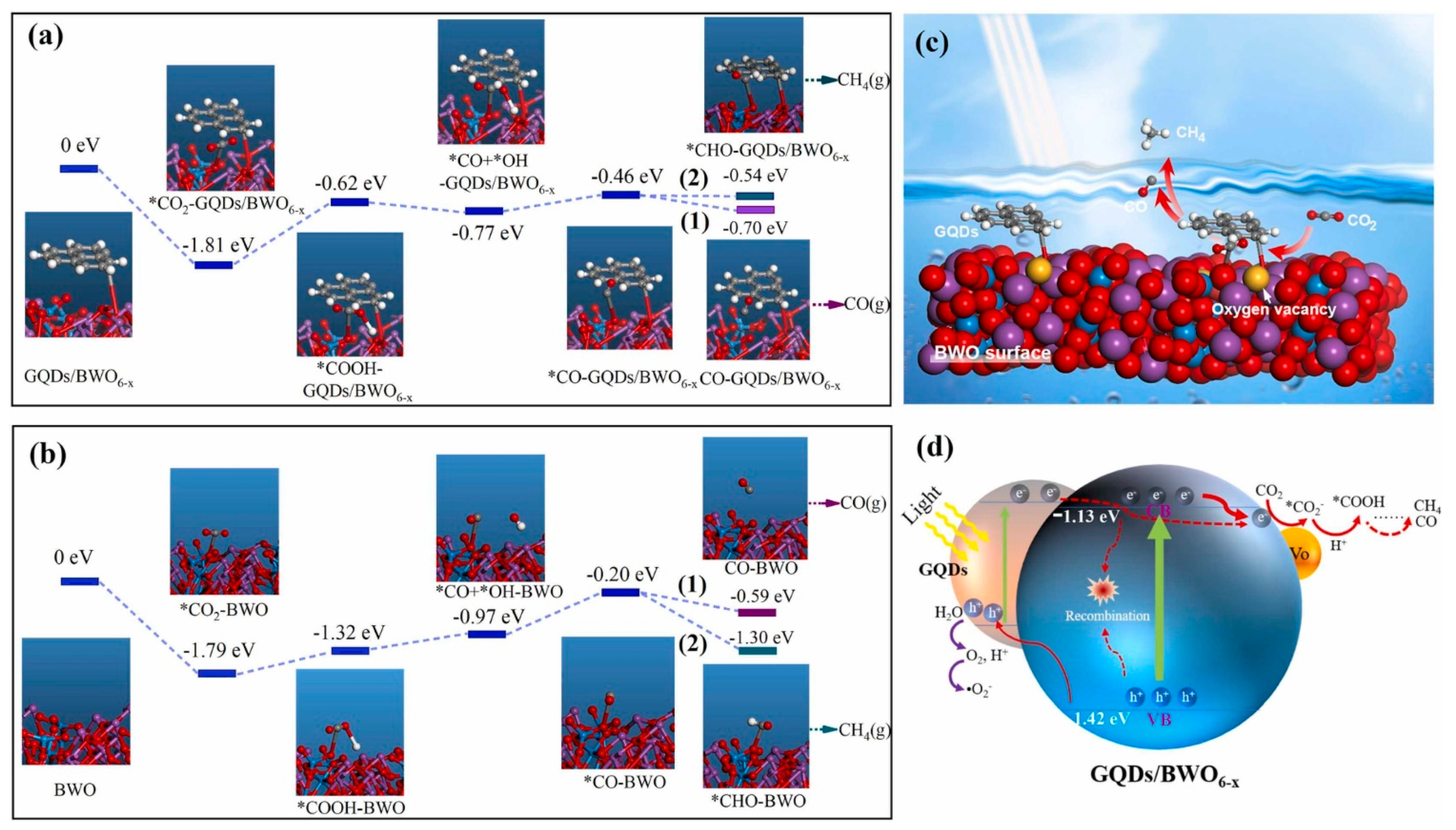
4.3. CO2 Reduction
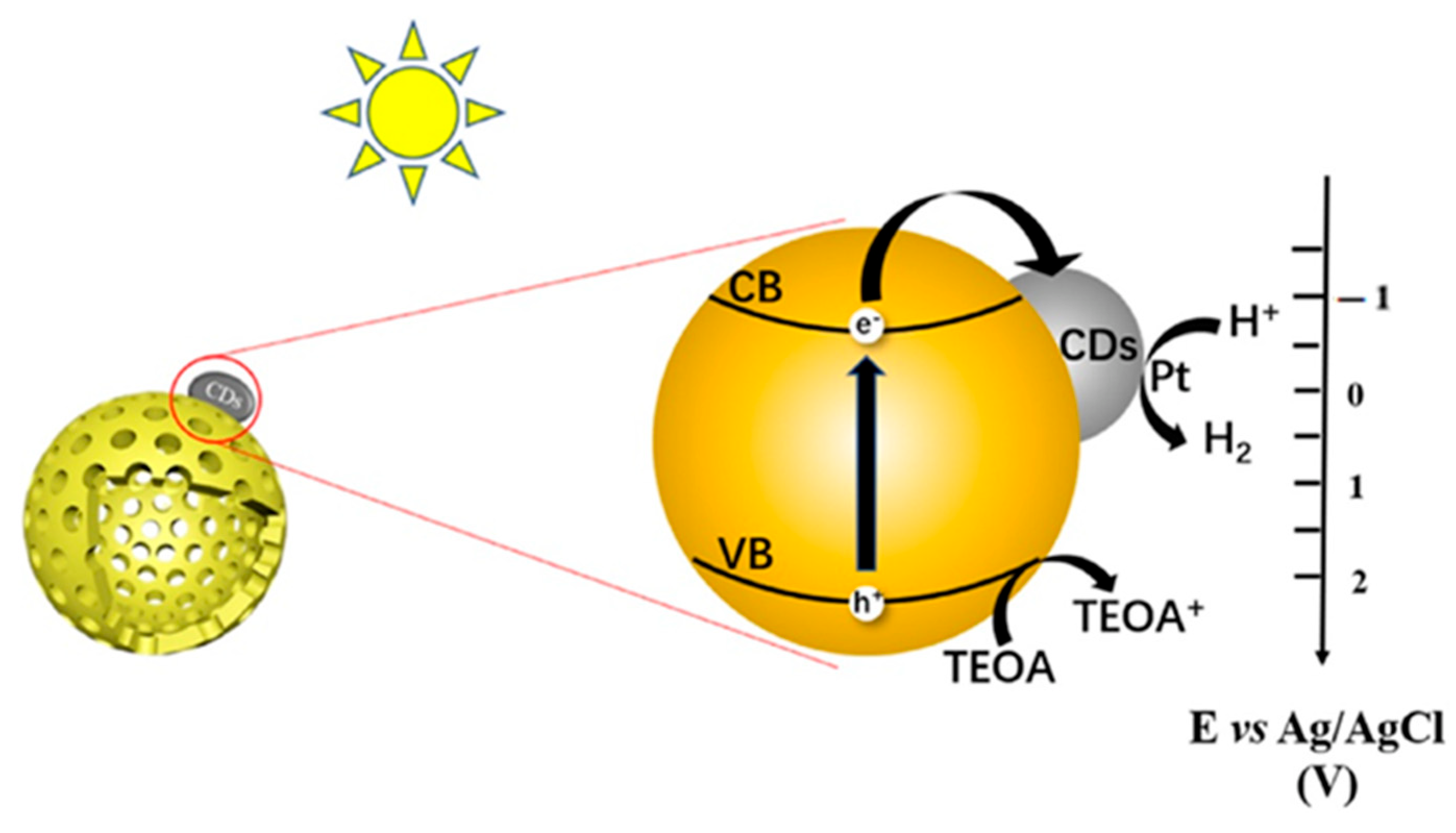
4.4. Hydrogen Evolution
4.5. Antimicrobial
4.5.1. Food Storage
4.5.2. Wound Healing
4.6. Cell Imaging
4.7. CDs for Pollutant Sensing
4.8. Possible Applications and Roles of Various CDs
5. Summary and Future Perspective
- (1)
- Commercial-scale fabrication:
- (2)
- Surface functional groups:
- (3)
- Structure/composition-property correlation:
- (4)
- Photoluminescence property:
- (5)
- Characterization techniques:
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ðorđević, L.; Arcudi, F.; Cacioppo, M.; Prato, M. A multifunctional chemical toolbox to engineer carbon dots for biomedical and energy applications. Nat. Nanotechnol. 2022, 17, 112–130. [Google Scholar] [CrossRef]
- Wu, J.; Chen, G.; Jia, Y.; Ji, C.; Wang, Y.; Zhou, Y.; Peng, Z. Carbon Dots Composites for Bioapplications: A Review. J. Mater. Chem. B 2022, 10, 843–869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, X.; Hou, Y.; Jia, B.; Fu, L.; Jia, M.; Kong, W. Recent advances in synthesis and modification of carbon dots for optical sensing of pesticides. J. Hazard. Mater. 2022, 422, 126881. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.; Garg, A.K.; Dalal, C.; Anand, S.R.; Sonkar, S.K.; Sonker, A.K.; Westman, G. Visible-Light-Promoted Photocatalytic Applications of Carbon Dots: A Review. ACS Appl. Nano Mater. 2022, 5, 3087–3109. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhang, K.; Lu, Y.; Chen, J.; Wang, S.; Wang, X. Application of carbon dots and their composite materials for the detection and removal of radioactive ions: A review. Chemosphere 2022, 287, 132313. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, B.; Shi, R.; Zhang, S.; Liu, Y.; Wang, B.; Lu, S. Carbon Dots as New Building Blocks for Electrochemical Energy Storage and Electrocatalysis. Adv. Energy Mater. 2022, 12, 2103426. [Google Scholar] [CrossRef]
- Han, Y.; Yang, W.; Luo, X.; He, X.; Zhao, H.; Tang, W.; Li, Z. Carbon dots based ratiometric fluorescent sensing platform for food safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 244–260. [Google Scholar] [CrossRef]
- Korah, B.K.; Chacko, A.R.; Abraham, T.; Mathew, B. Recent Progress and Future Perspectives of Carbon Dots in the Detection, Degradation, and Enhancement of Drugs. Part. Part. Syst. Charact. 2022, 39, 2100264. [Google Scholar] [CrossRef]
- Mohammadi, R.; Naderi-Manesh, H.; Farzin, L.; Vaezi, Z.; Ayarri, N.; Samandari, L.; Shamsipur, M. Fluorescence sensing and imaging with carbon-based quantum dots for early diagnosis of cancer: A review. J. Pharm. Biomed. 2022, 212, 114628. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Jiang, L.; Zuo, P.; Zhao, Y.; Wang, S.; Chen, X.; Liang, M.; Ma, L. Preparation of twin graphene quantum dots through the electric-field-assisted femtosecond laser ablation of graphene dispersions. Carbon 2021, 185, 384–394. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, S.; Huang, F.; Zhou, H.; Zhang, H.; Wang, S.; Zhou, S. Nitrogen-doped graphene quantum dots synthesized by femtosecond laser ablation in liquid from laser induced graphene. Nanotechnology 2021, 33, 115602. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Ding, J.; Wu, T.; Cai, S.; Zhang, W.; Cai, R.; Chen, C.; Yang, R. Synthesis of Carbon Quantum Dots for Application of Alleviating Amyloid-β Mediated Neurotoxicity. Colloids Surf. B 2022, 212, 112373. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Hoffman, J.; Morgiel, J.; Mościcki, T.; Stobiński, L.; Szymański, Z.; Małolepszy, A. Luminescent carbon dots synthesized by the laser ablation of graphite in polyethylenimine and ethylenediamine. Materials 2021, 14, 729. [Google Scholar] [CrossRef]
- Zhou, Q.; Yuan, G.; Lin, M.; Wang, P.; Li, S.; Tang, J.; Lin, J.; Huang, Y.; Zhang, Y. Large-scale electrochemical fabrication of nitrogen-doped carbon quantum dots and their application as corrosion inhibitor for copper. J. Mater. Sci. 2021, 56, 12909–12919. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, S.; Yuan, G.; Zhu, W.; Huang, Y.; Li, S.; Lin, M. Tailored graphene quantum dots to passivate defects and accelerate charge extraction for all-inorganic CsPbIBr2 perovskite solar cells. J. Alloy Compd. 2022, 895, 162529. [Google Scholar] [CrossRef]
- Danial, W.H.; Farouzy, B.; Abdullah, M.; Majid, Z.A. Facile one-step preparation and characterization of graphene quantum dots suspension via electrochemical exfoliation. Malays. J. Chem. 2021, 23, 127–135. [Google Scholar]
- Ng, H.M.; Lim, G.K.; Leo, C.P. Comparison between hydrothermal and microwave-assisted synthesis of carbon dots from biowaste and chemical for heavy metal detection: A review. Microchem. J. 2021, 165, 106116. [Google Scholar] [CrossRef]
- Saheeda, P.; Sabira, K.; Dhaneesha, M.; Jayaleksmi, S. Investigation on the pH-independent photoluminescence emission from carbon dots impregnated on polymer matrix. Luminescence 2018, 33, 22–28. [Google Scholar] [CrossRef]
- Sawalha, S.; Assali, M.; Nasasrah, A.; Salman, M.; Nasasrah, M.; Jitan, M.; Zyuod, A. Optical properties and photoactivity of carbon nanodots synthesized from olive solid wastes at different carbonization temperatures. RSC Adv. 2022, 12, 4490–4500. [Google Scholar] [CrossRef]
- Baluta, S.; Lesiak, A.; Cabaj, J. Simple and cost-effective electrochemical method for norepinephrine determination based on carbon dots and tyrosinase. Sensors 2020, 20, 4567. [Google Scholar] [CrossRef]
- Aggarwal, R.; Saini, D.; Singh, B.; Kaushik, J.; Garg, A.K.; Sonkar, S.K. Bitter apple peel derived photoactive carbon dots for the sunlight induced photocatalytic degradation of crystal violet dye. Sol. Energy 2020, 197, 326–331. [Google Scholar] [CrossRef]
- Lin, P.Y.; Hsieh, C.W.; Kung, M.L.; Chu, L.Y.; Huang, H.J.; Chen, H.T.; Wu, D.C.; Kuo, C.H.; Hsieh, S.L.; Hsieh, S. Eco-friendly synthesis of shrimp egg-derived carbon dots for fluorescent bioimaging. J. Biotechnol. 2014, 189, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Preethi, M.; Viswanathan, C.; Ponpandian, N. A metal-free, dual catalyst for the removal of Rhodamine B using novel carbon quantum dots from muskmelon peel under sunlight and ultrasonication: A green way to clean the environment. J. Photochem. Photobiol. A Chem. 2022, 426, 113765. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wu, M.; Feng, X.; Redfern, S.A.T.; Shang, Y.; Yong, X.; Feng, T.; Wu, K.; Liu, Z.; et al. Carbon-Quantum-Dots-Loaded Ruthenium Nanoparticles as an Efficient Electrocatalyst for Hydrogen Production in Alkaline Media. Adv. Mater. 2018, 30, 1800676. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, W.; Xu, C.; Liu, X.; Yang, K.; Li, D.; Dionysiou, D.D. Novel hierarchical carbon quantum dots-decorated BiOCl nanosheet/carbonized eggshell membrane composites for improved removal of organic contaminants from water via synergistic adsorption and photocatalysis. Chem. Eng. J. 2021, 420, 129582. [Google Scholar] [CrossRef]
- Mote, U.S.; Gore, A.H.; Panja, S.K.; Kolekar, G.B. Effect of Various Aqueous Extracting Agents on Fluorescence Properties of Waste Tea Residue Derived Carbon Dots (WTR-CDs): Comparative Spectroscopic Analysis. Luminescence 2022, 37, 440–447. [Google Scholar] [CrossRef]
- Koe, W.S.; Chong, W.C.; Pang, Y.L.; Koo, C.H.; Ebrahim, M.; Mohammad, A.W. Novel nitrogen and sulphur co-doped carbon quantum dots/titanium oxide photocatalytic membrane for in-situ degradation and removal of pharmaceutical compound. J. Water Process. Eng. 2020, 33, 101068. [Google Scholar] [CrossRef]
- Hong, W.T.; Yang, H.K. Luminescent properties of carbon dots originated from pine pollen foranti-counterfeiting application. Opt. LASER Technol. 2022, 145, 107452. [Google Scholar] [CrossRef]
- Duarah, R.; Karak, N. Hyperbranched polyurethane/reduced carbon dot-zinc oxide nanocomposite-mediated solar-assisted photocatalytic degradation of organic contaminant: An approach towards environmental remediation. Chem. Eng. J. 2019, 370, 716–728. [Google Scholar] [CrossRef]
- Kim, D.; Jo, G.; Chae, Y.; Subramani, S.; Lee, B.Y.; Kim, E.J.; Ji, M.K.; Sim, U.; Hyun, H. Bioinspired Camellia japonica carbon dots with high near-infrared absorbance for efficient photothermal cancer therapy. Nanoscale 2021, 13, 14426–14434. [Google Scholar] [CrossRef]
- Kozák, O.; Sudolská, M.; Pramanik, G.; Cígler, P.; Otyepka, M.; Zbořil, R. Photoluminescent carbon nanostructures. Chem. Mater. 2016, 28, 4085–4128. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, S.H. Carbon dots: Large-scale synthesis, sensing and Bioimaging. Mater. Today 2016, 19, 382–393. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis—A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Yuan, Y.; Wu, Y. Hydrothermal synthesis of fluorescent carbon dots from sodium citrate and polyacrylamide and their highly selective detection of lead and pyrophosphate. Carbon 2017, 115, 550–560. [Google Scholar] [CrossRef]
- Liao, X.; Chen, C.; Wang, P.; Zhou, R.; Zhao, X.; Fan, H.; Huang, Z. Carbon dots derived from cellobiose for temperature and phosalone detection. Mater. Res. Bull. 2022, 151, 111790. [Google Scholar] [CrossRef]
- Newman Monday, Y.; Abdullah, J.; Yusof, N.A.; Abdul Rashid, S.; Shueb, R.H. Facile Hydrothermal and Solvothermal Synthesis and Characterization of Nitrogen-Doped Carbon Dots from Palm Kernel Shell Precursor. Appl. Sci. 2021, 11, 1630. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Ning, Z.; Xu, Y. A catalytic microwave process for superfast preparation of high-quality reduced graphene oxide. Angew. Chem. Int. Ed. 2017, 56, 15677–15682. [Google Scholar] [CrossRef]
- Laddha, H.; Yadav, P.; Jain, Y.; Sharma, M.; Reza, M.; Agarwal, M.; Gupta, R. One-pot microwave-assisted synthesis of blue emissive multifunctional NSP co-doped carbon dots as a nanoprobe for sequential detection of Cr(VI) and ascorbic acid in real samples, fluorescent ink and logic gate operation. J. Mol. Liq. 2022, 346, 117088. [Google Scholar] [CrossRef]
- Uriarte, D.; Domini, C.; Garrido, M. New carbon dots based on glycerol and urea and its application in the determination of tetracycline in urine samples. Talanta 2019, 201, 143–148. [Google Scholar] [CrossRef]
- Balakrishnan, T.; Ang, W.L.; Mahmoudi, E.; Mohammad, A.W.; Sambudi, N.S. Formation mechanism and application potential of carbon dots synthesized from palm kernel shell via microwave assisted method. Carbon Resour. Convers. 2022; in press. [Google Scholar] [CrossRef]
- Olmos-Moya, P.M.; Velazquez-Martinez, S.; Pineda-Arellano, C.; Rangel-Mendez, J.R.; Chazaro-Ruiz, L.F. High added value functionalized carbon quantum dots synthetized from orange peels by assisted microwave solvothermal method and their performance as photosensitizer of mesoporous TiO2 photoelectrodes. Carbon 2022, 187, 216–229. [Google Scholar] [CrossRef]
- Bhatt, S.; Vyas, G.; Paul, P. Microwave-assisted synthesis of nitrogen-doped carbon dots using prickly pear as the carbon source and its application as a highly selective sensor for Cr(VI) and as a patterning agent. Anal. Methods 2022, 14, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Kalimuthu, R.; Kanagaraj, T.; Kulandaivelu, R.; Nagappan, R.; Pragasan, L.A.; Ponnusamy, V.K. Microwave-assisted green synthesis of multi-functional carbon quantum dots as efficient fluorescence sensor for ultra-trace level monitoring of ammonia in environmental water. Environ. Res. 2022, 206, 112589. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Hu, Z.; Peng, C.; Deng, L.; Liu, S. Effective adsorption and sensitive detection of Cr (VI) by chitosan/cellulose nanocrystals grafted with carbon dots composite hydrogel. Polymers 2021, 13, 3788. [Google Scholar] [CrossRef]
- El-Malla, S.F.; Elshenawy, E.; Hammad, S.; Mansour, F. Rapid microwave synthesis of N, S-doped carbon quantum dots as a novel turn off-on sensor for label-free determination of copper and etidronate disodium. Anal. Chim. Acta 2022, 1197, 339491. [Google Scholar] [CrossRef]
- Saleem, M.; Naz, M.Y.; Shukrullah, S.; Shujah, M.A.; Akhtar, M.; Ullah, S.; Ali, S. One-pot sonochemical preparation of carbon dots, influence of process parameters and potential applications: A review. Carbon Lett. 2022, 32, 39–55. [Google Scholar] [CrossRef]
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.U.; Yuan, C. Ultrasonic-assisted synthesis of N-doped, multicolor carbon dots toward fluorescent inks, fluorescence sensors, and logic gate operations. Nanomaterials 2022, 12, 312. [Google Scholar] [CrossRef]
- Kaimal, R.; Vinoth, V.; Salunke, A.S.; Valdés, H.; Mangalaraja, R.V.; Aljafari, B.; Anandan, S. Highly sensitive and selective detection of glutathione using ultrasonic aided synthesis of graphene quantum dots embedded over amine-functionalized silica nanoparticles. Ultrason. Sonochem. 2022, 82, 105868. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Ye, Y.; Guo, R.; Wang, A.; Zou, G.; Ji, X. Kilogram-scale synthesis and functionalization of carbon dots for superior electrochemical potassium storage. ACS Nano 2021, 15, 6872–6885. [Google Scholar] [CrossRef]
- Ji, C.; Han, Q.; Zhou, Y.; Wu, J.; Shi, W.; Gao, L.; Peng, Z. Phenylenediamine-derived near infrared carbon dots: The kilogram-scale preparation, formation process, photoluminescence tuning mechanism and application as red phosphors. Carbon 2022, 192, 198–208. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wang, B.; Song, H.; Liu, Z.; Lu, S.; Yang, B. Kilogram-scale synthesis of carbon quantum dots for hydrogen evolution, sensing and bioimaging. Chin. Chem. Lett. 2019, 30, 2323–2327. [Google Scholar] [CrossRef]
- Fang, L.; Wu, M.; Huang, C.; Liu, Z.; Liang, J.; Zhang, H. Industrializable synthesis of narrow-dispersed carbon dots achieved by microwave-assisted selective carbonization of surfactants and their applications as fluorescent nano-additives. J. Mater. Chem. A 2020, 8, 21317–21326. [Google Scholar] [CrossRef]
- Wang, X.; Yang, P.; Feng, Q.; Meng, T.; Wei, J.; Xu, C.; Han, J. Green preparation of fluorescent carbon quantum dots from cyanobacteria for biological imaging. Polymers 2019, 11, 616. [Google Scholar] [CrossRef] [Green Version]
- Kasprzyk, W.; Świergosz, T.; Bednarz, S.; Walas, K.; Bashmakova, N.V.; Bogdał, D. Luminescence phenomena of carbon dots derived from citric acid and urea-a molecular insight. Nanoscale 2018, 10, 13889–13894. [Google Scholar] [CrossRef]
- Yuan, J.M.; Zhao, R.; Wu, Z.J.; Li, W.; Yang, X.G. Graphene oxide quantum dots exfoliated from carbon fibers by microwave irradiation: Two photoluminescence centers and self-assembly behavior. Small 2018, 14, 1703714. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Chen, X.; Xu, Y.; Li, H. Mn(II)-coordinated fluorescent carbon dots: Preparation and discrimination of organic solvents. Opt. Mater. 2018, 78, 118–125. [Google Scholar] [CrossRef]
- Wu, Z.L.; Liu, Z.X.; Yuan, Y.H. Carbon dots: Materials, synthesis, properties and approaches to long-wavelength and multicolor emission. J. Mater. Chem. B 2017, 5, 3794–3809. [Google Scholar] [CrossRef]
- Li, L.; Shi, L.; Jia, J.; Eltayeb, O.; Lu, W.; Tang, Y.; Dong, C.; Shuang, S. Dual Photoluminescence Emission Carbon Dots for Ratiometric Fluorescent GSH Sensing and Cancer Cell Recognition. ACS Appl. Mater. Interfaces 2020, 12, 18250–18257. [Google Scholar] [CrossRef]
- Mohammed, L.J.; Omer, K.M. Dual functional highly luminescence B, N Co-doped carbon nanodots as nanothermometer and Fe3+/Fe2+ sensor. Sci. Rep. 2020, 10, 3028. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Tsai, Y.H.; Chang, C.W. Evaluation of the dialysis time required for carbon dots by HPLC and the properties of carbon dots after HPLC fractionation. New J. Chem. 2019, 43, 6153–6159. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef]
- Jia, X.; Han, Y.; Pei, M.; Zhao, X.; Tian, K.; Zhou, T.; Liu, P. Multi-functionalized hyaluronic acid nanogels crosslinked with carbon dots as dual receptor-mediated targeting tumor theranostics. Carbohydr. Polym. 2016, 152, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, Y.; Zhao, S.; Xiao, J.; Lan, M.; Wang, B.; Zhang, K.; Song, X.; Zeng, L. Metal ions-doped carbon dots: Synthesis, properties, and applications. Chem. Eng. J. 2022, 430, 133101. [Google Scholar] [CrossRef]
- Qing, W.; Chen, K.; Yang, Y.; Wang, Y.; Liu, X. Cu2+-doped carbon dots as fluorescence probe for specific recognition of Cr(VI) and its antimicrobial activity. Microchem. J. 2020, 152, 104262. [Google Scholar] [CrossRef]
- Wu, W.T.; Zhan, L.Y.; Fan, W.Y.; Song, J.Z.; Li, X.M.; Li, Z.T.; Wang, R.Q.; Zhang, J.Q.; Zheng, J.T.; Wu, M.B.; et al. Cu-N dopants boost electron transfer and photooxidation reactions of carbon dots. Angew. Chem. Int. Ed. 2015, 54, 6540–6544. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, P.; Wu, X.; Ma, C.; Luo, S.; Xu, M.; Li, W.; Liu, S. Nitrogen and copper(II) co-doped carbon dots for applications in ascorbic acid determination by non-oxidation reduction strategy and cellular imaging. Talanta 2020, 210, 120649. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhan, L.; Fan, W.; Song, J.; Li, X.; Li, Z.; Wang, R.; Zhang, J.; Zheng, J.; Wu, M.; et al. Cu-N Dopants Boost Electron Transfer and Photooxidation Reactions of Carbon Dots. Angew. Chem. Int. Ed. 2015, 127, 6640–6644. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Z.; Zhou, Y.; Tammina, S.K.; Yang, Y. A double carbon dot system composed of N, Cl-doped carbon dots and N, Cu-doped carbon dots as peroxidase mimics and as fluorescent probes for the determination of hydroquinone by fluorescence. Microchim. Acta 2020, 187, 350. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, S.; Sun, J.; He, P.; Yuan, N.; Ding, J.; Mo, R.; Wang, G.; Ding, G.; Xie, X. Deep ultraviolet emission photoluminescence and high luminescece efficiency of ferric passivated graphene quantum dots: Strong negative inductive effect of Fe. Synth. Met. 2015, 209, 468–472. [Google Scholar] [CrossRef]
- Yang, W.Q.; Huang, T.T.; Zhao, M.M.; Luo, F.; Weng, W.; Wei, Q.N.; Lin, Z.Y.; Chen, G.H. High peroxidase-like activity of iron and nitrogen co-doped carbon dots and its application in immunosorbent assay. Talanta 2017, 164, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Feng, T.; Chen, Y.; Zhao, X.; Yang, B. Ionic-State Cobalt and Iron Co-doped Carbon Dots with Superior Electrocatalytic Activity for the Oxygen Evolution Reaction. ChemElectroChem 2019, 6, 2088–2094. [Google Scholar] [CrossRef]
- Liu, Y.; Chao, D.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Yellow emissive carbon dots with quantum yield up to 68.6% from manganese ions. Carbon 2018, 135, 253–259. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, C.F.; Zhang, Y.; Yang, S.Y.; Chen, S. Zinc ion-doped carbon dots with strong yellow photoluminescence. RSC Adv. 2016, 6, 37189–37194. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, Y.; Xiao, S.; Wang, H.; Wang, J.H.; Feng, L. Rapid detection of Cr(VI) ions based on cobalt(II)-doped carbon dots. Biosens. Bioelectron. 2017, 87, 46–52. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Zhang, D.; Gan, L.; Zhao, P.; Zhang, Y.; Zhang, Q.; Hua, M.; Jia, C. Gadolinium-doped carbon quantum dots loaded magnetite nanoparticles as a bimodal nanoprobe for both fluorescence and magnetic resonance imaging. Magn. Reson. Imaging 2020, 68, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.L.; Kuzmanoski, A.; Wehner, T.; Müller-Buschbaum, K.; Feldmann, C. Microwave-assisted polyol synthesis of water dispersible red-emitting Eu3+-modified carbon dots. Materials 2016, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, Y.T.; Sun, X.F.; Zhang, G.P.; Dong, S.L.; Hao, J.C. Versatile selfassembly and biosensing applications of DNA and carbon quantum dots coordinated cerium ions. Chem. Eur. J. 2017, 23, 10413–10422. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, Z.X.; Zou, H.Y.; Huang, C.Z. Highly selective detection of 2,4,6-trinitrophenol by using newly developed terbium-doped blue carbon dots. Analyst 2016, 141, 2676–2681. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Yang, T.; Wang, J.; Dong Liu, X.; Huang, C.Z. One-pot carbonization synthesis of europium-doped carbon quantum dots for highly selective detection of tetracycline. Methods Appl. Fluoresc. 2017, 5, 015003. [Google Scholar] [CrossRef]
- Bera, K.; Sau, A.; Mondal, P.; Mukherjee, R.; Mookherjee, D.; Metya, A.; Kundu, A.K.; Mandal, D.; Satpati, B.; Chakrabarti, O.; et al. Metamorphosis of rutheniumdoped carbon dots: In search of the origin of photoluminescence and beyond. Chem. Mater. 2016, 28, 7404–7413. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Li, R.S.; Wang, Q.; Wu, Z.L.; Wang, J.; Liu, H.; Huang, C.Z. Germanium doped carbon dots as a new type of fluorescent probe for visualizing the dynamic invasions of mercury(II) ions into cancer cells. Nanoscale 2015, 7, 16841–16847. [Google Scholar] [CrossRef]
- Ma, G.; Ning, G.; Wei, Q. S-doped carbon materials: Synthesis, properties and applications. Carbon 2022, 195, 328–340. [Google Scholar] [CrossRef]
- Muthamma, K.; Sunil, D.; Shetty, P. Carbon dots as emerging luminophores in security inks for anti-counterfeit applications—An up-to-date review. Appl. Mater. Today 2021, 23, 101050. [Google Scholar] [CrossRef]
- Karuppusamy, N.; Mariyappan, V.; Chen, S.M.; Keerthi, M.; Ramachandran, R. A simple electrochemical sensor for quercetin detection based on cadmium telluride nanoparticle incorporated on boron, sulfur co-doped reduced graphene oxide composite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127094. [Google Scholar] [CrossRef]
- Shao, J.; Ma, C.; Zhao, J.; Wang, L.; Hu, X. Effective nitrogen and sulfur co-doped porous carbonaceous CO2 adsorbents derived from amino acid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127750. [Google Scholar] [CrossRef]
- Wang, H.B.; Tao, B.B.; Wu, N.N.; Zhang, H.D.; Liu, Y.M. Glutathione-stabilized copper nanoclusters mediated-inner filter effect for sensitive and selective determination of p-nitrophenol and alkaline phosphatase activity. Spectrochim. Acta A 2022, 271, 120948. [Google Scholar] [CrossRef]
- Qu, D.; Miao, X.; Wang, X.; Nie, C.; Li, Y.; Luo, L.; Sun, Z. Se & N co-doped carbon dots for high-performance fluorescence imaging agent of angiography. J. Mater. Chem. B 2017, 5, 4988–4992. [Google Scholar]
- Meng, A.; Huangfu, B.; Sheng, L.; Hong, X.; Li, Z. One-pot hydrothermal synthesis of boron and nitrogen co-doped carbon dots for copper ion assay and multicolor cell imaging using fluorescence quenchometric method. Microchem. J. 2022, 174, 106981. [Google Scholar] [CrossRef]
- Yu, N.; Huang, T.; Duan, T.; Bao, Y.; Gao, R.; Wang, X.; Han, C. Accurate detection and delineation boundary of renal cell carcinoma based on dual-targeted magnetic-fluorescent carbon dots. Chem. Eng. J. 2022, 440, 135801. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [Green Version]
- Mazrad, Z.A.I.; Kang, E.B.; In, I.; Park, S.Y. Preparation of carbon dot-based ratiometric fluorescent probes for cellular imaging from Curcuma longa. Luminescence 2018, 33, 40–46. [Google Scholar] [CrossRef]
- Tachi, S.; Morita, H.; Takahashi, M.; Okabayashi, Y.; Hosokai, T.; Sugai, T.; Kuwahara, S. Quantum yield enhancement in graphene quantum dots via esterification with benzyl alcohol. Sci. Rep. 2019, 9, 14115. [Google Scholar] [CrossRef] [Green Version]
- Meierhofer, F.; Dissinger, F.; Weigert, F.; Jungclaus, J.; Müller-Caspary, K.; Waldvogel, S.R.; Voss, T. Citric acid based carbon dots with amine type stabilizers: pH-specific luminescence and quantum yield characteristics. J. Phys. Chem. 2020, 124, 8894–8904. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Wang, B.; Liu, H.; Zhao, L.; Zhou, T.; Liu, M.; Huang, N.; Li, Y.; Ding, L.; et al. Microwave-assisted synthesis of carbon dots for” turn-on” fluorometric determination of Hg(II) via aggregation-induced emission. Microchim. Acta 2018, 185, 252. [Google Scholar] [CrossRef]
- Hu, S.; Liu, J.; Yang, J.; Wang, Y.; Cao, S. Laser synthesis and size tailor of carbon quantum dots. J. Nanopart. Res. 2011, 13, 7247–7252. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.F.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Jiang, Y.; Ji, C.; Wu, J.; Han, Q.; Cui, C.; Shi, W.; Peng, Z. Formation, photoluminescence and in vitro bioimaging of polyethylene glycol-derived carbon dots: The molecular weight effects. Polymer 2022, 243, 124625. [Google Scholar] [CrossRef]
- Kang, S.; Han, H.; Lee, K.; Kim, K.M. Ultrasensitive Detection of Fe3+ Ions Using Functionalized Graphene Quantum Dots Fabricated by a One-Step Pulsed Laser Ablation Process. ACS Omega 2022, 7, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jeong, Y.K.; Ryu, J.H.; Son, Y.; Kim, W.R.; Lee, B.; Jung, K.H.; Kim, K.M. Pulsed laser ablation based synthetic route for nitrogen-doped graphene quantum dots using graphite flakes. Appl. Surf. Sci. 2020, 506, 144998. [Google Scholar] [CrossRef]
- Jian, X.; Li, J.G.; Yang, H.M.; Cao, L.L.; Zhang, E.H.; Liang, Z.H. Carbon quantum dots reinforced polypyrrole nanowire via electrostatic self-assembly strategy for high-performance supercapacitors. Carbon 2017, 114, 533–543. [Google Scholar] [CrossRef]
- Essner, J.B.; Kist, J.A.; Polo-Parada, L.; Baker, G.A. Artifacts and errors associated with the ubiquitous presence of fluorescent impurities in carbon nanodots. Chem. Mater. 2018, 30, 1878–1887. [Google Scholar] [CrossRef]
- Hinterberger, V.; Damm, C.; Haines, P.; Guldi, D.M.; Peukert, W. Purification and structural elucidation of carbon dots by column chromatography. Nanoscale 2019, 11, 8464–8474. [Google Scholar] [CrossRef]
- Michaud, V.; Pracht, J.; Schilfarth, F.; Damm, C.; Platzer, B.; Haines, P.; Harreiß, C.; Guldi, D.M.; Spiecker, E.; Peukert, W. Well-separated water-soluble carbon dots via gradient chromatography. Nanoscale 2021, 13, 13116–13128. [Google Scholar] [CrossRef]
- He, Z.; Zhang, C.; Zhang, J.; Liu, S.; Sun, Y.; Chen, Q.; Chu, Z.; Ye, M.; Zhang, K. Concentration-dependent multi-color humic acid-based carbon dots for luminescent polymer composite films. J. Mater. Sci. 2022, 57, 1069–1083. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Xing, Y.; Wang, Z. Solvent-Dependent Red Emissive Carbon Dots and Their Applications in Sensing and Solid-State Luminescence. Sens. Actuators B 2022, 360, 131645. [Google Scholar] [CrossRef]
- Hu, S.; Tian, R.; Wu, L.; Zhao, Q.; Yang, J.; Liu, J.; Cao, S. Chemical regulation of carbon quantum dots from synthesis to photocatalytic activity. Chem. Asian J. 2013, 8, 1035–1041. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, H.; Bai, X.; Zhai, Y.; Zhu, Y.; Chen, X.; Pan, G.; Dong, B.; Xu, L.; Zhang, H.; et al. Modulation of the photoluminescence in carbon dots through surface modification: From mechanism to white light-emitting diodes. Nanotechnology 2018, 29, 245702. [Google Scholar] [CrossRef]
- Dey, A.; Ghosh, P.; Chandrabose, G.; Damptey, L.A.; Kuganathan, N.; Sainio, S.; Nordlund, D.; Selvaraj, V.; Chroneos, A.; Braithwaite, N.; et al. Ultrafast epitaxial growth of CuO nanowires using atmospheric pressure plasma with enhanced electrocatalytic and photocatalytic activities. Nano Sel. 2022, 3, 627–642. [Google Scholar] [CrossRef]
- Lanzilotto, V.; Silva, J.L.; Zhang, T.; Stredansky, M.; Grazioli, C.; Simonov, K.; Giangrisostomi, E.; Ovsyannikov, R.; Simone, M.; Coreno, M.; et al. Spectroscopic Fingerprints of Intermolecular H-Bonding Interactions in Carbon Nitride Model Compounds. Chem. Eur. J. 2018, 24, 14198–14206. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xia, T.; Wang, L.; Zheng, X.; Qi, Z.; Gao, C.; Zhu, J.; Li, Z.; Xu, H.; Xiong, Y. Enabling visible-light-driven selective CO2 reduction by doping quantum dots: Trapping electrons and suppressing H2 evolution. Angew. Chem. Int. Ed. 2018, 57, 16447–16451. [Google Scholar] [CrossRef] [PubMed]
- Pschunder, F.; Huergo, M.A.; Ramallo-López, J.M.; Kommula, B.; Requejo, F.G.; Bhattacharyya, S. Role of Intrinsic Atomic Features and Bonding Motifs from the Surface to the Deep Core on Multistate Emissive Properties of N, B-Codoped Carbon Dots. J. Phys. Chem. C 2019, 124, 1121–1128. [Google Scholar] [CrossRef]
- Moon, B.J.; Oh, Y.; Shin, D.H.; Kim, S.J.; Lee, S.H.; Kim, T.W.; Bae, S. Facile and purification-free synthesis of nitrogenated amphiphilic graphitic carbon dots. Chem. Mater. 2016, 28, 1481–1488. [Google Scholar] [CrossRef]
- Bokare, A.; Nordlund, D.; Melendrez, C.; Robinson, R.; Keles, O.; Wolcott, A.; Erogbogbo, F. Surface functionality and formation mechanisms of carbon and graphene quantum dots. Diam. Relat. Mater. 2020, 110, 108101. [Google Scholar] [CrossRef]
- Ren, J.; Achilleos, D.S.; Golnak, R.; Yuzawa, H.; Xiao, J.; Nagasaka, M.; Petit, T. Uncovering the charge transfer between carbon dots and water by in situ soft X-ray absorption spectroscopy. J. Phys. Chem. Lett. 2019, 10, 3843–3848. [Google Scholar] [CrossRef]
- Kumar, U.; Kuntail, J.; Kumar, A.; Prakash, R.; Pai, M.R.; Sinha, I. In-situ H2O2 production for tetracycline degradation on Ag/s-(Co3O4/NiFe2O4) visible light magnetically recyclable photocatalyst. Appl. Surf. Sci. 2022, 589, 153013. [Google Scholar] [CrossRef]
- Chang, C.J.; Hsu, M.H.; Weng, Y.C.; Tsay, C.Y.; Lin, C.K. Hierarchical ZnO nanorod-array films with enhanced photocatalytic performance. Thin Solid Film. 2013, 528, 167–174. [Google Scholar] [CrossRef]
- Chang, C.J.; Chao, P.Y.; Lin, K.S. Flower-like BiOBr decorated stainless steel wire-mesh as immobilized photocatalysts for photocatalytic degradation applications. Appl. Surf. Sci. 2019, 494, 492–500. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, X.; Guan, R.; Hu, Y.; Jiang, S.; Shao, X.; Yue, Q. Carbon dots as metal-free photocatalyst for dye degradation with high efficiency within nine minutes in dark. Opt. Mater. 2022, 123, 111914. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Zhang, R.; Zhao, W. Facile and low-energy-consumption synthesis of dual-functional carbon dots from Cornus walteri leaves for detection of p-nitrophenol and photocatalytic degradation of dyes. Colloids Surf. A 2022, 640, 128351. [Google Scholar] [CrossRef]
- Nu, T.T.V.; Tran, N.H.T.; Truong, P.L.; Phan, B.T.; Dinh, M.T.N.; Dinh, V.P.; Van Tran, V. Green synthesis of microalgae-based carbon dots for decoration of TiO2 nanoparticles in enhancement of organic dye photodegradation. Environ. Res. 2022, 206, 112631. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.B.; Gao, Y.T.; Jiang, L.; Lv, J.; Chang, S.; Li, D.W. Carbon dots induced in-situ formation of porous europium micro-networks with enhanced photocatalysis. J. Colloid Interface Sci. 2022, 606, 600–606. [Google Scholar] [CrossRef]
- Shen, S.; Chen, K.; Wang, H.; Fu, J. Construction of carbon dots-deposited TiO2 Photocatalysts with visible-light-induced photocatalytic activity for the elimination of pollutants. Diam. Relat. Mater. 2022, 124, 108896. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Li, Y.; Ghasemi, J.B.; Li, J.; Zhang, G. Visible-NIR light-responsive 0D/2D CQDs/Sb2WO6 nanosheets with enhanced photocatalytic degradation performance of RhB: Unveiling the dual roles of CQDs and mechanism study. J. Hazard. Mater. 2022, 424, 127595. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Mani, S.; Perumal, S.; Vinodh, R.; Thirunavukkarasu, S.; Lee, Y.R. Facile synthesis of a novel nitrogen-doped carbon dot adorned zinc oxide composite for photodegradation of methylene blue. Dalton Trans. 2020, 49, 17725–17736. [Google Scholar] [CrossRef]
- Teng, M.; Shi, J.; Qi, H.; Shi, C.; Wang, W.; Kang, F.; Huang, Z. Effective enhancement of electron migration and photocatalytic performance of nitrogen-rich carbon nitride by constructing fungal carbon dot/molybdenum disulfide cocatalytic system. J. Colloid Interface Sci. 2022, 609, 592–605. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A.; Seifzadeh, D.; Chand, H.; Krishnan, V. Visible-light-activated g-C3N4 nanosheet/carbon dot/FeOCl nanocomposites: Photodegradation of dye pollutants and tetracycline hydrochloride. Colloids Surf. A 2021, 617, 126424. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Q.; Wang, X.; Li, J.; Li, W.; Li, Y.; Zhang, G. Carbon dots modified bismuth antimonate for broad spectrum photocatalytic degradation of organic pollutants: Boosted charge separation, DFT calculations and mechanism unveiling. Chem. Eng. J. 2021, 418, 129460. [Google Scholar] [CrossRef]
- Bayan, E.M.; Pustovaya, L.E.; Volkova, M.G. Recent advances in TiO2-based materials for photocatalytic degradation of antibiotics in aqueous systems. Environ. Technol. Innov. 2021, 24, 101822. [Google Scholar] [CrossRef]
- González-González, R.B.; Sharma, A.; Parra-Saldívar, R.; Ramirez-Mendoza, R.A.; Bilal, M.; Iqbal, H.M. Decontamination of emerging pharmaceutical pollutants using carbon-dots as robust materials. J. Hazard. Mater. 2022, 423, 127145. [Google Scholar] [CrossRef]
- Liang, L.; Gao, S.; Zhu, J.; Wang, L.; Xiong, Y.; Xia, X.; Yang, L. The enhanced photocatalytic performance toward carbamazepine by nitrogen-doped carbon dots decorated on BiOBr/CeO2: Mechanism insight and degradation pathways. Chem. Eng. J. 2020, 391, 123599. [Google Scholar] [CrossRef]
- Mukherjee, I.; Cilamkoti, V.; Dutta, R.K. Sunlight-driven photocatalytic degradation of ciprofloxacin by carbon dots embedded in ZnO nanostructures. ACS Appl. Nano Mater. 2021, 4, 7686–7697. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Li, R.; Chen, P.; Chen, D.; Cai, M.; Liu, G. Synthesis of a carbon dots modified g-C3N4/SnO2 Z-scheme photocatalyst with superior photocatalytic activity for PPCPs degradation under visible light irradiation. J. Hazard. Mater. 2021, 401, 123257. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Jin, X.; Zheng, X.; Wang, Y.; Wei, D.; Liu, G. Highly active metal-free carbon dots/g-C3N4 hollow porous nanospheres for solar-light-driven PPCPs remediation: Mechanism insights, kinetics and effects of natural water matrices. Water Res. 2020, 172, 115492. [Google Scholar] [CrossRef]
- Song, B.; Wang, Q.; Wang, L.; Lin, J.; Wei, X.; Murugadoss, V.; Wei, S. Carbon nitride nanoplatelet photocatalysts heterostructured with B-doped carbon nanodots for enhanced photodegradation of organic pollutants. J. Colloid Interface Sci. 2020, 559, 124–133. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, A.; Sharma, G.; Du, B.; Naushad, M.; Stadler, F.J. Carbon quantum dots and reduced graphene oxide modified self-assembled S@C3N4/B@C3N4 metal-free nano-photocatalyst for high performance degradation of chloramphenicol. J. Mol. Liq. 2020, 300, 112356. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Q.; Liu, P.; Ma, S.; Xie, B.; Yang, K.; Zhao, Y. Novel up-conversion carbon quantum dots/α-FeOOH nanohybrids eliminate tetracycline and its related drug resistance in visible-light responsive Fenton system. Appl. Catal. B 2020, 263, 118336. [Google Scholar] [CrossRef]
- Aggarwal, R.; Saini, D.; Sonkar, S.K.; Sonker, A.K.; Westman, G. Sunlight promoted removal of toxic hexavalent chromium by cellulose derived photoactive carbon dots. Chemosphere 2022, 287, 132287. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Pei, Y.; Hou, J.; Zheng, Y.; Zhang, Y.; Wei, K.; Xi, B. Construction of a carbon dots-based Z-scheme photocatalytic electrode with enhanced visible-light-driven activity for Cr(VI) reduction and carbamazepine degradation in different reaction systems. Chem. Eng. J. 2021, 420, 127571. [Google Scholar] [CrossRef]
- Liang, Q.; Yan, X.; Li, Z.; Wu, Z.; Shi, H.; Huang, H.; Kang, Z. Replacing Ru complex with carbon dots over MOF-derived Co3O4/In2O3 catalyst for efficient solar-driven CO2 reduction. J. Mater. Chem. A 2022, 10, 4279–4287. [Google Scholar] [CrossRef]
- Xiong, S.; Bao, S.; Wang, W.; Hao, J.; Mao, Y.; Liu, P.; Ouyang, D. Surface oxygen vacancy and graphene quantum dots co-modified Bi2WO6 toward highly efficient photocatalytic reduction of CO2. Appl. Catal. B 2022, 305, 121026. [Google Scholar] [CrossRef]
- Wang, Y.; Godin, R.; Durrant, J.R.; Tang, J. Efficient Hole Trapping in Carbon Dot/Oxygen-Modified Carbon Nitride Heterojunction Photocatalysts for Enhanced Methanol Production from CO2 under Neutral Conditions. Angew. Chem. Int. Ed. 2021, 60, 20811–20816. [Google Scholar] [CrossRef]
- Lee, D.E.; Kim, D.J.; Moru, S.; Kim, M.G.; Jo, W.K.; Tonda, S. Highly-configured TiO2 hollow spheres adorned with N-doped carbon dots as a high-performance photocatalyst for solar-induced CO2 reduction to methane. Appl. Surf. Sci. 2021, 563, 150292. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Chen, H.; Weng, Y.X.; Tang, H.; Chen, Z.; Li, H. Unique Z-scheme carbonized polymer dots/Bi4O5Br2 hybrids for efficiently boosting photocatalytic CO2 reduction. Appl. Catal. B 2021, 293, 120182. [Google Scholar] [CrossRef]
- Sun, H.; Shi, Y.; Shi, W.; Guo, F. High-crystalline/amorphous g-C3N4 S-scheme homojunction for boosted photocatalytic H2 production in water/simulated seawater: Interfacial charge transfer and mechanism insight. Appl. Surf. Sci. 2022, 593, 153281. [Google Scholar] [CrossRef]
- Yu, B.; Chen, X.; Huang, C.; Yao, W. 2D CdS functionalized by NiS2-doped carbon nanosheets for photocatalytic H2 evolution. Appl. Surf. Sci. 2022, 592, 153259. [Google Scholar] [CrossRef]
- Chang, C.J.; Lee, Z.; Wei, M.; Chang, C.C.; Chu, K.W. Photocatalytic hydrogen production by magnetically separable Fe3O4@ ZnS and NiCo2O4@ ZnS core–shell nanoparticles. Int. J. Hydrogen Energy 2015, 40, 11436–11443. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, Z.; Deng, J.; Liu, Y.; Zhang, L.; Wang, K.; Cao, S. Construction of carbon dots modified hollow g-C3N4 spheres via in situ calcination of cyanamide and glucose for highly enhanced visible light photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 1568–1578. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, G.; Gao, Y.; Chen, Q.; Bi, J. Up-conversion fluorescent carbon quantum dots decorated covalent triazine frameworks as efficient metal-free photocatalyst for hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 8739–8748. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Jiang, Y.; Liu, Y.; Zhang, W.; Zhang, J.; Macauley, A.L.O. Morphology-engineered carbon quantum dots embedded on octahedral CdIn2S4 for enhanced photocatalytic activity towards pollutant degradation and hydrogen evolution. Environ. Res. 2022, 209, 112800. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, X.; Li, C.F.; Yu, W.; Wang, A.; Hu, Z.Y.; Hu, J. Carbon quantum dots modified TiO2 composites for hydrogen production and selective glucose photoreforming. J. Energy Chem. 2022, 64, 201–208. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Zhuang, J.; Li, Y.; Jia, L. High temperature hydrothermal etching of g-C3N4 for synthesis of N doped carbon quantum dots-supported CdS photocatalyst to enhance visible light driven hydrogen generation. Mol. Catal. 2022, 517, 111900. [Google Scholar] [CrossRef]
- Su, R.; Yan, H.; Jiang, X.; Zhang, Y.; Li, P.; Su, W. Orange-red to NIR emissive carbon dots for antimicrobial, bioimaging and bacteria diagnosis. J. Mater. Chem. B 2022, 10, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, S.; Tan, M. Green synthesis of fluorescent carbon dots with antibacterial activity and their application in Atlantic mackerel (Scomber scombrus) storage. Food Funct. 2022, 13, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.L.; Li, K.; Gu, S.; Wu, Y.; Zhang, J.; Wu, J.; Li, X. Antimicrobial carbon-dot-stabilized silver nanoparticles. New J. Chem. 2022, 46, 2546–2552. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Yu, L.; Wu, L.; Hao, X.; Liu, Q.; Lin, X. Quaternized carbon quantum dots with broad-spectrum antibacterial activity for the treatment of wounds infected with mixed bacteria. Acta Biomater. 2022, 138, 528–544. [Google Scholar] [CrossRef]
- Wu, L.N.; Yang, Y.J.; Huang, L.X.; Zhong, Y.; Chen, Y.; Gao, Y.R.; Liu, A.L. Levofloxacin-based carbon dots to enhance antibacterial activities and combat antibiotic resistance. Carbon 2022, 186, 452–464. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.W.; Molaei, R. Preparation of turmeric-derived sulfur-functionalized carbon dots: Antibacterial and antioxidant activity. J. Mater. Sci. 2022, 57, 2941–2952. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W.; Molaei, R.; Priyadarshi, R.; Roy, S.; Min, S.; Han, S. Preparation and characterization of B, S, and N-doped glucose carbon dots: Antibacterial, antifungal, and antioxidant activity. Sustain. Mater. Technol. 2022, 32, e00397. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.X.; Xie, X.F.; He, X.; Fan, J.T.; Chen, A.Y. Effect of ultra-trace Ag doping on the antibacterial performance of carbon quantum dots. J. Environ. Chem. Eng. 2022, 10, 107112. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, S.; Gu, Y.; Lu, H. Naphthalimide appended isoquinoline fluorescent probe for specific detection of Al3+ ions and its application in living cell imaging. Spectrochim. Acta Part A 2022, 265, 120364. [Google Scholar] [CrossRef]
- Wang, B.; Ji, Y.; Xia, Y.; Qin, K.; Li, B. The exploitation of thermophile resources in hot springs: Fluorescent carbon dots derived from Ureibacillus thermosphaericus for multicolour cellular imaging and selectivity detection of heavy metals. Anal. Methods 2021, 13, 1810–1815. [Google Scholar] [CrossRef]
- Durrani, S.; Zhang, J.; Yang, Z.; Pang, A.P.; Zeng, J.; Sayed, S.M.; Lin, F. Plant-derived Ca, N, S-Doped carbon dots for fast universal cell imaging and intracellular Congo red detection. Anal. Chim. Acta 2022, 1202, 339672. [Google Scholar] [CrossRef]
- Liu, B.; Wei, S.; Liu, E.; Zhang, H.; Lu, P.; Wang, J.; Sun, G. Nitrogen-doped carbon dots as a fluorescent probe for folic acid detection and live cell imaging. Spectrochim. Acta A 2022, 268, 120661. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Lee, Y.R. Morus nigra-derived hydrophilic carbon dots for the highly selective and sensitive detection of ferric ion in aqueous media and human colon cancer cell imaging. Colloids Surf. A Phys. Eng. Asp. 2022, 635, 128073. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, L.; Tao, Y.; Wang, D.; Zhang, K.; Tian, M.; Gao, D. Galli Gigerii Endothelium Corneum derived fluorescent carbon dots and their application as sensing platform for nitroimidazoles and cell imaging. Microchem. J. 2022, 174, 107089. [Google Scholar] [CrossRef]
- Atchudan, R.; Kishore, S.C.; Gangadaran, P.; Edison, T.N.J.I.; Perumal, S.; Rajendran, R.L.; Lee, Y.R. Tunable fluorescent carbon dots from biowaste as fluorescence ink and imaging human normal and cancer cells. Environ. Res. 2022, 204, 112365. [Google Scholar] [CrossRef]
- Yu, X.W.; Liu, X.; Jiang, Y.W.; Li, Y.H.; Gao, G.; Zhu, Y.X.; Wu, F.G. Rose Bengal-Derived Ultrabright Sulfur-Doped Carbon Dots for Fast Discrimination between Live and Dead Cells. Anal. Chem. 2022, 94, 4243–4251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.S.; Cheng, S.L.; Zhou, Z.D.; Du, B.; Shen, Y.Y.; Yu, B.Y. Bisligand-coordinated cadmium organic frameworks as fluorescent sensors to detect Ions, antibiotics and pesticides in aqueous solutions. Polyhedron 2022, 217, 115759. [Google Scholar] [CrossRef]
- Saha, A.; Das, S.; Devi, P.S. N-Doped Fluorescent Carbon Nanosheets as a Label-Free Platform for Sensing Bisphenol Derivatives. ACS Appl. Nano Mater. 2022, 5, 4908–4920. [Google Scholar] [CrossRef]
- Ji, Y.; Zou, X.; Chen, Q.; Tian, Y.; Gong, Z.; Fan, M. The design of aggregation-induced fluorescence sensor based on the cetyltrimethylammonium bromide-mediated nitrogen-doped carbon dots for selective detection of Hg2+. Dye. Pigm. 2022, 199, 110084. [Google Scholar] [CrossRef]
- Fu, W.J.; Peng, Z.X.; Dai, Y.; Yang, Y.F.; Song, J.Y.; Sun, W.; Yin, X.L. Highly fluorescent N doped C-dots as sensor for selective detection of Hg2+ in beverages. Spectrochim. Acta A 2022, 265, 120392. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Xu, J.; Zhang, H.; Zhao, T.; Jia, L. Formal analysis, Data curation Intelligent multicolor nano-sensor based on nontoxic dual fluoroprobe and MOFs for colorful consecutive detection of Hg2+ and cysteine. J. Hazard. Mater. 2022, 430, 128478. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Martinho, J.; Maçôas, E. A Fluorescent Nanosensor for Silver (Ag+) and Mercury (Hg2+) Ions Using Eu(III)-Doped Carbon Dots. Nanomaterials 2022, 12, 385. [Google Scholar] [CrossRef]
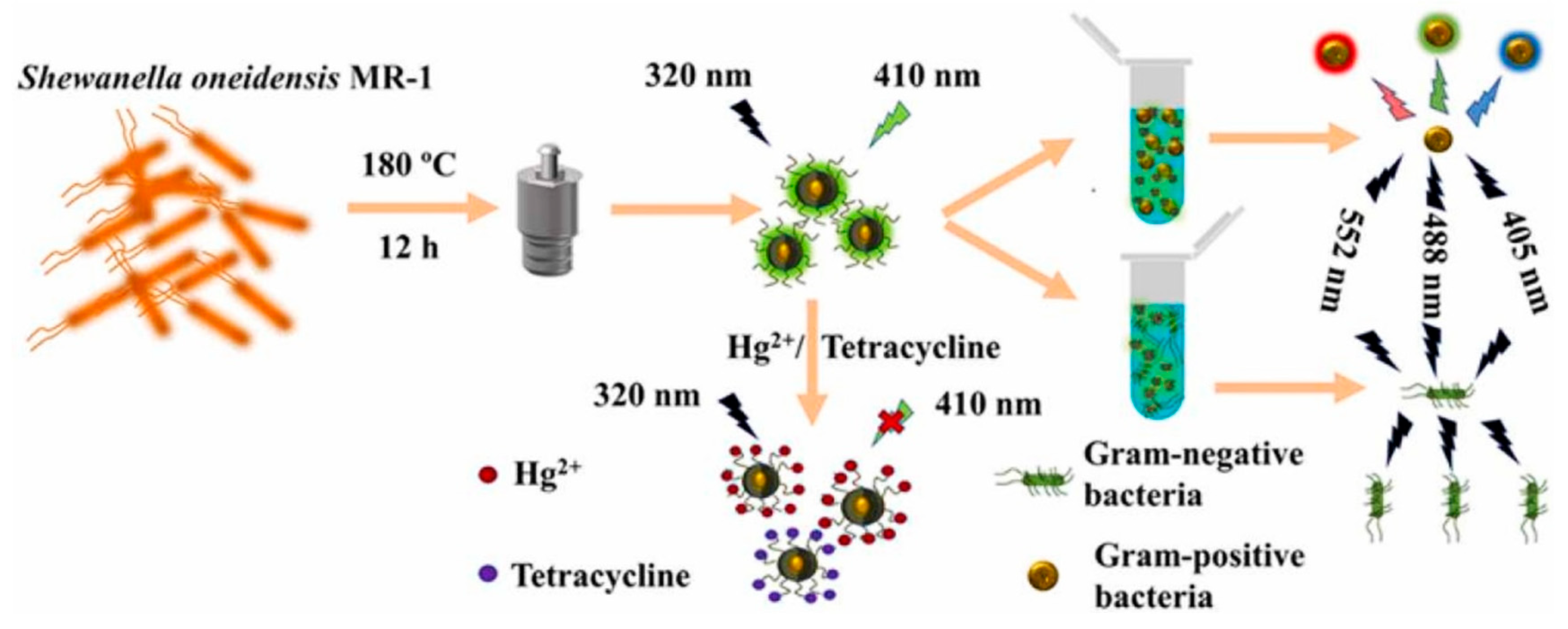
- Shen, C.; Dong, C.; Cheng, L.; Shi, X.; Bi, H. Fluorescent carbon dots from Shewanella oneidensis MR–1 for Hg2+ and tetracycline detection and selective fluorescence imaging of Gram–positive bacteria. J. Environ. Chem. Eng. 2022, 10, 107020. [Google Scholar] [CrossRef]
- Liang, A.; Zhang, R.; Huang, X.; Jiang, Z. A new Fe/N doped carbon dot naocatalytic amplification-aptamer SERS/RRS/Abs trimode assay platform for ultratrace Pb2+. Spectrochim. Acta A 2022, 272, 121008. [Google Scholar] [CrossRef]
- Wang, X.; Guo, H.; Wu, N.; Xu, M.; Zhang, L.; Yang, W. A dual-emission fluorescence sensor constructed by encapsulating double carbon dots in zeolite imidazole frameworks for sensing Pb2+. Colloids Surf. A Phys. Eng. Asp. 2021, 615, 126218. [Google Scholar] [CrossRef]
- Nandi, N.; Gaurav, S.; Sarkar, P.; Kumar, S.; Sahu, K. Hit Multiple Targets with One Arrow: Pb2+ and ClO− Detection by Edge Functionalized Graphene Quantum Dots and Their Applications in Living Cells. ACS Appl. Bio Mater. 2021, 4, 7605–7614. [Google Scholar] [CrossRef]
- Yang, X.C.; Yang, Y.L.; Xu, M.M.; Liang, S.S.; Pu, X.L.; Hu, J.F.; Zhang, Z.J. Metal-Ion-Cross-Linked Nitrogen-Doped Carbon Dot Hydrogels for Dual-Spectral Detection and Extractable Removal of Divalent Heavy Metal Ions. ACS Appl. Nano Mater. 2021, 4, 13986–13994. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, H.; Wang, M.; Yang, X.; Pang, S. N and S doped carbon dots as novel probes with fluorescence enhancement for fast and sensitive detection of Cr(VI). Colloids Surf. A Phys. Eng. Asp. 2022, 638, 128164. [Google Scholar] [CrossRef]
- Luo, Q.; Qin, K.; Liu, F.; Zheng, X.; Ding, Y.; Zhang, C.; Wei, Y. Carbon dots derived from kanamycin sulfate with antibacterial activity and selectivity for Cr6+ detection. Analyst 2021, 146, 1965–1972. [Google Scholar] [CrossRef]
- Guo, J.; Ye, S.; Li, H.; Chen, Y.; Liu, H.; Song, Y.; Qu, J. Novel fluorescent probes based on nitrogen–sulfur co-doped carbon dots for chromium ion detection. New J. Chem. 2021, 45, 4828–4834. [Google Scholar] [CrossRef]
- Si, J.; Wang, H.; Wu, B.; Wang, G.; Yang, J.; Huo, Z.; Han, S. Preparation of carbon dots with orange emission for Cr(III) detection and cellular imaging. Micro Nano Lett. 2021, 16, 58–63. [Google Scholar] [CrossRef]
- Limosani, F.; Bauer, E.M.; Cecchetti, D.; Biagioni, S.; Orlando, V.; Pizzoferrato, R.; Carbone, M. Top-down N-doped carbon quantum dots for multiple purposes: Heavy metal detection and intracellular fluorescence. Nanomaterials 2021, 11, 2249. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Yang, Y. Spectrofluorimetric determination of Cr(VI) and Cr(III) by quenching effect of Cr(III) based on the Cu-CDs with peroxidase-mimicking activity. Spectrochim. Acta A 2021, 244, 118882. [Google Scholar] [CrossRef]
- Elango, D.; Packialakshmi, J.S.; Manikandan, V.; Jayanthi, P. Synthesis of crab-shell derived CQDs for Cd2+ detection and antibacterial applications. Mater. Lett. 2022, 313, 131822. [Google Scholar] [CrossRef]
- Wang, X.; Duan, Q.; Zhang, B.; Cheng, X.; Wang, S.; Sang, S. Ratiometric fluorescence detection of Cd2+ based on N, S co-doped carbon quantum dots/Au nanoclusters. Microchem. J. 2021, 167, 106269. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.; Zhang, Z.; Zhang, J. “Off-on” fluorescence probe based on green emissive carbon dots for the determination of Cu2+ ions and glyphosate and development of a smart sensing film for vegetable packaging. Microchim. Acta 2022, 189, 131. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, J.; Quan, T.; Liu, S.; Deng, L.; Kang, X.; Gao, D. Liquid-liquid extraction and purification of oil red O derived nitrogen-doped highly photoluminescent carbon dots and their application as multi-functional sensing platform for Cu2+ and tetracycline antibiotics. Microchem. J. 2021, 168, 106391. [Google Scholar] [CrossRef]
- Guo, H.; Peng, L.; Wu, N.; Liu, B.; Wang, M.; Chen, Y.; Yang, W. A novel fluorescent Si/CDs for highly sensitive Hg2+ sensing in water environment. Colloids Surf. A Phys. Eng. Asp. 2022, 634, 128023. [Google Scholar] [CrossRef]
- Zhang, K.; Sang, Y.; Gao, Y.; Sun, Q.; Li, W. A fluorescence turn-on CDs-AgNPs composites for highly sensitive and selective detection of Hg2+. Spectrochim. Acta A 2022, 264, 120281. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Sun, X.; Jing, T.; Li, J.; Li, J. Integration detection of mercury(II) and GSH with a fluorescent “on-off-on” switch sensor based on nitrogen, sulfur co-doped carbon dots. RSC Adv. 2022, 12, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Dutta, R.K. N-Doped Carbon Dots Synthesized from Ethylene Glycol and β-Alanine for Detection of Cr(VI) and 4-Nitrophenol via Photoluminescence Quenching. ACS Appl. Nano Mater. 2021, 4, 3444–3454. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Luo, Z.; Duan, X.; Huang, W.Q.; Hu, W.; Huang, G.F. Amorphous B-doped graphitic carbon nitride quantum dots with high photoluminescence quantum yield of near 90% and their sensitive detection of Fe2+/Cd2+. Sci. China Mater. 2021, 64, 3037–3050. [Google Scholar] [CrossRef]
- Dai, J.; Peipei Wei, Y. Highly Efficient N-Doped Carbon Quantum Dots for Detection of Hg2+ and Cd2+ ions in Dendrobium huoshanense. Int. J. Electrochem. Sci. 2021, 16, 210716. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, D.; Zhu, Q.; Chao, D.; Liu, H.; Zhou, L. Preparation and characterisation of dual sensing carbon dots for water and Cu2+ detection. Dye. Pigm. 2022, 198, 110008. [Google Scholar] [CrossRef]
- Molkenova, A.; Kairova, M.; Zhussupbekova, A.; Zhussupbekov, K.; Duisenbayeva, B.; Shvets, I.V.; Atabaev, T.S. Carbon dots doped with barium as a novel contrast agent for efficient CT X-ray attenuation. Nano-Struct. Nano-Objects 2022, 29, 100839. [Google Scholar] [CrossRef]
- Su, Y.; Liu, S.; Guan, Y.; Xie, Z.; Zheng, M.; Jing, X. Renal clearable Hafnium-doped carbon dots for CT/Fluorescence imaging of orthotopic liver cancer. Biomaterials 2020, 255, 120110. [Google Scholar] [CrossRef]
- Phukan, K.; Sarma, R.R.; Dash, S.; Devi, R.; Chowdhury, D. Carbon dot based nucleus targeted fluorescence imaging and detection of nuclear hydrogen peroxide in living cells. Nanoscale Adv. 2022, 4, 138–149. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, J.; Yang, Z.; Zhao, M.; Long, H.; Wu, Q.; Nian, F. Tannin–Mn coordination polymer coated carbon quantum dots nanocomposite for fluorescence and magnetic resonance bimodal imaging. J. Mater. Sci. Mater. Med. 2022, 33, 16. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, L.; Wu, D.; Zhang, H.; Lian, H.; Zhao, X.; Zeng, L. Manganese-doped carbon dots with redshifted orange emission for enhanced fluorescence and magnetic resonance imaging. ACS Appl. Bio Mater. 2021, 4, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, H.; Fan, M.; Gao, S.; Fan, D.; Wang, Z.; Ge, K. Near-infrared-II photothermal ultra-small carbon dots promoting anticancer efficiency by enhancing tumor penetration. J. Colloid Interface Sci. 2022, 616, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, D.; Huang, X.; Guan, X.; Wang, F.; Wei, X. Extended π-Conjugative Carbon Nitride for Single 1064 nm Laser-Activated Photodynamic/Photothermal Synergistic Therapy and Photoacoustic Imaging. ACS Appl. Mater. Interfaces 2022, 14, 7626–7635. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yuan, H.; Qian, X.; Lu, Q.; Wang, L.; Hu, H.; Chen, Y. Municipal sludge-derived carbon dots-decorated, N-doped hierarchical biocarbon for the electrochemical reduction of carbon dioxide. Resour. Conserv. Recycl. 2022, 177, 105980. [Google Scholar] [CrossRef]
- Shi, W.; Hao, C.; Fu, Y.; Guo, F.; Tang, Y.; Yan, X. Enhancement of synergistic effect photocatalytic/persulfate activation for degradation of antibiotics by the combination of photo-induced electrons and carbon dots. Chem. Eng. J. 2022, 433, 133741. [Google Scholar] [CrossRef]
- Sarma, D.; Majumdar, B.; Sarma, T.K. Visible-light induced enhancement in the multi-catalytic activity of sulfated carbon dots for aerobic carbon–carbon bond formation. Green Chem. 2019, 21, 6717–6726. [Google Scholar] [CrossRef]
- Rosso, C.; Filippini, G.; Prato, M. Use of Nitrogen-doped carbon nanodots for the photocatalytic fluoroalkylation of organic compounds. Chem. A Eur. J. 2019, 25, 16032–16036. [Google Scholar] [CrossRef]
- Cailotto, S.; Negrato, M.; Daniele, S.; Luque, R.; Selva, M.; Amadio, E.; Perosa, A. Carbon dots as photocatalysts for organic synthesis: Metal-free methylene–oxygen-bond photocleavage. Green Chem. 2020, 22, 1145–1149. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Yang, Y.; Liu, X.; Qiu, J.; Tian, Y. Tunable full-color solid-state fluorescent carbon dots for light emitting diodes. Carbon 2022, 190, 22–31. [Google Scholar] [CrossRef]
- Dai, R.; Chen, X.; Ouyang, N.; Hu, Y. A pH-controlled synthetic route to violet, green, and orange fluorescent carbon dots for multicolor light-emitting diodes. Chem. Eng. J. 2022, 431, 134172. [Google Scholar] [CrossRef]
- Chen, M.; Liu, C.; An, Y.; Li, Y.; Zheng, Y.; Tian, H.; Lin, X. Red, green, and blue light-emitting carbon dots prepared from gallic acid for white light-emitting diode applications. Nanoscale Adv. 2022, 4, 14–18. [Google Scholar] [CrossRef]
- Han, Y.; Huang, X.; Liu, J.; Ni, J.; Bai, Y.; Zhao, B.; Zhang, C. Seeking eye protection from biomass: Carbon dot-based optical blocking films with adjustable levels of blue light blocking. J. Colloid Interface Sci. 2022, 617, 44–52. [Google Scholar] [CrossRef]
- Xie, F.; Liu, Z.; Wang, C.; Chen, D.; Zhu, W.; Li, X.; Wang, G. Graphene quantum dots assisted synthesis of highconcentration nitrogen doped graphene for infrared photodetectors. Diam. Relat. Mater. 2022, 121, 108774. [Google Scholar] [CrossRef]
- Hsiao, P.H.; Lai, Y.C.; Chen, C.Y. Dual-sized carbon quantum dots enabling outstanding silicon-based photodetectors. Appl. Surf. Sci. 2021, 542, 148705. [Google Scholar] [CrossRef]
- Surana, K.; Mehra, R.M.; Soni, S.S.; Bhattacharya, B. Real-time photovoltaic parameters assessment of carbon quantum dots showing strong blue emission. RSC Adv. 2022, 12, 1352–1360. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, A.; Devi, S.; Tyagi, S.; Kaur, D. Relevant photovoltaic effect in N-doped CQDs/MoS2 (0D/2D) quantum dimensional heterostructure. Ceram. Int. 2022, 48, 14107–14116. [Google Scholar] [CrossRef]
- Qu, K.; Chen, M.; Wang, W.; Yang, S.; Jing, S.; Guo, S.; Huang, Z. Biomass-derived carbon dots regulating nickel cobalt layered double hydroxide from 2D nanosheets to 3D flower-like spheres as electrodes for enhanced asymmetric supercapacitors. J. Colloid Interface Sci. 2022, 616, 584–594. [Google Scholar] [CrossRef]
- Sinha, R.; Roy, N.; Mandal, T.K. SWCNT/ZnO nanocomposite decorated with carbon dots for photoresponsive supercapacitor applications. Chem. Eng. J. 2022, 431, 133915. [Google Scholar] [CrossRef]
- El-Shamy, A.G. Acido-treatment of PEDOT: PSS/Carbon Dots (CDots) nano-composite films for high thermoelectric power factor performance and generator. Mater. Chem. Phys. 2021, 257, 123762. [Google Scholar] [CrossRef]
- El-Shamy, A.G. The role of nitrogen-carbon dots (NC) nano-particles in enhancing thermoelectric power functions of PEDOT: PSS/Te nano-composite films. Chem. Eng. J. 2021, 417, 129212. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, Z.; Dong, W.; Liu, Y.; Song, S.; Hu, Q.; Gong, X. Intelligently design primary aromatic amines derived carbon dots for optical dual-mode and smartphone imaging detection of nitrite based on specific diazo coupling. J. Hazard. Mater. 2022, 430, 128393. [Google Scholar] [CrossRef]
- Singh, S.; Kansal, S.K. Dual Fluorometric Detection of Fe3+ and Hg2+ Ions in an Aqueous Medium Using Carbon Quantum Dots as a “Turn-off” Fluorescence Sensor. J. Fluoresc. 2022, 32, 1143–1154. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Zhao, P.; Wang, C.; Fei, J.; Xie, Y. Ultrasensitive luteolin electrochemical sensor based on zeolitic imidazolate frameworks-derived cobalt trioxide@ nitrogen doped carbon nanotube/amino-functionalized graphene quantum dots composites modified glass carbon electrode. Sens. Actuators B 2022, 351, 130938. [Google Scholar] [CrossRef]
- Ji, W.; Yu, J.; Cheng, J.; Fu, L.; Zhang, Z.; Li, B.; Wang, X. Dual-Emissive Near-Infrared Carbon Dot-Based Ratiometric Fluorescence Sensor for Lysozyme. ACS Appl. Nano Mater. 2022, 5, 1656–1663. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Chen, D.; Tong, Q.X. Carbon Dots/α-Fe2O3-Fe3O4 nanocomposite: Efficient synthesis and application as a novel electrochemical aptasensor for the ultrasensitive determination of aflatoxin B1. Food Chem. 2022, 373, 131415. [Google Scholar] [CrossRef]
- Tejwan, N.; Kundu, M.; Ghosh, N.; Chatterjee, S.; Sharma, A.; Singh, T.A.; Sil, P.C. Synthesis of green carbon dots as bioimaging agent and drug delivery system for enhanced antioxidant and antibacterial efficacy. Inorg. Chem. Commun. 2022, 139, 109317. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Yuan, T.; Zhu, J.; Yang, Y. Influence of the iodine content of nitrogen-and iodine-doped carbon dots as a peroxidase mimetic nanozyme exhibiting antifungal activity against C. albicans. Biochem. Eng. J. 2021, 175, 108139. [Google Scholar] [CrossRef]
- Kanthi Gudimella, K.; Gedda, G.; Kumar, P.S.; Babu, B.K.; Yamajala, B.; Rao, B.V.; Sharma, A. Novel synthesis of fluorescent carbon dots from bio-based Carica Papaya Leaves: Optical and structural properties with antioxidant and anti-inflammatory activities. Environ. Res. 2022, 204, 111854. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Unnikrishnan, B.; Lehman, C.W.; Wang, P.H.; Tseng, Y.J.; Harroun, S.G.; Huang, C.C. Exploring molecular moieties on carbonized polymer dots from flavonoid glycosides with activity against enterovirus A71. Carbon 2022, 192, 285–294. [Google Scholar] [CrossRef]
- Łoczechin, A.; Séron, K.; Barras, A.; Giovanelli, E.; Belouzard, S.; Chen, Y.T.; Szunerits, S. Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 42964–42974. [Google Scholar] [CrossRef] [PubMed]
- Kainth, S.; Sharma, V.; Bhagat, M.; Basu, S. Yellow emissive carbon dots in ludox silica matrix with anticancer activity for enhanced imaging of developed sweat latent fingermarks. Mater. Today Chem. 2022, 23, 100659. [Google Scholar] [CrossRef]
- Saikia, M.; Singh, A.; Dihingia, A.; Khare, P.; Kalita, J.; Saikia, B.K. Scalable production, cell toxicity assessment, and plant growth promotion activities of carbon quantum dots derived from low-quality coal feedstock. Chem. Eng. J. 2022, 433, 133633. [Google Scholar] [CrossRef]
- Xiong, Z.; Dai, L.; Wang, Y.; Qu, K.; Xia, Y.; Lei, L.; Xu, Z. Two-dimensional sub-nanometer confinement channels enabled by functional carbon dots for ultra-permeable alcohol dehydration. J. Membr. Sci. 2022, 644, 120069. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Hameed, A.; Snari, R.M.; Shah, R.; Alfi, A.A.; El-Metwaly, N.M. Development of fluorescent carbon dots ink from rice straw waste toward security authentication. J. Mol. Liq. 2022, 354, 118927. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Dai, S.; Lu, H. Remarkably boosting the lubricity of polyalphaolefin by loading amphiphilic carbon dots stabilized by Span-80. Diam. Relat. Mater. 2022, 124, 108924. [Google Scholar] [CrossRef]
- Ezati, P.; Roy, S.; Rhim, J.W. Pectin/gelatin-based bioactive composite films reinforced with sulfur functionalized carbon dots. Colloids Surf. A 2022, 636, 128123. [Google Scholar] [CrossRef]
- Gu, W.; Dong, Z.; Zhang, A.; Ma, T.; Hu, Q.; Wei, J.; Wang, R. Functionalization of PET with carbon dots as copolymerizable flame retardants for the excellent smoke suppressants and mechanical properties. Polym. Degrad. Stab. 2022, 195, 109766. [Google Scholar] [CrossRef]







| Type * | CDs-Based Photocatalyst | CDs Synthesis Method | CD Precursor | Pollutant Removed | Main Active Species | Role of CDs | Efficiency (%/min of Irradiation) | Reference/Year |
|---|---|---|---|---|---|---|---|---|
| 1 | CDs | Solvothermal | Glyoxal and ethanol | Indigo carmine (IC) | •O2•−, h+, •OH | e−-h+ pair separation | 91/4.5 | [119] 2022 |
| 1 | CDs | Carbonization | Bitter apple peel | Crystal violet (CV) | h+, •OH | efficient e−/h+ separation | 100/90 | [21] 2020 |
| 1 | CNDs | Carbonization (pyrolysis) | Olive solid wastes | MB | O2•− •OH | e−-h+ pair separation | 100/120 | [19] 2022 |
| 1 | CQDs | Stirrer-assisted | Muskmelon peel | RhB | •OH | Up-conversion charge separation | 99.11/35 | [23] 2022 |
| 1 | G-CDs | Hydrothermal | Cornus walteri leaves | MG | O2•− | e−-h+ pair separation | 98.0/40 | [120] 2022 |
| MO | •OH | 97.1/50 | ||||||
| MV | 63.6/90 | |||||||
| 2 | CDs/TNs | Hydrothermal | Ammonium citrate (AC) | CR RhB TC | •OH | e−-h+ pair separation | 85.9/120 | [123] 2022 |
| 2 | TiO2-MCDs | Microwave-assisted | Microalgae tablet | MB | O2•− •OH | e− trapping, Up-conversion | 83/120 | [121] 2022 |
| 2 | CDs-BiSbO4 | Hydrothermal | Citric acid and urea | Rh B Ciprofloxacin | O2•−, | Up-conversion, e− trapping, | 91/100 | [128] 2021 |
| •OH, | 43/100 | |||||||
| 2 | CQDs/Sb2WO6 | Hydrothermal | Urea ascorbic acid | RhB | h+, •OH | Up-conversion, efficient e−/h+ separation | 83/120 | [125] 2022 |
| 2 | N-CDs@ZnO composite | Hydrothermal | Malus floribunda fruits | MB | h+, •OH | e− trapping, Up-conversion | 99/60 | [126] 2020 |
| 2 | RCD-ZnO nanohybrid | Hydrothermal | Colocasia esculenta leaves | Dodecylbenzene sulfonate commercial detergent | h+, •OH | e−/h+ separation | 96.7/110 94.8/150 | [29] 2019 |
| 2 | N-CDs on BiOBr/CeO2 | Hydrothermal | C6H5O7 (NH4)3 and ethylenediamine | Carbamazepine | O2•−, h+, •OH | Accelerating the migration and separation of the charge carries | 97/120 | [131] 2020 |
| 2 | N,S-CQDs/TiO2 on polysulfone membrane | Thermal treatment | Egg yolk | Diclofenac | O2•−, •OH, | Up-conversion, efficient e−/h+ separation | 62.3/150 | [27] 2020 |
| 2 | CQDs on BiOCl/carbonized eggshell membrane | Thermal treatment | Eggshell membranes | Tetracycline hydrochloride | O2•−, h+, •OH | Electron trapping | 97.39/60 | [25] 2021 |
| 2 | ZnO/CD nanocomposites | Hydrothermal | Trisodium citrate dihydrate and ammonium carbonate | Ciprofloxacin | h+, O2•−, •OH | Up-conversion, efficient e−/h+ separation | 98/110 | [132] 2021 |
| 2 | CDs modified g-C3N4/SnO2 | Hydrothermal | Citric acid and urea | Indomethacin | O2•−, h+ | Up-conversion, efficient e−/h+ separation | 90.8/80 | [133] 2021 |
| 3 | CDs/MoS2/p-C3N5 | Hydrothermal | Fungal | MB | •OH | e− trapping, charge transfer | 93.51/120 | [124] 2022 |
| 4 | Fe, N-CDs | Hydrothermal | Citric acid urea and ferric citric | MB | O2•−, h+, •OH | Charge separation | 100/60 | [120] 2022 |
| 4 | CDs@P-Eu-MNs | Hydrothermal | Citric acid Cysteine | Rhodamine 6G | O2•−, h+ | Charge separation | 95/160 | [122] 2022 |
| 4 | C3N4-NS/CD/FeOCl | microwave-assisted | Citric acid and urea | RhB | O2•−, h+, | Charge separation | 100/60 100/45 | [127] 2021 |
| Tetracycline | •OH | |||||||
| hydrochloride | ||||||||
| 4 | CDs/hollow g-C3N4 nanospheres | Hydrothermal | Citric acid and urea | Naproxen | O2•− | Up-conversion, efficient e−/h+ separation | 98.6/25 | [134] 2020 |
| Indomethacin | ~100/25 | |||||||
| Norfloxacin | ~80/25 | |||||||
| Diclofenac | ~50/25 | |||||||
| 4 | B-CDs on C3N4 | Hydrothermal | Carbon fibers | Tetracycline hydrochloride | O2•−, h+ | enlarged surface absorption, light-harvesting ability, and charge separation and transfer | 65.82/180 | [135] 2020 |
| 4 | CQDs and reduced graphene oxide layers on S@g-C3N4/B@g-C3N4 | Ultrasonic method | Glucose | Chloramphenicol | O2•−, •OH, | Transmission of charge | 99.1/90 | [136] 2020 |
| 4 | (CQD) incorporated goethite (α-FeOOH) nanohybrids | Hydrothermal | Citric acid | Tetracycline | O2•−, •OH, 1O2 | Up-conversion | 94.5/60 | [137] 2020 |
| Material | Precursors | Method | QY (%) | Analyte | Linear Range | LOD | Reference/Year |
|---|---|---|---|---|---|---|---|
| Si/CDs | (3-Aminopropyl) triethoxysilane and citric acid | Solvothermal | - | Hg2+ | 0–200 µM | 26.7 nM | [190] 2022 |
| CTAB/NCDs | Citric acid and urea | Solvothermal | 32% | Hg2+ | 0.16–10.24 µM | 85.71 nM | [171] 2022 |
| NCDs | Citric acid and ethylenediamine | Hydrothermal | 67.4% | Hg2+ | 0.3–2.0 µM | 0.24 µM | [172] 2022 |
| CDs/InPQDs@ZIF-8 | Kelp powder | Hydrothermal | - | Hg2+ | 0–5 µM | 8.68 nM | [173] 2022 |
| CDs-AgNPs | Melamine and citric acid | Hydrothermal | - | Hg2+ | 100–160 µM | 2.22 × 10−8 M | [191] 2022 |
| NS-CDs | Aurine and citric acid | Thermal Lysis | 68.94% | Hg2+ | 0–100 µM | 50 nM | [192] 2022 |
| Eu-CDs | Citric acid and urea | Hydrothermal | 0.013% | Hg2+ | 0–80 µM | 4 µM | [174] 2022 |
| N-CDs/R-CDs@ZIF-8 | Citric acid, urea, and spinach extract | Hydrothermal Solvothermal | - | Pb2+ | 0.05–50 µM | 4.78 nM | [177] 2021 |
| Functionalized-GQD | Graphite flakes | Ultrasonication | 13.4% | Pb2+ | 0–300 µM | 1.2 µM | [178] 2021 |
| N-CDs | Sodium alginate and urea | Thermal sintering | - | Pb2+ | - | 3 ppb | [179] 2021 |
| Cu2+ | - | 15 ppb | |||||
| CDs-HS18 | Ureibacillus thermosphaericus | Hydrothermal | 17.3% | Cr6+ | 0–9 µM | 36 nM | [163] 2021 |
| N and S doped CDs | O-phenylenediamine and dl-Thioctic acid | Hydrothermal | 21.82% | Cr6+ | 0–60 µM | 0.64 µM | [180] 2022 |
| CDs-Kan | Kanamycin sulfate | Hydrothermal | 5.26% | Cr6+ | 0–33 μM | 0.36 μM | [181] 2021 |
| N and S doped CDs | Glycine and 4-sulfophthalic acid | Hydrothermal | - | Cr3+ | 0–40 μM | 7.8 nM | [182] 2021 |
| Orange emission CDs | 1,2,4-Triaminobenzene and p-aminobenzenesulphonic acid | Hydrothermal | 14.9% | Cr3+ | 1–96 μM | 0.38 μM | [183] 2021 |
| N doped CDs | Ethylene glycol and β-alanine | Heating in an oil bath | 14.3% | Cr6+ | 0.5–500 μM | 0.29 μM | [193] 2021 |
| 4-NP | 1–250 μM | 0.4 μM | |||||
| N-doped CQDs | Fullerene, H2O2, and NH4OH | Hydroxy radical | 10% | Cr3+ | 0–100 μM | 2 μM | [184] 2021 |
| CQDs | Crab-shell waste | Hydrothermal | - | Cd2+ | 50–250 µM | - | [186] 2022 |
| N,S-CDs | Citric acid and thiourea | sonication | - | Cd2+ | 0–2.1 µM | 62 nM | [187] 2021 |
| B doped CNQDs | Urea, boric acid, and citric acid | Hydrothermal | 87.4% | Cd2+ | 0–20 µM | 1.1 nM | [194] 2021 |
| Fe2+ | 0–20 µM | 2.3 nM | |||||
| N-doped CQDs | Auricularia auricular and ethylenediamine | Hydrothermal | 28.4% | Cd2+ | 0–50 µM | 101.55 nM | [195] 2021 |
| Hg2+ | 0–50 µM | 77.21 nM | |||||
| CDs | 1,4-Dihydroxyanthraquinone | Solvothermal | 41.3% | Cu2+ glyphosate | 50–300 ng·mL−1 | 22.65 nM 5 nM | [188] 2022 |
| h-CDs | Hydroquinone, o-phenylenediamine, and terephthalic acid | Solvothermal | 30.8% | Cu2+ H2O | 0–0.01 mM | 1.8 × 10−4 mM | [196] 2022 |
| NCDs | Oil red O | Solvothermal | 68% | Cu2+ | 0–50 μM 0–100 μM | 4 nM | [189] 2021 |
| DMC * | 50 pM | ||||||
| TC | 500 pM | ||||||
| MC | 5 nM | ||||||
| DC | 50 nM | ||||||
| OTC | 100 nM |
| Applications | Types of CDs | Function of CDs | Reference/Year |
|---|---|---|---|
| Bioimaging | |||
| Computer tomography (CT) | Barium-doped (Ba-CDs) | Contrast agents | [197] 2022 |
| Hafnium-doped (Hf-CDs) | Contrast agents | [198] 2020 | |
| Fluorescent imaging (FI) | Boron-doped p-phenylenediamine-based carbon quantum dots (B-PPD CDs) | Cell labeling agent | [199] 2022 |
| Kiwi-fruit-peel carbon dots (KFP-CDs) | Cell labeling agent | [167] 2022 | |
| Magnetic resonance imaging (MRI) | Manganese-doped blue emission carbon quantum dots (BCQD@Mn) composite | Contrast agents | [200] 2022 |
| Manganese-doped CDs (Mn-CDs) | Contrast agents | [201] 2021 | |
| Photoacoustic imaging (PAI) | Permeable carbon dots (PCDs) | PAI agent, Tumor ablation (laser irradiated at 1064 nm) | [202] 2022 |
| Carbon nitride nanoparticles (CN-NPs) | PAI agent, Tumor-growth inhibition (laser irradiated at 1064 nm) | [203] 2022 | |
| Catalysis | |||
| CO2 reduction | N-doped carbon and carbon dots (CDs) | CO2 adsorbent and active N-sites to generate CH4/CH3OH by radical •CO2 | [204] 2022 |
| CD-modified Co3O4/In2O3 composite | Electron and hole transfer processes | [142] 2022 | |
| Degradation of pollutants | Vis/CDs–ZIS/PS ((visible light CDs, ZnIn2S4 (ZIS), persulfate (PS)) | Photoinduced charge separation | [205] 2022 |
| H2 evolution | CQDs/CTF (carbon quantum dots/covalent triazine–based framework) | Up-conversion | [149] 2022 |
| Organic synthesis | Carbon dots decorated with hydrogen sulfate groups (S-CDs) | Photocatalyst ((dehydrogenative cross-coupling (C-C bond formation) reactions)) | [206] 2019 |
| Amine-rich N-doped carbon nanodots (NCNDs) | Photocatalyst (C-C bond formation reactions) | [207] 2019 | |
| Citric acid–derived carbon dots (CACDs) | Photocatalyst (C-O bond photocleavage reactions) | [208] 2020 | |
| Energy-associated application | |||
| Light-emitting diode (LED) | Phloroglucinol and urea precursor–based CDs, with emissions of blue (B-CDs), green (G-CDs), yellow (Y-CDs), orange (O-CDs), red (R-CDs) | Solid-state fluorescence and multicolor light emission | [209] 2022 |
| 2,3-Diaminopyridine based CDs | Solid-state fluorescence and multicolor light emission | [210] 2022 | |
| Gallic acid and o-phthalaldehyde–based red, green, and blue CDs | Solid-state fluorescence, multicolor light emission (CDs dispersed into epoxy resin to form multicolor LEDs) | [211] 2022 | |
| Bio-CDs (microcrystalline cellulose and ethylenediamine) | Optical blocking films (OBF) prepared by mixing of Bio-CDs and polyvinyl alcohol (PVA) blocks the blue light | [212] 2022 | |
| Photodetectors | Nitrogen-doped graphene quantum dots (N-GQDs) | Mixing of n-type N-GQDs and SiO2/Si substrate to prepare the Photodetector | [213] 2022 |
| Pure glucose–based dual-sized CQDs | The dual-sized CQDs films directly formed on Si substrates, supporting as self-powered photodetectors. | [214] 2021 | |
| Photovoltaics | Citric acid and uric acid–based nitrogen-doped carbon quantum dots (N-CQDs) | Used as a co-sensitizer | [215] 2022 |
| N-CQDs (carbon and nitrogen source from Aminobenzene-dicarboxylic acid) | Hole transporter, an electron blocker | [216] 2022 | |
| Supercapacitors | CDs/NCLDH ((2D nickel–cobalt layered double hydroxide (NCLDH) nanosheets are regulated to form 3D flower-like spheres by fungus bran-derived carbon dots (CDs)) | As a bridge for charge transfer | [217] 2022 |
| SWCNT/ZnO nanocomposite decorated with carbon dots (CDs- Citric acid, Ethylenediamine, SWCNT- single-walled carbon nanotube) | Reactive to UV light, electron-hole pairs generation | [218] 2022 | |
| Thermoelectric devices | CDs/PEDOT:PSS (poly(3,4-ethylene-dioxythiophene), poly (styrenesulphonate) nano-composite films | generation of an increased level of charge carrier concentration | [219] 2021 |
| PEDOT:PSS/NC@Te films ((NC- Nitrogen doped Carbon nano-dots, decorated Telluride (Te) nano-rods embedding into Poly(3,4-ethylene-dioxythiophene), Poly(styrenesulphonate)) | the formation of conductive paths within the films, as well as an increase in carrier mobility and carrier concentration | [220] 2021 | |
| Sensing | |||
| Colorimetric | PAA-CDs (primary aromatic amines derived CDs) | Detection of NO2− ions with LOD of 0.024 μM and 0.16 μM by colorimetric and fluorimetric methods, respectively. | [221] 2022 |
| Fluorescent | CQDs (citric acid and ethylenediamine) | Detection of Fe3+ and Hg2+ with LOD of 0.406 µM and 0.934 µM, respectively. | [222] 2022 |
| Electrochemical | Co3O4@N-CNTs/NH2-GQDs/GCE composite (N-CNTs, nitrogen-doped carbon nanotubes; NH2-GQDs, amine-functionalized GQDs) | Detection of luteolin with a LOD of 0.1 nM | [223] 2022 |
| CDs/α-Fe2O3-Fe3O4 composite (CDs from 5-sulfosalicylic acid and diethylene glycol) | Detection of aflatoxin B1 With an LOD of 0.5 pM | [224] 2022 | |
| Ratiometric | dNIR-CDs (dual emission near-infrared carbon dots from glutathione and polyethylenimine) | Detection of Lysozyme with an LOD of 7 nM | [225] 2022 |
| Therapy | |||
| Antibacterial | Boron-doped glucose carbon dots (BGCDs), Sulfur-doped glucose carbon dots (SGCDs), Nitrogen-doped glucose carbon dots (NGCDs), Glucose carbon dots (GCDs) | Antibacterial activity against Escherichia coli and Listeria monocytogenes | [159] 2022 |
| CDs (red Korean ginseng root extract), CDs-RUT nanohybrid) | Inhibiting the growth of Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) | [226] 2022 | |
| Antifungal | Nitrogen-doped glucose carbon dots (NGCDs), Sulfur-doped glucose carbon dots (SGCDs), Boron-doped glucose carbon dots (BGCDs) | Inhibiting the growth of A. fumigatus, F. solani, P. citrinum, C. Albicans, and R. Rubra. | [159] 2022 |
| Nitrogen and iodine-doped (I-CDs), i.e., I-CDs-3 (iopromide and EDA) | Inhibiting the growth of C. Albicans | [227] 2021 | |
| Antioxidant | Glucose carbon dots (GCDs), Nitrogen-doped Glucose carbon dots (NGCDs) | Free radical scavenging | [159] 2022 |
| CDs (Red Korean ginseng root extract), CDs-RUT nanohybrid | Free radical scavenging | [226] 2022 | |
| Anti-inflammatory | FCDs (fluorescent carbon dots synthesized from Carica Papaya Leaves) | Prevent red blood cells (RBC) lysis caused by induced hypotonicity. | [228] 2022 |
| Antiviral | Hsd-CPDs (carbonized polymer dots from hesperidin (Hsd)) | Hsd-CPDs surface contains bioactive moieties of apocynin, and guaiacol binds with the proteins of enterovirus A71 (EV-A71), thus blocking the viral attachment in neonatal mice. | [229] 2022 |
| CQDs (carbon quantum dots ethylenediamine/citric acid/boronic acid ligands) | The human coronavirus HCoV-229E is inactivated using a concentration-dependent method. | [230] 2019 | |
| Anticancer | Nano-powder of Ludox@CDs (CDs prepared from cetylpyridinium chloride) | Acts as cytotoxic to cancer cells by persuading apoptosis | [231] 2022 |
| Others | |||
| Fertilizer for Plant | nitrogen and sulfur co-doped CQDs (NS-CQDs) | Carriers of nutrients and microbes for plant growth promotion | [232] 2022 |
| Separation of water from alcohol (alcohol dehydration) | SCQDs (sulfonated carbon quantum dots) with GO (graphene oxide) | SCQDs act as water transporter | [233] 2022 |
| Security authentication (covertness under daylight) | NCDs printing ink (Nitrogen-doped CDs from rice straw waste) | Reversible photochromism (printed cellulose papers: no color under daylight conditions, but blue emissions under UV light) | [234] 2022 |
| Nano-powder of Ludox@CDs (CDs prepared from cetylpyridinium chloride) | Fingermark imaging under UV light (wide range of emission) | [231] 2022 | |
| Anti-counterfeit agent (covertness under daylight) | CDs ink (pine pollen) | Reversible photochromism (printed cellulose papers do not exhibit any color under daylight conditions, but blue emission is demonstrated under UV light) | [28] 2022 |
| Lubricants | A-CDs (Amphiphilic CDs synthesized from TWEEN-80) | A-CDs stabilized with Span-80 are used as lubricant additives of polyalphaolefin | [235] 2022 |
| Food packing | S-CD (Sulfur functionalized turmeric-derived carbon dots) | S-CD is used as combined material with pectin/gelatin film due to antibacterial activity against the foodborne pathogenic bacteria | [236] 2022 |
| Flame retardant | gCDs-PET co-polyester ((gelatin based CDs as a co-polymerizable flame retardants for PET (poly(ethylene terephthalate)) | Thermal decomposition of PET is catalyzed by gCDs | [237] 2022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pundi, A.; Chang, C.-J. Recent Advances in Synthesis, Modification, Characterization, and Applications of Carbon Dots. Polymers 2022, 14, 2153. https://doi.org/10.3390/polym14112153
Pundi A, Chang C-J. Recent Advances in Synthesis, Modification, Characterization, and Applications of Carbon Dots. Polymers. 2022; 14(11):2153. https://doi.org/10.3390/polym14112153
Chicago/Turabian StylePundi, Arul, and Chi-Jung Chang. 2022. "Recent Advances in Synthesis, Modification, Characterization, and Applications of Carbon Dots" Polymers 14, no. 11: 2153. https://doi.org/10.3390/polym14112153
APA StylePundi, A., & Chang, C.-J. (2022). Recent Advances in Synthesis, Modification, Characterization, and Applications of Carbon Dots. Polymers, 14(11), 2153. https://doi.org/10.3390/polym14112153