Synthesis of Thermoplastic Polyurethanes Containing Bio-Based Polyester Polyol and Their Fiber Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Thermoplastic Polyurethanes
2.3. Preparation of Thermoplastic Polyurethane Fibers
2.4. Characterization
3. Results and Discussion
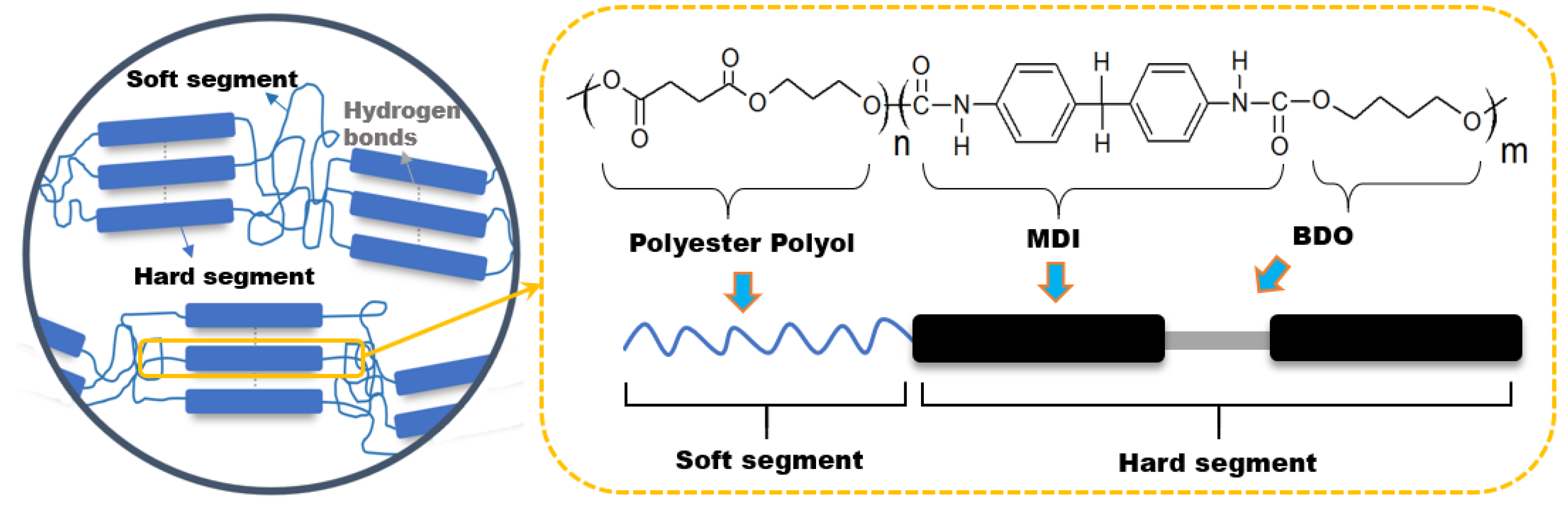
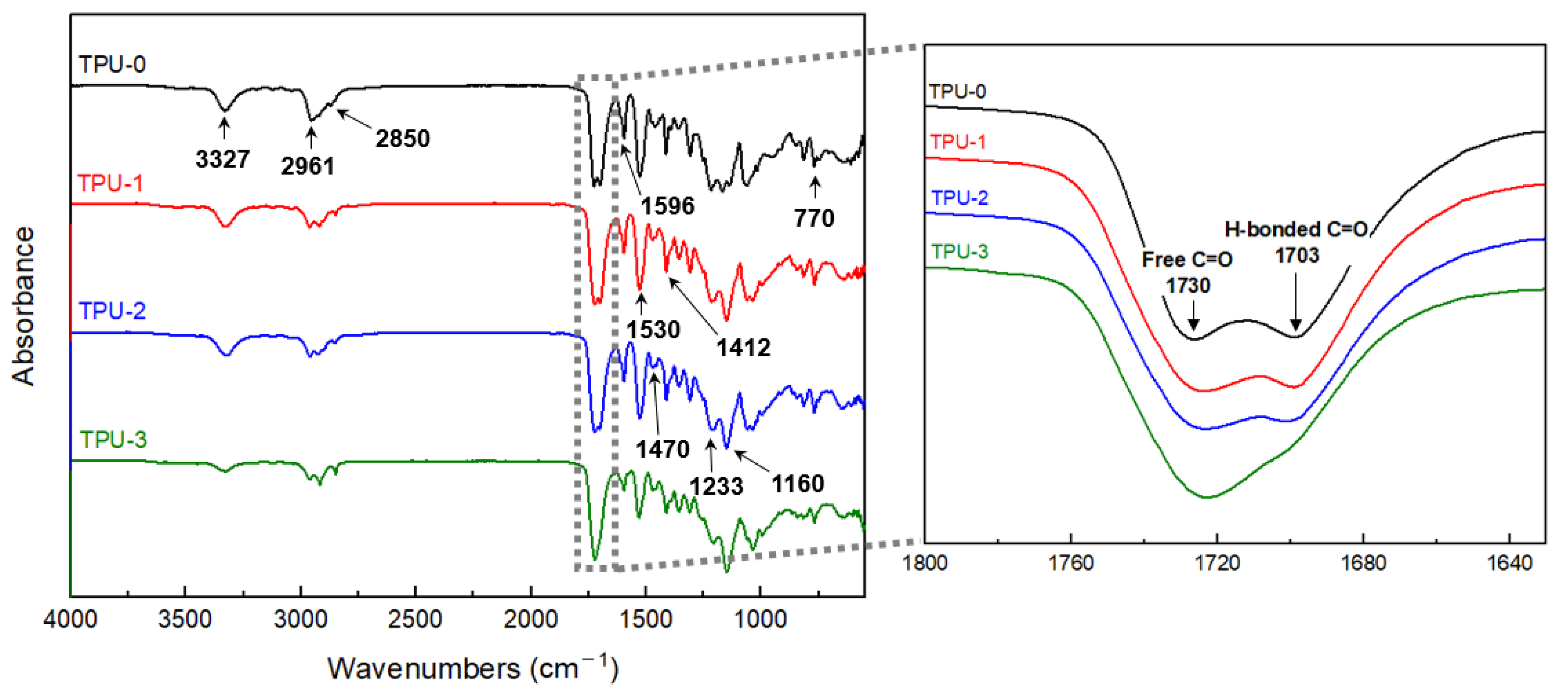
3.1. Structural Characterisation of Thermoplastic Polyurethanes
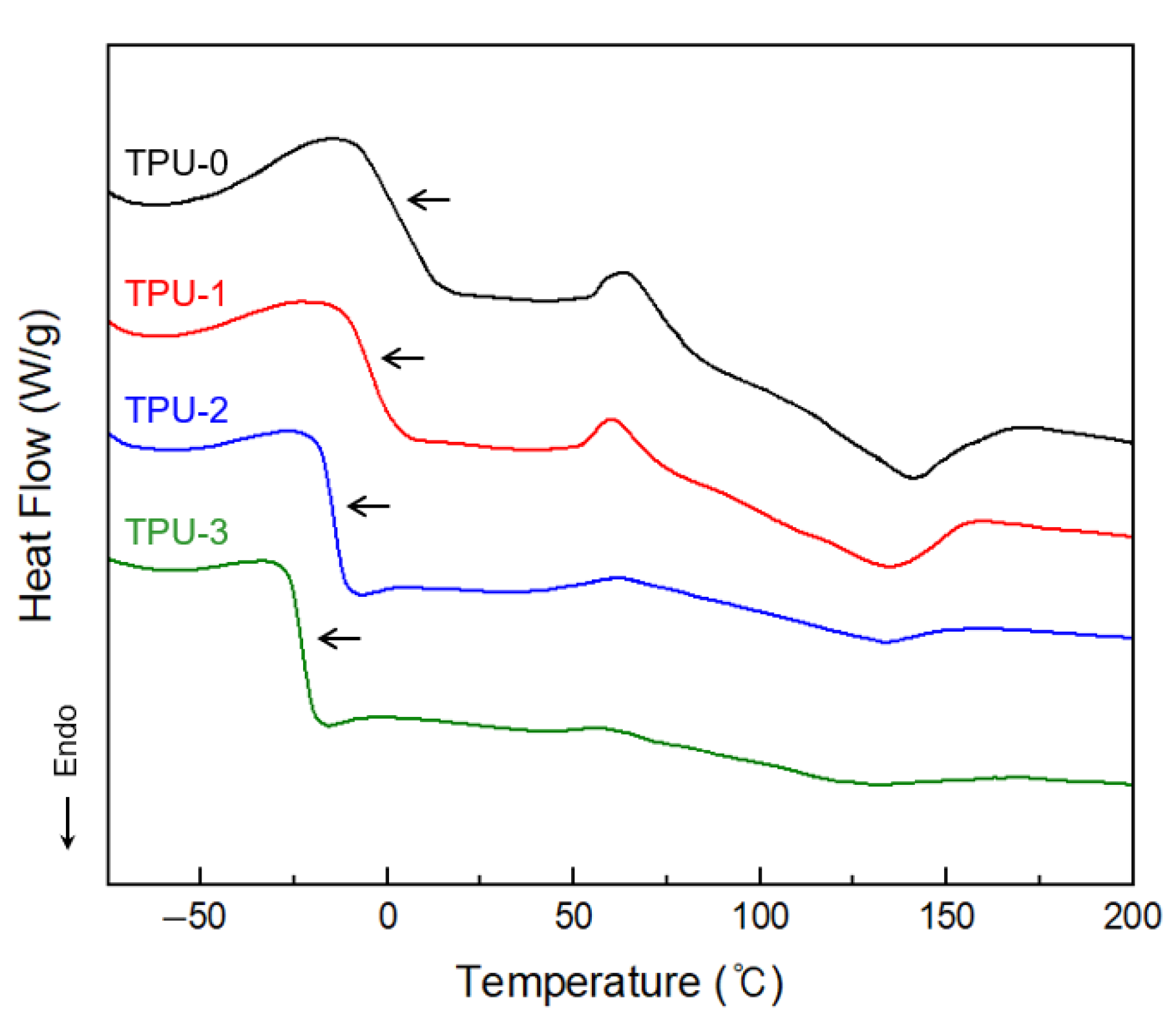
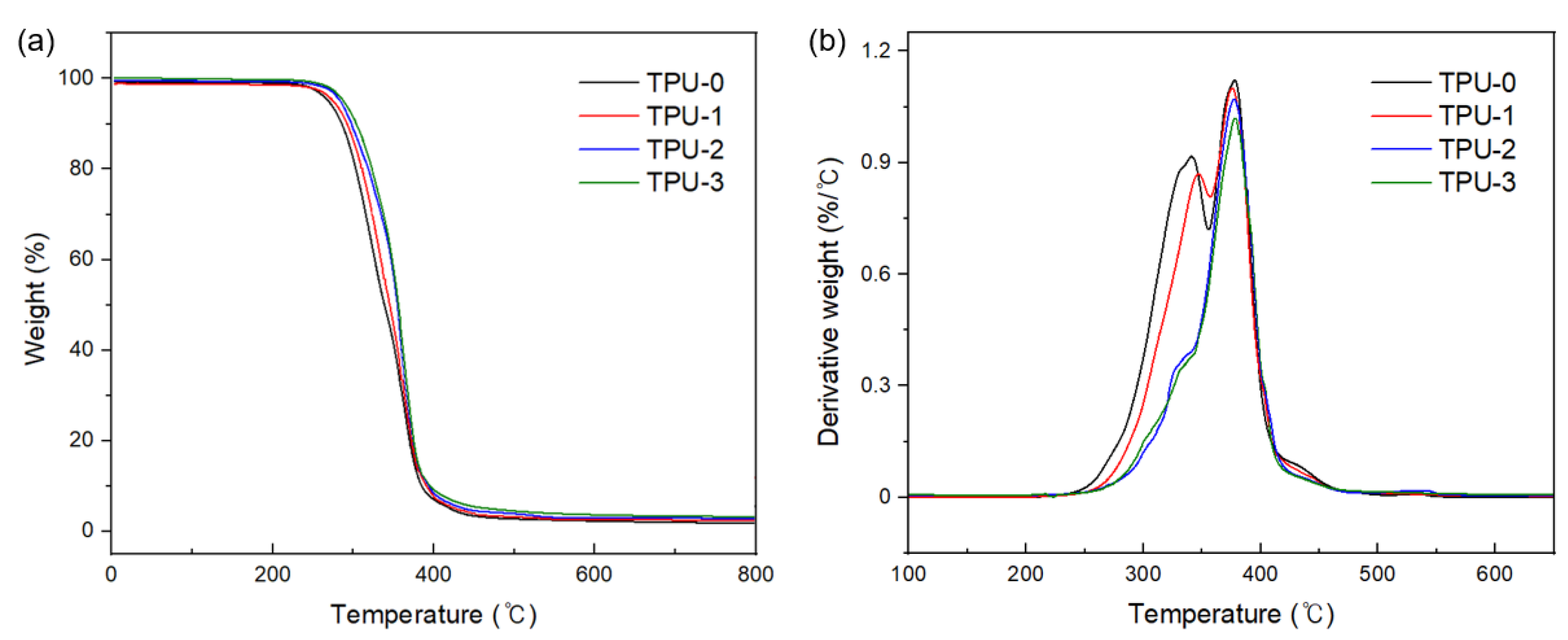
3.2. Thermal Characterisation of Thermoplastic Polyurethanes
3.3. Mechanical Properties of Thermoplastic Polyurethane Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charlon, M.; Heinrich, B.; Matter, Y.; Couzigné, E.; Donnio, B.; Avérous, L. Synthesis, structure and properties of fully biobased thermoplastic polyurethanes, obtained from a diisocyanate based on modified dimer fatty acids, and different renewable diols. Eur. Polym. J. 2014, 61, 197–205. [Google Scholar] [CrossRef]
- Cai, W.; Wang, B.; Liu, L.; Zhou, X.; Chu, F.; Zhan, J.; Hu, Y.; Kan, Y.; Wang, X. An operable platform towards functionalization of chemically inert boron nitride nanosheets for flame retardancy and toxic gas suppression of thermoplastic polyurethane. Compos. Part B 2019, 178, 107462. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Jiao, C. Synergistic effects between hollow glass microsphere and ammonium polyphosphate on flame-retardant thermoplastic polyurethane. J. Therm. Anal. Calorim. 2014, 117, 857–866. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, T.K.; Varadarajan, K.M. Strong, stretchable and ultrasensitive mwcnt/tpu nanocomposites for piezoresistive strain sensing. Compos. Part B 2019, 177, 107285. [Google Scholar] [CrossRef]
- Martin, D.J.; Meijs, G.F.; Gunatillake, P.A.; McCarthy, S.J.; Renwick, G.M. The effect of average soft segment length on morphology and properties of a series of polyurethane elastomers. II. Saxs-dsc annealing study. J. Appl. Polym. Sci. 1997, 64, 803–817. [Google Scholar] [CrossRef]
- Gupta, T.K.; Singh, B.P.; Tripathi, R.K.; Dhakate, S.R.; Singh, V.N.; Panwar, O.S.; Mathur, R.B. Superior nano-mechanical properties of reduced graphene oxide reinforced polyurethane composites. RSC Adv. 2015, 5, 16921–16930. [Google Scholar] [CrossRef]
- Miller, J.A.; Lin, S.B.; Hwang, K.K.S.; Wu, K.S.; Gibson, P.E.; Cooper, S.L. Properties of polyether-polyurethane block copolymers: Effects of hard segment length distribution. Macromolecules 1985, 18, 32–44. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. J. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Bueno-Ferrer, C.; Hablot, E.; Perrin-Sarazin, F.; Garrigós, M.C.; Jiménez, A.; Averous, L. Structure and morphology of new bio-based thermoplastic polyurethanes obtained from dimeric fatty acids. Macromol. Mater. Eng. 2012, 297, 777–784. [Google Scholar] [CrossRef]
- Bueno-Ferrer, C.; Hablot, E.; Garrigós, M.D.C.; Bocchini, S.; Averous, L.; Jiménez, A. Relationship between morphology, properties and degradation parameters of novative biobased thermoplastic polyurethanes obtained from dimer fatty acids. Polym. Degrad. Stab. 2012, 97, 1964–1969. [Google Scholar] [CrossRef]
- Hepburn, C. Property-structure relationships in polyurethanes. In Polyurethane Elastomers, 1st ed.; Springer: Dordrecht, The Netherlands, 1992; pp. 51–106. [Google Scholar]
- Lin, S.B.; Hwang, K.S.; Tsay, S.Y.; Cooper, S.L. Segmental orientation studies of polyether polyurethane block copolymers with different hard segment lengths and distributions. Colloid Polym. Sci. 1985, 263, 128–140. [Google Scholar] [CrossRef]
- Hundiwale, D.G.; Kapadi, U.R.; Pandya, M.V. Effect of macroglycol structure and its molecular weight on physicomechanical properties of polyurethanes. J. Appl. Polym. Sci. 1995, 55, 1329–1333. [Google Scholar] [CrossRef]
- Seymour, R.W.; Estes, G.M.; Cooper, S.L. Infrared studies of segmented polyurethan elastomers. I. Hydrogen bonding. Macromolecules 1970, 3, 579–583. [Google Scholar] [CrossRef]
- Abouzahr, S.; Wilkes, G.L. Structure property studies of polyester- and polyether-based mdi–bd segmented polyurethanes: Effect of one- vs. Two-stage polymerization conditions. J. Appl. Polym. Sci. 1984, 29, 2695–2711. [Google Scholar] [CrossRef]
- Ahn, T.O.; Choi, I.S.; Jeong, H.M.; Cho, K. Thermal and mechanical properties of thermoplastic polyurethane elastomers from different polymerization methods. Polym. Int. 1993, 31, 329–333. [Google Scholar] [CrossRef]
- Pattamaprom, C.; Wu, C.-H.; Chen, P.-H.; Huang, Y.-L.; Ranganathan, P.; Rwei, S.-P.; Chuan, F.-S. Solvent-free one-shot synthesis of thermoplastic polyurethane based on bio-poly(1,3-propylene succinate) glycol with temperature-sensitive shape memory behavior. ACS Omega 2020, 5, 4058–4066. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wu, B.; Jiang, Y.; Lei, J.; Zhou, C.; Zhang, J.; Wang, J. Solvent-free and self-catalysis synthesis and properties of waterborne polyurethane. Polymer 2018, 143, 129–136. [Google Scholar] [CrossRef]
- Kim, S.G.; Lee, D.S. Effect of polymerization procedure on thermal and mechanical properties of polyether based thermoplastic polyurethanes. Macromol. Res. 2002, 10, 365–368. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lebarbé, T.; Grau, E.; Cramail, H. Structure-properties relationship of fatty acid-based thermoplastics as synthetic polymer mimics. Polym. Chem. 2013, 4, 5472–5517. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased polyurethanes prepared from different vegetable oils. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. [Google Scholar] [CrossRef]
- Petrović, Z.S. Polyurethanes from vegetable oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Sharma, V.; Kundu, P.P. Addition polymers from natural oils—A review. Prog. Polym. Sci. 2006, 31, 983–1008. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Plant oils as platform chemicals for polyurethane synthesis: Current state-of-the-art. Biomacromolecules 2010, 11, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Can, E.; Küsefoğlu, S.; Wool, R.P. Rigid, thermosetting liquid molding resins from renewable resources. I. Synthesis and polymerization of soy oil monoglyceride maleates. J. Appl. Polym. Sci. 2001, 81, 69–77. [Google Scholar] [CrossRef]
- Kaushik, A.; Ahuja, D.; Salwani, V. Synthesis and characterization of organically modified clay/castor oil based chain extended polyurethane nanocomposites. Compos. Part A 2011, 42, 1534–1541. [Google Scholar] [CrossRef]
- Hablot, E.; Zheng, D.; Bouquey, M.; Avérous, L. Polyurethanes based on castor oil: Kinetics, chemical, mechanical and thermal properties. Macromol. Mater. Eng. 2008, 293, 922–929. [Google Scholar] [CrossRef]
- Parcheta, P.; Głowińska, E.; Datta, J. Effect of bio-based components on the chemical structure, thermal stability and mechanical properties of green thermoplastic polyurethane elastomers. Eur. Polym. J. 2020, 123, 109422. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Głowińska, E.; Datta, J. Structure and properties comparison of poly(ether-urethane)s based on nonpetrochemical and petrochemical polyols obtained by solvent free two-step method. Eur. Polym. J. 2021, 157, 110673. [Google Scholar] [CrossRef]
- Haliloglu, T.; Erman, B.; Bahar, I. Dependence of segmental orientation on polymer conformational characteristics. Polymer 1995, 36, 4131–4134. [Google Scholar] [CrossRef]
- Bower, D.I. Orientation distribution functions for biaxially oriented polymers. Polymer 1982, 23, 1251–1255. [Google Scholar] [CrossRef]
- Haverly, M.R.; Fenwick, S.R.; Patterson, F.P.K.; Slade, D.A. Biobased carbon content quantification through ams radiocarbon analysis of liquid fuels. Fuel 2019, 237, 1108–1111. [Google Scholar] [CrossRef]
- Chang, C.-W.; Liao, J.-Y.; Lu, K.-T. Syntheses and characteristics of urushiol-based waterborne uv-cured wood coatings. Polymers 2021, 13, 4005. [Google Scholar] [CrossRef] [PubMed]
- Marín, R.; Alla, A.; Ilarduya, A.M.; Muñoz-Guerra, S. Carbohydrate-based polyurethanes: A comparative study of polymers made from isosorbide and 1,4-butanediol. J. Appl. Polym. Sci. 2012, 123, 986–994. [Google Scholar] [CrossRef]
- Ng, H.N.; Allegrezza, A.E.; Seymour, R.W.; Cooper, S.L. Effect of segment size and polydispersity on the properties of polyurethane block polymers. Polymer 1973, 14, 255–261. [Google Scholar] [CrossRef]
- Hu, W.; Koberstein, J.T. The effect of thermal annealing on the thermal properties and molecular weight of a segmented polyurethane copolymer. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 437–446. [Google Scholar] [CrossRef]
- Kwei, T.K. Phase separation in segmented polyurethanes. J. Appl. Polym. Sci. 1982, 27, 2891–2899. [Google Scholar] [CrossRef]
- Wilhelm, C.; Gardette, J.-L. Infrared analysis of the photochemical behaviour of segmented polyurethanes: 1. Aliphatic poly(ester-urethane). Polymer 1997, 38, 4019–4031. [Google Scholar] [CrossRef]
- Irusta, L.; Fernandez-Berridi, M.J. Aromatic poly(ester–urethanes): Effect of the polyol molecular weight on the photochemical behaviour. Polymer 2000, 41, 3297–3302. [Google Scholar] [CrossRef]
- Kaushiva, B.D.; McCartney, S.R.; Rossmy, G.R.; Wilkes, G.L. Surfactant level influences on structure and properties of flexible slabstock polyurethane foams. Polymer 2000, 41, 285–310. [Google Scholar] [CrossRef]
- Senich, G.A.; MacKnight, W.J. Fourier transform infrared thermal analysis of a segmented polyurethane. Macromolecules 1980, 13, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.F.; Joseph, M.D.; Davidson, C.; Deporter, C.D.; Dais, V.A. Polyurethane elastomers based on molecular weight advanced poly(ethylene ether carbonate) diols. II. Effects of variations in hard segment concentration. J. Appl. Polym. Sci. 1990, 41, 509–525. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Javni, I. The effect of soft-segment length and concentration on phase separation in segmented polyurethanes. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 545–560. [Google Scholar] [CrossRef]
- Son, T.W.; Lee, D.W.; Lim, S.K. Thermal and phase behavior of polyurethane based on chain extender, 2,2-bis-[4-(2-hydroxyethoxy)phenyl]propane. Polymer 1999, 31, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Takahara, A.; Tashita, J.-I.; Kajiyama, T.; Takayanagi, M.; MacKnight, W.J. Microphase separated structure, surface composition and blood compatibility of segmented poly(urethaneureas) with various soft segment components. Polymer 1985, 26, 987–996. [Google Scholar] [CrossRef]
- Wang, C.B.; Cooper, S.L. Morphology and properties of segmented polyether polyurethaneureas. Macromolecules 1983, 16, 775–786. [Google Scholar] [CrossRef]
- Yemul, O.S.; Petrović, Z.S. Thermoplastic polyurethane elastomers from modified oleic acid. Polym. Int. 2014, 63, 1771–1776. [Google Scholar] [CrossRef]
- Orgilέs-Calpena, E.; Arán-Aís, F.; Torró-Palau, A.M.; Orgilέs-Barceló, C. Sustainable polyurethane adhesives derived from carbon dioxide. Polym. Renew. Resour. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Paul, U.C.; Bayer, G.; Grasselli, S.; Malchiodi, A.; Bayer, I.S. Biodegradable, stretchable and transparent plastic films from modified waterborne polyurethane dispersions. Polymers 2022, 14, 1199. [Google Scholar] [CrossRef]
- Pigott, K.A.; Frye, B.F.; Allen, K.R.; Steingiser, S.S.; Darr, W.C.; Saunders, J.H.; Hardy, E.E. Development of cast urethane elastomers for ultimate properties. J. Chem. Eng. Data 1960, 5, 391–395. [Google Scholar] [CrossRef]
- Macknight, W.J.; Yang, M. Property-structure relationships in poly-urethanes: Infrared studies. J. Polym. Sci. Polym. Symp. 1973, 42, 817–832. [Google Scholar] [CrossRef]
- Abouzahr, S.; Wilkes, G.L.; Ophir, Z. Structure-property behaviour of segmented polyether-mdi-butanediol based urethanes: Effect of composition ratio. Polymer 1982, 23, 1077–1086. [Google Scholar] [CrossRef]
- Hespe, H.F.; Zembrod, A.; Cama, F.J.; Lantman, C.W.; Seneker, S.D. Influence of molecular weight on the thermal and mechanical properties of polyurethane elastomers based on 4,4′-diisocyanato dicyclohexylmethane. J. Appl. Polym. Sci. 1992, 44, 2029–2035. [Google Scholar] [CrossRef]
- Brunette, C.M.; Hsu, S.L.; MacKnight, W.J. Hydrogen-bonding properties of hard-segment model compounds in polyurethane block copolymers. Macromolecules 1982, 15, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Lee, N.W.; Ihm, D.W.; Paik, K.H. Segmental orientation behavior of poly(butyleneterephthalate-co-tetramethylene oxide) upon uniaxial deformation. Macromolecules 1994, 27, 4364–4370. [Google Scholar] [CrossRef]
- Briber, R.M.; Thomas, E.L. The structure of mdi/bdo-based polyurethanes: Diffraction studies on model compounds and oriented thin films. J. Polym. Sci. Polym. Phys. 1985, 23, 1915–1932. [Google Scholar] [CrossRef]
| Samples | Type of Polyol | Mn of Polyol (g/mol) | Polyol:BDO:MDI (Molar Ratio) | HS Content (wt%) |
|---|---|---|---|---|
| TPU-0 | Petroleum | 1000 | 1:1:2 | 39.0 |
| TPU-1 | Biomass | 1000 | 1:1:2 | 38.8 |
| TPU-2 | Biomass | 2000 | 1:1:2 | 22.3 |
| TPU-3 | Biomass | 4000 | 1:1:2 | 13.3 |
| Samples | Mw (g/mol) | Mn (g/mol) | PDI (Mw/Mn) | Biobased C (%) |
|---|---|---|---|---|
| TPU-0 | 121,855 | 96,155 | 1.2 | 0 |
| TPU-1 | 120,158 | 88,215 | 1.3 | 54.1 |
| TPU-2 | 113,738 | 84,906 | 1.3 | 53.0 |
| TPU-3 | 107,693 | 81,606 | 1.3 | 51.5 |
| Samples | Tg,SS (°C) | Tg,HS (°C) | Tm,HS (°C) | ∆Hm,HS (J/g) |
|---|---|---|---|---|
| TPU-0 | −0.67 | 95.31 | 135.08 | 10.80 |
| TPU-1 | −4.86 | 93.57 | 133.25 | 9.31 |
| TPU-2 | −10.93 | 89.33 | 131.22 | 4.39 |
| TPU-3 | −18.54 | 86.18 | 130.80 | 4.04 |
| Samples | T5% (°C) | Tmax% (°C) | Td1 (°C) | Td2 (°C) | Char Residue at 600 °C (wt%) |
|---|---|---|---|---|---|
| TPU-0 | 300.05 | 408.22 | 346.81 | 385.64 | 1.1 |
| TPU-1 | 301.32 | 408.50 | 346.68 | 386.40 | 1.3 |
| TPU-2 | 302.84 | 408.74 | 348.75 | 386.09 | 1.3 |
| TPU-3 | 302.91 | 409.18 | 344.07 | 388.57 | 1.5 |
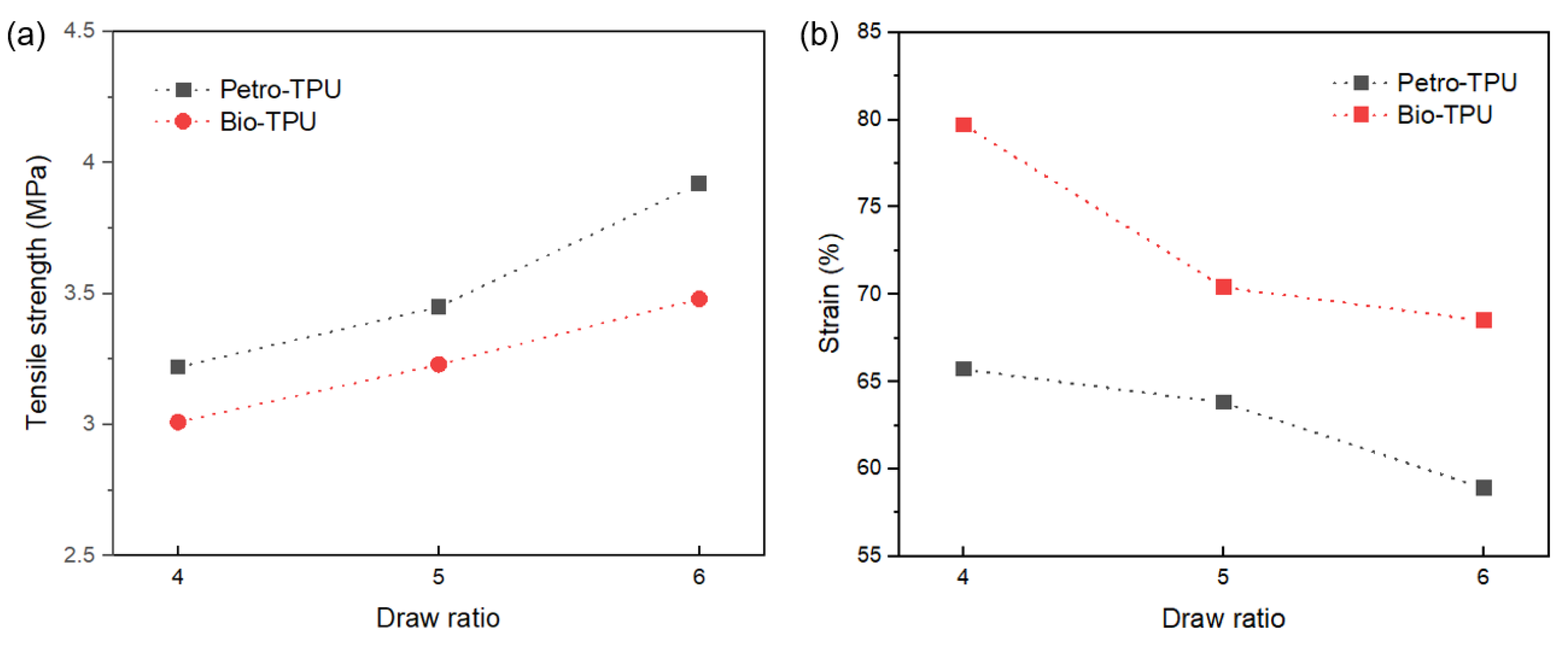
| Samples | 4 Times (Draw Ratio) | 5 Times (Draw Ratio) | 6 Times (Draw Ratio) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | Elastic Recovery (%) | Tensile Strength (MPa) | Elongation at Break (%) | Elastic Recovery (%) | Tensile Strength (MPa) | Elongation at Break (%) | Elastic Recovery (%) | |
| Petro-TPU | 3.22 ± 0.05 | 65.7 ± 0.2 | 100 | 3.45 ± 0.05 | 63.8 ± 0.2 | 100 | 3.92 ± 0.04 | 58.9 ± 0.1 | 100 |
| Bio-TPU | 3.01 ± 0.08 | 79.7 ± 0.3 | 100 | 3.23 ± 0.06 | 70.4 ± 0.3 | 100 | 3.48 ± 0.05 | 68.5 ± 0.2 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Kim, Y.K.; Hwang, S.-H.; Kim, H.-C.; Jung, J.-H.; Jeon, C.-H.; Kim, J.; Lim, S.K. Synthesis of Thermoplastic Polyurethanes Containing Bio-Based Polyester Polyol and Their Fiber Property. Polymers 2022, 14, 2033. https://doi.org/10.3390/polym14102033
Oh J, Kim YK, Hwang S-H, Kim H-C, Jung J-H, Jeon C-H, Kim J, Lim SK. Synthesis of Thermoplastic Polyurethanes Containing Bio-Based Polyester Polyol and Their Fiber Property. Polymers. 2022; 14(10):2033. https://doi.org/10.3390/polym14102033
Chicago/Turabian StyleOh, Jiyeon, Young Kwang Kim, Sung-Ho Hwang, Hyun-Chul Kim, Jae-Hun Jung, Cho-Hyun Jeon, Jongwon Kim, and Sang Kyoo Lim. 2022. "Synthesis of Thermoplastic Polyurethanes Containing Bio-Based Polyester Polyol and Their Fiber Property" Polymers 14, no. 10: 2033. https://doi.org/10.3390/polym14102033
APA StyleOh, J., Kim, Y. K., Hwang, S.-H., Kim, H.-C., Jung, J.-H., Jeon, C.-H., Kim, J., & Lim, S. K. (2022). Synthesis of Thermoplastic Polyurethanes Containing Bio-Based Polyester Polyol and Their Fiber Property. Polymers, 14(10), 2033. https://doi.org/10.3390/polym14102033






