Facile Fabrication of Fluorine-Free, Anti-Icing, and Multifunctional Superhydrophobic Surface on Wood Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Superhydrophobic PMHOS
2.3. Synthesis of the SiO2/PMHOS Solution
2.4. Surface Modification of the Wood Samples
2.5. Characterization
3. Results and Discussion
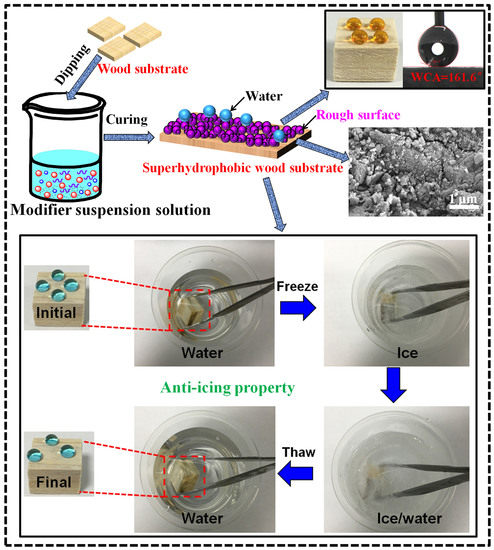
3.1. Fabrication of Superhydrophobic Wood
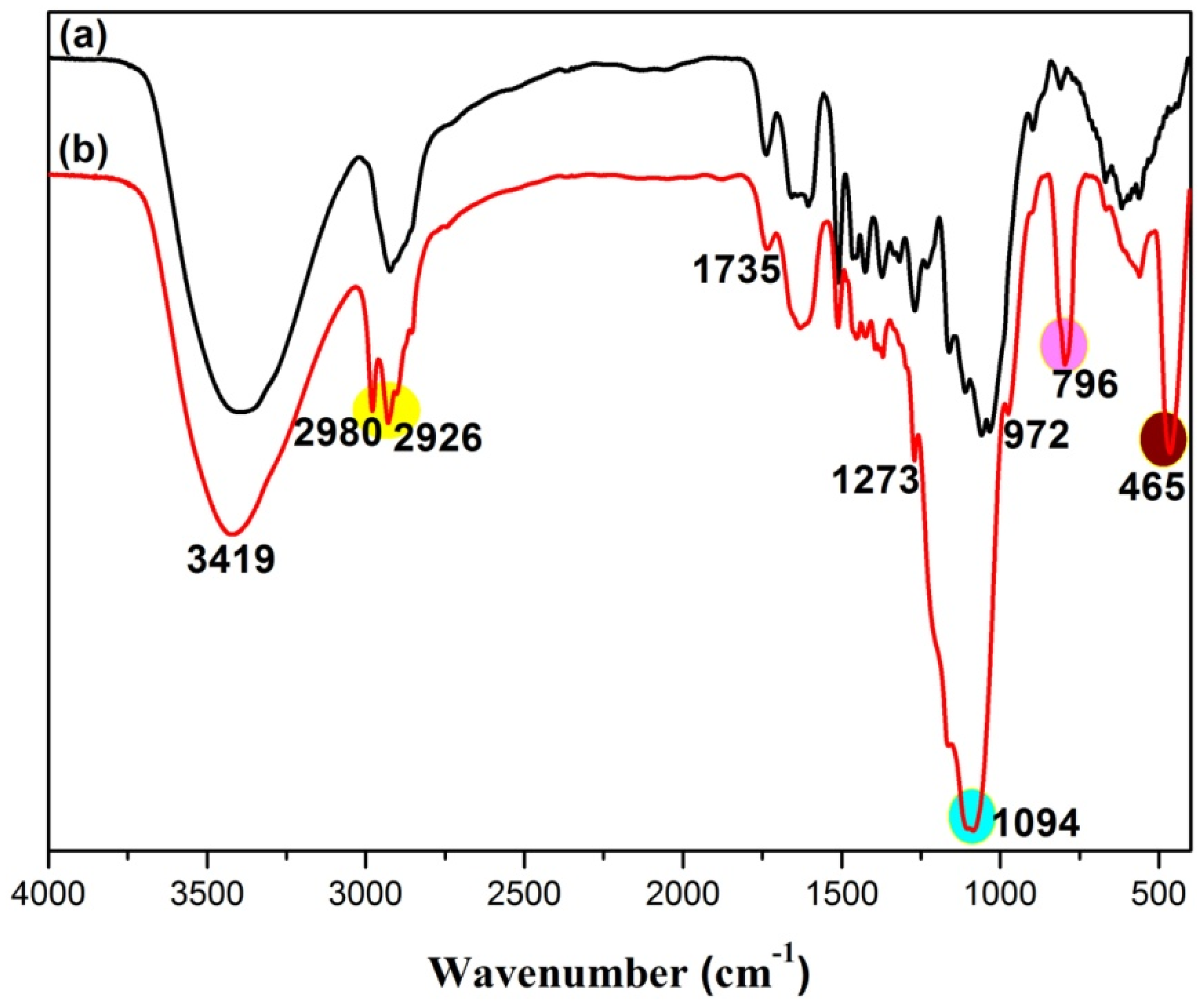
3.2. Fourier Transform Infrared (FTIR) Characterization
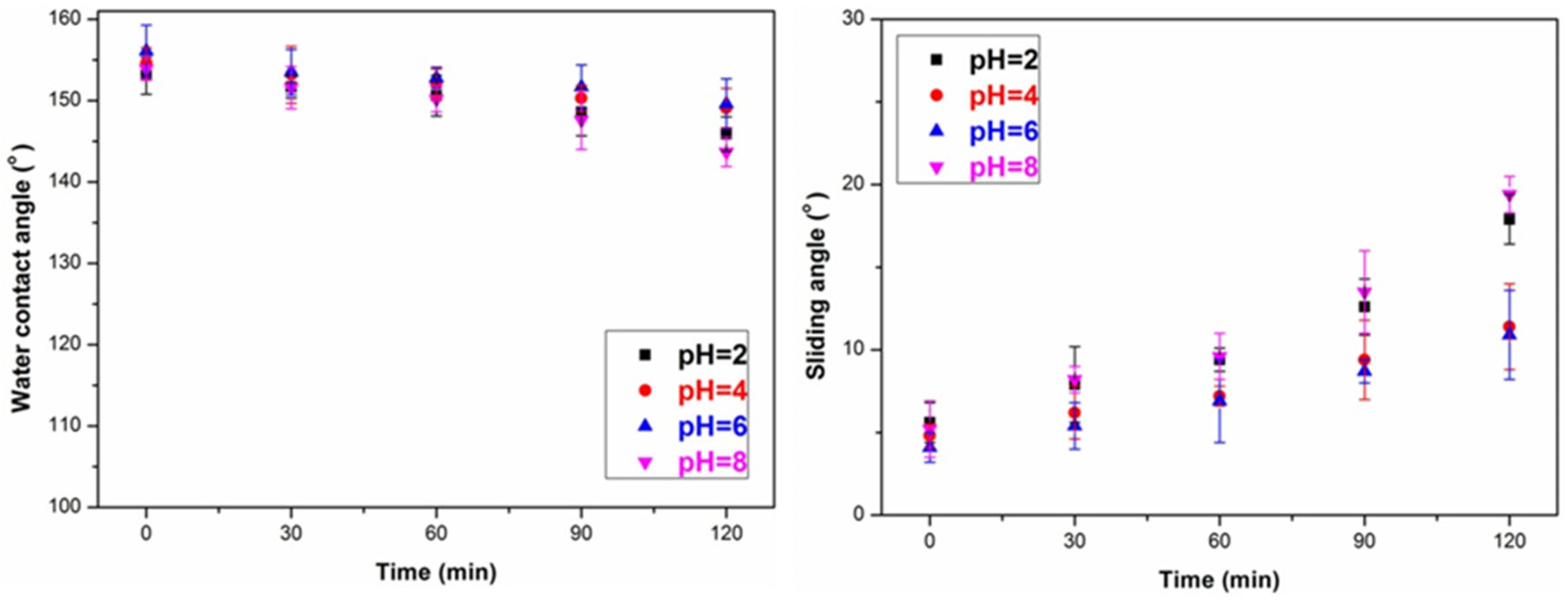
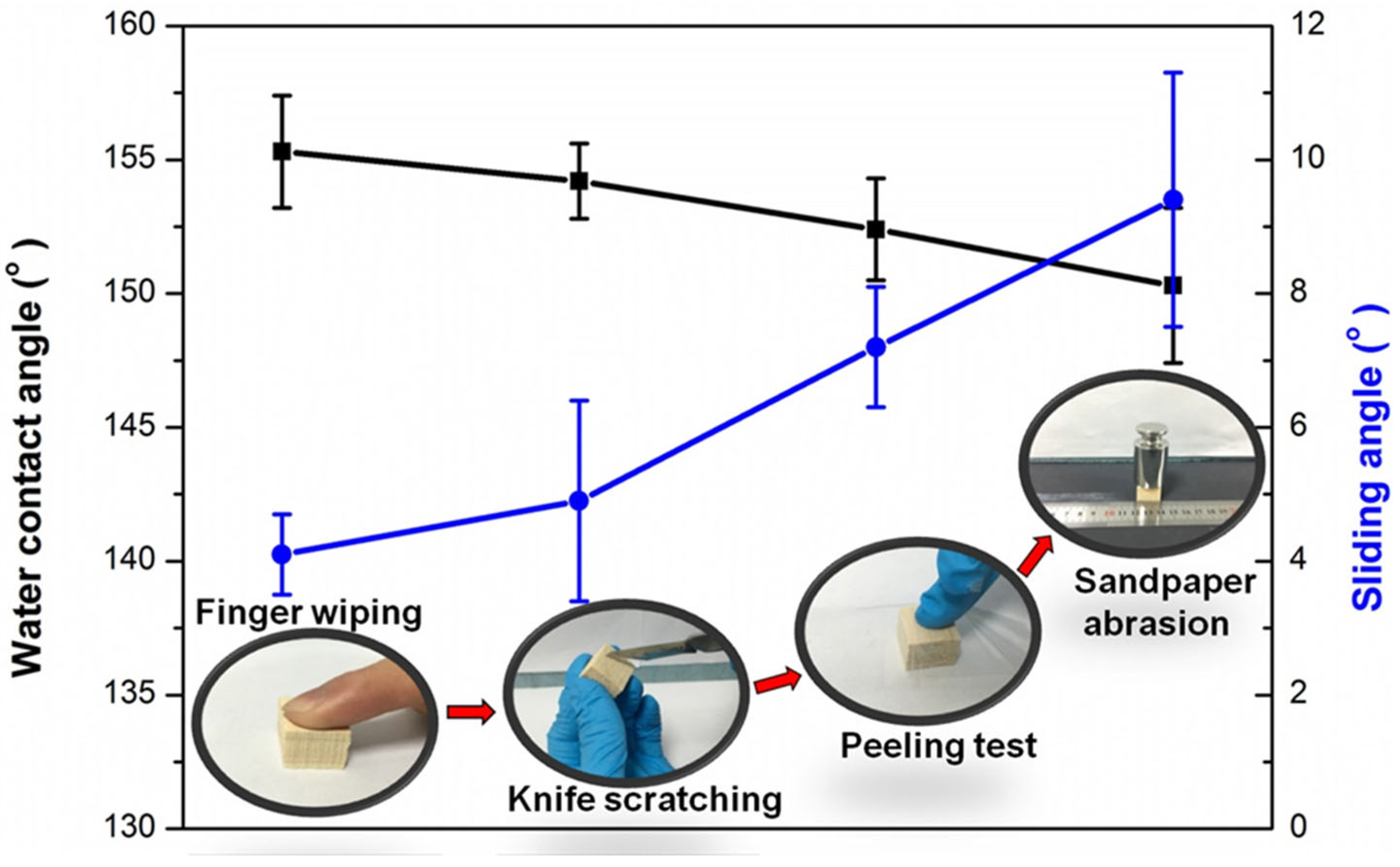
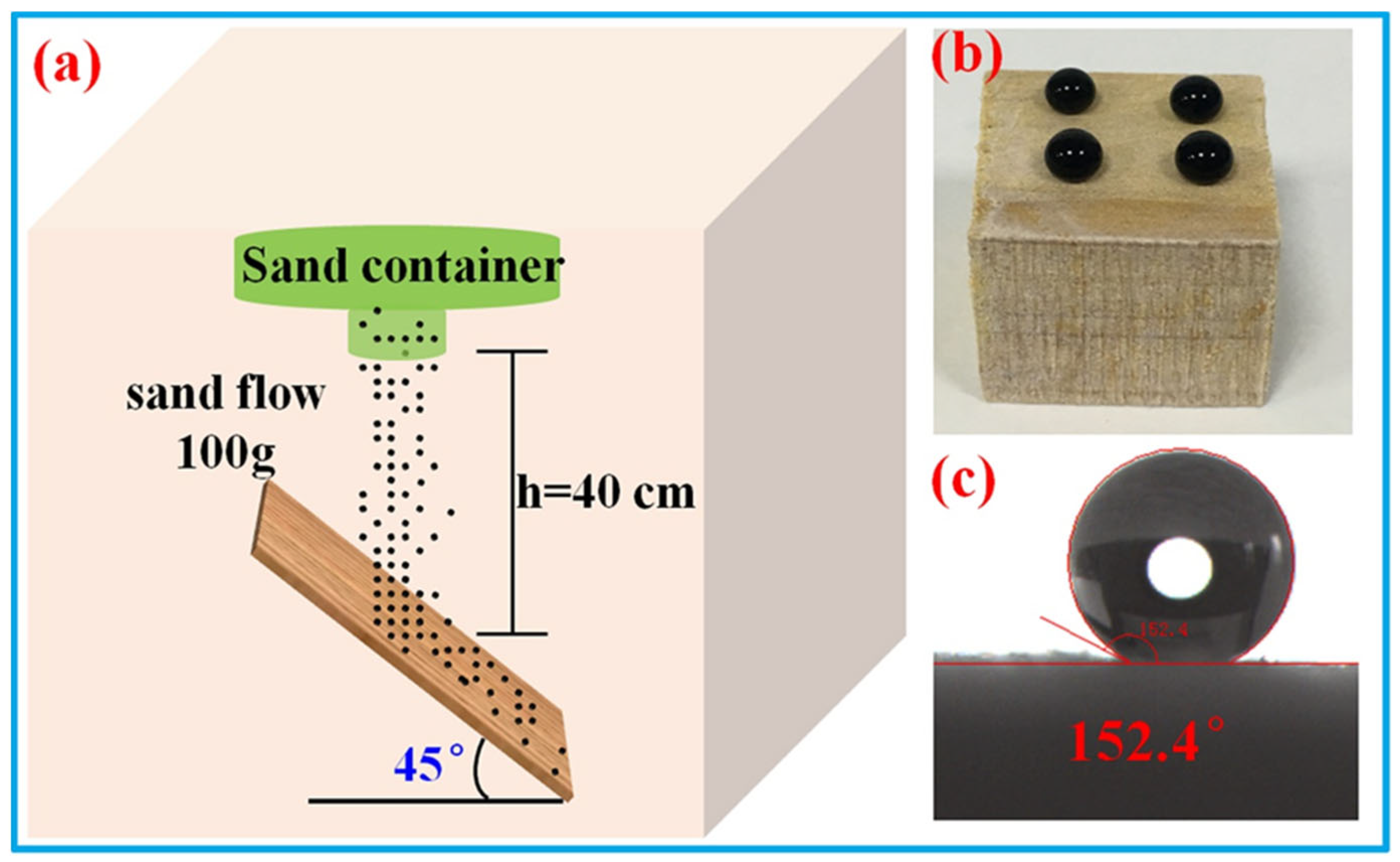
3.3. The Durability of the Superhydrophobic Wood
3.4. Multifunctional Wood Treated with SiO2/PMHOS
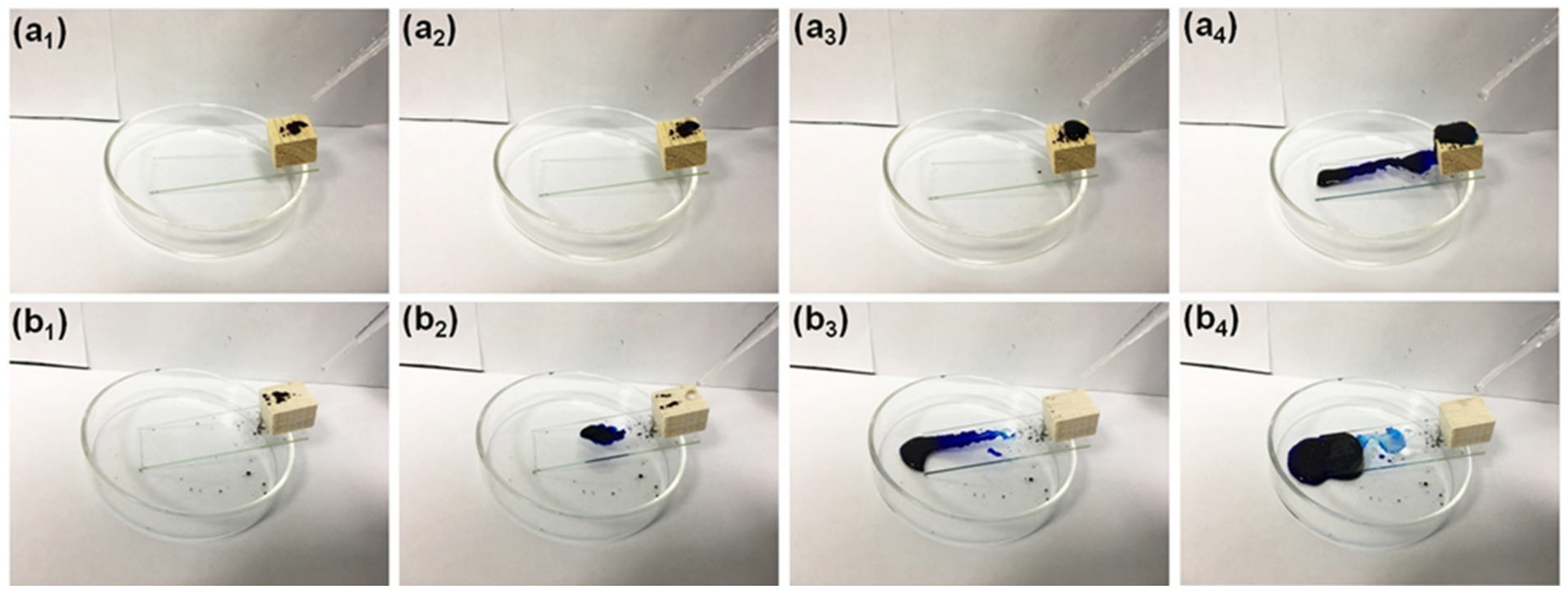
3.4.1. Self-Cleaning and Anti-Fouling Property
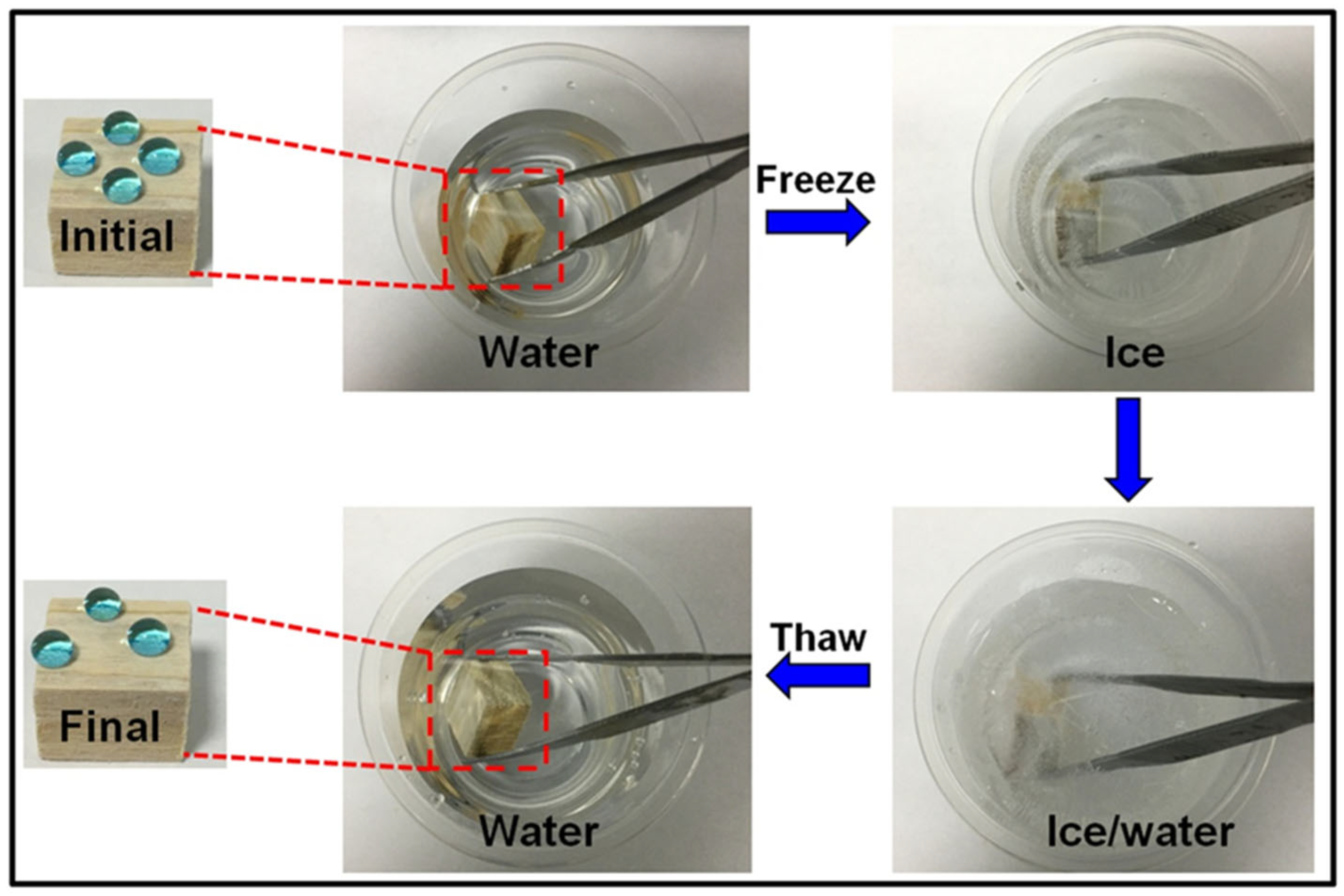
3.4.2. Anti-Icing Property
3.5. Water Repellency and Dimensional Stability
3.6. The Formation Mechanism of the Superhydrophobic Wood Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poaty, B.; Riedl, B.; Blanchet, P.; Blanchard, V.; Stafford, L. Improved water repellency of black spruce wood surfaces after treatment in carbon tetrafluoride plasmas. Wood Sci. Technol. 2013, 47, 411–422. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, M.; Zhan, H. Preparation of SiO2-wood composites by an ultrasonic-assisted sol-gel technique. Cellulose 2014, 21, 4393–4403. [Google Scholar] [CrossRef]
- Kumar, A.; Richter, J.; Tywoniak, J.; Hajek, P.; Adamopoulos, S.; Segedin, U.; Petric, M. Surface modification of Norway spruce wood by octadecyltrichlorosilane (OTS) nanosol by dipping and water vapour diffusion properties of the OTS-modified wood. Holzforschung 2017, 72, 45–56. [Google Scholar] [CrossRef]
- Han, X.; Yin, Y.; Zhang, Q.; Li, R.; Pu, J. Improved wood properties via two-step grafting with itaconic acid (IA) and nano-SiO2. Holzforschung 2018, 72, 499–506. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Y.; Li, J.; Liu, Z.; Yang, W.; Li, R.; Chen, H.; Zhang, X. Preparation of highly hydrophobic and anti-fouling wood using poly (methylhydrogen) siloxane. Cellulose 2018, 25, 7341–7353. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Biophysics: Water-repellent legs of water striders. Nature 2004, 432, 36–39. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Extrand, C.W. Repellency of the Lotus Leaf: Resistance to Water Intrusion under Hydrostatic Pressure. Langmuir 2011, 27, 6920–6925. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Bioinspired rice leaf and butterfly wing surface structures combining shark skin and lotus effects. Soft Matter 2012, 8, 11271–11284. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, S.; Qing, Y.; Luo, S.; Liu, M. A versatile and efficient method to fabricate durable superhydrophobic surfaces on wood, lignocellulosic fiber, glass, and metal substrates. J. Mater. Chem. A 2016, 4, 14111–14121. [Google Scholar] [CrossRef]
- Tu, K.; Wang, X.; Kong, L.; Guan, H. Facile preparation of mechanically durable, self-healing and multifunctional superhydrophobic surfaces on solid wood. Mater. Des. 2018, 140, 30–36. [Google Scholar] [CrossRef]
- Deng, S.; Jia, S.; Deng, X.; Yan, Q.; Wu, Y. New insight into island-like structure driven from hydroxyl groups for high-performance superhydrophobic surfaces. Chem. Eng. J. 2021, 416, 129078. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-Icing Superhydrophobic Coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Maslakov, K.I. Anti-icing properties of a superhydrophobic surface in a salt environment: An unexpected increase in freezing delay times for weak brine droplets. Phys. Chem. Chem. Phys. 2016, 18, 3131–3136. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Shi, L.; Li, J.; Xin, Y.; Yang, T.; Guo, Z. Transparent superhydrophobic/superhydrophilic coatings for self-cleaning and anti-fogging. Appl. Phys. Lett. 2012, 101, 033701. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, Y.; Gong, J.; Gong, D.; Chi, L.; Lin, C.; Chen, Z. Transparent superhydrophobic/superhydrophilic TiO2-based coatings for self-cleaning and anti-fogging. J. Mater. Chem. 2012, 22, 7420–7426. [Google Scholar] [CrossRef]
- Cho, E.C.; Changjian, C.W.; Chen, H.C.; Chuang, K.S.; Zheng, J.H.; Hsiao, Y.; Lee, K.C.; Huang, J.H. Robust multifunctional superhydrophobic coatings with enhanced water/oil separation, self-cleaning, anti-corrosion, and anti-biological adhesion. Chem. Eng. J. 2017, 314, 347–357. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, X.; Cai, Q.; Yang, W.; Chen, H. Dehydrogenation-driven assembly of transparent and durable superhydrophobic ORMOSIL coatings on cellulose-based substrates. Cellulose 2020, 27, 7805–7821. [Google Scholar] [CrossRef]
- Lee, C.; Kim, C.J. Underwater Restoration and Retention of Gases on Superhydrophobic Surfaces for Drag Reduction. Phys. Rev. Lett. 2011, 106, 014502. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, Z.; Xu, X.; Guo, F.; Zhu, X.; Men, X.; Ge, B. Robust and Durable Superhydrophobic Cotton Fabrics for Oil/Water Separation. ACS Appl. Mater. Inter. 2013, 5, 7208–7214. [Google Scholar] [CrossRef]
- Mai, Z.; Shu, X.; Li, G.; Chen, D.; Liu, M.; Xu, W.; Zhang, H. One-step fabrication of flexible, durable and fluorine-free superhydrophobic cotton fabrics for efficient oil/water separation. Cellulose 2019, 26, 7208–7214. [Google Scholar] [CrossRef]
- Kong, L.; Tu, K.; Guan, H.; Wang, X. Growth of high-density ZnO nanorods on wood with enhanced photostability, flame retardancy and water repellency. Appl. Surf. Sci. 2017, 407, 479–484. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, S.; Wang, S.; Qing, Y.; Yan, N.; Wang, Q.; Meng, T. A facile and novel emulsion for efficient and convenient fabrication of durable superhydrophobic materials. Chem. Eng. J. 2017, 328, 186–196. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Liu, G.; Zhang, M.; Li, J.; Wang, C. Fabrication of superhydrophobic wood surface by a sol–gel process. Appl. Surf. Sci. 2011, 258, 806–810. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Liu, C.; Zhang, M.; Ma, H.; Li, J. Fabrication of superhydrophobic spherical-like α-FeOOH films on the wood surface by a hydrothermal method. Colloid Surf. A 2012, 403, 29–34. [Google Scholar] [CrossRef]
- Wu, Y.; Qing, Y.; Liang, J.; Liu, M.; Luo, S. Facile fabrication of superhydrophobic surfaces on wood substrates via a one-step hydrothermal process. Appl. Surf. Sci. 2015, 330, 332–338. [Google Scholar]
- Xie, L.; Wang, H.; Dai, Q.; Guanben, D.U. Fabrication of superhydrophobic wood by plasma etching and deposition of diamond-like carbon films. J. For. Eng. 2016, 1, 10–14. [Google Scholar]
- Yue, D.; Feng, Q.; Huang, X.; Zhang, X.; Chen, H. In Situ Fabrication of a Superhydrophobic ORMOSIL Coating on Wood by an Ammonia–HMDS Vapor Treatment. Coatings 2019, 9, 556. [Google Scholar] [CrossRef] [Green Version]
- Xi, L.; Hu, Y. Layer-by-layer Deposition of TiO2 Nanoparticles in the Wood Surface and its Superhydrophobic Performance. Bioresources 2016, 11, 4605–4620. [Google Scholar]
- Tu, K.; Wang, X.; Kong, L.; Chang, H.; Liu, J. Fabrication of robust, damage-tolerant superhydrophobic coatings on naturally micro-grooved wood surfaces. Rsc. Adv. 2016, 6, 701–707. [Google Scholar] [CrossRef]
- Wang, K.; Dong, Y.; Yan, Y.; Zhang, W.; Qi, C.; Han, C.; Li, J.; Zhang, S. Highly hydrophobic and self-cleaning bulk wood prepared by grafting long-chain alkyl onto wood cell walls. Wood Sci. Technol. 2017, 51, 395–411. [Google Scholar] [CrossRef]
- Brassard, J.; Sarkar, D.K.; Perron, J. Synthesis of Monodisperse Fluorinated Silica Nanoparticles and Their Superhydrophobic Thin Films. ACS Appl. Mater. Inter. 2011, 3, 3583–3588. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xia, B.; Ding, B.; Zhang, Y.; Luo, J.; Jiang, B. Ultra-fast surface hydrophobic modification of sol–gel silica antireflective coating with enhanced abrasion-resistance. Mater. Lett. 2013, 104, 31–33. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Tang, M.; Lin, W.; Ding, Z.; Cai, S.; Chen, H.; Zhang, X. Facile Fabrication of Fluorine-Free, Anti-Icing, and Multifunctional Superhydrophobic Surface on Wood Substrates. Polymers 2022, 14, 1953. https://doi.org/10.3390/polym14101953
Cao M, Tang M, Lin W, Ding Z, Cai S, Chen H, Zhang X. Facile Fabrication of Fluorine-Free, Anti-Icing, and Multifunctional Superhydrophobic Surface on Wood Substrates. Polymers. 2022; 14(10):1953. https://doi.org/10.3390/polym14101953
Chicago/Turabian StyleCao, Mengting, Mingwei Tang, Wensheng Lin, Zehao Ding, Shuang Cai, Hanxian Chen, and Xinxiang Zhang. 2022. "Facile Fabrication of Fluorine-Free, Anti-Icing, and Multifunctional Superhydrophobic Surface on Wood Substrates" Polymers 14, no. 10: 1953. https://doi.org/10.3390/polym14101953
APA StyleCao, M., Tang, M., Lin, W., Ding, Z., Cai, S., Chen, H., & Zhang, X. (2022). Facile Fabrication of Fluorine-Free, Anti-Icing, and Multifunctional Superhydrophobic Surface on Wood Substrates. Polymers, 14(10), 1953. https://doi.org/10.3390/polym14101953