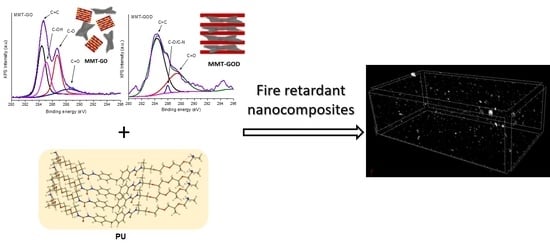
Layered Clay–Graphene Oxide Nanohybrids for the Reinforcement and Fire-Retardant Properties of Polyurea Matrix
Abstract
:1. Introduction
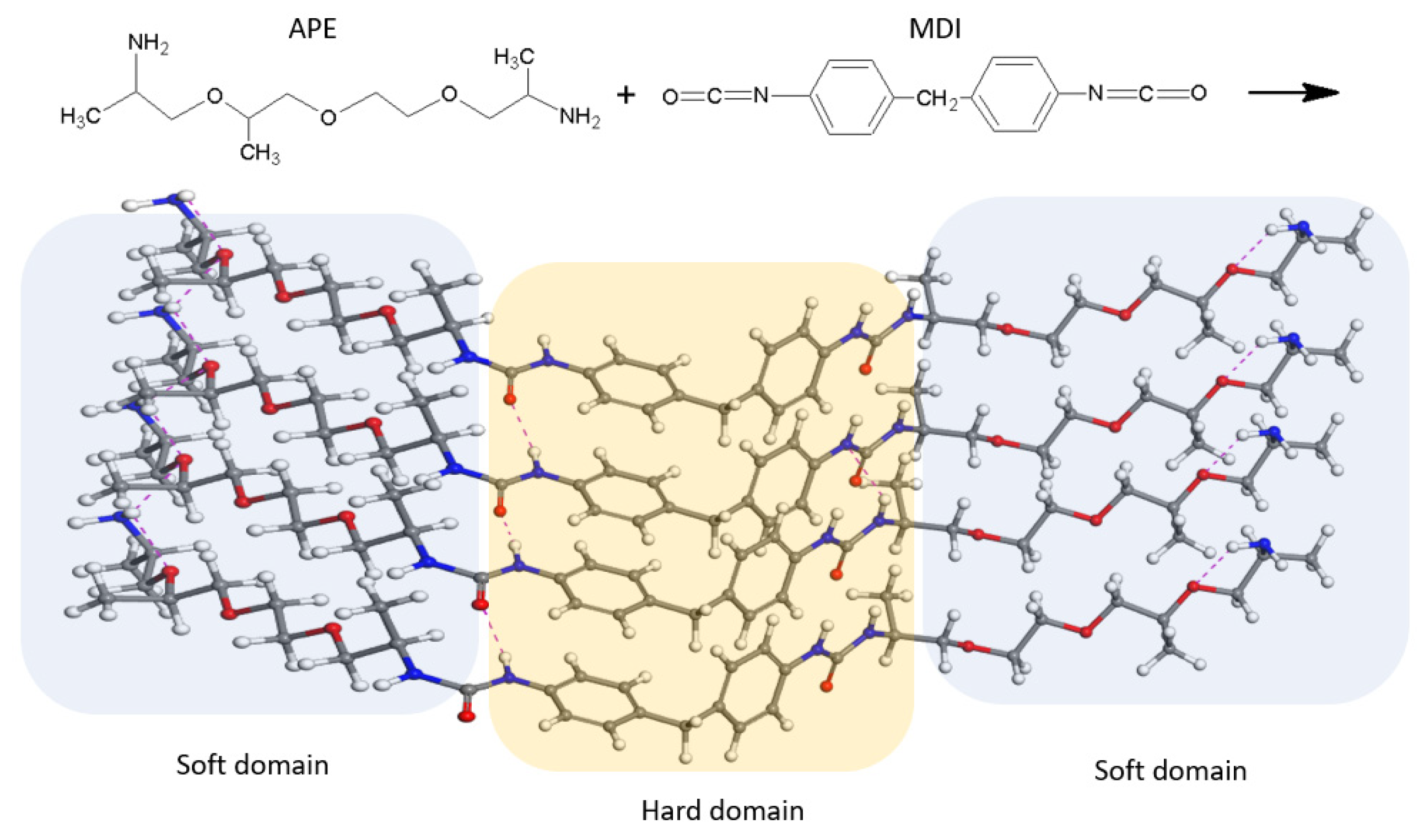
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
3. Results and Discussions
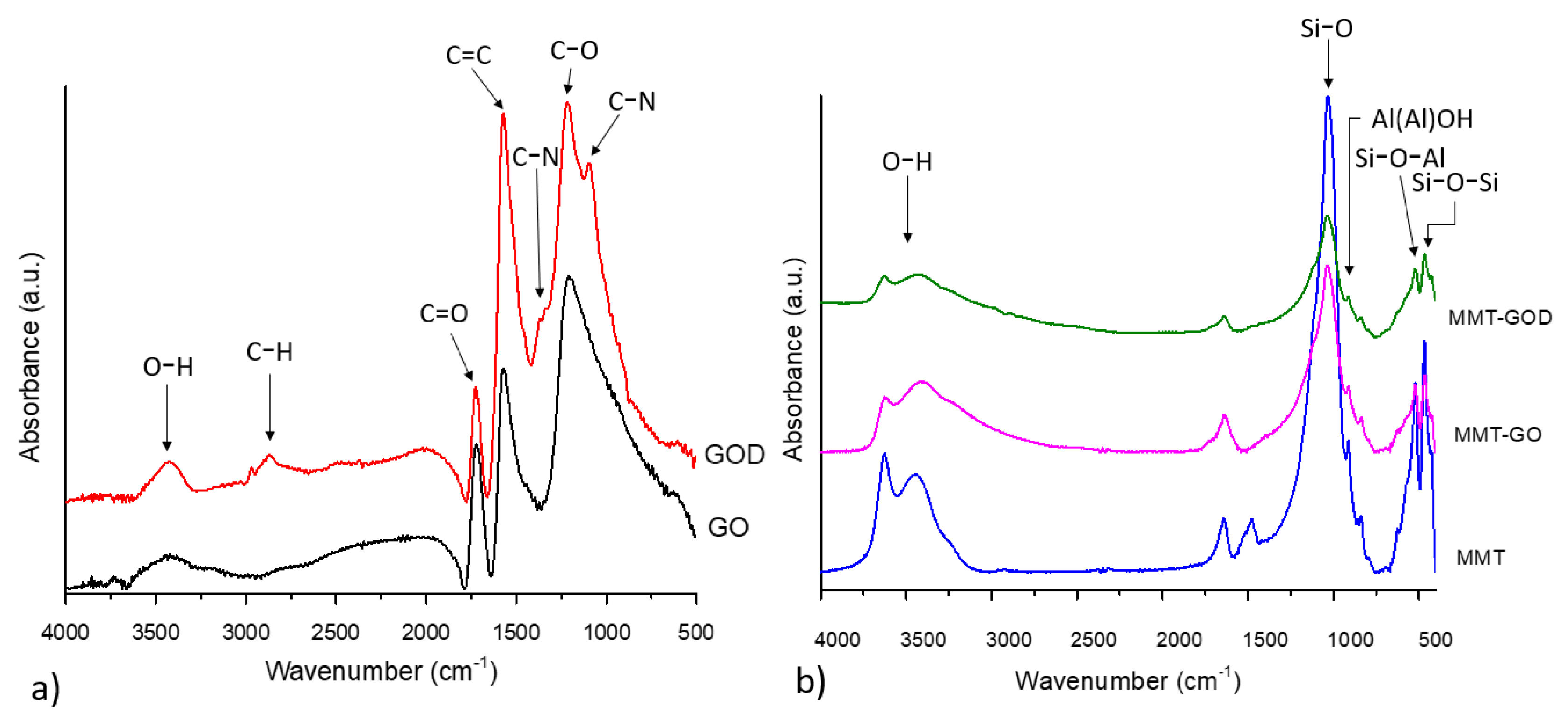
3.1. Nanostructures Characterization
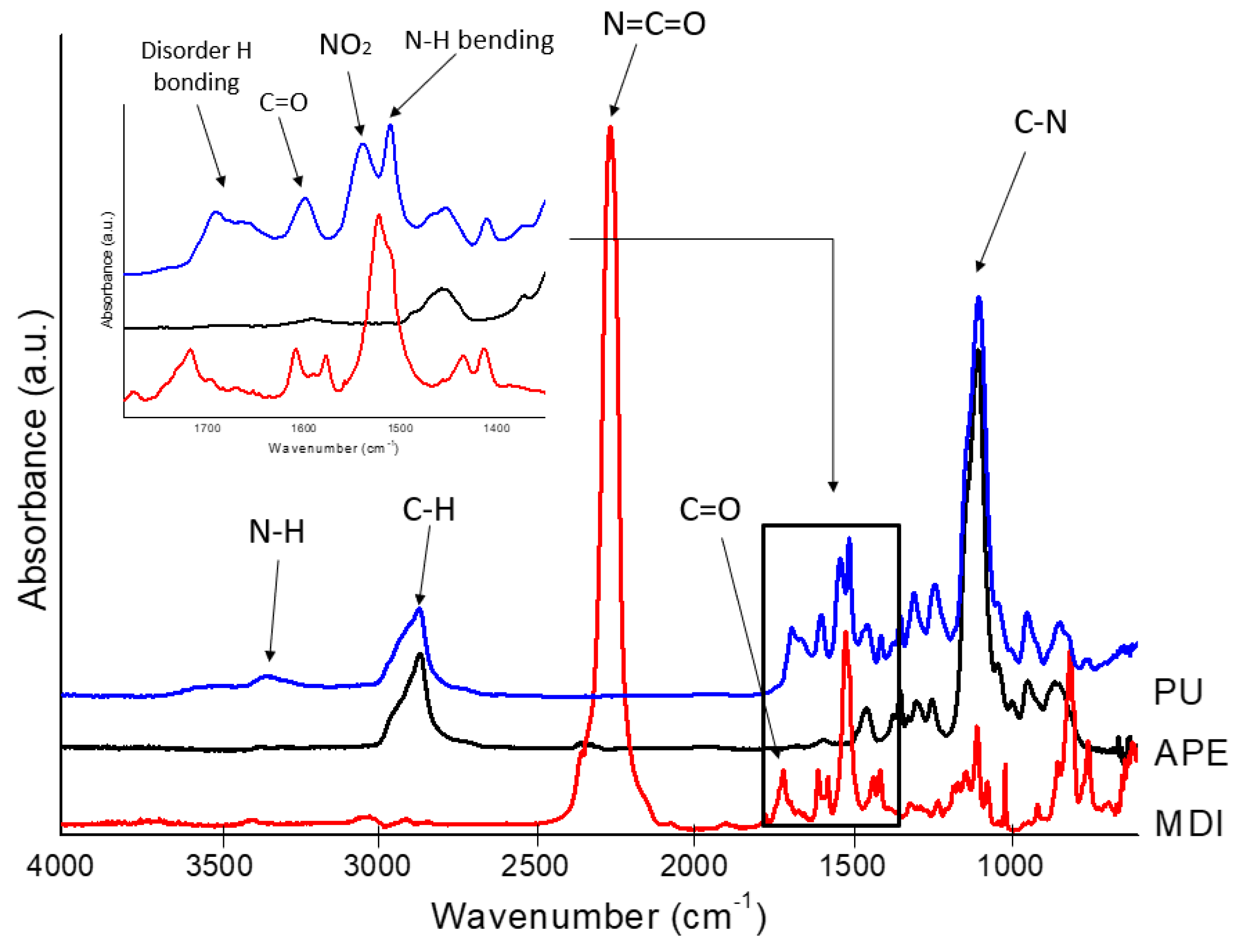
3.2. Composite Materials Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.; Zhang, C.; Xie, Z.; Xu, J.; Guo, B.-H. A multi-scale investigation on effects of hydrogen bonding on micro-structure and macro-properties in a polyurea. Polymer 2018, 145, 261–271. [Google Scholar] [CrossRef]
- Qiao, J.; Amirkhizi, A.V.; Schaaf, K.; Nemat-Nasser, S.; Wu, G. Dynamic mechanical and ultrasonic properties of polyurea. Mech. Mater. 2011, 43, 598–607. [Google Scholar] [CrossRef]
- Mattia, J.; Painter, P. A Comparison of Hydrogen Bonding and Order in a Polyurethane and Poly(urethane−urea) and Their Blends with Poly(ethylene glycol). Macromolecules 2007, 40, 1546–1554. [Google Scholar] [CrossRef]
- Castagna, A.M.; Pangon, A.; Choi, T.; Dillon, G.P.; Runt, J. The Role of Soft Segment Molecular Weight on Microphase Separation and Dynamics of Bulk Polymerized Polyureas. Macromolecules 2012, 45, 8438–8444. [Google Scholar] [CrossRef]
- Shim, J.; Mohr, D. Using split Hopkinson pressure bars to perform large strain compression tests on polyurea at low, intermediate and high strain rates. Int. J. Impact Eng. 2009, 36, 1116–1127. [Google Scholar] [CrossRef]
- Raman, S.; Ngo, T.; Lu, J.; Mendis, P. Experimental investigation on the tensile behavior of polyurea at high strain rates. Mater. Des. 2013, 50, 124–129. [Google Scholar] [CrossRef]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nano-materials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Awad, W.H.; Wilkie, C.A. Further study on the flammability of polyurea: The effect of intumescent coating and additive flame retardants. Polym. Adv. Technol. 2011, 22, 1297–1304. [Google Scholar] [CrossRef]
- Arunkumar, T.; Ramachandran, S.; Sebastian, P.J.; Vipin Raj, C. Thermal and fire retardant behaviour of polyurea. Int. J. Appl. Eng. Res. 2015, 10, 10159–10162. [Google Scholar]
- Khobragade, P.S.; Hansora, D.P.; Naik, J.B.; Chatterjee, A. Flame retarding performance of elastomeric nanocomposites: A review. Polym. Degrad. Stab. 2016, 130, 194–244. [Google Scholar] [CrossRef]
- Emin ÇETİN, M. Investigation of carbon nanotube reinforcement to polyurethane adhesive for improving impact per-formance of carbon fiber composite sandwich panels. Int. J. Adhes. Adhes. 2022, 112, 103002. [Google Scholar] [CrossRef]
- Elmergawy, F.H.; Nassif, M.S.; El-Borady, O.M.; Mabrouk, M.; El-Korashy, D.I. Physical and mechanical evaluation of dental resin composite after modification with two different types of Montmorillonite nanoclay. J. Dent. 2021, 112, 103731. [Google Scholar] [CrossRef] [PubMed]
- Fadil, Y.; Thickett, S.C.; Agarwal, V.; Zetterlund, P.B. Synthesis of graphene-based polymeric nanocomposites using emulsion techniques. Prog. Polym. Sci. 2021, 125, 101476. [Google Scholar] [CrossRef]
- Nizam Uddin, M.; Le, L.; Nair, R.; Asmatulu, R. Effects of graphene oxide thin films and nanocomposite coatings on flame retardancy and thermal stability of aircraft composites: A comparative study. J. Eng. Mater. Technol. Trans ASME 2019, 141, 031004. [Google Scholar]
- Hong, S.; Yoo, S.S.; Yoo, P.J. Binder-free heat dissipation films assembled with reduced graphene oxide and alumina nano-particles for simultaneous high in-plane and cross-plane thermal conductivities. J. Mater. Chem. C 2019, 7, 9380–9388. [Google Scholar] [CrossRef]
- Ciobotaru, C.C.; Damian, C.M.; Matei, E.; Iovu, H. Covalent functionalization of graphene oxide with cisplatin. Mater Plast. 2014, 51, 75–80. [Google Scholar]
- Sánchez-Ferrer, A.; Rogez, D.; Martinoty, P. Synthesis and Characterization of New Polyurea Elastomers by Sol/Gel Chemistry. Macromol. Chem. Phys. 2010, 211, 1712–1721. [Google Scholar] [CrossRef]
- Arabkhani, P.; Asfaram, A.; Ateia, M. Easy-to-prepare graphene oxide/sodium montmorillonite polymer nanocomposite with enhanced adsorption performance. J. Water Process. Eng. 2020, 38, 101651. [Google Scholar] [CrossRef]
- Kim, D.; Mittal, G.; Kim, M.; Kim, S.H.; Rhee, K.Y. Surface modification of MMT and its effect on fatigue and fracture behavior of basalt/epoxy based composites in a seawater environment. Appl. Surf. Sci. 2018, 473, 55–58. [Google Scholar] [CrossRef]
- Chen, D.; Chen, J.; Luan, X.; Ji, H.; Xia, Z. Characterization of anion–cationic surfactants modified montmorillonite and its application for the removal of methyl orange. Chem. Eng. J. 2011, 171, 1150–1158. [Google Scholar] [CrossRef]
- Nimita Jebaranjitham, J.; Mageshwari, C.; Saravanan, R.; Mu, N. Fabrication of amine functionalized graphene oxide—AgNPs nanocomposite with improved dispersibility for reduction of 4-nitrophenol. Compos. Part B Eng. 2019, 171, 302–309. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, B.; Xiao, W.Q.; Yan, L.; Wang, Z.J.; Zhang, J.J.; Lin, H.L.; Bian, J.; Lu, Y. Morphology and properties of shape memory thermoplastic polyurethane composites incorporating graphene-montmorillonite hybrids. J. Appl. Polym. Sci. 2017, 135, 46149. [Google Scholar] [CrossRef]
- Wang, C.; Ge, X.; Jiang, Y. Synergistic effect of graphene oxide/montmorillonite-sodium carboxymethycellulose ternary mimic-nacre nanocomposites prepared via a facile evaporation and hot- pressing technique. Carbohydr. Polym. 2019, 222, 115026. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Wu, J.; He, Q.; Li, Y.; Yang, W.; Zhou, Y. Preparation and characterization of APTES modified magnetic MMT capable of using as anisotropic nanoparticles. Appl. Surf. Sci. 2018, 447, 393–400. [Google Scholar] [CrossRef]
- Stanly, S.; Jelmy, E.; Nair, C.; John, H. Carbon dioxide adsorption studies on modified montmorillonite clay/reduced graphene oxide hybrids at low pressure. J. Environ. Chem. Eng. 2019, 7, 103344. [Google Scholar] [CrossRef]
- Ganjaee Sari, M.; Shamshiri, M.; Ramezanzadeh, B. Fabricating an epoxy composite coating with enhanced corrosion re-sistance through impregnation of functionalized graphene oxide-co-montmorillonite Nanoplatelet. Corros Sci. 2017, 129, 38–53. [Google Scholar] [CrossRef]
- Ali, M.E. Preparation of graphene nanosheets by electrochemical exfoliation of a graphite-nanoclay composite electrode: Application for the adsorption of organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 107–116. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak-Orłowska, B.; Małolepszy, A.; Mazurkiewicz-Pawlicka, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Yang, S.; Ren, X.; Zhao, G.; Shi, W.; Montavon, G.; Grambow, B.; Wang, X. RETRACTED: Competitive sorption and selective sequence of Cu(II) and Ni(II) on montmorillonite: Batch, modeling, EPR and XAS studies. Geochim. Cosmochim. Acta 2015, 166, 129–145. [Google Scholar] [CrossRef]
- Ying, Z.; Wu, C.; Zhang, C.; Jiang, S.; Shi, R.; Cheng, H.; Zhang, B.; Li, Y.; Zhao, F. Synthesis of polyureas with CO 2 as carbonyl building block and their high performances. J. CO2 Util. 2017, 19, 209–213. [Google Scholar] [CrossRef]
- Arunkumar, T.; Ramachandran, S. Surface coating and characterisation of polyurea for liquid storage. Int. J. Ambient. Energy 2016, 38, 781–787. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. Part A Polym. Chem. 2011, 50, 1026–1036. [Google Scholar] [CrossRef]
- Holzworth, K.; Jia, Z.; Amirkhizi, A.; Qiao, J.; Nemat-Nasser, S. Effect of isocyanate content on thermal and mechanical properties of polyurea. Polymer 2013, 54, 3079–3085. [Google Scholar] [CrossRef]
- Qian, X.; Song, L.; Tai, Q.; Hu, Y.; Yuen, R.K. Graphite oxide/polyurea and graphene/polyurea nanocomposites: A comparative investigation on properties reinforcements and mechanism. Compos. Sci. Technol. 2013, 74, 228–234. [Google Scholar] [CrossRef]
- Casalini, R.; Bogoslovov, R.; Qadri, S.; Roland, C. Nanofiller reinforcement of elastomeric polyurea. Polymer 2012, 53, 1282–1287. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. High mechanical performance polyurea/organoclay nanocomposites. Compos. Sci. Technol. 2014, 103, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Fragiadakis, D.; Gamache, R.; Bogoslovov, R.; Roland, C. Segmental dynamics of polyurea: Effect of stoichiometry. Polymer 2010, 51, 178–184. [Google Scholar] [CrossRef]
- Grujicic, M.; He, T.; Pandurangan, B.; Svingala, F.R.; Settles, G.S.; Hargather, M.J. Experimental characterization and materi-al-model development for microphase-segregated polyurea: An overview. J. Mater. Eng. Perform. 2012, 21, 2–16. [Google Scholar] [CrossRef]
- Awad, W.H.; Wilkie, C.A. Investigation of the thermal degradation of polyurea: The effect of ammonium polyphosphate and expandable graphite. Polymer 2010, 51, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Cai, W.; Chu, F.; Zhou, F.; Liang, S.; Ma, C.; Hu, Y. Hydroxyapatite/polyurea nanocomposite: Preparation and multiple performance enhancements. Compos. Part A Appl. Sci. Manuf. 2019, 128, 105681. [Google Scholar] [CrossRef]
- Roland, C.M.; Twigg, J.N.; Vu, Y.; Mott, P.H. High strain rate mechanical behavior of polyurea. Polymer 2007, 48, 574–578. [Google Scholar] [CrossRef]
- Mott, P.H.; Giller, C.B.; Fragiadakis, D.; Rosenberg, D.A.; Roland, C.M. Deformation of polyurea: Where does the energy go? Polymer 2016, 105, 227–233. [Google Scholar] [CrossRef]
- Wolk, A.; Rosenthal, M.; Weiß, J.; Voigt, M.; Wesendahl, J.-N.; Hartmann, M.; Grundmeier, G.; Wilhelm, R.; Meschut, G.; Tiemann, M.; et al. Graphene oxide as flexibilizer for epoxy amine resins. Prog. Org. Coat. 2018, 122, 280–289. [Google Scholar] [CrossRef]
- Thi, N.H.; Pham, D.L.; Hanh, N.T.; Oanh, H.T.; Yen Duong, T.H.; Nguyen, T.N.; Tuyen, N.D.; Phan, D.L.; Trinh, H.T.; Nguyen, H.T.; et al. Influence of Organoclay on the Flame Re-tardancy and Thermal Insulation Property of Expandable Graphite/Polyurethane Foam. J. Chem. 2019, 2019, 19–21. [Google Scholar] [CrossRef]
- Giraud, S.; Bourbigot, S.; Rochery, M.; Vroman, I.; Tighzert, L.; Delobel, R.; Poutch, F. Flame retarded polyurea with micro-encapsulated ammonium phosphate for textile coating. Polym. Degrad. Stab. 2005, 88, 106–113. [Google Scholar] [CrossRef]








| No. | Sample | Name |
|---|---|---|
| 1 | PU–APE | PU |
| 2 | PU–APE–GO | PUG |
| 3 | PU–APE–GOD | PUGD |
| 4 | PU–APE–MMT | PUM |
| 5 | PU–APE–MMT–GO | PUMG |
| 6 | PU–APE–MMT–GOD | PUMGD |
| Atomic % | GO | GOD | MMT | MMT–GO | MMT–GOD |
|---|---|---|---|---|---|
| C1s | 79.42 | 76.75 | 5.13 | 15.54 | 13.01 |
| O1s | 20.58 | 18.75 | 55.94 | 53.35 | 53.77 |
| N1s | - | 4.50 | - | - | 1.24 |
| Si2p | - | - | 23.57 | 20.43 | 21.20 |
| Mg1s | - | - | 2.74 | 3.33 | 2.76 |
| Al2p | - | - | 7.36 | 6.34 | 6.86 |
| Ca2p | - | - | 2.15 | 1.01 | 1.16 |
| Na1S | - | - | 3.11 | - | - |
| Sample | Thermal Decomposition Range | Char (%) | Td3% (°C) | ||
|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | |||
| GO | 331–752 °C | - | - | 4 | 125 |
| GOD | 96–238 °C | 238–491 °C | - | 46 | 102 |
| MMT | 350–714 °C | - | - | 92 | 493 |
| MMT–GO | 45–149 °C | 149–263 °C | 263–898 °C | 83 | 94 |
| MMT–GOD | 36–140 °C | 140–245 °C | 491–899 °C | 85 | 60 |
| Sample | Tg Soft (°C) | Tg Hard (°C) |
|---|---|---|
| PU | −30 | 32 |
| PUG | −47 | 37 |
| PUGD | −36 | 54 |
| PUM | −39 | 40 |
| PUMG | −32 | 44 |
| PUMGD | −32 | 65 |
| Sample | Air | Nitrogen | ||||
|---|---|---|---|---|---|---|
| Td5%(°) 1 | Td10%(°) 2 | Tmax(°) 3 | Td5%(°) | Td10%(°) | Tmax(°) | |
| PU | 296 | 329 | 406 | 304 | 340 | 404 |
| PUG | 296 | 325 | 391 | 304 | 345 | 400 |
| PUGD | 294 | 324 | 401 | 301 | 334 | 403 |
| PUM | 300 | 328 | 386 | 305 | 337 | 400 |
| PUMG | 299 | 326 | 395 | 305 | 335 | 400 |
| PUMGD | 283 | 321 | 392 | 297 | 337 | 399 |
| Sample | E (MPa) | Tensile Stress (%) |
|---|---|---|
| PU | 0.105 | 42.2 |
| PUG | 0.610 | 61.4 |
| PUGD | 0.555 | 47.3 |
| PUM | 0.268 | 44.5 |
| PUMG | 1.034 | 40.2 |
| PUMGD | 0.235 | 29.6 |
| Sample | Limiting Oxygen Index (LOI) 1 (%) | Residual Mass 2 (%)—Air | Residual Mass 2 (%)—Nitrogen |
|---|---|---|---|
| PU | 32.4 | 0.98 | 12.33 |
| PUG | 33.6 | 1.16 | 15.19 |
| PUGD | 33.8 | 0.92 | 13.22 |
| PUM | 33.8 | 1.98 | 13.31 |
| PUMG | 35.2 | 1.29 | 11.25 |
| PUMGD | 35.4 | 2.57 | 12.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Necolau, M.I.; Damian, C.M.; Fierăscu, R.C.; Chiriac, A.-L.; Vlăsceanu, G.M.; Vasile, E.; Iovu, H. Layered Clay–Graphene Oxide Nanohybrids for the Reinforcement and Fire-Retardant Properties of Polyurea Matrix. Polymers 2022, 14, 66. https://doi.org/10.3390/polym14010066
Necolau MI, Damian CM, Fierăscu RC, Chiriac A-L, Vlăsceanu GM, Vasile E, Iovu H. Layered Clay–Graphene Oxide Nanohybrids for the Reinforcement and Fire-Retardant Properties of Polyurea Matrix. Polymers. 2022; 14(1):66. https://doi.org/10.3390/polym14010066
Chicago/Turabian StyleNecolau, Mădălina Ioana, Celina Maria Damian, Radu Claudiu Fierăscu, Anita-Laura Chiriac, George Mihail Vlăsceanu, Eugeniu Vasile, and Horia Iovu. 2022. "Layered Clay–Graphene Oxide Nanohybrids for the Reinforcement and Fire-Retardant Properties of Polyurea Matrix" Polymers 14, no. 1: 66. https://doi.org/10.3390/polym14010066
APA StyleNecolau, M. I., Damian, C. M., Fierăscu, R. C., Chiriac, A.-L., Vlăsceanu, G. M., Vasile, E., & Iovu, H. (2022). Layered Clay–Graphene Oxide Nanohybrids for the Reinforcement and Fire-Retardant Properties of Polyurea Matrix. Polymers, 14(1), 66. https://doi.org/10.3390/polym14010066