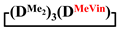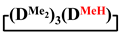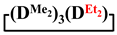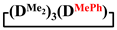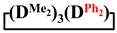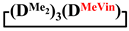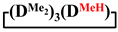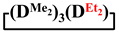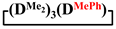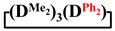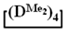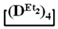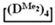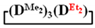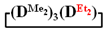Abstract
This paper reports a method for the synthesis of 1,1,3,3,5,5-hexamethyl-7,7-diorganocyclotetrasiloxanes by the interaction of 1,5-disodiumoxyhexamethylsiloxane with dichlorodiorganosilanes such as methyl-, methylvinyl-, methylphenyl-, diphenyl- and diethyl dichlorosilanes. Depending on the reaction conditions, the preparative yield of the target cyclotetrasiloxanes is 55–75%. Along with mixed cyclotetrasiloxanes, the proposed method leads to the formation of polymers with regular alternation of diorganosylil and dimethylsylil units. For example, in the case of dichlorodiethylsilane, 70% content of linear poly(diethyl)dimethylsiloxanes with regular alternation of units can be achieved in the reaction product. Using 7,7-diethyl-1,1,3,3,5,5-hexamethylcyclotetrasiloxane as an example, the prospects of the mixed cycle in copolymer preparation in comparison with the copolymerization of octamethyl- and octaethylcyclotetrasiloxanes are shown.
Keywords:
mixed cyclosiloxanes; 1,1,3,3,5,5,7-heptamethyl-7-vinylcyclotetrasiloxane; 7-hydro-1,1,3,3,5,5,7-heptamethylcyclotetrasiloxane; 1,1,3,3,5,5,7-heptamethyl-7-phenylcyclotetrasiloxanes; 7,7-diethyl-1,1,3,3,5,5-hexamethylcyclotetrasiloxane; 1,1,3,3,5,5-hexamethyl-7,7-diphenylcyclotetrasiloxane; 1,5-disodiumoxyhexamethylsiloxane; poly(diethyl)(dimethyl)siloxane 1. Introduction
Cyclosiloxanes can be used as an initial reagent for the preparation of siloxane homo- and copolymer rubbers and liquids [1,2,3,4], as well as functional precursors for molecular design [5,6,7,8,9], cross-linking reagents [10], flame retardants [11], components of compositions for dry cleaning and detergents [12,13,14], solvents for coloring fabrics [15,16,17,18,19] and in cosmetics for various purposes, including skin and hair care products, deodorants/antiperspirants, makeup products, etc. [20,21,22,23].
Cyclosiloxanes of mixed composition are of particular interest in modifying polydimethylsiloxane [24,25,26,27] to provide it with the required properties and to obtain a linear functional matrix containing reactive groups in the chain for further transformations and obtaining new polymers with a determined structure and a required set of characteristics [28,29]. Substituents of the silicon atom have a strong effect on the polymerization rate of cyclosiloxanes; as a result, it is difficult to obtain copolymers by polymerization of a mixture of cyclosiloxanes with different groups at the silicon level [30,31,32]. This problem can be solved by using mixed dimethylcyclotetrasiloxanes. In this regard, the development of simple and effective methods for their preparation is an urgent task. Until very recently, no effective methods for the synthesis of mixed cyclotetrasiloxanes could be observed in the literature. For instance, mixed dimethylcyclotetrasiloxanes containing one silicon atom with different substituents can be synthesized via the cohydrolysis of dichlorodimethylsilane and the corresponding dichlorodiorganosilane, but the yield of the target cyclosiloxane does not exceed 30% [33,34,35]. Another approach is the heterofunctional condensation of hexamethyltrisiloxanes with terminal chloro- [36], hydro- [37,38] and hydroxy-groups [39,40] and the corresponding diorganosilanediols, diorganodialkoxy- or chlorosilanes. Mixed dimethylcyclotetrasiloxanes can form selectively using either diorganosilanediols or trisiloxanes with terminal functional groups. While the stability of diorganosilanediols limits the former approach [41,42,43,44], the multistep preparation and complexity of the method limit the latter [19,45,46,47,48].
Selective synthesis of 1,5-sodiumoxyhexamethyltrisiloxanes from dimethylsiloxanes of cyclic and linear structure [49] opens up opportunities for the directed production of mixed dimethylcyclotetrasiloxanes, including the transition to “green” chemistry methods. On one hand, the salt yield does not depend on the structure of the initial reagent; thus, low-molecular-weight debris from polydimethylsiloxane rubber production can become the raw material for its production. On the other hand, using an acceptor of hydrogen chloride, which is a standard component of hydrolytic and condensation processes with chlorsilanes and the presence of which has a significant effect on the cyclotetrasiloxane formation, is unnecessary [39,47].
Thus, this work aims to obtain mixed dimethylcyclotetrasiloxanes by reacting 1,5-sodiumoxyhexamethyltrisiloxanes with a number of dichlorodiorganosilanes. We further demonstrate the preparation of copolymers using dimethylcyclotetrasiloxanes.
2. Materials and Methods
2.1. Materials
The following reagents and organic solvents were used in the work: hexane, tetrahydrofuran (THF), methyl-tret-butyl ether (MTBE), anhydrous sodium hydroxide and potassium hydroxide from OOO “Component-Reaktiv”, Russia; methanol and pyridine from OOO “SpektrChem”, Russia; α,ω-dihydroxypolydimethylsiloxane brand SKTN A (PDMS) from OOO “Penta-91”, Russia; dichloromethylsilane 97%, dichloromethylsilane 97%, dichlorodiethylsilane 97%, dichloromethylphenylsilane 98%, chlorotrimethylsilane 97%, dichlorodiphenylsilane 98%, octamethylcyclotetrasiloxane, octaethylcyclotetrasiloxane from Reatorg, Russia.
All reagents were subjected to preliminary preparation in accordance with generally accepted methods [50]. Chlorosilanes were distilled immediately before use. Pyridine was dried over barium oxide. The toluene, THF and MTBE were distilled on a rotary evaporator and dried over calcium hydride.
1,5-disodiumoxyhexamethyltrisiloxane was obtained immediately before use by the interaction of sodium hydroxy and PDMS according to the procedure described in [51].
Synthesis of 1,1,3,3,5,5,7-heptamethyl-7-vinylcyclotetrasiloxane in MTBE medium (№ 1, Table 1). First, 20 g (0.07 mol) 1,5-disodiumoxyhexamethyltrisiloxane, 480 mL of anhydrous THF and 1 mL of anhydrous pyridine were added into a 2 L round-bottom flask equipped with a thermometer, reflux condenser and mechanical stirrer in an argon flow. Then, the reaction mass was heated to 66 °C with vigorous stirring and allowed to cool to room temperature. In this case, the salt completely dissolved and a clear solution was formed. After this, a solution of 11.9 g (0.08 mol) of dichloromethylvinylsilane in 268 mL of anhydrous THF was rapidly added to the reaction mixture and cooled to −60 °C with vigorous stirring. The reaction mass was stirred until room temperature (for 1 h). The pH of the reaction mass was 5–7. After this, the excess THF was distilled off on a rotary evaporator and MTBE was added. Then, the reaction mixture was washed with water to remove the precipitate, and the excess MTBE was distilled off on a rotary evaporator. The obtained siloxane product was analyzed by gas–liquid (GLC) and gel permeation chromatography (GPC). The results are shown in Table 1 (№ 1). Then, the product was distilled. As a result, 11.8 g containing of 98% of 1,1,3,3,5,5,7-hexamethyl-7-vinylcyclotetrasiloxane was isolated by 85 °C/20 mm Hg. The 1,1,3,3,5,5,7-hexamethyl-7-vinylcyclotetrasiloxane yield was 55%.

Table 1.
Reaction conditions in THF and product characteristics.
In addition, 7-hydro-1,1,3,3,5,5,7-heptamethylcyclotetrasiloxane, 7,7-diethyl-1,1,3,3,5,5-hexamethylcyclotetrasiloxane, 1,1,3,3,5,5,7-heptamethyl-7-phenylcyclotetrasiloxane and 1,1,3,3,5,5-hexamethyl-7,7-diphenylcyclotetrasiloxane were obtained analogously to this procedure in THF medium. The experimental results are presented in Table 1 (№ 3, 4, 5, 6, respectively).
Synthesis of 1,1,3,3,5,5,7-heptamethyl-7-vinylcyclotetrasiloxane in MTBE medium (№ 2, Table 1). First, 20 g (0.07 mol) 1,5-disodiumoxyhexamethyltrisiloxane, 341 mL of anhydrous THF and 1 mL of anhydrous pyridine were added into a 1 L round-bottom flask equipped with a thermometer, reflux condenser and mechanical stirrer in an argon flow. Then, the reaction mass was heated to 66 °C with vigorous stirring and allowed to cool to room temperature. A solution of 11.9 g (0.08 mol) of dichloromethylvinylsilane in 341 mL of anhydrous THF was prepared in a separate flask. Then, into another 2 L flask equipped with 2 reflux condensers, a thermometer and a mechanical stirrer, with vigorous stirring and cooling to −60 °C, a salt solution in THF and a solution of chlorosilane in THF were added simultaneously and at the same rate. The reaction mass was stirred until room temperature (for 1 h). The pH of the reaction mass was 5–7. After this, the excess THF was distilled off on a rotary evaporator and MTBE was added. Then, the reaction mixture was washed with water to remove the precipitate, and the excess MTBE was distilled off on a rotary evaporator. The obtained siloxane product was analyzed by GLC and GPC. The results are shown in Table 1 (№ 1). Then, the product was distilled. As a result, 9.9 g containing of 96% of 1,1,3,3,5,5,7-hexamethyl-7-vinylcyclotetrasiloxane was isolated. The 1,1,3,3,5,5,7-hexamethyl-7-vinylcyclotetrasiloxane yield was 45%.
Synthesis of 1,1,3,3,5,5,7-heptamethyl-7-vinylcyclotetrasiloxane in MTBE medium (№ 7, Table 1). First, 20 g (0.07 mol) 1,5-disodiumoxyhexamethyltrisiloxane, 577 mL of anhydrous MTBE and 1 mL of anhydrous pyridine were added into a 2 L round-bottom flask equipped with a thermometer, dropping funnel and mechanical stirrer in an argon flow. A solution of 11.9 g (0.08 mol) of dichloromethylvinylsilane in 322 mL of anhydrous MTBE was rapidly added to the reaction mixture and cooled to −60 °C with vigorous stirring. The reaction mass was stirred until room temperature (for 1 h). The pH of the reaction mass was 5-7. Then, the reaction mixture was washed with water to remove the precipitate, and the excess MTBE was distilled off on a rotary evaporator. The obtained siloxane product was analyzed by GLC and GPC. The results are shown in Table 1 (№ 1). Then, the product was distilled. As a result, 16.3 g containing of 97% of 1,1,3,3,5,5,7-hexamethyl-7-vinylcyclotetrasiloxane was isolated by 85 °C/20 mm Hg. The 1,1,3,3,5,5,7-hexamethyl-7-vinylcyclotetrasiloxane yield was 75%.
In addition, 7-hydro-1,1,3,3,5,5,7-heptamethylcyclotetrasiloxane, 7,7-diethyl-1,1,3,3,5,5-hexamethylcyclotetrasiloxane, 1,1,3,3,5,5,7-heptamethyl-7-phenylcyclotetrasiloxane and 1,1,3,3,5,5-hexamethyl-7,7-diphenylcyclotetrasiloxane were obtained analogously to this procedure in MTBE medium. The experimental results are presented in Table 2 (№ 9, 10, 11, 12, respectively).

Table 2.
Reaction conditions in MTBE and product characteristics.
Synthesis of 1,1,3,3,5,5,7-heptamethyl-7-vinylcyclotetrasiloxane in MTBE medium (№ 8, Table 1). First, 11.9 g (0.08 mol) of dichloromethylvinylsilane, in 899 mL of anhydrous MTBE, and 1 mL of anhydrous pyridine were added into a 2 L round-bottom flask equipped with a thermometer, dropping funnel and mechanical stirrer in an argon flow. Then, 20 g (0.07 mol) dry 1,5-disodiumoxyhexamethyltrisiloxane was rapidly added to the reaction mixture and cooled to −60 °C with vigorous stirring. The reaction mass was stirred until room temperature (for 1 h). The pH of the reaction mass was 5–7. Then, the reaction mixture was washed with water to remove the precipitate, and the excess MTBE was distilled off on a rotary evaporator. The obtained siloxane product was analyzed by GLC and GPC. The results are shown in Table 1 (№ 1). Then, the product was distilled. As a result, 15.2 g containing of 97% of 1,1,3,3,5,5,7-hexamethyl-7-vinylcyclotetrasiloxane was isolated. The 1,1,3,3,5,5,7-hexamethyl-7-vinylcyclotetrasiloxane yield was 70%.
All obtained cycles were characterized by 1H and 29Si nuclear magnetic resonance (NMR)-1,1,3,3,5,5,7-hexamethyl-7-vinylcyclotetrasiloxane 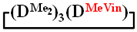 . 1H NMR, δ, ppm: 5.67–6.19 m (3H, ((CH2=CH)Si), 0.15 m (12H, Si(CH3)2). 29Si NMR, δ ppm: −18.48 (2Si, Si(CH3)2O2/2), −18.89 (1Si, Si(CH3)2O2/2), −33.47 (1Si, Si(CH3)(CH2=CH)O2/2).
. 1H NMR, δ, ppm: 5.67–6.19 m (3H, ((CH2=CH)Si), 0.15 m (12H, Si(CH3)2). 29Si NMR, δ ppm: −18.48 (2Si, Si(CH3)2O2/2), −18.89 (1Si, Si(CH3)2O2/2), −33.47 (1Si, Si(CH3)(CH2=CH)O2/2).
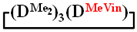 . 1H NMR, δ, ppm: 5.67–6.19 m (3H, ((CH2=CH)Si), 0.15 m (12H, Si(CH3)2). 29Si NMR, δ ppm: −18.48 (2Si, Si(CH3)2O2/2), −18.89 (1Si, Si(CH3)2O2/2), −33.47 (1Si, Si(CH3)(CH2=CH)O2/2).
. 1H NMR, δ, ppm: 5.67–6.19 m (3H, ((CH2=CH)Si), 0.15 m (12H, Si(CH3)2). 29Si NMR, δ ppm: −18.48 (2Si, Si(CH3)2O2/2), −18.89 (1Si, Si(CH3)2O2/2), −33.47 (1Si, Si(CH3)(CH2=CH)O2/2).7-hydro-1,1,3,3,5,5,7-heptamethylcyclotetrasiloxane 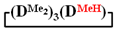 . 1H NMR, δ, ppm: 4.70 s (1H, SiH), 0.15 m (21H, Si(CH3)2). 29Si NMR, δ, ppm: −17.65 (2Si, Si(CH3)2O2/2), −18.79 (1Si, Si(CH3)2O2/2), −34.79 (1Si, Si(CH3)(H)O2/2).
. 1H NMR, δ, ppm: 4.70 s (1H, SiH), 0.15 m (21H, Si(CH3)2). 29Si NMR, δ, ppm: −17.65 (2Si, Si(CH3)2O2/2), −18.79 (1Si, Si(CH3)2O2/2), −34.79 (1Si, Si(CH3)(H)O2/2).
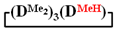 . 1H NMR, δ, ppm: 4.70 s (1H, SiH), 0.15 m (21H, Si(CH3)2). 29Si NMR, δ, ppm: −17.65 (2Si, Si(CH3)2O2/2), −18.79 (1Si, Si(CH3)2O2/2), −34.79 (1Si, Si(CH3)(H)O2/2).
. 1H NMR, δ, ppm: 4.70 s (1H, SiH), 0.15 m (21H, Si(CH3)2). 29Si NMR, δ, ppm: −17.65 (2Si, Si(CH3)2O2/2), −18.79 (1Si, Si(CH3)2O2/2), −34.79 (1Si, Si(CH3)(H)O2/2).7,7-diethyl-1,1,3,3,5,5-hexamethylcyclotetrasiloxane 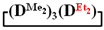 1H NMR, δ, ppm: 0.91–0.96 t (6H, Si(CH2CH3)2), 0.49–0.52 q (4H, Si(CH2CH3)2), 0.08–0.10 d (18H (Si(CH3)2). 29Si NMR, δ ppm: −19.24 (1Si, Si(CH2CH3)2O2/2), −19.45 (1Si, Si(CH3)2O2/2), −19.58 (2Si, Si(CH3)2O2/2).
1H NMR, δ, ppm: 0.91–0.96 t (6H, Si(CH2CH3)2), 0.49–0.52 q (4H, Si(CH2CH3)2), 0.08–0.10 d (18H (Si(CH3)2). 29Si NMR, δ ppm: −19.24 (1Si, Si(CH2CH3)2O2/2), −19.45 (1Si, Si(CH3)2O2/2), −19.58 (2Si, Si(CH3)2O2/2).
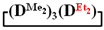 1H NMR, δ, ppm: 0.91–0.96 t (6H, Si(CH2CH3)2), 0.49–0.52 q (4H, Si(CH2CH3)2), 0.08–0.10 d (18H (Si(CH3)2). 29Si NMR, δ ppm: −19.24 (1Si, Si(CH2CH3)2O2/2), −19.45 (1Si, Si(CH3)2O2/2), −19.58 (2Si, Si(CH3)2O2/2).
1H NMR, δ, ppm: 0.91–0.96 t (6H, Si(CH2CH3)2), 0.49–0.52 q (4H, Si(CH2CH3)2), 0.08–0.10 d (18H (Si(CH3)2). 29Si NMR, δ ppm: −19.24 (1Si, Si(CH2CH3)2O2/2), −19.45 (1Si, Si(CH3)2O2/2), −19.58 (2Si, Si(CH3)2O2/2).1,1,3,3,5,5,7-heptamethyl-7-phenylcyclotetrasiloxane 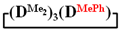 . 1H NMR, δ, ppm: m 7.25-7.57 (5H, Si(C6H5)), 0.24 s (3H, Si(CH3)), 0.03–0.07 m (18H, Si(CH3)2). 29Si NMR, δ ppm: −18.07 (2Si, Si(CH3)2O2/2), −18.61 (1Si, Si(CH3)2O2/2), −32.59 (1Si, Si(CH3)(C6H5)O2/2).
. 1H NMR, δ, ppm: m 7.25-7.57 (5H, Si(C6H5)), 0.24 s (3H, Si(CH3)), 0.03–0.07 m (18H, Si(CH3)2). 29Si NMR, δ ppm: −18.07 (2Si, Si(CH3)2O2/2), −18.61 (1Si, Si(CH3)2O2/2), −32.59 (1Si, Si(CH3)(C6H5)O2/2).
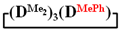 . 1H NMR, δ, ppm: m 7.25-7.57 (5H, Si(C6H5)), 0.24 s (3H, Si(CH3)), 0.03–0.07 m (18H, Si(CH3)2). 29Si NMR, δ ppm: −18.07 (2Si, Si(CH3)2O2/2), −18.61 (1Si, Si(CH3)2O2/2), −32.59 (1Si, Si(CH3)(C6H5)O2/2).
. 1H NMR, δ, ppm: m 7.25-7.57 (5H, Si(C6H5)), 0.24 s (3H, Si(CH3)), 0.03–0.07 m (18H, Si(CH3)2). 29Si NMR, δ ppm: −18.07 (2Si, Si(CH3)2O2/2), −18.61 (1Si, Si(CH3)2O2/2), −32.59 (1Si, Si(CH3)(C6H5)O2/2).1,1,3,3,5,5-hexamethyl-7,7-diphenylcyclotetrasiloxane 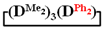 . 1H NMR, δ, ppm: 7.25–7.58 m (10H, Si(C6H5)2), 0.01–0.06 m (18H, (Si(CH3)2). 29Si NMR, δ ppm: −17.49 (2Si, Si(CH3)2O2/2), −18.51 (1Si, Si(CH3)2O2/2), −46.19 (1Si, Si(C6H5)2O2/2).
. 1H NMR, δ, ppm: 7.25–7.58 m (10H, Si(C6H5)2), 0.01–0.06 m (18H, (Si(CH3)2). 29Si NMR, δ ppm: −17.49 (2Si, Si(CH3)2O2/2), −18.51 (1Si, Si(CH3)2O2/2), −46.19 (1Si, Si(C6H5)2O2/2).
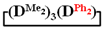 . 1H NMR, δ, ppm: 7.25–7.58 m (10H, Si(C6H5)2), 0.01–0.06 m (18H, (Si(CH3)2). 29Si NMR, δ ppm: −17.49 (2Si, Si(CH3)2O2/2), −18.51 (1Si, Si(CH3)2O2/2), −46.19 (1Si, Si(C6H5)2O2/2).
. 1H NMR, δ, ppm: 7.25–7.58 m (10H, Si(C6H5)2), 0.01–0.06 m (18H, (Si(CH3)2). 29Si NMR, δ ppm: −17.49 (2Si, Si(CH3)2O2/2), −18.51 (1Si, Si(CH3)2O2/2), −46.19 (1Si, Si(C6H5)2O2/2).Polymerization of 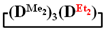 . First, 5 g (0.015 mol) of 7,7-diethyl-1,1,3,3,5,5-hexamethylcyclotetrasiloxane and 0.16 g (2.85 mmol) of KOH were stirred at 140 °C for 1 h. At the end of the polymerization, a colorless, highly viscous product was obtained. After this, at 5 °C, 13.5 mL anhydrous toluene, 1.5 g (0.014 mol) of chlorotrimethylsilane and 1.1 g (0.014 mol) pyridine were added to the reaction mixture. The resulting product was washed to neutral pH of the aqueous layer, and the solution was dried over anhydrous sodium sulfate. Then, the excess solvent was removed on a rotary evaporator and the polymer was dried at 1 mmHg. The resulting product was characterized by a bimodal molecular weight distribution. The high-molecular-weight part was separated using preparative gel permeation chromatography. The product was analyzed by GPC and NMR methods. The copolymerizations of octamethylcyclotetrasiloxane
. First, 5 g (0.015 mol) of 7,7-diethyl-1,1,3,3,5,5-hexamethylcyclotetrasiloxane and 0.16 g (2.85 mmol) of KOH were stirred at 140 °C for 1 h. At the end of the polymerization, a colorless, highly viscous product was obtained. After this, at 5 °C, 13.5 mL anhydrous toluene, 1.5 g (0.014 mol) of chlorotrimethylsilane and 1.1 g (0.014 mol) pyridine were added to the reaction mixture. The resulting product was washed to neutral pH of the aqueous layer, and the solution was dried over anhydrous sodium sulfate. Then, the excess solvent was removed on a rotary evaporator and the polymer was dried at 1 mmHg. The resulting product was characterized by a bimodal molecular weight distribution. The high-molecular-weight part was separated using preparative gel permeation chromatography. The product was analyzed by GPC and NMR methods. The copolymerizations of octamethylcyclotetrasiloxane 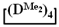 with octaethylcyclotetrasiloxane
with octaethylcyclotetrasiloxane 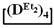 or
or 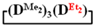 were performed in a similar manner to this procedure. The copolymerization conditions and results are shown in Table 3.
were performed in a similar manner to this procedure. The copolymerization conditions and results are shown in Table 3.
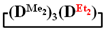 . First, 5 g (0.015 mol) of 7,7-diethyl-1,1,3,3,5,5-hexamethylcyclotetrasiloxane and 0.16 g (2.85 mmol) of KOH were stirred at 140 °C for 1 h. At the end of the polymerization, a colorless, highly viscous product was obtained. After this, at 5 °C, 13.5 mL anhydrous toluene, 1.5 g (0.014 mol) of chlorotrimethylsilane and 1.1 g (0.014 mol) pyridine were added to the reaction mixture. The resulting product was washed to neutral pH of the aqueous layer, and the solution was dried over anhydrous sodium sulfate. Then, the excess solvent was removed on a rotary evaporator and the polymer was dried at 1 mmHg. The resulting product was characterized by a bimodal molecular weight distribution. The high-molecular-weight part was separated using preparative gel permeation chromatography. The product was analyzed by GPC and NMR methods. The copolymerizations of octamethylcyclotetrasiloxane
. First, 5 g (0.015 mol) of 7,7-diethyl-1,1,3,3,5,5-hexamethylcyclotetrasiloxane and 0.16 g (2.85 mmol) of KOH were stirred at 140 °C for 1 h. At the end of the polymerization, a colorless, highly viscous product was obtained. After this, at 5 °C, 13.5 mL anhydrous toluene, 1.5 g (0.014 mol) of chlorotrimethylsilane and 1.1 g (0.014 mol) pyridine were added to the reaction mixture. The resulting product was washed to neutral pH of the aqueous layer, and the solution was dried over anhydrous sodium sulfate. Then, the excess solvent was removed on a rotary evaporator and the polymer was dried at 1 mmHg. The resulting product was characterized by a bimodal molecular weight distribution. The high-molecular-weight part was separated using preparative gel permeation chromatography. The product was analyzed by GPC and NMR methods. The copolymerizations of octamethylcyclotetrasiloxane 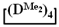 with octaethylcyclotetrasiloxane
with octaethylcyclotetrasiloxane 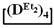 or
or 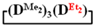 were performed in a similar manner to this procedure. The copolymerization conditions and results are shown in Table 3.
were performed in a similar manner to this procedure. The copolymerization conditions and results are shown in Table 3.
Table 3.
Polymerization conditions and characteristics of products.
2.2. Methods
GLC analysis was performed on a Chromatek Analytic 5000 chromatograph (Russia), a katharometer detector, a helium carrier gas, 2 m × 3 mm columns and a stationary phase SE-30 (5%) printed on Chromaton-H-AW. Registration and calculation of data were carried out using the program “Chromatek Analyst” (Russia).
GPC analysis was performed on a chromatographic system consisting of a STAYER series 2 high-pressure pump (Aquilon, Russia), a RIDK 102 refractometric detector (Czech Republic) (using eluent—toluene) and a JETSTREAM 2 PLUS column thermostat (KNAUER, Berlin, Germany). Eluents—toluene + 2% THF, flow rate—1.0 mL/min. Columns 300 mm long and 7.8 mm in diameter (300 × 7.8 mm) were filled with the Phenogel sorbent (Phenomenex, Torrance, CA, USA), the particle size was 5 mm, and the pore size was 103A and 104A (the passport separation range was up to 75,000 Da and up to 500,000 Da, respectively). The registration and calculation of data were performed using the UniChrom 4.7 program (Belarus).
1H and 29Si NMR spectra of products were recorded using a Bruker Avance II 300 spectrometer. CDCl3 was used as the internal standard with a chemical shift of δ = 7.25 ppm.
Infrared (IR) spectra were recorded on an IR Fourier spectrometer—Nicolet iS50 (Thermo Scientific, Waltham, MA, USA)—with a built-in ATR (crystal-diamond) attachment. Measurement conditions: resolution—4 cm−1, number of scans—32.
Differential scanning calorimetry (DSC) of samples was performed on the differential scanning calorimeter DSC-3 (Mettler-Toledo, Switzerland) at a heating rate of 10°/min in an argon atmosphere (60 mL/min).
3. Results and Discussion
3.1. Synthesis of 1,1,3,3,5,5-Hexamethyl-7,7-diorganocyclotetrasiloxanes
The general scheme of interaction of 1,5-sodiumoxyhexamethyltrisiloxane with dichlorodiorganosilanes is shown in Figure 1.
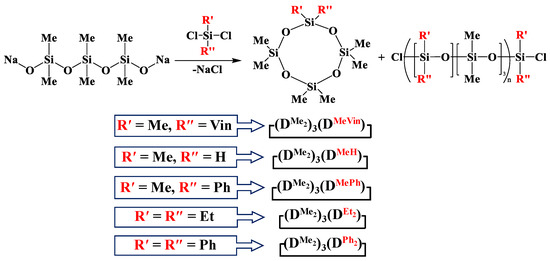
Figure 1.
Scheme of interaction of 1,5-disodiumoxyhexamethyltrisiloxane and diorganodichlorosilanes.
Methylvinyl- and methyldichlorosilanes were used as dichlorodiorganosilane for the synthesis of functional mixed dimethylcyclotetrasiloxanes. To study the effect of the substituent type, the interaction of 1,5-disodiumoxyhexamethyltrisiloxane and methylphenyl-, diphenyl- and diethyldichlorosilanes was also investigated.
Firstly, 1,5-disodiumoxyhexamethyltrisiloxane is a white hygroscopic powder, practically insoluble in most organic solvents. Its dissolution in tetrahydrofuran or pyridine is achieved only at temperatures up to 50–60 °C, but, even in this case, the solubility of the salt does not exceed 5 wt.%. Therefore, it was of interest to compare the process under homogeneous and heterogeneous conditions at the same concentration of reagent (5 wt.%) in the reaction mixture. THF was used for homogeneous conditions; MTBE was used for heterogeneous conditions. Regardless of other conditions, the reaction was carried out at −60 °C to prevent the processes of cleavage of the siloxane bond under the action of silanolate end groups. The reaction mixture was intensively stirred for 1 h after adding the reagents. If the pH was neutral or slightly acidic, the reaction mixture was stirred until room temperature. The siloxane product was isolated and analyzed by GPC, and the 1,1,3,3,5,5-hexamethyl-7,7-diorganocyclotetrasiloxane was isolated by distillation at reduced pressure. The purity and structure of the mixed cyclotetrasiloxanes were confirmed by a combination of GLC and 1H and 29Si NMR spectroscopy methods. The reaction conditions and the composition of the products are shown in Table 1 and Table 2.
GLC data indicate the absence of side processes with the silanolate ends’ participation. In all cases, depending on the type of diorganodichlorosilane and solvent, volatile products consisted of the target cyclotetrasiloxane by 85–98% (Figure 2a,b, Table 1 and Table 2). In comparison, Figure 2c shows the GLC curve of the volatile products of the 1,5-disodiumoxyhexamethyltrisiloxane and dichlorodiethylsilane interaction under homogeneous conditions, where the processes of siloxane bond cleavage and rearrangement of the resulting products were found.
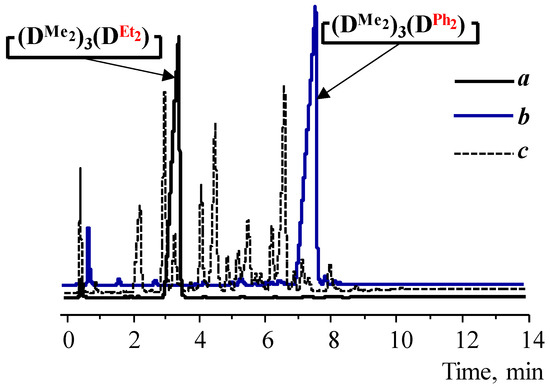
The effect of adding reagents on the reaction mixture was studied in the case of dichloromethylvinylsilane and 1,5-disodiumoxyhexamethyltrisiloxane. It was found that the order of reagent injection under homogeneous conditions and heterogeneous conditions did not significantly affect the yield of the target 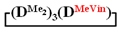 . Thus, under homogeneous conditions, the target cycle was formed in 45–55% yield, both when adding chlorosilane in THF to a solution of salt in THF (№ 1, Table 1) and with the simultaneous injection solutions of salt and chlorosilane in THF with the same molarity (№ 2, Table 1). In this case, the sequence of reagent addition affected only the molecular weight distribution of linear oligomers (Figure 3). Under heterogeneous conditions, salt was added to a chlorosilane in MTBE (№ 4, Table 1) or chlorosilane to a suspension of salt in MTBE, and the yield of the product was 70–75% (№ 8, Table 1).
. Thus, under homogeneous conditions, the target cycle was formed in 45–55% yield, both when adding chlorosilane in THF to a solution of salt in THF (№ 1, Table 1) and with the simultaneous injection solutions of salt and chlorosilane in THF with the same molarity (№ 2, Table 1). In this case, the sequence of reagent addition affected only the molecular weight distribution of linear oligomers (Figure 3). Under heterogeneous conditions, salt was added to a chlorosilane in MTBE (№ 4, Table 1) or chlorosilane to a suspension of salt in MTBE, and the yield of the product was 70–75% (№ 8, Table 1).
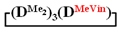 . Thus, under homogeneous conditions, the target cycle was formed in 45–55% yield, both when adding chlorosilane in THF to a solution of salt in THF (№ 1, Table 1) and with the simultaneous injection solutions of salt and chlorosilane in THF with the same molarity (№ 2, Table 1). In this case, the sequence of reagent addition affected only the molecular weight distribution of linear oligomers (Figure 3). Under heterogeneous conditions, salt was added to a chlorosilane in MTBE (№ 4, Table 1) or chlorosilane to a suspension of salt in MTBE, and the yield of the product was 70–75% (№ 8, Table 1).
. Thus, under homogeneous conditions, the target cycle was formed in 45–55% yield, both when adding chlorosilane in THF to a solution of salt in THF (№ 1, Table 1) and with the simultaneous injection solutions of salt and chlorosilane in THF with the same molarity (№ 2, Table 1). In this case, the sequence of reagent addition affected only the molecular weight distribution of linear oligomers (Figure 3). Under heterogeneous conditions, salt was added to a chlorosilane in MTBE (№ 4, Table 1) or chlorosilane to a suspension of salt in MTBE, and the yield of the product was 70–75% (№ 8, Table 1).Further interactions were carried out by adding a solution of dichlorodiorganosilane to a solution or suspension of the salt in THF or MTBE, respectively.
Analysis of the data in Table 1 and Table 2 allowed us to divide all cases into two groups. Vinylmethyl- and methyldichlorosilanes showed the highest preparative yield of 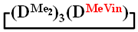 and
and 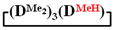 in MTBE, which is 55 and 75%, respectively (№ 3 and 6, Table 1). The opposite situation was observed using more sterically hindered chlorosilyl end groups such as diethyl-, methylphenyl- and diphenyldichlorosilanes: the highest yields of
in MTBE, which is 55 and 75%, respectively (№ 3 and 6, Table 1). The opposite situation was observed using more sterically hindered chlorosilyl end groups such as diethyl-, methylphenyl- and diphenyldichlorosilanes: the highest yields of 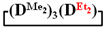 ,
, 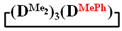 and
and 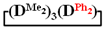 were achieved under homogeneous conditions, equal to up to 65, 67 and 70%, respectively (№ 8, 10, 12, Table 2). Such differences in the yields of products indicate significant opportunities for further optimization of the yield of each specific mixed cycle.
were achieved under homogeneous conditions, equal to up to 65, 67 and 70%, respectively (№ 8, 10, 12, Table 2). Such differences in the yields of products indicate significant opportunities for further optimization of the yield of each specific mixed cycle.
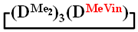 and
and 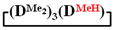 in MTBE, which is 55 and 75%, respectively (№ 3 and 6, Table 1). The opposite situation was observed using more sterically hindered chlorosilyl end groups such as diethyl-, methylphenyl- and diphenyldichlorosilanes: the highest yields of
in MTBE, which is 55 and 75%, respectively (№ 3 and 6, Table 1). The opposite situation was observed using more sterically hindered chlorosilyl end groups such as diethyl-, methylphenyl- and diphenyldichlorosilanes: the highest yields of 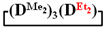 ,
, 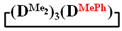 and
and 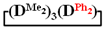 were achieved under homogeneous conditions, equal to up to 65, 67 and 70%, respectively (№ 8, 10, 12, Table 2). Such differences in the yields of products indicate significant opportunities for further optimization of the yield of each specific mixed cycle.
were achieved under homogeneous conditions, equal to up to 65, 67 and 70%, respectively (№ 8, 10, 12, Table 2). Such differences in the yields of products indicate significant opportunities for further optimization of the yield of each specific mixed cycle.All dimethylcyclotetrasiloxanes were isolated with a purity of at least 95% according to GLC data; the structure of the obtained products was confirmed by 1H, 29Si NMR and IR spectroscopy. The relevant data are given in the Supplementary Materials (Figures S1–S15). The IR spectroscopy data of the isolated cycles indicate the absence of an absorption band in the region of 3400–3600 cm−1, which is characteristic of silanol groups and confirms the cyclic structure of the isolated compounds (Figures S1–S5). The 1H and 29Si NMR spectroscopy data of the isolated fractions indicate that the integral intensities of the protons signals of corresponding substituents at silicon atoms and silicon atoms themselves conform to the calculated values (Figures S11–S15).
The data in Table 1 and Table 2 show that the main reaction product may be a linear oligomer with a regular arrangement of modifying units under certain conditions. In particular, in sample 10 (Table 2), the product contained 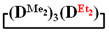 along with the linear poly(diethyl)(dimethyl)siloxane with Mp = 1900 and content of 70%. The product was blocked with chlorodimethylvinylsilane to confirm the linear structure (Figure 4) and its composition and molecular weight characteristics were determined by 1H NMR spectroscopy and GPC methods (Figure 5).
along with the linear poly(diethyl)(dimethyl)siloxane with Mp = 1900 and content of 70%. The product was blocked with chlorodimethylvinylsilane to confirm the linear structure (Figure 4) and its composition and molecular weight characteristics were determined by 1H NMR spectroscopy and GPC methods (Figure 5).
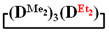 along with the linear poly(diethyl)(dimethyl)siloxane with Mp = 1900 and content of 70%. The product was blocked with chlorodimethylvinylsilane to confirm the linear structure (Figure 4) and its composition and molecular weight characteristics were determined by 1H NMR spectroscopy and GPC methods (Figure 5).
along with the linear poly(diethyl)(dimethyl)siloxane with Mp = 1900 and content of 70%. The product was blocked with chlorodimethylvinylsilane to confirm the linear structure (Figure 4) and its composition and molecular weight characteristics were determined by 1H NMR spectroscopy and GPC methods (Figure 5).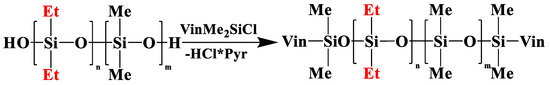
Figure 4.
Scheme of blocking linear poly(diethyl)(dimethyl)siloxane (№ 10, Table 2).
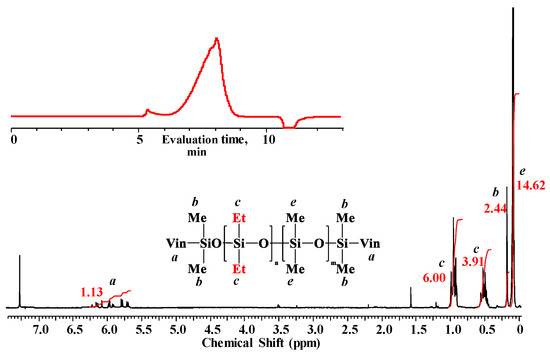
Figure 5.
1H NMR spectrum and GPC curve of blocked linear poly(diethyl)(dimethyl)siloxane (№ 10, Table 2).
The correlation of the integral intensities of proton signals of ethyl, vinyl and methyl groups in the backbone and terminal silicon atoms allowed us to determine by the 1H NMR spectrum that the unit composition of the obtained product corresponded to the following formula: VinMe2SiO-{[Et2SiO]1[Me2SiO]2,4}5,3-SiMe2Vin with Mn equal to ~1640. The number-average molecular weights of the polymer calculated from the NMR and determined by the GPC method (Mn = 1800, Mw = 2300, Mw/Mn = 1.3) were consistent and confirmed the linear structure of poly(diethyl)(dimethyl)siloxane.
Thus, the interactions of 1,5-disodiumoxyhexamethyltrisiloxane with diorganodichlorosilanes were investigated to obtain 1,1,3,3,5,5-hexamethyl-7,7-diorganocyclotetrasiloxanes. For the first time, it was shown that mixed dimethylcyclotetrasiloxanes can be obtained with a yield of 55 to 75% by this method. The ratio of linear and cyclic products of a mixed structure can be controlled within wide limits by selecting the reaction conditions. Using dichlorodiethylsilane as an example, it was shown that this method can be a promising means of obtaining linear oligomers with alternating diethyl- and dimethylsiloxane units.
3.2. Preparation of Poly(diethyl)(dimethyl)siloxane
A simple and cheap method for the preparation of 1,1,3,3,5,5-hexamethyl-7,7-diorganocyclotetrasiloxanes opens up new prospects for the preparation of polydimethyldiorganosiloxanes with a controlled content of diorganosilyl groups via polymerization methods. It is known that, in order to obtain polydiethylsiloxanes, hexaethylcyclotrisiloxane is polymerized [51,52] since octaethylcyclotetrasiloxane 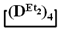 is practically not polymerized. To obtain poly(diethyl)(dimethyl)siloxane copolymers, catalytic rearrangement of the cohydrolysis products of dimethyl- and diethyldichlorosilanes is carried out [53]. In our study, we paid attention to the prospects of using mixed
is practically not polymerized. To obtain poly(diethyl)(dimethyl)siloxane copolymers, catalytic rearrangement of the cohydrolysis products of dimethyl- and diethyldichlorosilanes is carried out [53]. In our study, we paid attention to the prospects of using mixed 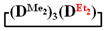 , in contrast to
, in contrast to 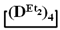 , for the preparation of (diethyl)(dimethyl)siloxane copolymers. Anionic polymerization of
, for the preparation of (diethyl)(dimethyl)siloxane copolymers. Anionic polymerization of 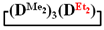 , its copolymerization with
, its copolymerization with 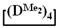 and copolymerization of
and copolymerization of 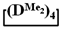 and
and 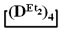 in the presence of potassium hydroxide were carried out to illustrate this statement (Figure 6a–c, respectively). The duration of anionic polymerization was 1 h at 140 °C. Trimethylchlorosilane was used as a termination agent.
in the presence of potassium hydroxide were carried out to illustrate this statement (Figure 6a–c, respectively). The duration of anionic polymerization was 1 h at 140 °C. Trimethylchlorosilane was used as a termination agent.
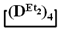 is practically not polymerized. To obtain poly(diethyl)(dimethyl)siloxane copolymers, catalytic rearrangement of the cohydrolysis products of dimethyl- and diethyldichlorosilanes is carried out [53]. In our study, we paid attention to the prospects of using mixed
is practically not polymerized. To obtain poly(diethyl)(dimethyl)siloxane copolymers, catalytic rearrangement of the cohydrolysis products of dimethyl- and diethyldichlorosilanes is carried out [53]. In our study, we paid attention to the prospects of using mixed 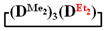 , in contrast to
, in contrast to 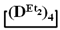 , for the preparation of (diethyl)(dimethyl)siloxane copolymers. Anionic polymerization of
, for the preparation of (diethyl)(dimethyl)siloxane copolymers. Anionic polymerization of 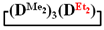 , its copolymerization with
, its copolymerization with 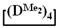 and copolymerization of
and copolymerization of 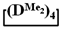 and
and 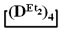 in the presence of potassium hydroxide were carried out to illustrate this statement (Figure 6a–c, respectively). The duration of anionic polymerization was 1 h at 140 °C. Trimethylchlorosilane was used as a termination agent.
in the presence of potassium hydroxide were carried out to illustrate this statement (Figure 6a–c, respectively). The duration of anionic polymerization was 1 h at 140 °C. Trimethylchlorosilane was used as a termination agent.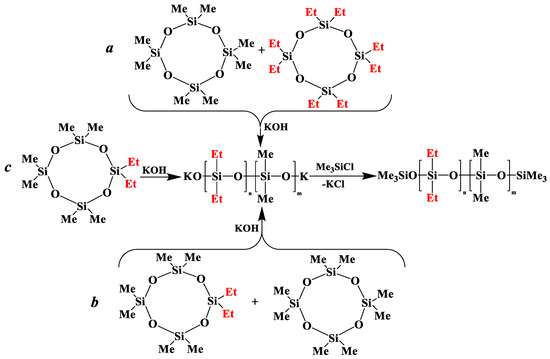
Figure 6.
Polymerization schematics of 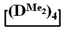 and
and 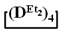 (a), of
(a), of 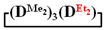 and
and 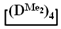 (b) and of
(b) and of 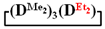 (c).
(c).
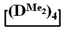 and
and 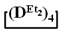 (a), of
(a), of 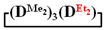 and
and 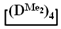 (b) and of
(b) and of  (c).
(c).
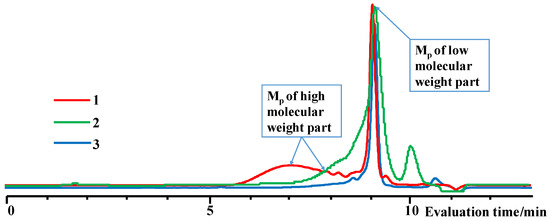
The content of the high-molecular and low-molecular parts of the products was determined by the GPC method (Table 3, Figure 7 and Figure 8). The high-molecular-weight part was separated using preparative GPC, and its composition and molecular weight characteristics were analyzed by 1H NMR spectroscopy and GPC methods (Figures S16–S18). The characteristics of the obtained products are shown in Table 3.
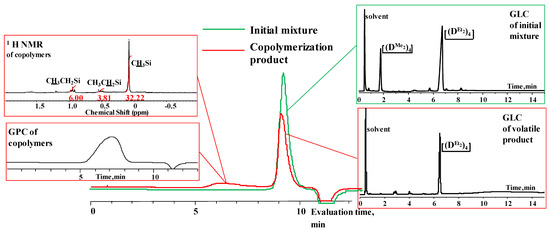
Figure 7.
Characteristics of the initial mixture of 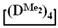 /
/ 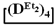 and the product of their copolymerization (№ 1, Table 3): GPC data for the initial mixture (green curve) and the product (red curve); GLC curves of the initial mixture of monomers (top right) and volatile fraction after copolymerization (bottom right); 1H NMR spectrum (top left) and GPC curve (bottom left) of obtained copolymer.
and the product of their copolymerization (№ 1, Table 3): GPC data for the initial mixture (green curve) and the product (red curve); GLC curves of the initial mixture of monomers (top right) and volatile fraction after copolymerization (bottom right); 1H NMR spectrum (top left) and GPC curve (bottom left) of obtained copolymer.
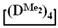 /
/ 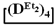 and the product of their copolymerization (№ 1, Table 3): GPC data for the initial mixture (green curve) and the product (red curve); GLC curves of the initial mixture of monomers (top right) and volatile fraction after copolymerization (bottom right); 1H NMR spectrum (top left) and GPC curve (bottom left) of obtained copolymer.
and the product of their copolymerization (№ 1, Table 3): GPC data for the initial mixture (green curve) and the product (red curve); GLC curves of the initial mixture of monomers (top right) and volatile fraction after copolymerization (bottom right); 1H NMR spectrum (top left) and GPC curve (bottom left) of obtained copolymer.
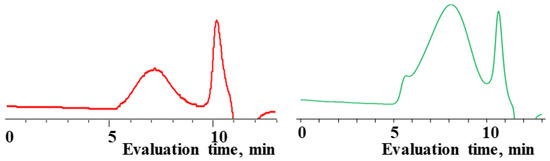
Figure 8.
GPC curves of copolymerization 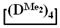 and
and 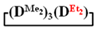 (on left) and polymerization
(on left) and polymerization 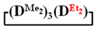 (on right) products.
(on right) products.
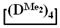 and
and 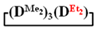 (on left) and polymerization
(on left) and polymerization 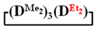 (on right) products.
(on right) products.
As expected, the content of the high-molecular part was three times higher in the case of the copolymerization of 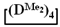 and
and 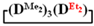 (№ 2, Table 3) than in the copolymerization of homocycles
(№ 2, Table 3) than in the copolymerization of homocycles 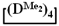 and
and 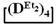 with various substituents (№ 1, Table 3), where low conversion of the
with various substituents (№ 1, Table 3), where low conversion of the 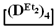 was observed. It follows from a comparison of the GLC data for the initial mixture of monomers and the low-molecular-weight fraction of the products (Figure 7). Analysis of the high-molecular-weight fractions of the products showed the correspondence of the structural unit of the copolymer obtained by the copolymerization of
was observed. It follows from a comparison of the GLC data for the initial mixture of monomers and the low-molecular-weight fraction of the products (Figure 7). Analysis of the high-molecular-weight fractions of the products showed the correspondence of the structural unit of the copolymer obtained by the copolymerization of 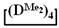 and mixed
and mixed 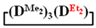 to the calculated value, in contrast to the copolymerization of
to the calculated value, in contrast to the copolymerization of 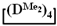 and
and 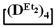 , where the polymer composition was enriched with dimethylsilyl units.
, where the polymer composition was enriched with dimethylsilyl units.
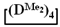 and
and 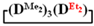 (№ 2, Table 3) than in the copolymerization of homocycles
(№ 2, Table 3) than in the copolymerization of homocycles 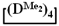 and
and 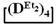 with various substituents (№ 1, Table 3), where low conversion of the
with various substituents (№ 1, Table 3), where low conversion of the 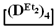 was observed. It follows from a comparison of the GLC data for the initial mixture of monomers and the low-molecular-weight fraction of the products (Figure 7). Analysis of the high-molecular-weight fractions of the products showed the correspondence of the structural unit of the copolymer obtained by the copolymerization of
was observed. It follows from a comparison of the GLC data for the initial mixture of monomers and the low-molecular-weight fraction of the products (Figure 7). Analysis of the high-molecular-weight fractions of the products showed the correspondence of the structural unit of the copolymer obtained by the copolymerization of 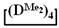 and mixed
and mixed 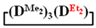 to the calculated value, in contrast to the copolymerization of
to the calculated value, in contrast to the copolymerization of 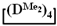 and
and 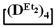 , where the polymer composition was enriched with dimethylsilyl units.
, where the polymer composition was enriched with dimethylsilyl units.The polymerization of mixed 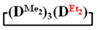 forms poly(diethyl)dimethylsiloxane with a Mn close to the calculated value, a broad molecular weight distribution and a structural unit composition corresponding to the calculated one (№3, Table 3, Figure 8 (on right)). According to DSC data (Figures S19 and S20), the obtained poly(diethyl)dimethylsiloxanes (№ 2 and 3 of Table 3) had a low glass transition temperature of −132 °C~–131 °C and the absence of crystallization.
forms poly(diethyl)dimethylsiloxane with a Mn close to the calculated value, a broad molecular weight distribution and a structural unit composition corresponding to the calculated one (№3, Table 3, Figure 8 (on right)). According to DSC data (Figures S19 and S20), the obtained poly(diethyl)dimethylsiloxanes (№ 2 and 3 of Table 3) had a low glass transition temperature of −132 °C~–131 °C and the absence of crystallization.
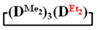 forms poly(diethyl)dimethylsiloxane with a Mn close to the calculated value, a broad molecular weight distribution and a structural unit composition corresponding to the calculated one (№3, Table 3, Figure 8 (on right)). According to DSC data (Figures S19 and S20), the obtained poly(diethyl)dimethylsiloxanes (№ 2 and 3 of Table 3) had a low glass transition temperature of −132 °C~–131 °C and the absence of crystallization.
forms poly(diethyl)dimethylsiloxane with a Mn close to the calculated value, a broad molecular weight distribution and a structural unit composition corresponding to the calculated one (№3, Table 3, Figure 8 (on right)). According to DSC data (Figures S19 and S20), the obtained poly(diethyl)dimethylsiloxanes (№ 2 and 3 of Table 3) had a low glass transition temperature of −132 °C~–131 °C and the absence of crystallization.Thus, firstly, the advantages of 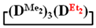 used for the preparation of poly(diethyl)(dimethyl)siloxanes with a controlled unit composition were demonstrated in comparison with the mixture of
used for the preparation of poly(diethyl)(dimethyl)siloxanes with a controlled unit composition were demonstrated in comparison with the mixture of 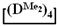 and
and 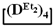 .
.
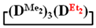 used for the preparation of poly(diethyl)(dimethyl)siloxanes with a controlled unit composition were demonstrated in comparison with the mixture of
used for the preparation of poly(diethyl)(dimethyl)siloxanes with a controlled unit composition were demonstrated in comparison with the mixture of 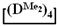 and
and 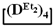 .
.4. Conclusions
Mixed tetrasiloxane cycles have high potential for practical application; however, the lack of selective methods for its preparation has been a limiting factor for the realization of this potential for a long time. This work shows that high selectivity of mixed cycle synthesis can be achieved based on 1,5-disodiumoxyhexamethyltrisiloxanes, a unique reagent that we described earlier [49]. The yield of these cyclosiloxanes in the best experiments reaches 70%. It is important that linear alternating oligomers are formed as by-products, which can be used independently. Moreover, the ratio between linear and cyclic products can be changed within wide limits.
The second part of the article demonstrates the advantages of mixed cyclosiloxane polymerization in comparison with a mixture of two cyclosiloxanes with a homogeneous structure. This result is a consequence of the low reactivity of 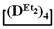 in comparison with the high reactivity mixed cycle in anionic polymerization.
in comparison with the high reactivity mixed cycle in anionic polymerization.
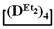 in comparison with the high reactivity mixed cycle in anionic polymerization.
in comparison with the high reactivity mixed cycle in anionic polymerization.We believe that the considered method opens up new prospects both for expanding the range of cyclic siloxane products with a specific composition and structure, which have many different applications, and for obtaining linear polymers with a controlled content of modifying units and new materials based on them. Mixed cycles have many other applications, including fluids with controlled properties. The realization of these and other potential applications requires further research and is an important subject of current research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14010028/s1, Figures S1–S5: IR spectra of all obtained cycles; Figures S6–S10: GLC curves of all obtained cycles; Figures S11–S15: 1H (top) and 29Si (bottom) NMR spectra for mixed cyclotetrasiloxanes; Figures S16–S18: 1H NMR spectra for high-molecular-weight copolymers; Figures S19 and S20: DSC thermograms for high-molecular-weight copolymers.
Author Contributions
Conceptualization, A.M.M.; Funding acquisition, A.A.K.; Investigation, E.V.T., E.V.C., A.G.K., M.A.O. and G.V.C.; Resources, E.V.T.; Validation, E.V.T. and E.V.C.; Visualization, A.A.K.; Writing—original draft, E.V.T., A.A.K., A.G.K.; Writing—review and editing, A.M.M. and A.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by RFBR (No. 20-33-70228). Molecular wt. distribution studies and recording of the NMR spectra were performed with financial support from the Ministry of Science and Higher Education of the Russian Federation (FFSM-2021-0004) using the equipment of Collaborative Access Center “Center for Polymer Research” of ISPM RAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yang, X.; Chen, Z.; Liu, J.; Chen, Q.; Liu, Q.; Luo, M.; Lai, G. A convenient method for preparation of hydroxyl silicone oils with ring opening polymerization of octamethylcyclotetrasiloxane (D4). Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 117–122. [Google Scholar] [CrossRef]
- Barnes, Q.; Longuet, C.; Ganachaud, F. Cationic Polymerization of Hexamethylcyclotrisiloxane in Excess Water. Molecules 2021, 26, 4402. [Google Scholar] [CrossRef]
- Fuchise, K.; Sato, K.; Igarashi, M. Precise Synthesis of Side-Chain-Functionalized Linear Polysiloxanes by Organocatalytic Ring-Opening Polymerization of Monofunctional Cyclotrisiloxanes. Macromolecules 2021, 54, 5204–5217. [Google Scholar] [CrossRef]
- Goff, J.; Sulaiman, S.; Arkles, B. Applications of Hybrid Polymers Generated from Living Anionic Ring Opening Polymerization. Molecules 2021, 26, 2755. [Google Scholar] [CrossRef]
- Katarzhnova, E.Y.; Ignatyeva, G.M.; Kalinina, A.A.; Talalaeva, E.V.; Tereshchenko, A.S. Synthesis and properties of hybrid carbosilane dendrimers with cyclosiloxane external shells. INEOS OPEN 2020, 3, 219–225. [Google Scholar] [CrossRef]
- Migulin, D.; Vysochinskaya, Y.; Buzin, M.; Bakirov, A.; Cherkaev, G.; Shchegolikhina, O. Stereoregular hybrid azobenzene-cyclosiloxanes with photoinduced reversible solid to liquid transition properties. J. Photochem. Photobiol. A Chem. 2021, 407, 113033. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Anisimov, A.A.; Peregudov, A.S.; Dubovik, A.S.; Orlov, V.N.; Malakhova, Y.N.; Stupnikov, A.A.; Buzin, M.I.; Nikiforova, G.G.; Vasil’ev, V.G.; et al. Star-shaped siloxane polymers with various cyclic cores: Synthesis and properties. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1233–1246. [Google Scholar] [CrossRef]
- Anisimov, A.A.; Kononevich, Y.N.; Buzin, M.I.; Peregudov, A.S.; Shchegolikhina, O.I.; Muzafarov, A.M. Convenient synthesis of new Si-H and Si-Vinyl functionalized stereospecific 8-, 12-and 24-membered cyclosiloxanes. Macroheterocycles 2016, 9, 442–452. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Akkus, B.; Gao, Y.; Liu, Y.; Yamamoto, S.; Matsui, J.; Mitsuishi, M. Regioselective synthesis of eight-armed cyclosiloxane amphiphile for functional 2D and 3D assembly motifs. ACS Appl. Mater. Interfaces 2017, 9, 28144–28150. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wei, X.; Peng, Q.; Fan, L.; Li, X.; Hu, H.; Yang, J. Silacyclobutane-functionalized cyclosiloxanes as photoactive precursors for high thermal stability, low dielectric constant and low dielectric loss polymers. J. Appl. Polym. Sci. 2021, 138, 51376. [Google Scholar] [CrossRef]
- Kong, D.; Liu, J.; Zhang, Z.; Wang, S.; Lu, Z. Preparation of synergistic silicon, phosphorus and nitrogen flame retardant based on cyclosiloxane and its application to cotton fabric. Cellulose 2021, 28, 8115–8128. [Google Scholar] [CrossRef]
- Hasenclever, K.D. Verwendung von Polydialkylcyclosiloxanen als Loesemittel Fuer die Chemischreinigung. Patent of Germany DE3739711, 8 June 1989. [Google Scholar]
- Gaidau, C.; Martinescu, T.; Simion, D.; Mocioiu, A.M.; Sendrea, C.; Fleancu, M.; Niculescu, M. Study on Dry Cleaning with Decamethylcyclopentasiloxane as Ecological Alternative for Leathers and Furskins. Rev. Chim. 2014, 65, 411–415. [Google Scholar]
- Liu, J. Composite of Dry Cleaning Solvent. Patent of China CN101735906, 16 June 2010. [Google Scholar]
- Lee, C.H.; Tang, Y.L.; Wang, Y.; Kan, C.W. Dyeing of Cotton Fabric in Decamethylcyclopentasiloxane Using Alkyl Polyglucoside-based Reverse Micelle as Reactive Dye Carrier. Fibers Polym. 2021, 1–12. [Google Scholar] [CrossRef]
- Pei, L.; Liu, J.; Cai, G.; Wang, J. Study of hydrolytic kinetics of vinyl sulfone reactive dye in siloxane reverse micro-emulsion. Text. Res. J. 2017, 87, 2368–2378. [Google Scholar] [CrossRef]
- Liu, J.Q.; Miao, H.L.; Li, S.Z. Non-aqueous dyeing of reactive dyes in D5. Adv. Mater. Res. 2012, 441, 138–144. [Google Scholar] [CrossRef]
- Fan, J.; Shao, M.; Miao, J.; Ma, J.; Hu, M.; An, Y.; Shao, J. Thermodynamic properties of cotton dyeing with indigo dyes in non-aqueous media of liquid paraffin and D5. Text. Res. J. 2021, 91, 2692–2704. [Google Scholar] [CrossRef]
- Loretz, L.J.; Api, A.M.; Babcock, L.; Barraj, L.M.; Burdick, J.; Cater, K.C.; Jarrett, G.; Mann, S.; Pan, Y.H.L.; Re, T.A.; et al. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem. Toxicol. 2008, 46, 1516–1524. [Google Scholar] [CrossRef]
- Loretz, L.J.; Api, A.M.; Barraj, L.M.; Burdick, J.; Dressler, W.E.; Gettings, S.D.; Hsu, H.H.; Pan, Y.H.L.; Re, T.A.; Renskers, K.J.; et al. Exposure data for cosmetic products: Lipstick, body lotion, and face cream. Food Chem. Toxicol. 2005, 43, 279–291. [Google Scholar] [CrossRef]
- Loretz, L.; Api, A.M.; Barraj, L.; Burdick, J.; Davis, D.A.; Dressler, W.; Gilberti, E.; Jarrett, G.; Mann, S.; Pen, Y.H.L.; et al. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem. Toxicol. 2006, 44, 2008–2018. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, L. Anti-Wrinkle Cosmetic Composition and Preparation Method Thereof. Patent of China CN112220703, 15 January 2021. [Google Scholar]
- Nasu, A.; Otsubo, Y. Rheology and UV protection properties of suspensions of fine titanium dioxides in a silicone oil. J. Colloid Interface Sci. 2006, 296, 558–564. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. The effect of phenyl content on the degradation of poly (dimethyl diphenyl) siloxane copolymers. Polym. Degrad. Stab. 2001, 74, 363–370. [Google Scholar] [CrossRef]
- Madsen, P.J.; Yu, L.; Boucher, S.; Skov, A.L. Enhancing the electro-mechanical properties of polydimethylsiloxane elastomers through blending with poly (dimethylsiloxane-co-methylphenylsiloxane) copolymers. RSC Adv. 2018, 8, 23077–23088. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, D.; Wu, L.; Fan, H.; Wang, D.; Li, B.G. Ring-Opening Copolymerization of Mixed Cyclic Monomers: A Facile, Versatile and Structure-Controllable Approach to Preparing Poly (methylphenylsiloxane) with Enhanced Thermal Stability. Ind. Eng. Chem. Res. 2017, 56, 7120–7130. [Google Scholar] [CrossRef]
- Jha, P.; Way, J.D. Concentration and temperature dependence on diffusivities of CO2 and N2 for poly (dimethyl, methylphenyl siloxane). AIChE J. 2008, 54, 143–149. [Google Scholar] [CrossRef]
- Rodchenko, S.; Amirova, A.; Milenin, S.; Ryzhkov, A.; Talalaeva, E.; Kalinina, A.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Amphiphilic molecular brushes with regular polydimethylsiloxane backbone and poly-2-isopropyl-2-oxazoline side chains. 1. Synthesis, characterization and conformation in solution. Eur. Polym. J. 2020, 140, 110035. [Google Scholar] [CrossRef]
- Gorodov, V.V.; Milenin, S.A.; Demchenko, N.V.; Muzafarov, A.M. Carboxyl-containing polydimethylsiloxanes: Synthesis and properties. INEOS OPEN 2020, 3, 43–54. [Google Scholar] [CrossRef]
- Babu, G.N.; Christopher, S.S.; Newmark, R.A. Poly(dimethylsiloxane-co-diphenylsiloxanes): Synthesis, characterization, and sequence analysis. Macromolecules 1987, 20, 2654–2659. [Google Scholar] [CrossRef]
- Andrianov, K.A.; Zavin, B.G.; Sablina, G.F. Anionic copolymerization of octamethyl and octaphenylcyclotetrasiloxanes. Polym. Sci. USSR 1972, 14, 1294–1302. [Google Scholar] [CrossRef]
- Ziemelis, M.J.; Saam, J.C. Sequence distribution in poly(dimethyl-siloxane-co-methylvinylsiloxanes). Macromolecules 1989, 22, 2111–2116. [Google Scholar] [CrossRef]
- Andrianov, K.A.; Khananashvili, L.M.; Konopchenko, Y.F. Synthesis of eight-membered mixed organocyclosiloxanes and their polymerization. Polym. Sci. USSR 1960, 2, 719–727. [Google Scholar]
- Andrianov, K.A.; Yakushkina, S.Y.; Guniava, L.N. Polymerization of mixed diphenyldimethylcyclosiloxanes. Polym. Sci. USSR 1966, 8, 2398–2404. [Google Scholar] [CrossRef]
- Andrianov, K.A.; Yakushkina, S.E. Synthesis of cyclic polyorganosiloxanes containing various groups in the ring. Bull. Acad. Sci. USSR Div. Chem. Sci. 1960, 9, 425–427. [Google Scholar] [CrossRef]
- Juengst, C.D.; Weber, W.P.; Manuel, G. Synthesis of spirocyclosiloxanes by flash vacuum pyrolysis of 2, 7-dimethyl-2, 3: 7, 8-diepoxy-5-silaspiro [4.4] nonane and cyclosiloxanes. J. Organomet. Chem. 1986, 308, 187–194. [Google Scholar] [CrossRef]
- Chrusciel, J.; Lasocki, Z. Dehydrocondensation of organic hydrosilanes with silanols. 1. Kinetics and mechanism of the reaction in dimethylformamide. Pol. J. Chem. 1983, 57, 113–120. [Google Scholar]
- Yu, J.; Liu, Y. Cyclic polysiloxanes with linked cyclotetrasiloxane subunits. Angew. Chem. Int. Ed. 2017, 56, 8706–8710. [Google Scholar] [CrossRef]
- Makarova, N.N.; Astapova, T.V.; Lavrukhin, B.D. Synthesis of organosiloxanes with reactive groups at silicon atoms. Russ. Chem. Bull. 1996, 45, 914–919. [Google Scholar] [CrossRef]
- Unno, M.; Tanaka, R. Silanols and silsesquioxanes. In Efficient Methods for Preparing Silicon Compounds; Academic Press: Cambridge, MA, USA, 2016; pp. 399–440. [Google Scholar]
- Mitsudome, T.; Arita, S.; Mori, H.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Supported Silver-Nanoparticle-Catalyzed Highly Efficient Aqueous Oxidation of Phenylsilanes to Silanols. Angew. Chem. 2008, 120, 8056–8058. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, J.; Jiang, Y.; Zhang, M.; Wang, C.; Xue, D.; Li, C. Selective Manganese-Catalyzed Oxidation of Hydrosilanes to Silanols under Neutral Reaction Conditions. Angew. Chem. 2019, 131, 6446–6450. [Google Scholar] [CrossRef]
- Schamschurin, A.; Uhrig, D.; Fisher, M.; Clarke, S.; Matisons, J. The synthesis and characterisation of novel dimethyl-and diphenyl-silanediolates. Silicon Chem. 2008, 3, 313–325. [Google Scholar] [CrossRef]
- Urayama, T.; Mitsudome, T.; Maeno, Z.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. O2-enhanced catalytic activity of gold nanoparticles in selective oxidation of hydrosilanes to silanols. Chem. Lett. 2015, 44, 1062–1064. [Google Scholar] [CrossRef]
- Teng, C.J.; Weber, W.P.; Cai, G. Anionic and cationic ring-opening polymerization of 2, 2, 4, 4, 6, 6-hexamethyl-8, 8-divinylcyclotetrasiloxane. Macromolecules 2003, 36, 5126–5130. [Google Scholar] [CrossRef]
- Andrianov, K.A.; Astakhin, V.V.; Pyzhov, V.K. Synthesis and properties of α, ω-dihydroxydimethylsiloxanes. Bull. Acad. Sci. USSR Div. Chem. Sci. 1962, 11, 2144–2146. [Google Scholar] [CrossRef]
- Hafner, T.; Torvisco, A.; Uhlig, F. Building blocks for oligomeric siloxanes–selective chlorination of hydrido-siloxanes. J. Organomet. Chem. 2018, 875, 1–4. [Google Scholar] [CrossRef]
- Basenko, S.V.; Maylyan, A.A.; Soldatenko, A.S. New Approach to the Synthesis of Symmetrical 1,3-Dichloro-1,1,3,3-Tetraorganyl-and 1,1,3,3-Tetrachloro-1,3-Diorganyldisiloxanes. Silicon 2018, 10, 465–470. [Google Scholar] [CrossRef]
- Talalaeva, E.V.; Kalinina, A.A.; Vasilenko, N.G.; Demchenko, N.V.; Cherkaev, G.V.; Goloveshkin, A.S.; Muzafarov, A.M. Selective formation of 1,5-disodiumoxyhexamethyltrisiloxane in the reaction of dimethylsiloxanes and sodium hydroxide. J. Organomet. Chem. 2020, 906, 121050. [Google Scholar] [CrossRef]
- Armarego, W.L.; Perrin, D.D. Purification of Laboratory Chemicals; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Molenberg, A.; Siffrin, S.; Möller, M.; Boileau, S.; Teyssié, D. Well defined columnar liquid crystalline polydiethylsiloxane. Macromol. Symp. 1996, 102, 199–207. [Google Scholar] [CrossRef]
- Zavin, B.G.; Rabkina, A.; Kuteinikova, L.I.; Blagodatskikh, I.V.; Dubovik, I.I. Anionic polymerization of diethylcyclosiloxanes: Formation of linear oligodiethylsiloxanes and their phase transitions. Polym. Sci. 1995, 37, 355–362. [Google Scholar]
- Filimonova, L.V.; Makarova, L.I.; Voronina, A.A.; Strelkova, T.V.; Barakovskaya, I.G.; Zavin, B.G.; Papkov, V.S. Polymerization of hexaethylcyclotrisiloxane during the synthesis of carbofunctional oligo (diethylsiloxane diols). Polym. Sci. Ser. B 2013, 55, 266–270. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).