Preparation of PVA–CS/SA–Ca2+ Hydrogel with Core–Shell Structure
Abstract
1. Introduction
2. Experimental Method
2.1. Raw Materials and Reagents
2.2. Preparation of PVA–CS/SA–Ca2+ Core–Shell Hydrogels
2.3. Structural Characterization and Performance Testing of PVA–CS/SA–Ca2+ Core–Shell Hydrogels
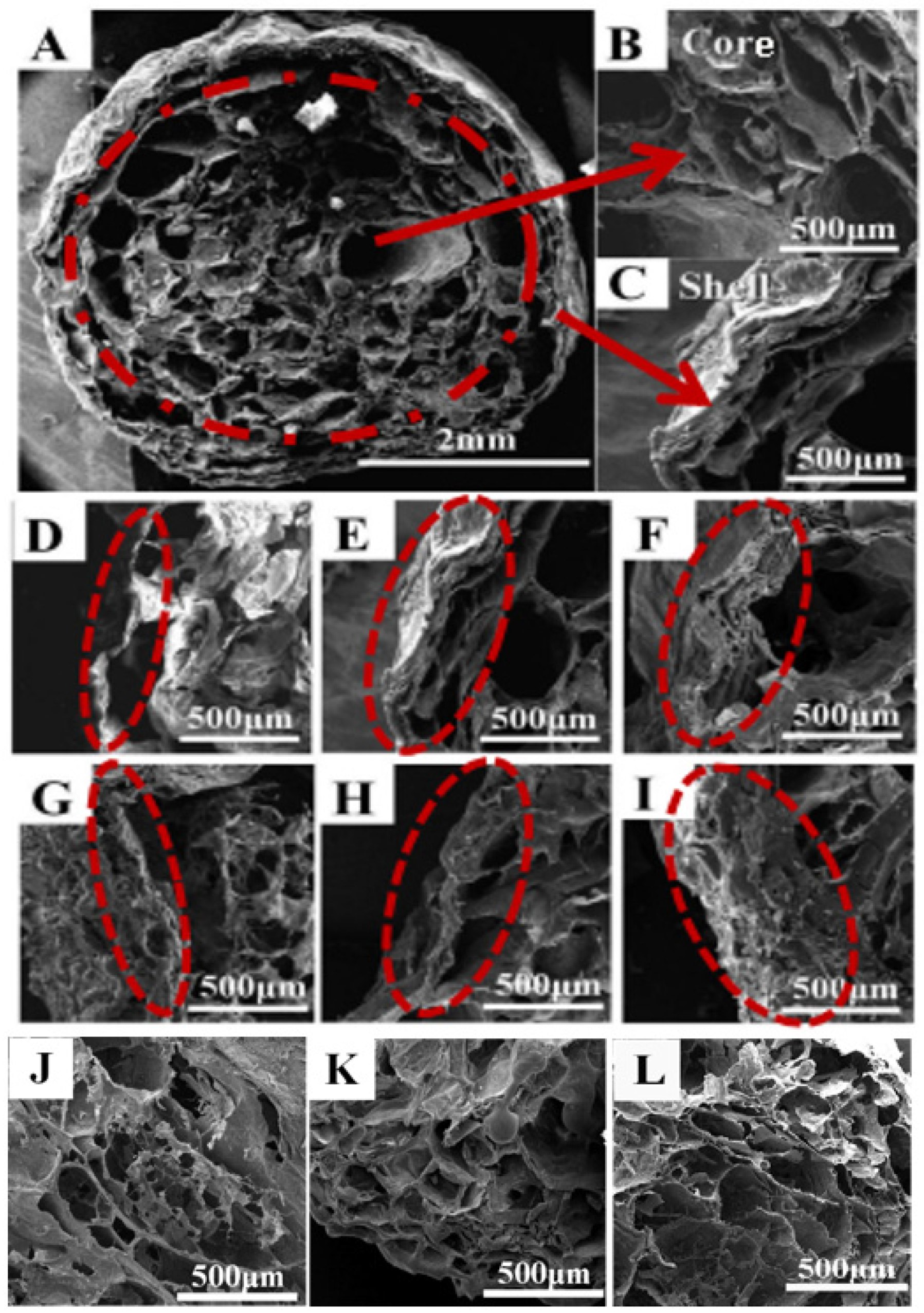
2.3.1. Microscopic Morphology of PVA–CS/SA–Ca2+ Core–Shell Hydrogels (SEM)
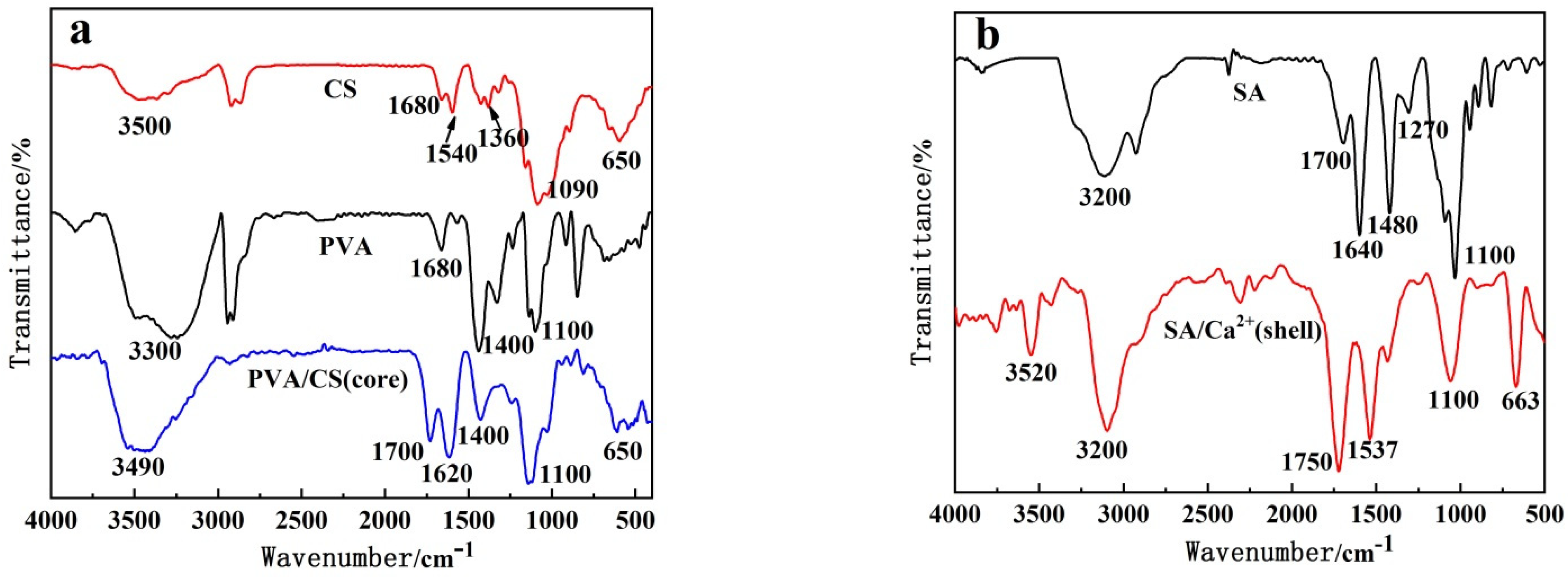
2.3.2. Structure of PVA–CS/SA–Ca2+ Core–Shell Hydrogels (FTIR)
2.3.3. Swelling Properties of PVA–CS/SA–Ca2+ Core–Shell Hydrogels
2.4. Statistical Analysis
3. Results and Analysis
3.1. Microscopic Morphology of PVA–CS/SA–Ca2+ Core–Shell Hydrogels
3.2. Structure of Core–Shell Hydrogel
3.3. Swelling Properties of PVA–CS/SA–Ca2+ Core–Shell Hydrogels
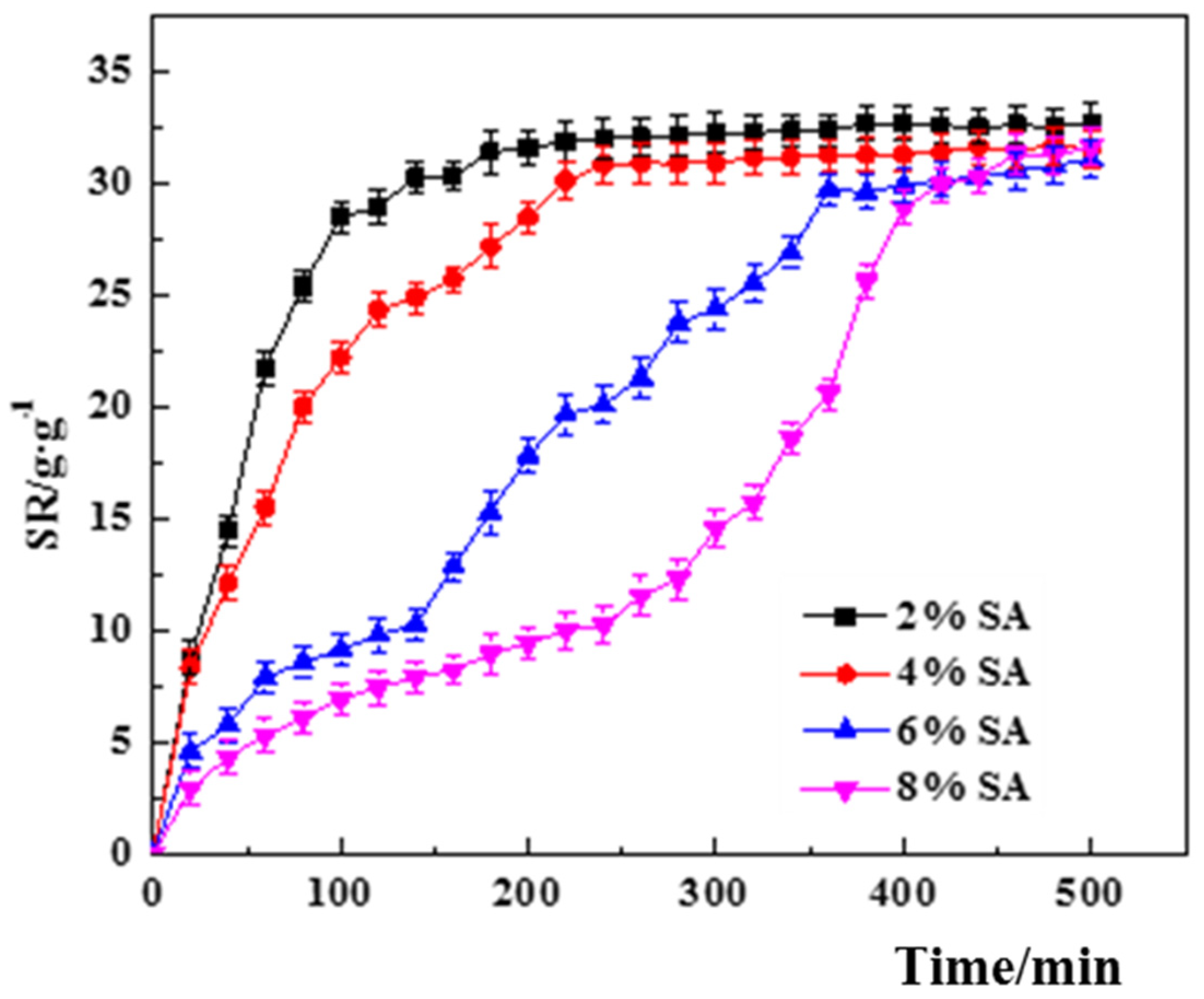
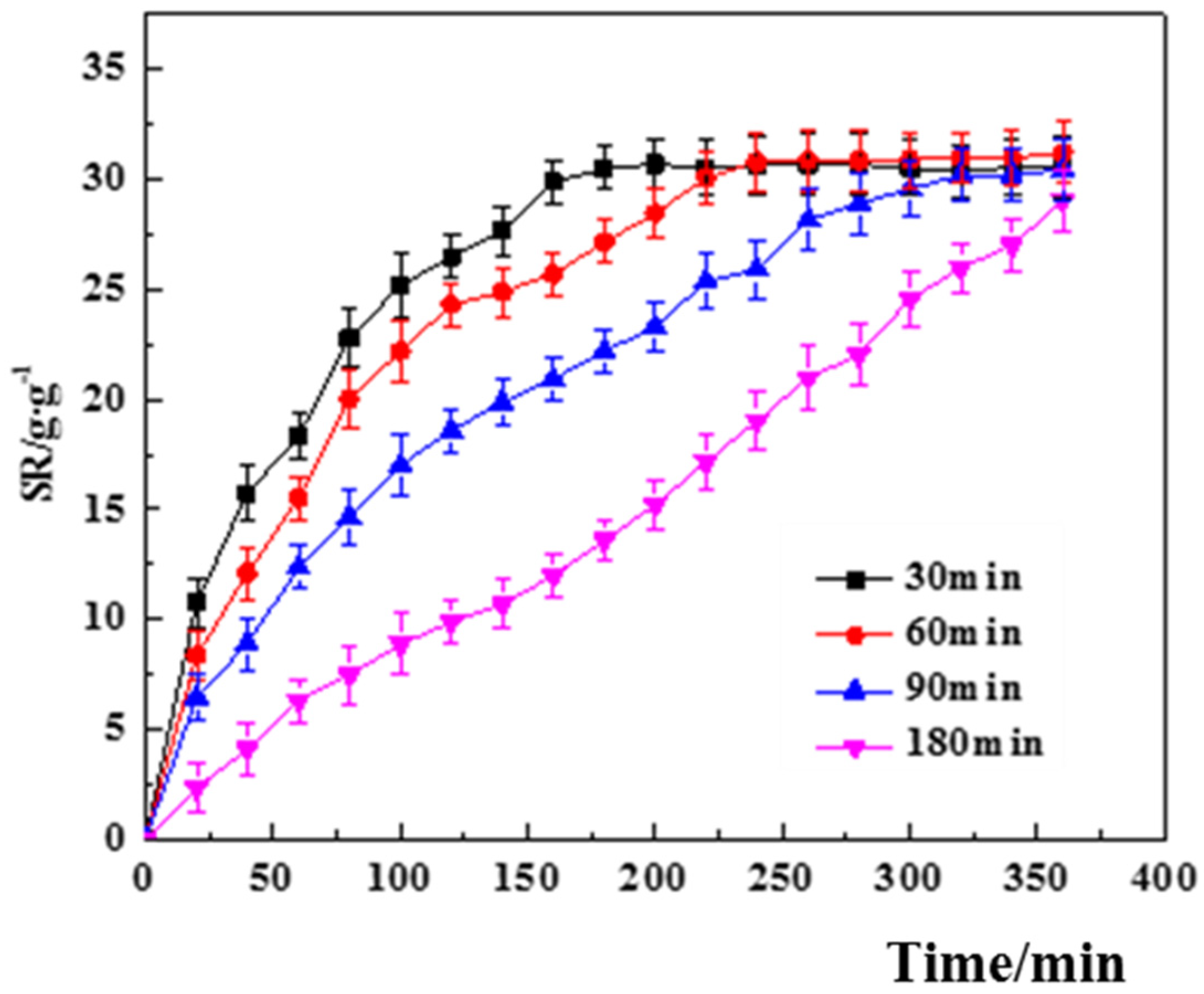
3.3.1. Single Factor Screening for Swelling Performance
- (1)
- The effect of SA concentration.
- (2)
- Effect of SA/Ca2+ cross-linking time.
- (3)
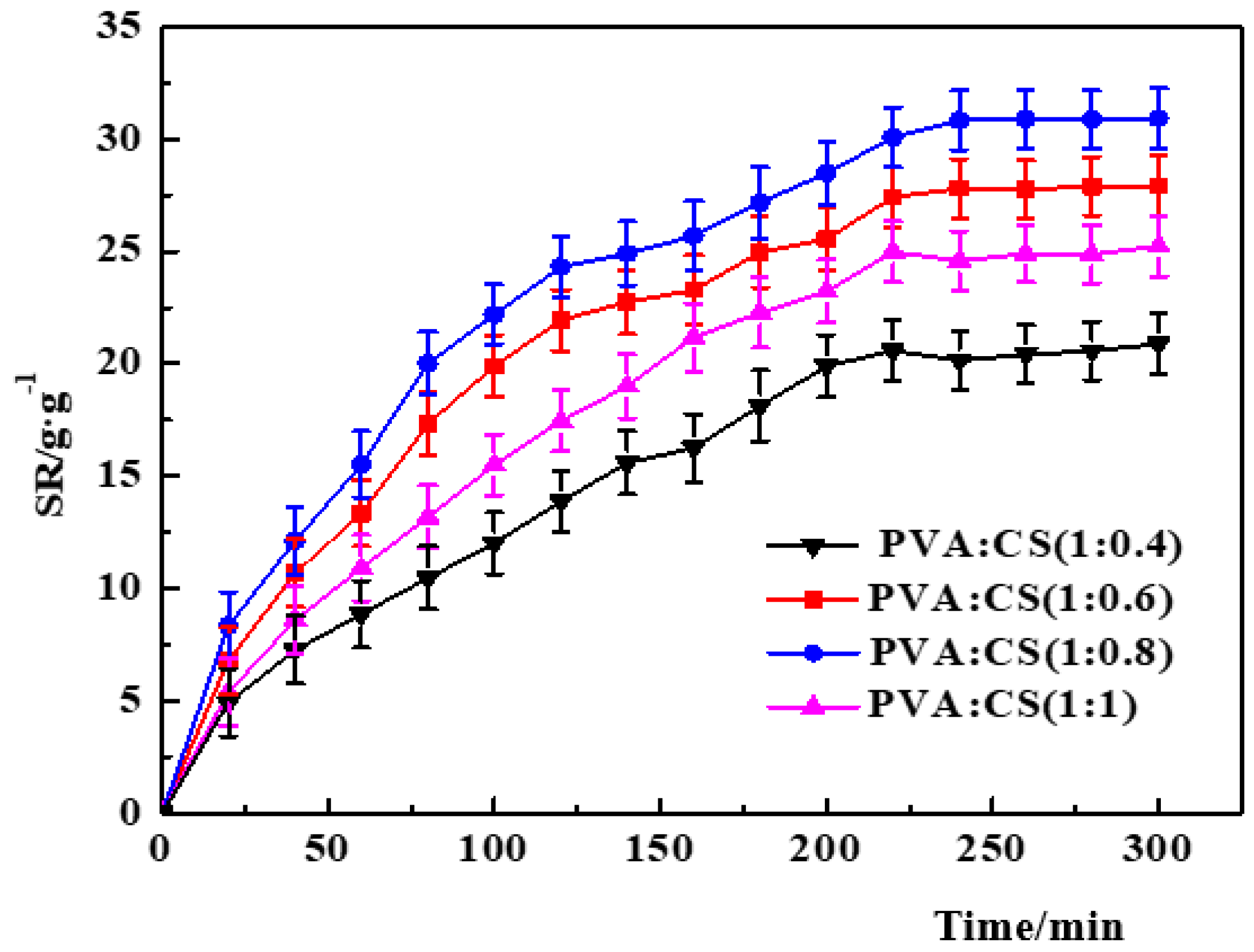
- Effect of PVA/CS ratio.
3.3.2. Response Surface Analysis
- (1)
- Response modeling.
- (2)
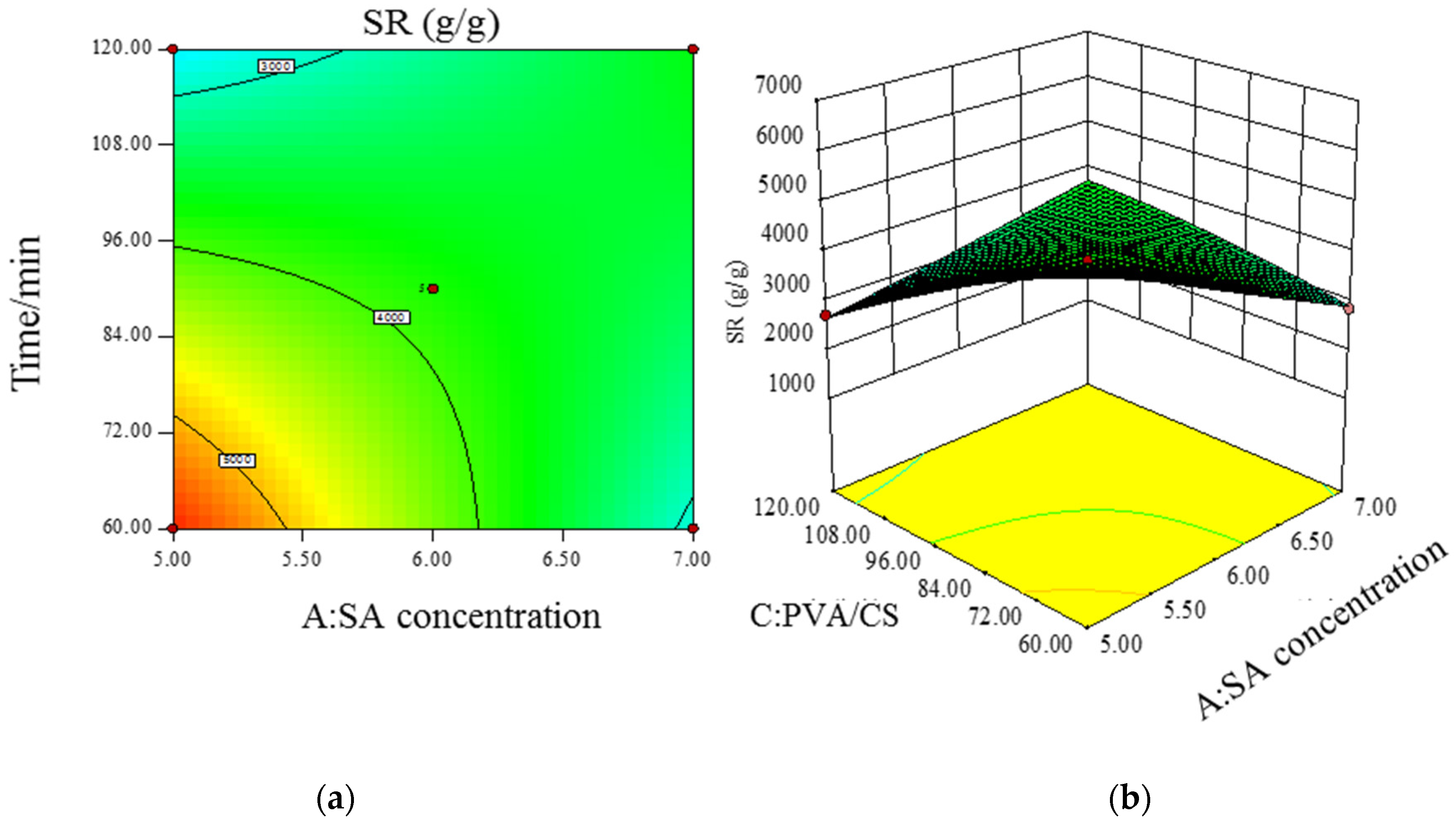
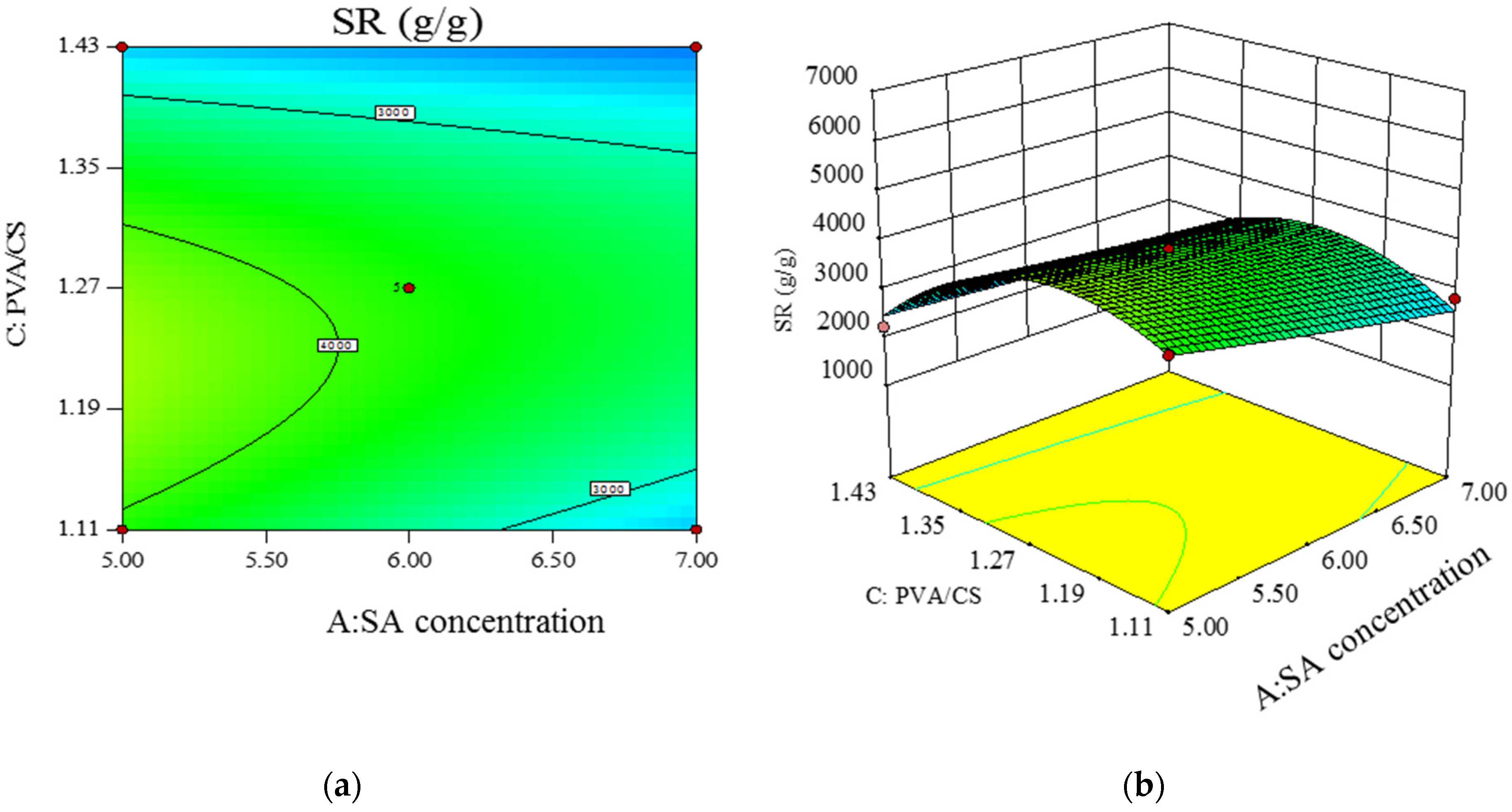
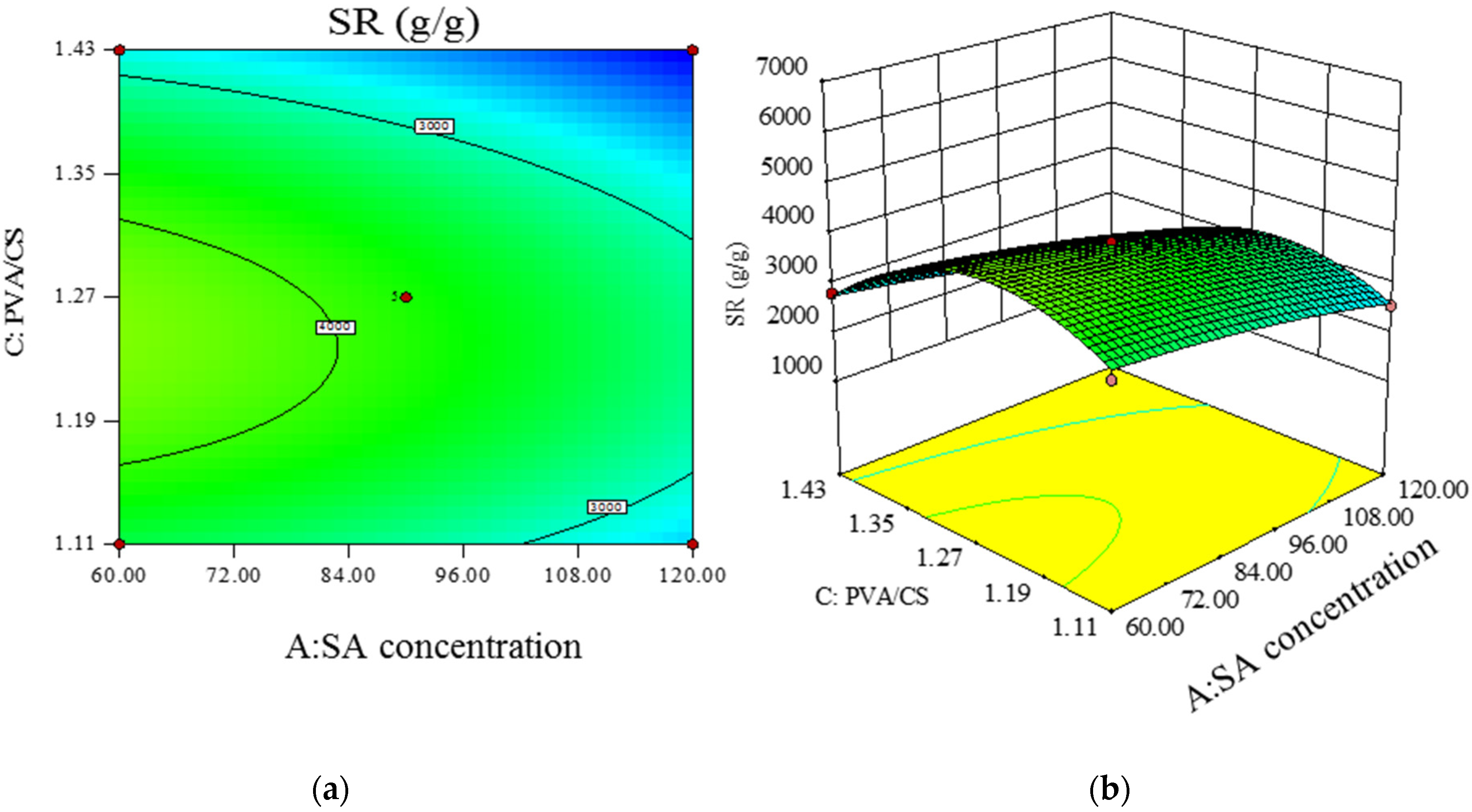
- Model significance and response surface analysis.
C + 0.237412 × A2 − 0.00146488 × B2 − 425.35 × C2
- (3)
- Prediction of optimal experimental conditions for swelling degree.
4. Discussions
4.1. Swelling Properties of Hydrogels
4.2. Dual Loading Function of PVA–CS/SA–Ca2+ Core–Shell Hydrogels
5. Conclusions
- (1)
- The PVA–CS/SA–Ca2+ hydrogels have obvious nucleation and shell structures as observed by SEM. The SA concentration and SA/Ca2+ cross-linking time are positively correlated with the thickness of the shell structure; the PVA/CS mass ratio affects the structural characteristics of the nucleation structure, and a higher CS content means a more obvious three-dimensional network of the hydrogels’ structure.
- (2)
- The optimal experimental conditions for the swelling of the core–shell hydrogels were obtained by single-factor screening and surface response analysis, which were an SA concentration of 5%, SA/Ca2+ cross-linking time of 90 min, PVA/CS mass ratio of 1:0.7 and maximum swelling of 50 g/g.
Author Contributions
Funding
Conflicts of Interest
References
- Xu, N.; Ma, N.; Yang, X.; Ling, G.; Yu, J.; Zhang, P. Preparation of intelligent DNA hydrogel and its applications in biosensing. Eur. Polym. J. 2020, 137, 109951. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Syuuhei, K. Fabrication of thermoresponsive degradable hydrogel made by radical polymerization of 2-methylene-1,3-dioxepane: Unique thermal coacervation in hydrogel. Polymer 2019, 179, 121633. [Google Scholar]
- Dixit, A.; Bag, D.S.; Kalra, S. Synthesis of strong and stretchable double network (DN) hydrogels of PVA-borax and P(AM-HEMA) and study of their swelling kinetics and mechanical properties. Polymer 2017, 119, 263–273. [Google Scholar] [CrossRef]
- Tao, G.; Wang, Y.; Cai, R.; Chang, H.; Song, K.; Zuo, H.; Zhao, P.; Xia, Q.; He, H. Design and performance of sericin/poly(vinyl alcohol) hydrogel as a drug delivery carrier for potential wound dressing application. Mater. Sci. Eng. 2019, 101, 341–351. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, S.; Zhu, T.; Tang, N.; Bai, X.; Zheng, C. Preparation of printable double-network hydrogels with rapid self-healing and high elasticity based on hyaluronic acid for controlled drug release. Polymer 2020, 186, 121994. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef]
- Qi, X.; Su, T.; Tong, X.; Xiong, W.; Zeng, Q.; Qian, Y.; Zhou, Z.; Wu, X.; Li, Z.; Shen, L.; et al. Facile formation of salecan/agarose hydrogels with tunable structural properties for cell culture. Carbohydr. Polym. 2019, 224, 115208. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Polat, T.G.; Duman, O.; Tun, S. Agar/κ-carrageenan/montmorillonite nanocomposite hydrogels for wound dressing applications. Int. J. Biol. Macromol. 2020, 164, 4591–4602. [Google Scholar] [CrossRef]
- Seo, J.W.; Moon, J.H.; Jang, G.; Jung, W.K.; Park, Y.H.; Park, K.T.; Bae, H. Cell-Laden Gelatin Methacryloyl Bioink for the Fabrication of Z-Stacked Hydrogel Scaffolds for Tissue Engineering. Polymers 2020, 12, 3027. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, R.; Chahal, G.K. Synthesis of lignin-based hydrogels and their applications in agriculture: A review. Chem. Pap. 2021, 75, 4465–4478. [Google Scholar] [CrossRef]
- Bojarczuk, K.; Karliński, L.; Hazubska-Przybył, T.; Kieliszewska-Rokicka, B. Influence of mycorrhizal inoculation on growth of micropropagated Populus×canescens lines in metal-contaminated soils. New For. 2015, 46, 195–215. [Google Scholar] [CrossRef][Green Version]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Hydrogel adsorbent in industrial wastewater treatment and ecological environment protection. Environ. Technol. Innov. 2020, 20, 101107. [Google Scholar] [CrossRef]
- Gajewski, P.; Lewandowska, A.; Szcześniak, K.; Przesławski, G.; Marcinkowska, A. Optimization of the Properties of Photocured Hydrogels for Use in Electrochemical Capacitors. Polymers 2021, 13, 3495. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, 6337. [Google Scholar] [CrossRef] [PubMed]
- Giweta, M.; Garedew, E. Hydrogel-A Promising Technology for Optimization of Nutrients and Water in Agricultural and Forest Ecosystems. Int. J. Environ. Sci. Nat. Resour. 2020, 23, 106–111. [Google Scholar]
- Alizadehgiashi, M.; Khuu, N.; Khabibullin, A.; Henry, A.; Tebbe, M.; Suzuki, T.; Kumacheva, E. Nanocolloidal hydrogel for heavy metal scavenging. ACS Nano 2018, 12, 8160–8168. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Xi, X.; Nie, S.; Ke, H.; Liu, Y.; Tong, S.; Chen, Z. A versatile TOCN/CGG self-assembling hydrogel for integrated wastewater treatment. Cellulose 2020, 27, 915–925. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Jia, F.; Gao, G. A flexible, adhesive and self-healable hydrogel-based wearable strain sensor for human motion and physiological signal monitoring. J. Mater. Chem. B 2019, 7, 4638–4648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liang, Y.; Ren, L.; Yu, Z.; Zhang, Z.; Ren, L. Bionic intelligent hydrogel actuators with multimodal deformation and locomotion. Nano Energy 2018, 51, 621–631. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Wang, X.; Xing, L.; Shi, L.; Ran, R. Stable, strain-sensitive conductive hydrogel with antifreezing capability, remoldability, and reusability. ACS Appl. Mater. Interfaces 2018, 10, 44000–44010. [Google Scholar] [CrossRef]
- Matsuda, T.; Kawakami, R.; Namba, R.; Nakajima, T.; Gong, J.P. Mechanoresponsive self-growing hydrogels inspired by muscle training. Science 2019, 363, 504–508. [Google Scholar] [CrossRef]
- Sharma, B.; Thakur, S.; Mamba, G.; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J. Environ. Chem. Eng. 2020, 9, 104608. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wan, Y.; Wen, Y.; Li, C.; Kanwal, A. A novel Poly (vinyl alcohol)/carboxymethyl cellulose/yeast double degradable hydrogel with yeast foaming and double degradable property. Ecotoxicol. Environ. Saf. 2020, 187, 109765. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhao, Y.; Sun, X.; Hu, B. Developing high strength, antiseptic and swelling-resistant polyvinyl alcohol/chitosan hydrogels for tissue engineering material. Mater. Lett. 2020, 280, 128499. [Google Scholar] [CrossRef]
- Qin, C.; Ou, K.; Dong, X.; Ji, X.; He, J. Preparation and properties of calcium alginate/polyvinyl alcohol hydrogel. J. Mater. Sci. Eng. 2018, 36, 739–744. [Google Scholar]
- Zhang, J.; Chen, D.; Liang, G.; Xu, W.; Tao, Z. Biosynthetic Polymalic Acid as a Delivery Nanoplatform for Translational Cancer Medicine. Trends Biochem. Sci. 2020, 46, 213–224. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F.; Chen, H.; Zheng, J.; Ma, J.; Chen, J. Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity. J. Mater. Chem. A 2016, 4, 10885–10892. [Google Scholar] [CrossRef]
- Arslan, H.; Pfaff, A.; Lu, Y.; Stepanek, P.; Müller, A.H. Stimuli-R esponsive Spherical Brushes Based on d-G alactopyranose and 2-(Dimethylamino) ethyl Methacrylate. Macromol. Biosci. 2014, 14, 81–91. [Google Scholar] [CrossRef] [PubMed]

| Sample | PVA/mL | CS/mL | SA/% | SA/Ca2+ Cross-Linking Time/min |
|---|---|---|---|---|
| 1 | 10 | 20 | 4 | 30 |
| 2 | 10 | 20 | 4 | 60 |
| 3 | 10 | 20 | 4 | 90 |
| 4 | 10 | 20 | 2 | 30 |
| 5 | 10 | 20 | 6 | 30 |
| 6 | 10 | 15 | 4 | 30 |
| 7 | 10 | 25 | 4 | 30 |
| Level | SA Concentration | SA/Ca2+ Cross-Linking Time | PVA/CS |
|---|---|---|---|
| 1 | 5 | 60 | 1:0.7 |
| 2 | 6 | 90 | 1:0.8 |
| 3 | 7 | 120 | 1:0.9 |
| Variation Source | Sum of Squares | Freedom | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1414.60 | 9 | 157.18 | 40.20 | <0.0001 | ** |
| A | 131.263 | 1 | 131.23 | 33.56 | 0.0007 | ** |
| B | 222.81 | 1 | 222.81 | 56.98 | 0.0001 | ** |
| C | 166.01 | 1 | 166.01 | 42.45 | 0.0003 | ** |
| AB | 352.96 | 1 | 352.96 | 0.40 | 0.5460 | |
| AC | 24.86 | 1 | 24.86 | 6.36 | 0.0397 | * |
| BC | 1.57 | 1 | 1.57 | 90.26 | <0.0001 | ** |
| A2 | 0.24 | 1 | 0.24 | 0.061 | 0.8125 | |
| B2 | 7.32 | 1 | 7.32 | 1.87 | 0.2136 | |
| C2 | 499.24 | 1 | 499.24 | 127.67 | <0.0001 | ** |
| Residual | 27.37 | 7 | 3.91 | |||
| Spurious term | 27.37 | 3 | 9.12 | |||
| Pure error | 0.00 | 4 | 0.00 | |||
| Total value | 1441.98 | 16 | ||||
| Model determination coefficient | R2 = 0.9810 | |||||
| Model adjustment determination Coefficient | R2adj = 0.9566 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wan, Y.; Yuan, W.; Zhang, Y.; Zhou, Z.; Zhang, M.; Wang, L.; Wang, R. Preparation of PVA–CS/SA–Ca2+ Hydrogel with Core–Shell Structure. Polymers 2022, 14, 212. https://doi.org/10.3390/polym14010212
Zhang S, Wan Y, Yuan W, Zhang Y, Zhou Z, Zhang M, Wang L, Wang R. Preparation of PVA–CS/SA–Ca2+ Hydrogel with Core–Shell Structure. Polymers. 2022; 14(1):212. https://doi.org/10.3390/polym14010212
Chicago/Turabian StyleZhang, Shuai, Yu Wan, Weijie Yuan, Yaoxiang Zhang, Ziyuan Zhou, Min Zhang, Luzhen Wang, and Ran Wang. 2022. "Preparation of PVA–CS/SA–Ca2+ Hydrogel with Core–Shell Structure" Polymers 14, no. 1: 212. https://doi.org/10.3390/polym14010212
APA StyleZhang, S., Wan, Y., Yuan, W., Zhang, Y., Zhou, Z., Zhang, M., Wang, L., & Wang, R. (2022). Preparation of PVA–CS/SA–Ca2+ Hydrogel with Core–Shell Structure. Polymers, 14(1), 212. https://doi.org/10.3390/polym14010212





