Enhanced Performance of Cyclopentadithiophene-Based Donor-Acceptor-Type Semiconducting Copolymer Transistors Obtained by a Wire Bar-Coating Method
Abstract
:1. Introduction
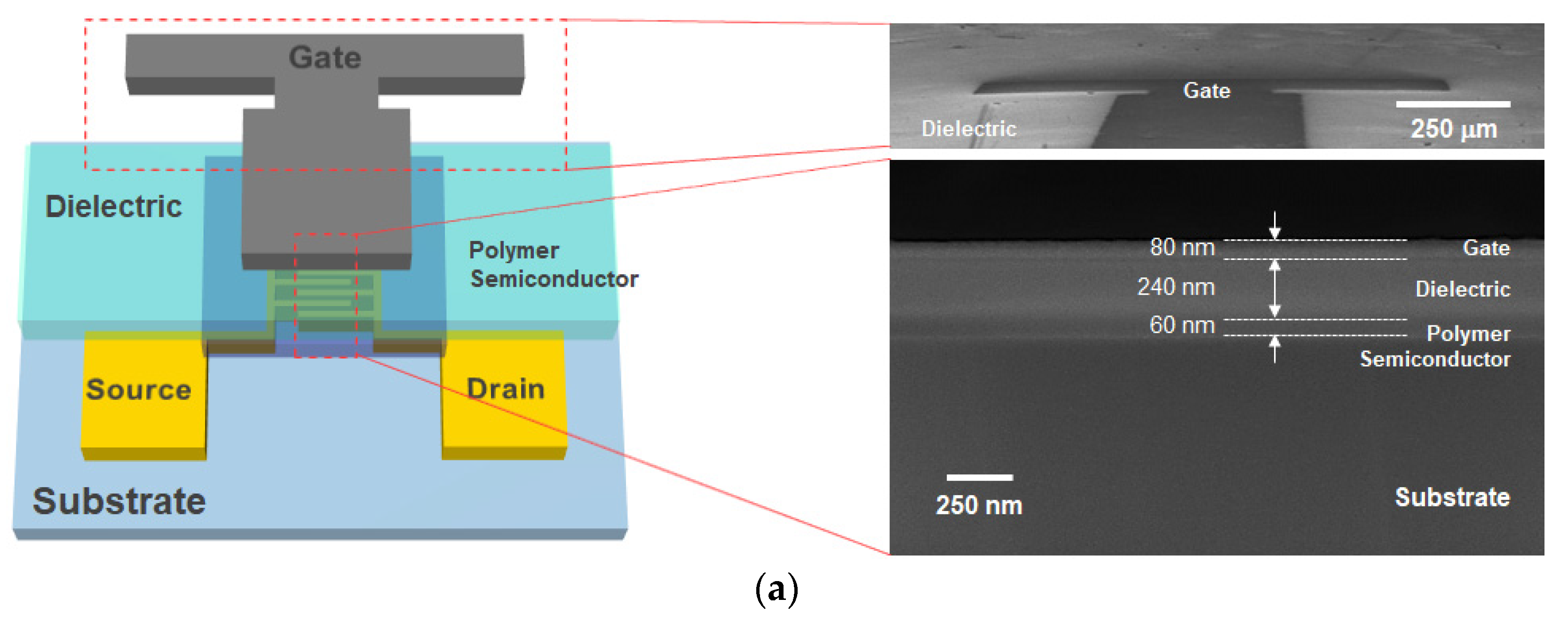
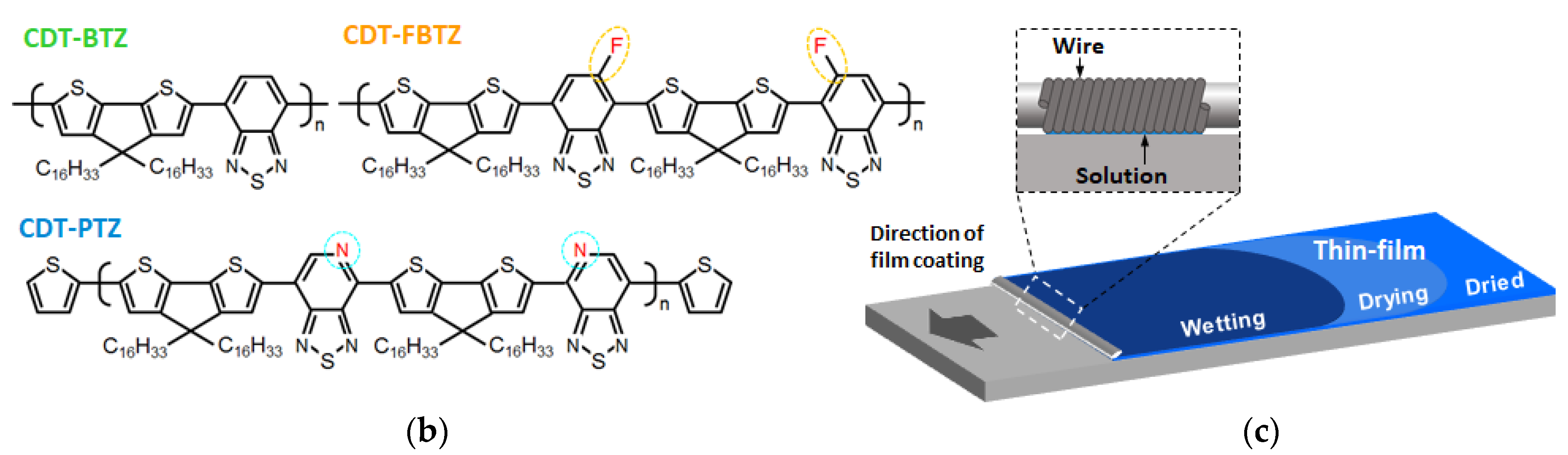
2. Experimental Methods
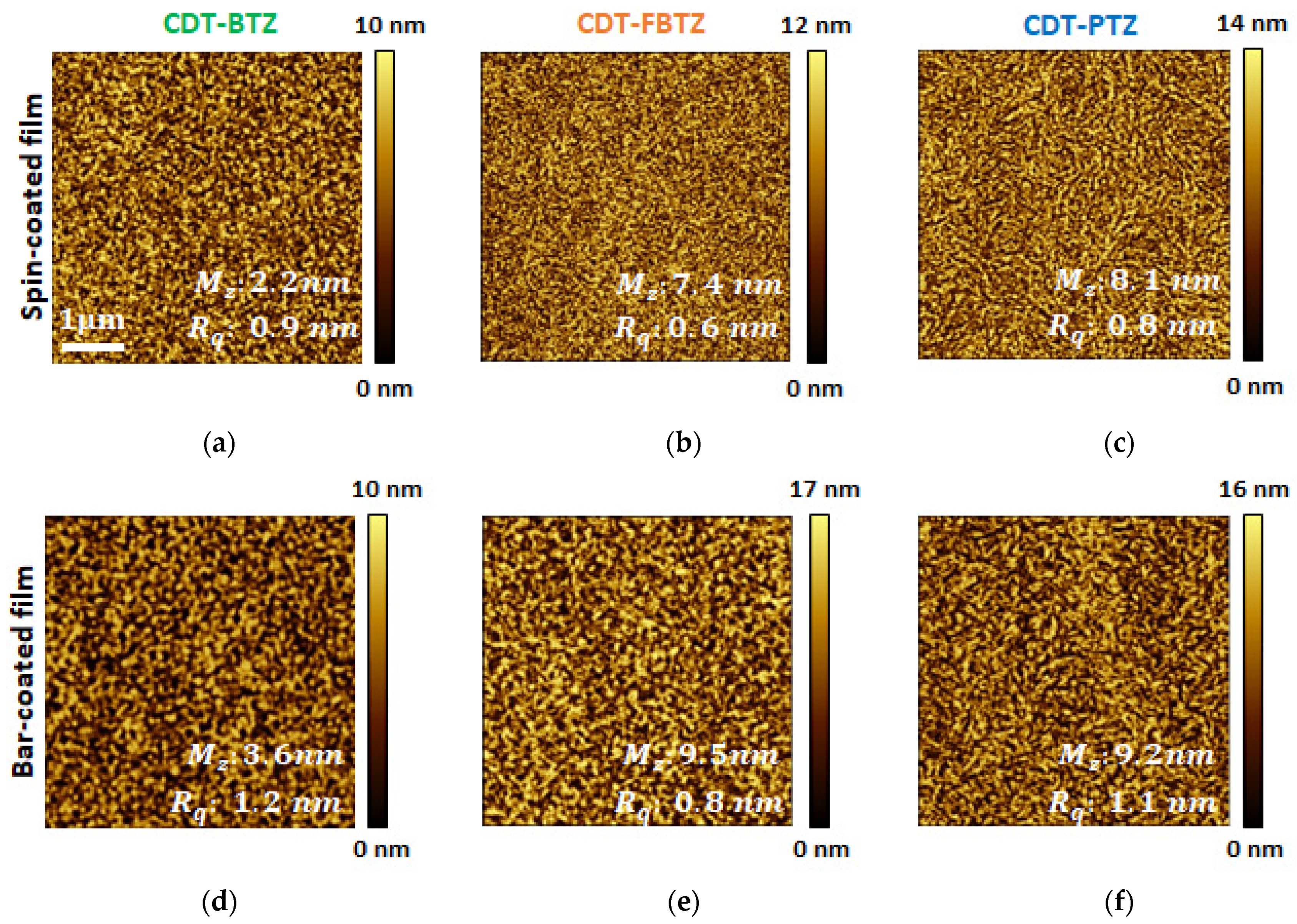
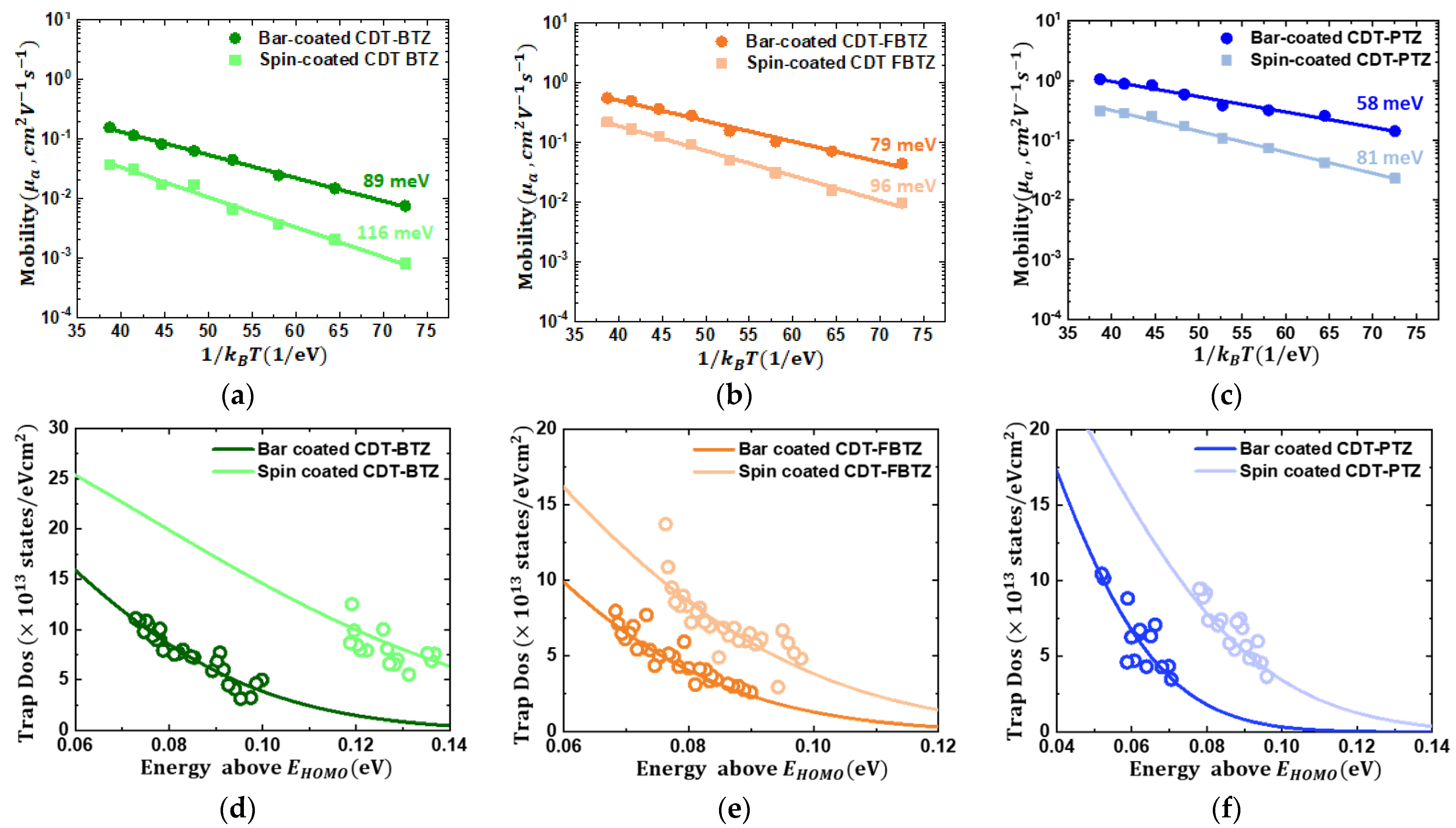
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wadsworth, A.; Chen, H.; Thorley, K.J.; Cendra, C.; Nikolka, M.; Bristow, H.; Moser, M.; Salleo, A.; Anthopoulos, T.D.; Sirringhaus, H.; et al. Modification of indacenodithiophene-based polymers and its impact on charge carrier mobility in organic thin-film transistors. J. Am. Chem. Soc. 2020, 142, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, H.; Angunawela, I.; Zhou, J.; Du, J.; Liebman-Pelaez, A.; Zhu, C.; Zhang, Z.; Meng, L.; Xie, Z.; et al. Effects of short-axis Alkoxy substituents on molecular self-assembly and photovoltaic performance of indacenodithiophene-based acceptors. Adv. Funct. Mater. 2020, 30, 1906855. [Google Scholar] [CrossRef]
- Zhifeng, D.; Taotao, A.; Rui, L.; Wei, Y.; Kaili, Z.; Huiling, D.; Haichang, Z. Conjugated polymers containing building blocks 1,3,4,6-Tetraarylpyrrolo[3,2-b]pyrrole-2,5-dione (isoDPP), benzodipyrrolidone (BDP) or naphthodipyrrolidone (NDP): A review. Polymers 2019, 11, 1683. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Guo, Y.; Yu, G.; Zhao, Y.; Zhang, J.; Gao, D.; Liu, H.; Liu, Y. Highly π-extended copolymers with diketopyrrolopyrrole moieties for high-performance field-effect transistors. Adv. Mater. 2012, 24, 4618–4622. [Google Scholar] [CrossRef]
- Estrada, L.A.; Stalder, R.; Abboud, K.A.; Risko, C.; Brédas, J.L.; Reynolds, J.R. Understanding the electronic structure of isoindigo in conjugated systems: A combined theoretical and experimental approach. Macromolecules 2013, 46, 8832–8844. [Google Scholar] [CrossRef]
- Deng, P.; Zhang, Q. Recent developments on isoindigo-based conjugated polymers. Polym. Chem. 2014, 5, 3298–3305. [Google Scholar] [CrossRef]
- Szumilo, M.M.; Gann, E.H.; Mcneill, C.R.; Lemaur, V.; Oliver, Y.; Thomsen, L.; Vaynzof, Y.; Sommer, M.; Sirringhaus, H. Structure influence on charge transport in naphthalenediimide−thiophene copolymers. Chem. Mater. 2014, 26, 6796–6804. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Y.; Wang, C.; Ma, J.; Wu, F.; Zhang, Y.; Ba, X. Synthesis and characterization of fully conjugated ladder naphthalene bisimide copolymers. Polymers 2018, 10, 790. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, D.; Giovannitti, A.; Sun, H.; Biskup, T.; Hofmann, A.; Koopmans, M.; Cendra, C.; Weber, S.; Anton Koster, L.J.; Olsson, E.; et al. Enhanced n-doping efficiency of a naphthalenediimide-based copolymer through polar side chains for organic thermoelectrics. ACS Energy Lett. 2018, 3, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedzialek, D.; Lemaur, V.; Dudenko, D.; Shu, J.; Hansen, M.R.; Andreasen, J.W.; Pisula, W.; Müllen, K.; Cornil, J.; Beljonne, D. Probing the relation between charge transport and supramolecular organization down to Ångström resolution in a benzothiadiazole- cyclopentadithiophene copolymer. Adv. Mater. 2013, 25, 1939–1947. [Google Scholar] [CrossRef]
- Um, J.G.; Habibpour, S.; Jun, Y.S.; Elkamel, A.; Yu, A. Development of π-π interaction-induced functionalized graphene oxide on mechanical and anticorrosive properties of reinforced polyurethane composites. Ind. Eng. Chem. Res. 2020, 59, 3617–3628. [Google Scholar] [CrossRef]
- Horie, M.; Majewski, L.A.; Fearn, M.J.; Yu, C.Y.; Luo, Y.; Song, A.; Saunders, B.R.; Turner, M.L. Cyclopentadithiophene based polymers—A comparison of optical, electrochemical and organic field-effect transistor characteristics. J. Mater. Chem. 2010, 20, 4347–4355. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.; Sheng, P.; Zhao, G.; Zhu, D. Organic donor-acceptor complexes as novel organic semiconductors. Acc. Chem. Res. 2017, 50, 1654–1662. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, R.A. An investigation on single crystal growth, structural, thermal and optical properties of a series of organic D-π-a push-pull materials. RSC Adv. 2015, 5, 38591–38600. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, J.; Guo, Y.; Han, G.; Yi, Y. Regulation of molecular orientations of A-D-A nonfullerene acceptors for organic photovoltaics: The role of end-group π-π stacking. Adv. Funct. Mater. 2021, 2108551. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Zhang, M.; Wang, P. Theoretical investigation of the donor group related electronic structure properties in push-pull organic sensitizers. RSC Adv. 2013, 3, 6030–6035. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, G.; Zhang, D. Modification of side chains of conjugated molecules and polymers for charge mobility enhancement and sensing functionality. Acc. Chem. Res. 2018, 51, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Niskala, J.R.; Unruh, D.A.; Chu, C.K.; Lee, O.P.; Fréchet, J.M.J. Control of polymer-packing orientation in thin films through synthetic tailoring of backbone coplanarity. Chem. Mater. 2013, 25, 4088–4096. [Google Scholar] [CrossRef]
- Mukherjee, T.; Sinha, S.; Mukherjee, M. Electronic structure of twisted and planar rubrene molecules: A density functional study. Phys. Chem. Chem. Phys. 2018, 20, 18623–18629. [Google Scholar] [CrossRef] [PubMed]
- Khim, D.; Han, H.; Baeg, K.J.; Kim, J.; Kwak, S.W.; Kim, D.Y.; Noh, Y.Y. Simple bar-coating process for large-area, high-performance organic field-effect transistors and ambipolar complementary integrated circuits. Adv. Mater. 2013, 25, 4302–4308. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kang, B.; Kim, D.; Park, C.; Kim, S.; Lee, M.; Lee, W.B.; Cho, K. Motion-programmed bar-coating method with controlled gap for high-speed scalable preparation of highly crystalline organic semiconductor thin films. ACS Appl. Mater. Interfaces 2019, 11, 47153–47161. [Google Scholar] [CrossRef]
- Pang, B.; Tang, Z.; Li, Y.; Meng, H.; Xiang, Y.; Li, Y.; Huang, J. Synthesis of conjugated polymers containing B N bonds with strong electron affinity and extended absorption. Polymers 2019, 11, 1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Peng, B.; Ji, X.; Pei, K.; Chan, P.K.L. Marangoni-effect-assisted bar-coating method for high-quality organic crystals with compressive and tensile strains. Adv. Funct. Mater. 2017, 27, 1703443. [Google Scholar] [CrossRef]
- Kanimozhi, C.; Naik, M.; Yaacobi-Gross, N.; Burnett, E.K.; Briseno, A.L.; Anthopoulos, T.D.; Patil, S. Controlling conformations of diketopyrrolopyrrole-based conjugated polymers: Role of torsional angle. J. Phys. Chem. C 2014, 118, 11536–11544. [Google Scholar] [CrossRef]
- Kim, N.K.; Jang, S.Y.; Pace, G.; Caironi, M.; Park, W.T.; Khim, D.; Kim, J.; Kim, D.Y.; Noh, Y.Y. High-performance organic field-effect transistors with directionally aligned conjugated polymer film deposited from pre-aggregated solution. Chem. Mater. 2015, 27, 8345–8353. [Google Scholar] [CrossRef]
- Bencheikh, F.; Duché, D.; Ruiz, C.M.; Simon, J.J.; Escoubas, L. Study of optical properties and molecular aggregation of conjugated low band gap copolymers: PTB7 and PTB7-Th. J. Phys. Chem. C 2015, 119, 24643–24648. [Google Scholar] [CrossRef]
- Kamatham, N.; Ibraikulov, O.A.; Durand, P.; Wang, J.; Boyron, O.; Heinrich, B.; Heiser, T.; Lévêque, P.; Leclerc, N.; Méry, S. On the impact of linear siloxanated side chains on the molecular self-assembling and charge transport properties of conjugated polymers. Adv. Funct. Mater. 2021, 31, 2007734. [Google Scholar] [CrossRef]
- Schwarze, M.; Gaul, C.; Scholz, R.; Bussolotti, F.; Hofacker, A.; Schellhammer, K.S.; Nell, B.; Naab, B.D.; Bao, Z.; Spoltore, D.; et al. Molecular parameters responsible for thermally activated transport in doped organic semiconductors. Nat. Mater. 2019, 18, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Fratini, S.; Ciuchi, S. Bandlike motion and mobility saturation in organic molecular semiconductors. Phys. Rev. Lett. 2009, 103, 266601. [Google Scholar] [CrossRef] [Green Version]
- Lee, J. Physical modeling of charge transport in conjugated polymer field-effect transistors. J. Phys. D Appl. Phys. 2021, 54, 3255. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the arrhenius equation. J. Chem. Educ. 1984, 61, 494–498. [Google Scholar] [CrossRef]
- Kalb, W.L.; Batlogg, B. Calculating the trap density of states in organic field-effect transistors from experiment: A comparison of different methods. Phys. Rev. B 2010, 81, 035327. [Google Scholar] [CrossRef] [Green Version]
- Lang, D.V.; Chi, X.; Siegrist, T.; Sergent, A.M.; Ramirez, A.P. Amorphouslike density of gap states in single-crystal pentacene. Phys. Rev. Lett. 2004, 93, 8–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirringhaus, H. Device physics of solution-processed organic field-effect transistors. Adv. Mater. 2005, 17, 2411–2425. [Google Scholar] [CrossRef]
- Park, K.S.; Kwok, J.J.; Dilmurat, R.; Qu, G.; Kafle, P.; Luo, X.; Jung, S.H.; Olivier, Y.; Lee, J.K.; Mei, J.; et al. Tuning conformation, assembly, and charge transport properties of conjugated polymers by printing flow. Sci. Adv. 2019, 5, eaaw7757. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Coating Method | Device Parameter | DOS Parameter | |||||
|---|---|---|---|---|---|---|---|
| gm (×10−6 S) | mlin (cm2 V−1 s−1) | Vth (V) | EA (meV) | N (cm−2) | σ (meV) | ||
| CDT-BTZ | Bar-coating | 3.1 | 0.16 | −25 | 89 | 4.2 × 1013 | 95 |
| Spin-coating | 0.6 | 0.04 | −33 | 116 | 6.6 × 1013 | 153 | |
| CDT-FBTZ | Bar-coating | 4.9 | 0.28 | −28 | 79 | 3.1 × 1013 | 79 |
| Spin-coating | 3 | 0.16 | −32 | 96 | 4.3 × 1013 | 94 | |
| CDT-PTZ | Bar-coating | 17 | 0.95 | −30 | 58 | 3.0 × 1013 | 65 |
| Spin-coating | 6.2 | 0.31 | −33 | 81 | 4.0 × 1013 | 93 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Yoon, M.; Lee, J. Enhanced Performance of Cyclopentadithiophene-Based Donor-Acceptor-Type Semiconducting Copolymer Transistors Obtained by a Wire Bar-Coating Method. Polymers 2022, 14, 2. https://doi.org/10.3390/polym14010002
Kim D, Yoon M, Lee J. Enhanced Performance of Cyclopentadithiophene-Based Donor-Acceptor-Type Semiconducting Copolymer Transistors Obtained by a Wire Bar-Coating Method. Polymers. 2022; 14(1):2. https://doi.org/10.3390/polym14010002
Chicago/Turabian StyleKim, Doyeon, Minho Yoon, and Jiyoul Lee. 2022. "Enhanced Performance of Cyclopentadithiophene-Based Donor-Acceptor-Type Semiconducting Copolymer Transistors Obtained by a Wire Bar-Coating Method" Polymers 14, no. 1: 2. https://doi.org/10.3390/polym14010002
APA StyleKim, D., Yoon, M., & Lee, J. (2022). Enhanced Performance of Cyclopentadithiophene-Based Donor-Acceptor-Type Semiconducting Copolymer Transistors Obtained by a Wire Bar-Coating Method. Polymers, 14(1), 2. https://doi.org/10.3390/polym14010002







