Material Analysis and a Visual Guide of Degradation Phenomena in Historical Synthetic Polymers as Tools to Follow Ageing Processes in Industrial Heritage Collections
Abstract
:1. Introduction
1.1. Describing Damage in Plastics: State of the Art
1.2. Relevance, Occurrence and Identification of Rubbers in Heritage Collections
1.3. The Analysis of Degradation Phenomena on Historical Polymers: Nature and Occurrence of Blooming in Polymeric Materials
1.4. Aim of This Work
2. Materials and Methods
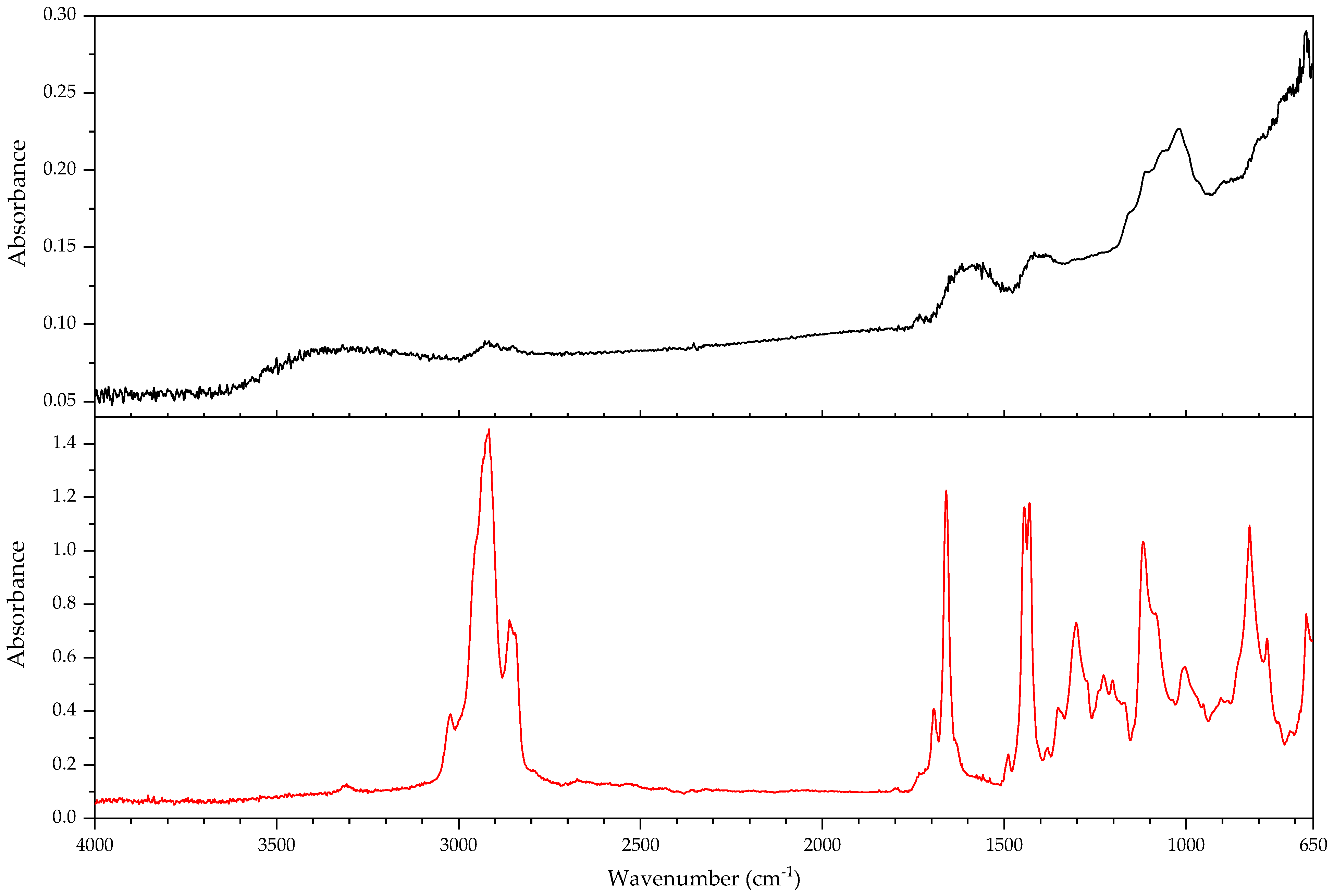
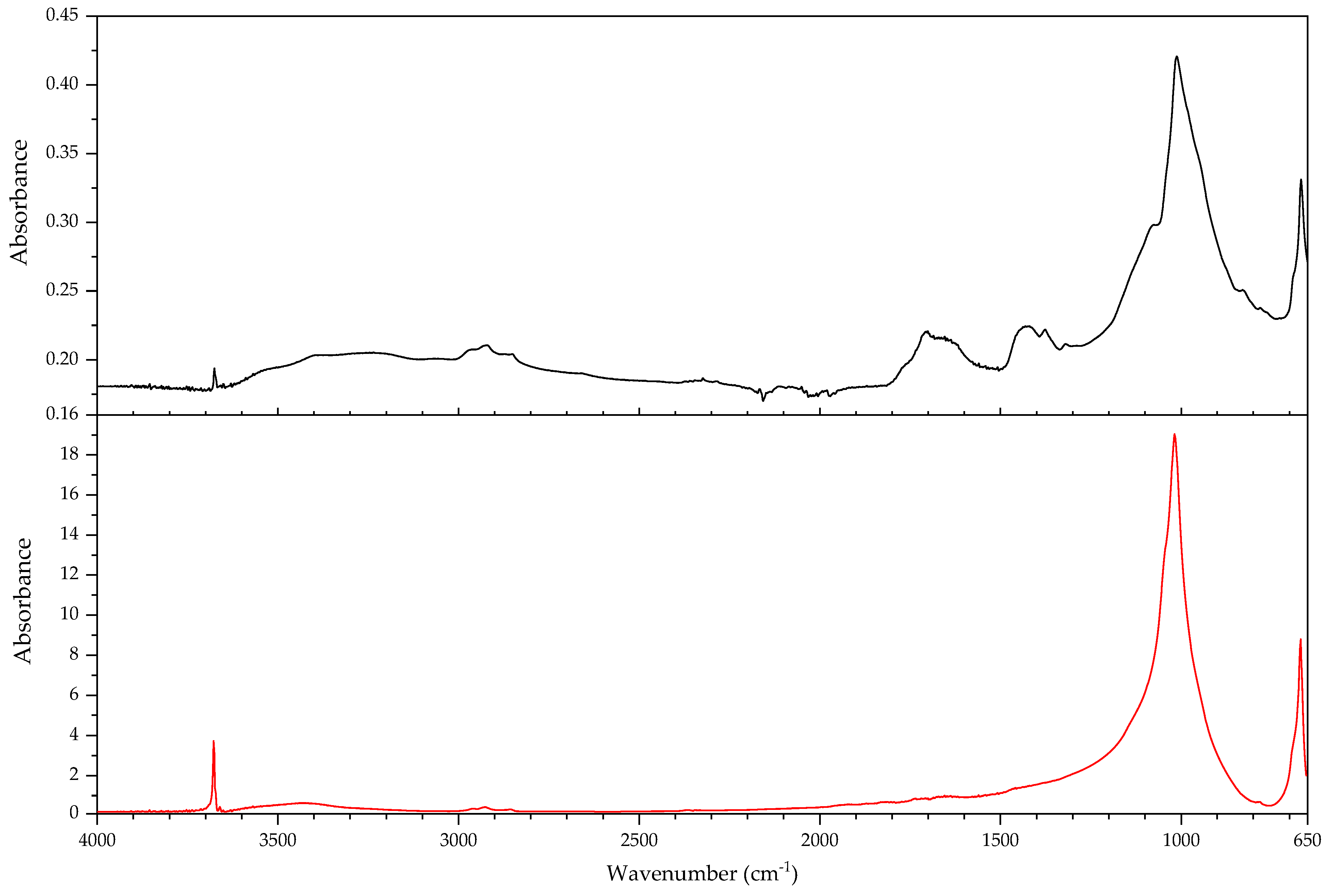
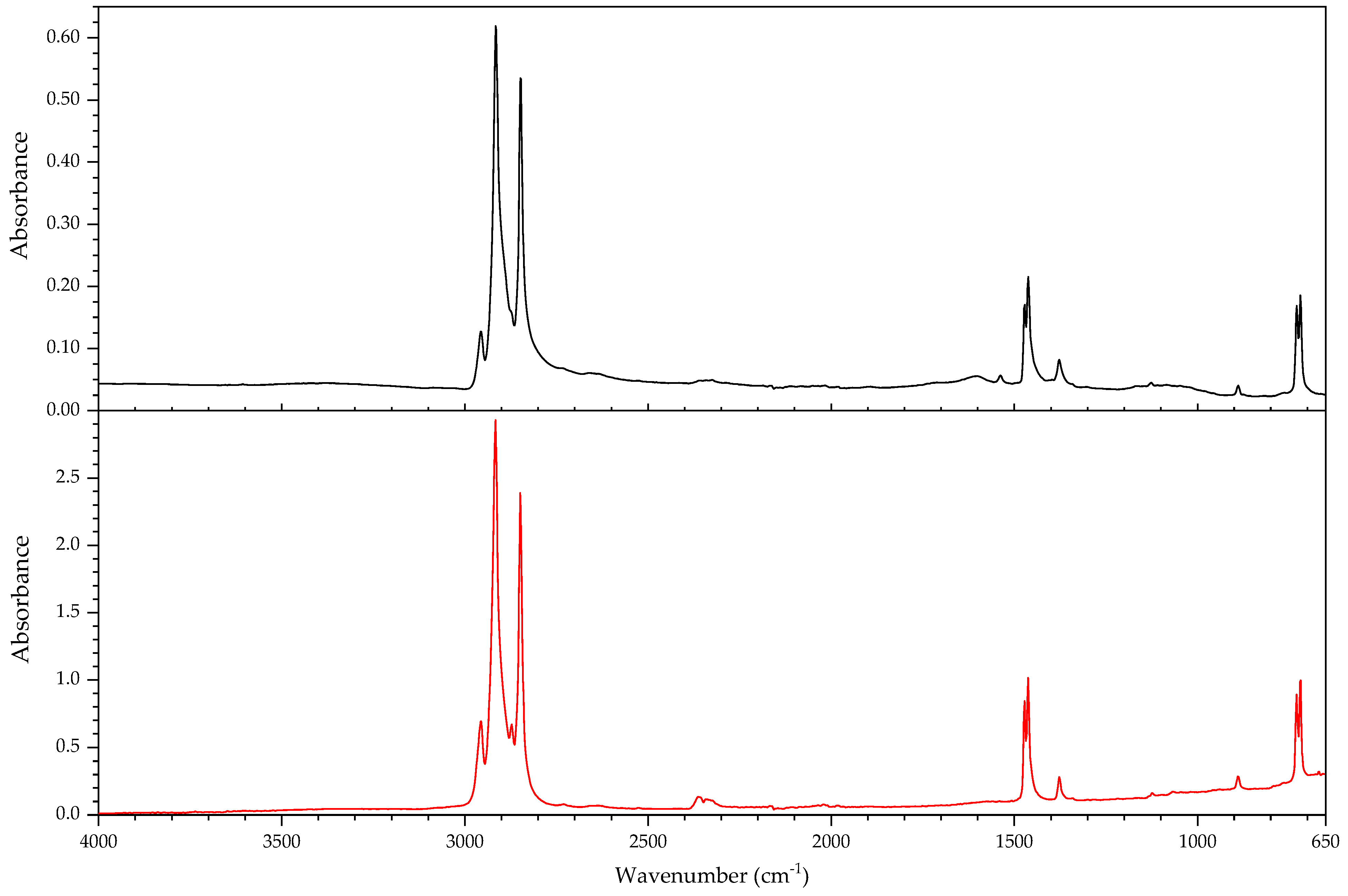
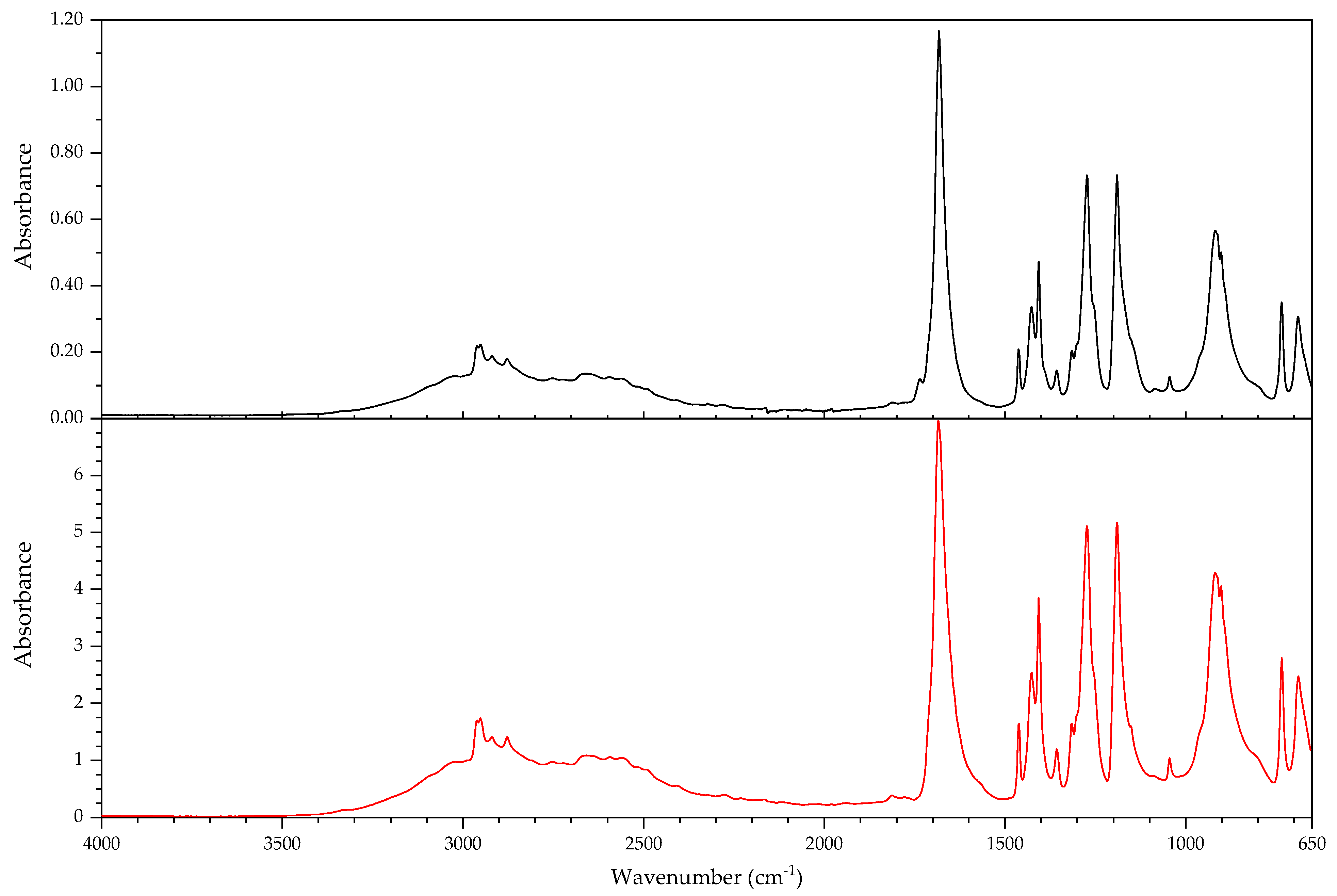
2.1. Infrared Spectroscopy
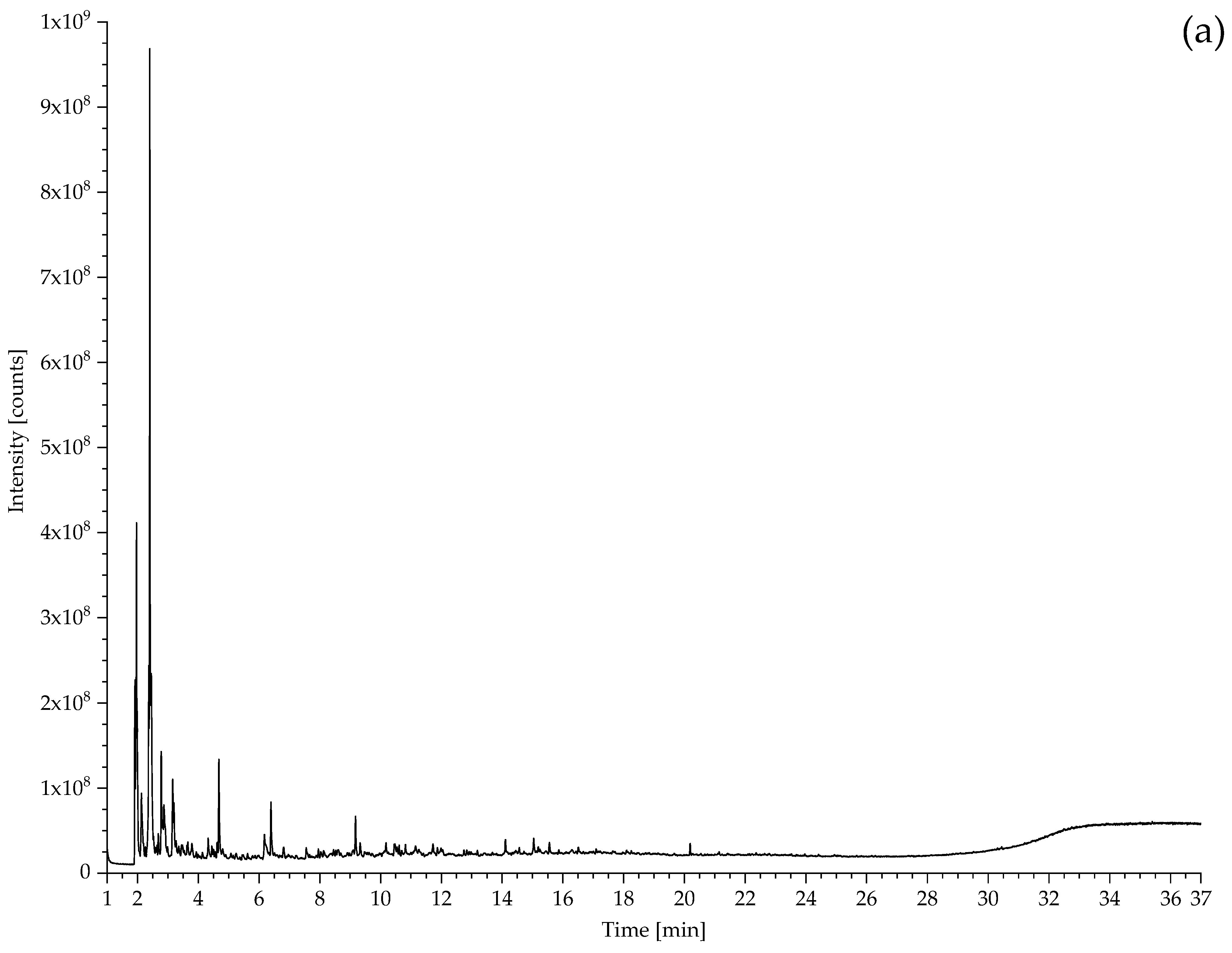
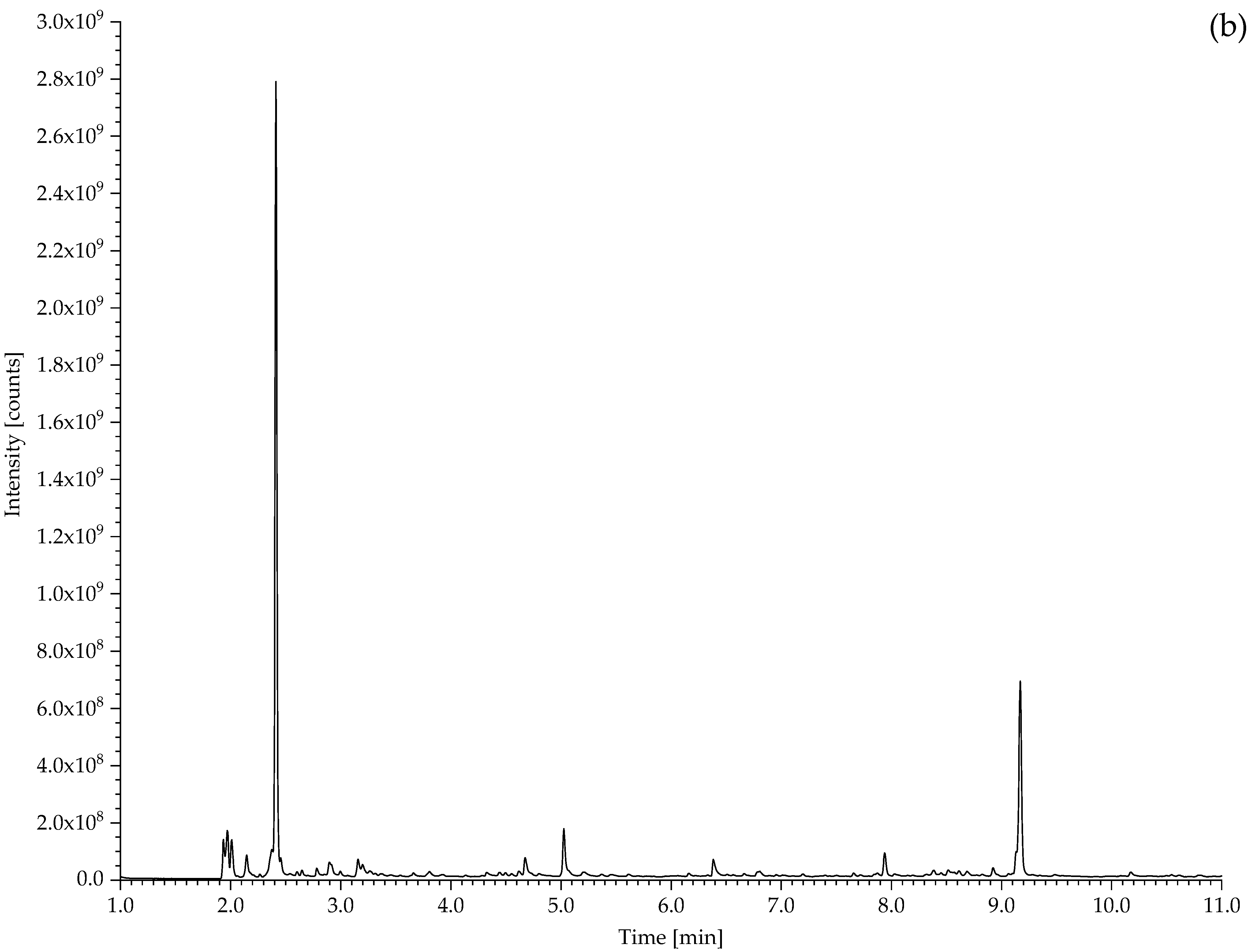
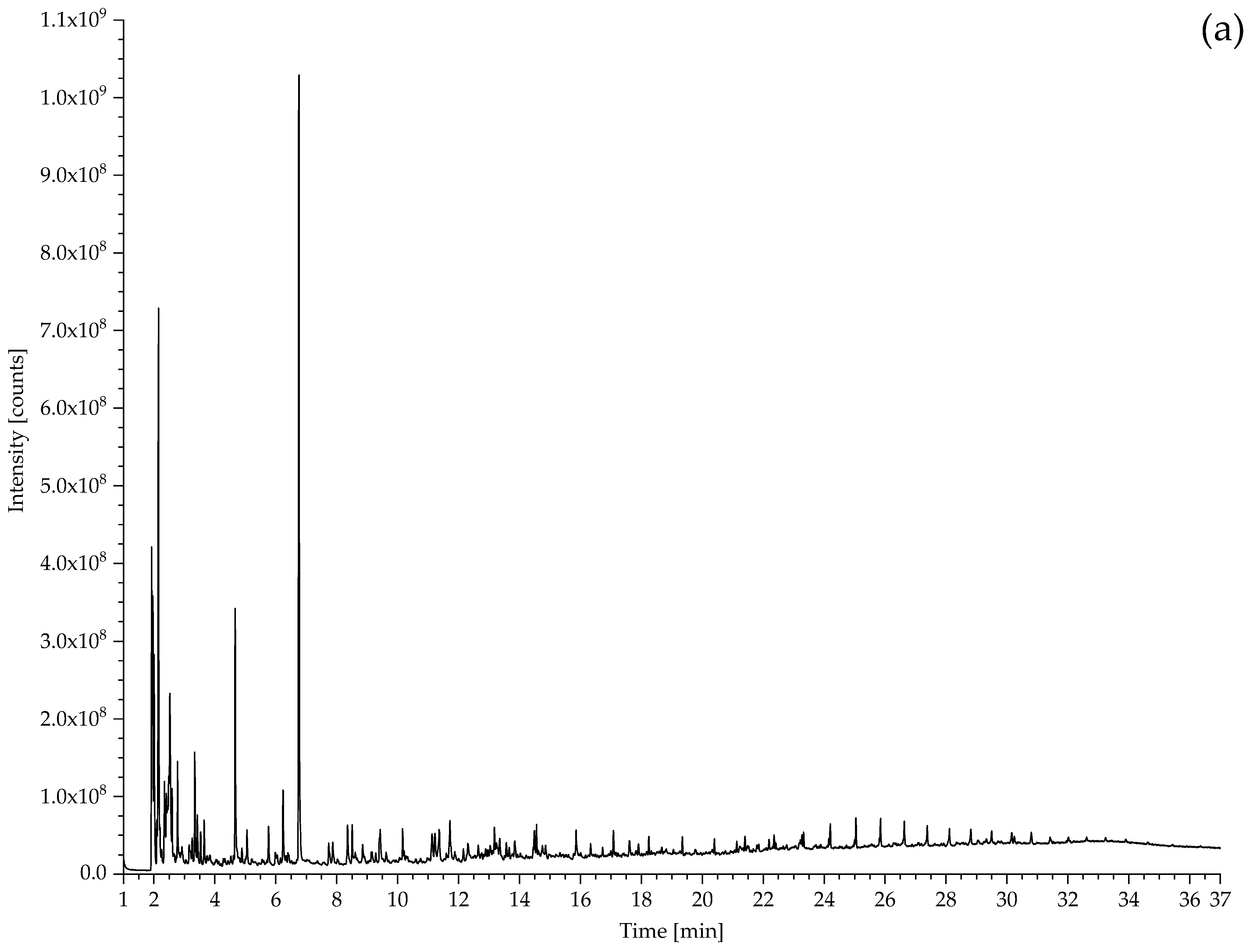
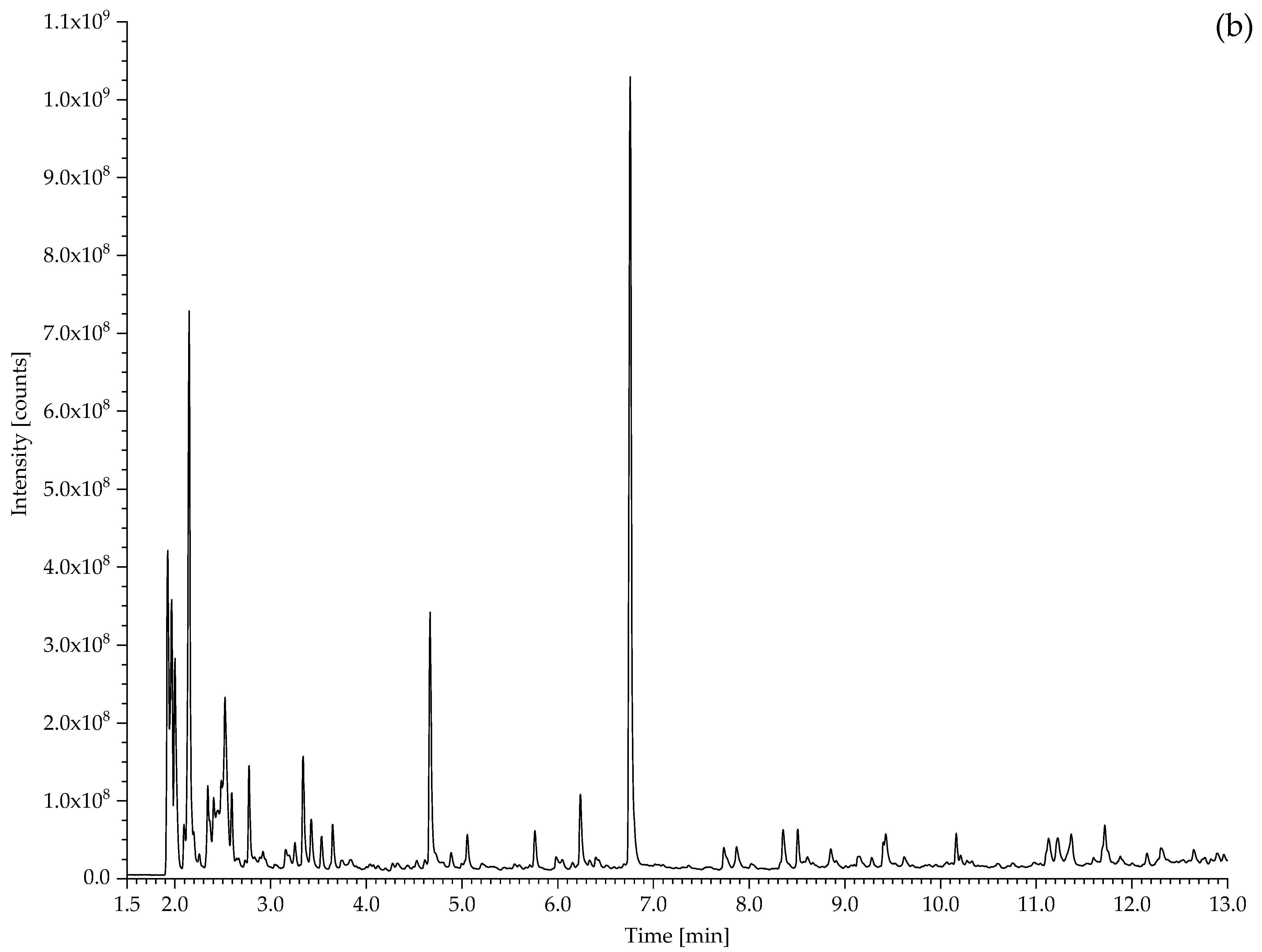
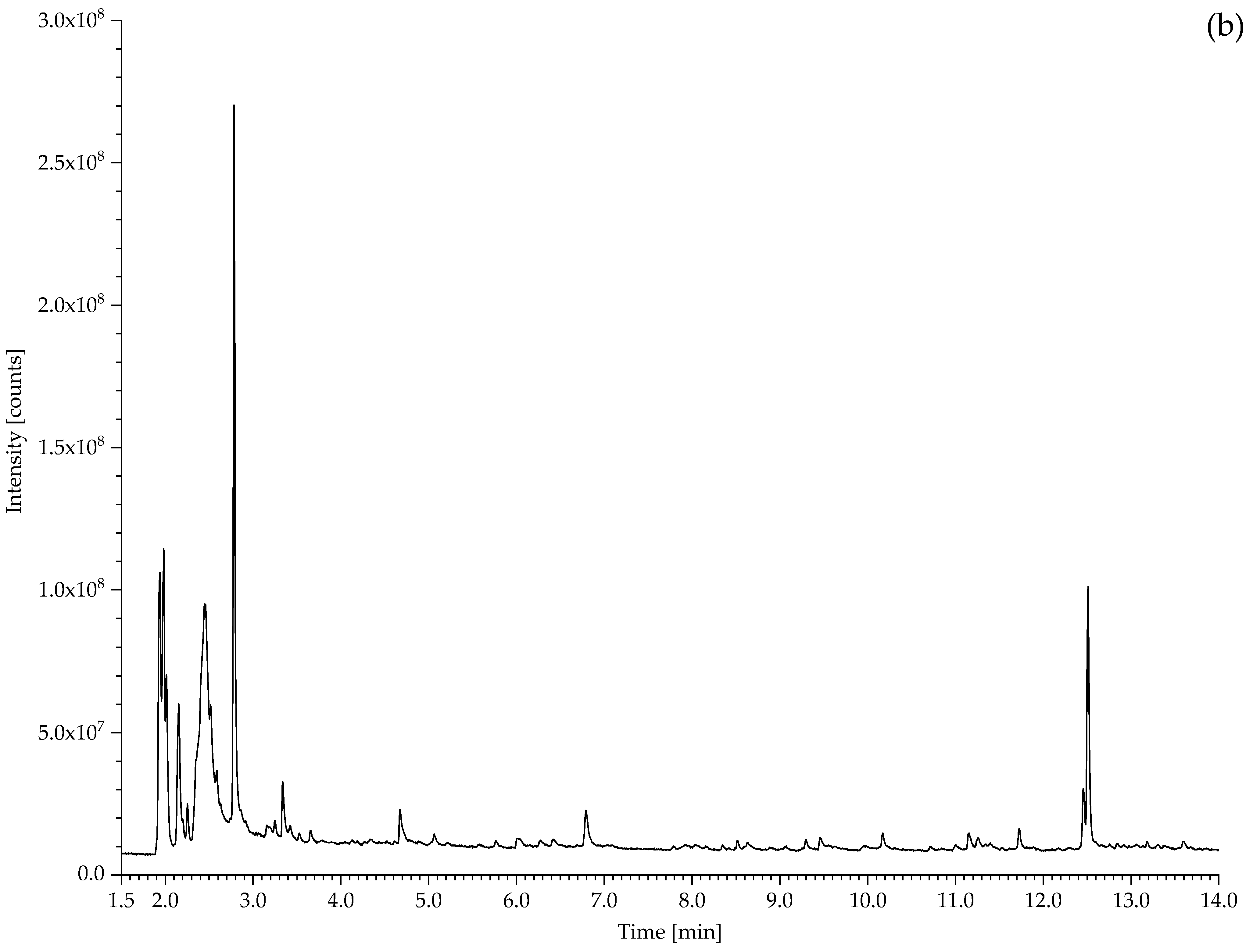
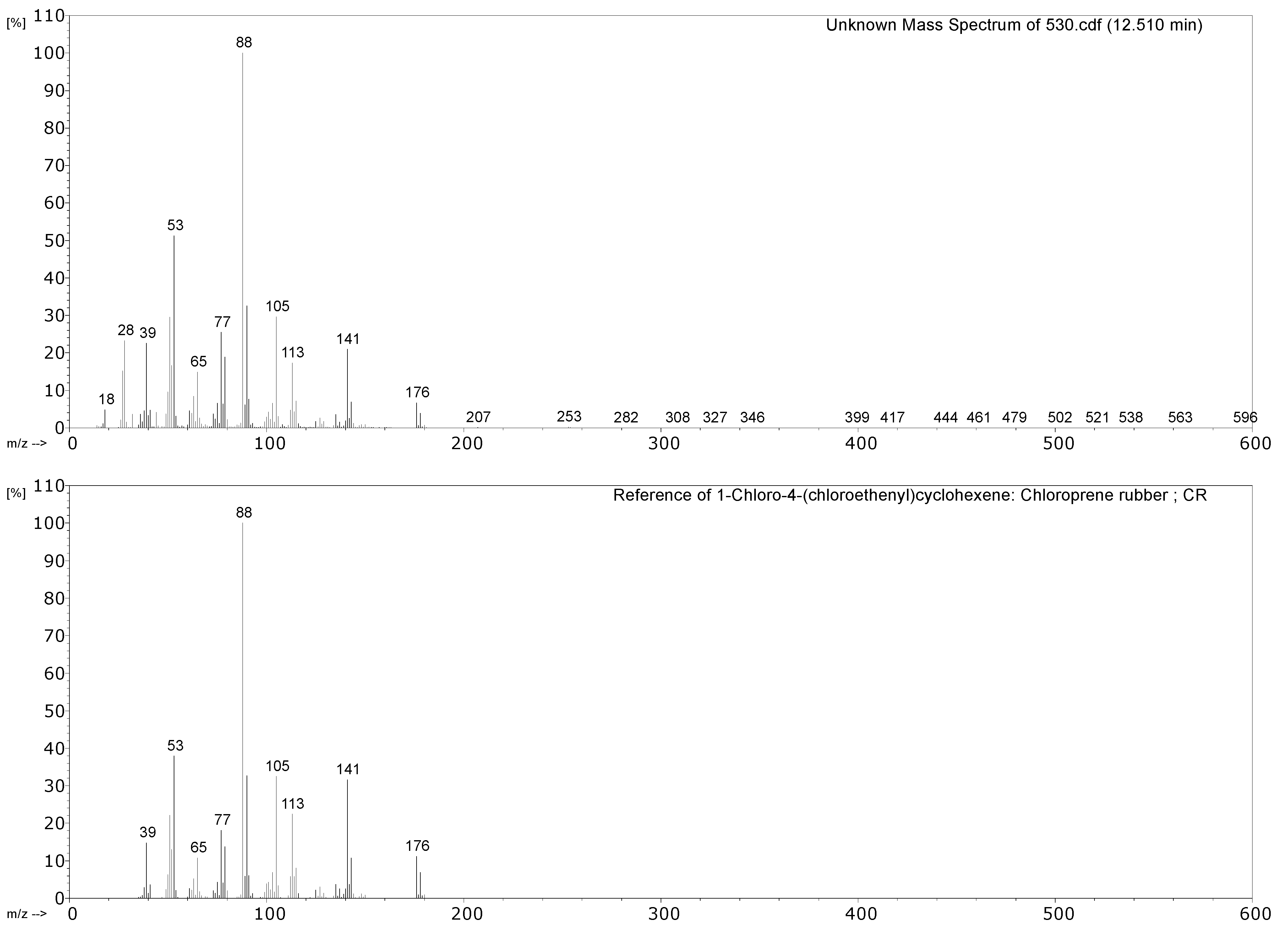
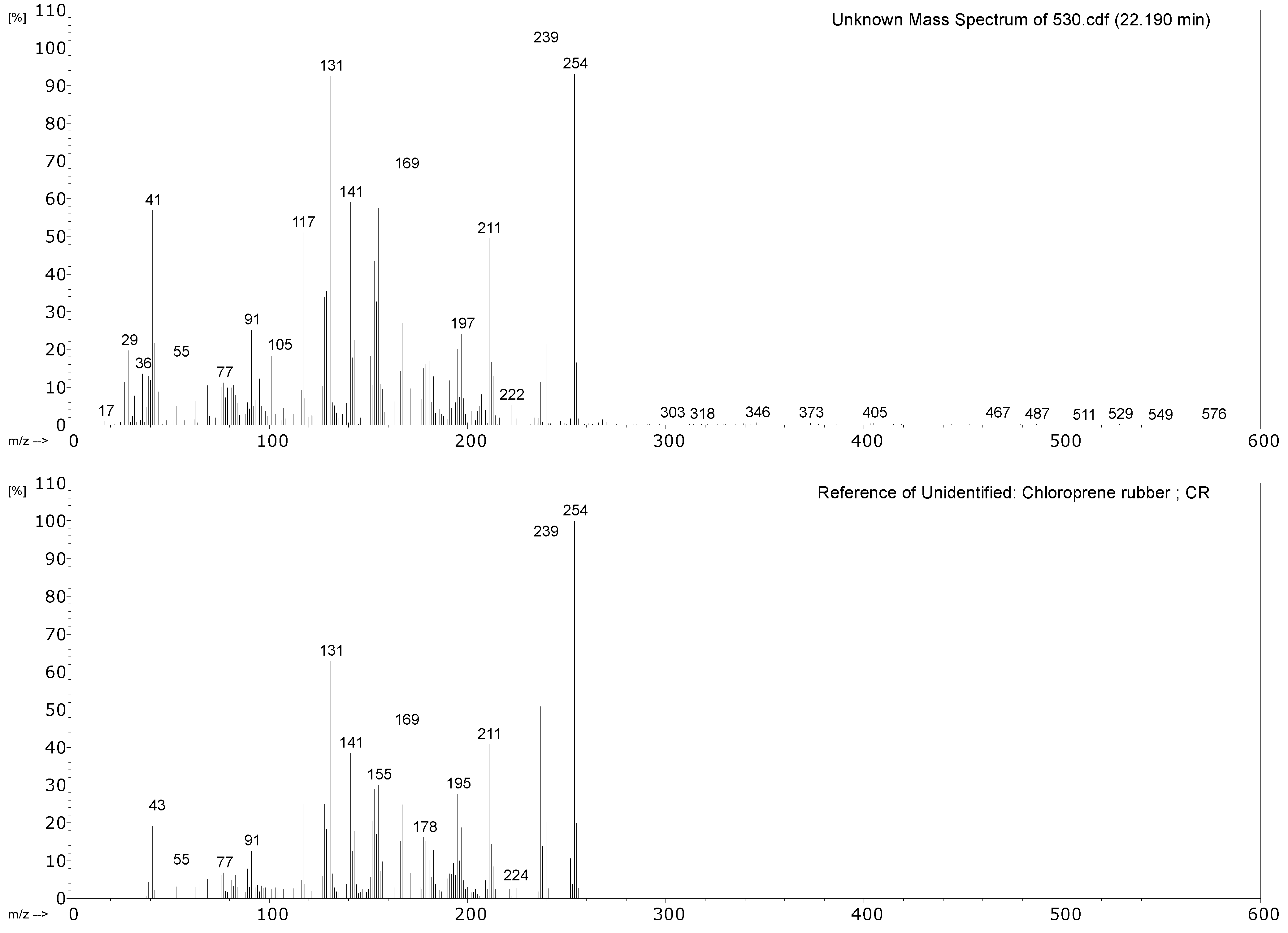
2.2. Pyrolysis–Gas Chromatography–Mass Spectrometry
3. Results and Discussion
3.1. Description of Damage Phenomena
| Keneghan (2001) | Shashoua (2008) | POPART Project (2012) | This Work | ||
|---|---|---|---|---|---|
| (a) | (b) | (c) | |||
| DEPOSITS Solid deposits | |||||
| x | x | x | x | x | Bloom. Crystalline |
| - | Chalking | Powdery | - | - | Other |
| - | - | - | - | - | Pox |
| - | - | x | x | x | Dust |
| x | x | x | x | x | Stain |
| - | - | x | x | x | Dirt |
| Liquid deposits | |||||
| - | - | - | Fat | - | Smeary |
| x, Wax | x | x, Exudation, | x, Exudation, | x, Exudation, | Sweating |
| Droplet | Droplet | Droplet | |||
| - | - | Unidentified | Other deposits | - | |
| CHROMATICITY/ TRANSPARENCY Colour change | |||||
| x | x | x | x, Darkening | x | Yellowing |
| - | - | x | x | x | Fading |
| x | x | x | - | x | Discolouration |
| - | - | x | x | x | Loss of transparency |
| DEFORMATION | |||||
| - | - | x | x | Distortion | Warping |
| - | - | x | x | x | Fold |
| - | x | x | x | x | Dent |
| - | x | x | x | x | Shrinkage |
| - | - | - | - | - | Channeling |
| - | - | x | x | x | Blistering |
| - | - | - | - | - | Softening |
| - | - | - | Deformation, loss of original shape | - | - |
| LOSS OF INTEGRITY Usually involving loss of material | |||||
| x | x | - | - | - | Abrasion |
| x | x | x | x | x | Chip |
| - | - | - | - | - | Flaking |
| - | Missing | x | x | x | Loss of material |
| - | x | - | x | - | Crumbling |
| x | x | x | x | x | Scratch |
| - | Pitting | - | - | - | - |
| Not usually or necessarily involving loss of material | |||||
| x | x | x | - | x | Brittleness |
| x, Torn | x | x, Tear | x, Tear | x, Tear | Crack |
| - | - | - | - | - | Craquelure |
| x | x | x | x | x | Crazing |
| - | - | - | x | - | Hardening |
| - | x | - | - | - | Loose |
| x | - | x | x | x | Peeling |
| x | x | x | x, Split of parts | x | Break |
| SMELL | |||||
| - | - | - | x | - | Vinegar |
| - | - | - | x | - | Acrid |
| - | - | - | - | - | Rubber |
| - | - | - | x | - | Camphor |
| - | - | - | - | - | Naphthalene |
| - | - | - | Paraffin | - | - |
| OTHER/MISCEL-LANEOUS FEATURES | |||||
| - | - | - | - | - | Overpaint |
| - | x | x | x | x | Corrosion (metal part) |
| - | - | - | - | - | Adhesive tape |
| - | - | - | x | - | Sticky |
| - | x | x, Insect, Mould | x, Insect, Mould | x, Insect (‘Pest’), Moulding | Biological attack |
| - | Surface damage | Inclusion | Growing tree | - | - |
3.1.1. Deposits. Table and Discussion
| Deposits | |||
|---|---|---|---|
| Solid deposits | Blooming | Blooming (crystalline) Crystalline efflorescence, where the crystalline character is identifiable macroscopically, e.g., through the identification of small crystalline particles and/or, depending on light incidence, a shiny reflection; typically white. |  |
| Blooming (other) Amorphous deposit, macros-copically non-crystalline, of matte, powdery or waxy aspect, or (cloudy) film affecting large surface areas; typically whitish-greyish. |  | ||
| Dried fluid | Solid material left behind as a residue after the drying of a liquid deposit. It usually appears in the form of a coloured, highly glossy semitransparent substance that sometimes shows some visual feature hinting to the previous liquid state of the substance (e.g., drop form). |  | |
| Pox | Small protrusions or elevations with the form of a truncated pyramid with round edges, which may show a different form, colour or texture from the surrounding material. | 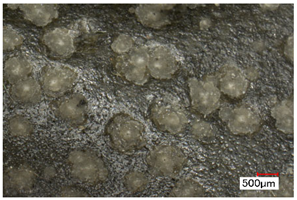 | |
| Dust | Fine air-borne particles that deposit more or less extensively, and typically uniformly, in surfaces. Dust can be found either simply lying or adhered to these in combination with other substances (e.g., dirt deposition such as fat). |  | |
| Dirt | Solid soiling other than dust or stains, regardless of whether it can be found localised or extended in a wide area. |  | |
| Stain | Transfer of foreign material from an external object to the surface of study through rubbing off, spill or other process, causing changes in colour, texture and/or glossiness in a localised area, e.g., dash of colour. |  | |
| Liquid deposits | Sweating | Liquid phase occurring either in the form of drops (moisture) or of a film, of origin unknown; increased glossiness possible. Any liquid deposit that does not definitely have an external origin. |  |
| Smeary | Oily or fatty residues, typically as a greasy film but not necessarily, coming from a known, external source (e.g., lamp oil); increased glossiness possible. |  |
3.1.2. Changes in Chromaticity and Transparency. Table and Discussion
| Changes in Chromaticity and Transparency | |||
|---|---|---|---|
| Colour change | Fading | Reduced colour brightness or intensity. |  |
| Yellowing | Colour change to a yellow tinge/hue, which can sometimes be darker (red/brown). Easily visible in clear or lightly coloured objects. |  | |
| Discolouration | Any change in colour of the original material other than fading or yellowing. |  | |
| Loss of transparency | Increase of the opacity of a transparent or translucent object. |  | |
3.1.3. Deformations. Table and Discussion
| Deformations | ||
|---|---|---|
| Blistering | Raised area, bulge or bubble on an objects’ surface, often between adjoining layers of different materials [9]. |  |
| Channeling | Channel-like detachment between a foil or coating and their undercoat. | |
| Dent | Three-dimensional form change appearing as a hollow mark, buckle or bump; indentation. |  |
| Fold | A bend, crease or kink of an object on itself, causing after a certain time stress in the material, eventually leading to partially irreversible damage. |  |
| Shrinkage | Dimensional reduction of the volume of a material; it may appear together with cracks and breakage. | |
| Softening | Loss of hardness or firmness of the material, in extreme cases up to the point of liquefaction, which may have happened at some point in the material life. A (re)solidification may have taken place meanwhile, but the loss of original form, surface texture, glossiness or other changes hint towards a previous softening. |  |
| Warping | Three-dimensional form change appearing as concave and/or convex distortion or twisting, sometimes in the form of a wave, so that the material/object surface is no longer flat. |  |
3.1.4. Loss of Integrity. Table and Discussions
3.1.5. Smell. Table and Discussions
3.1.6. Other/Miscellaneous Features. Table and Discussions
3.2. Analytical Challenges of Surveying Industrial Heritage Collections
3.2.1. Identification of Rubbers
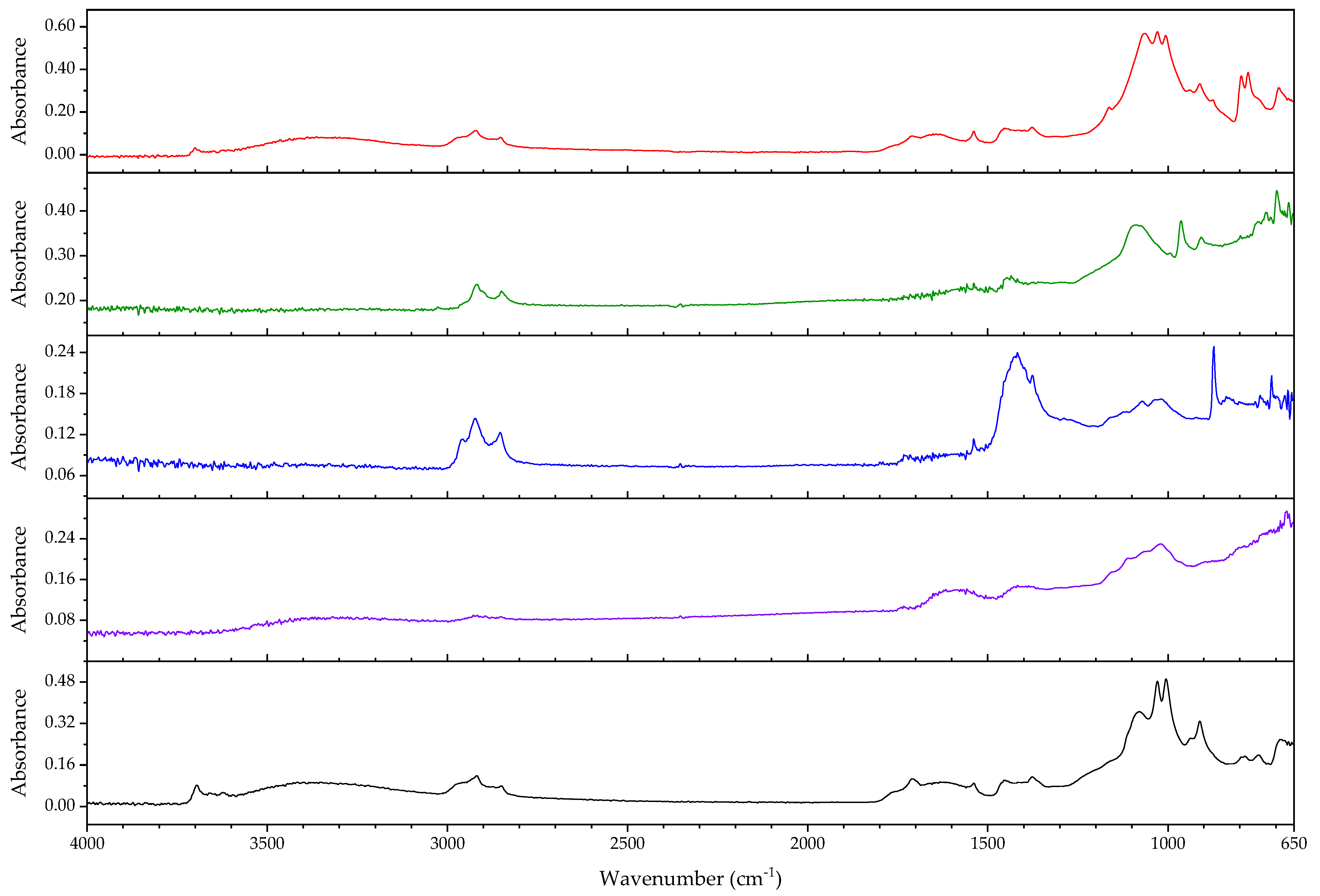
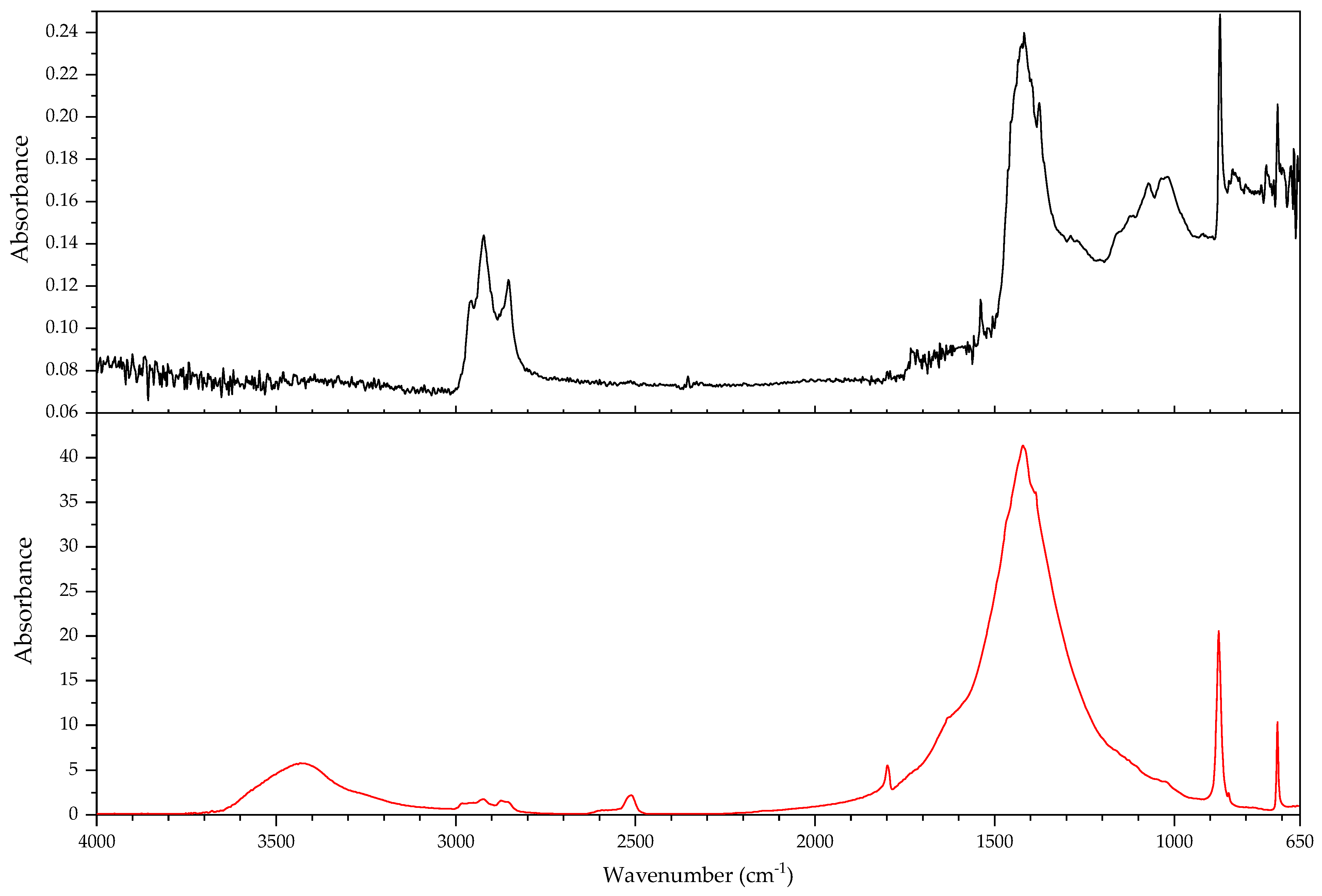
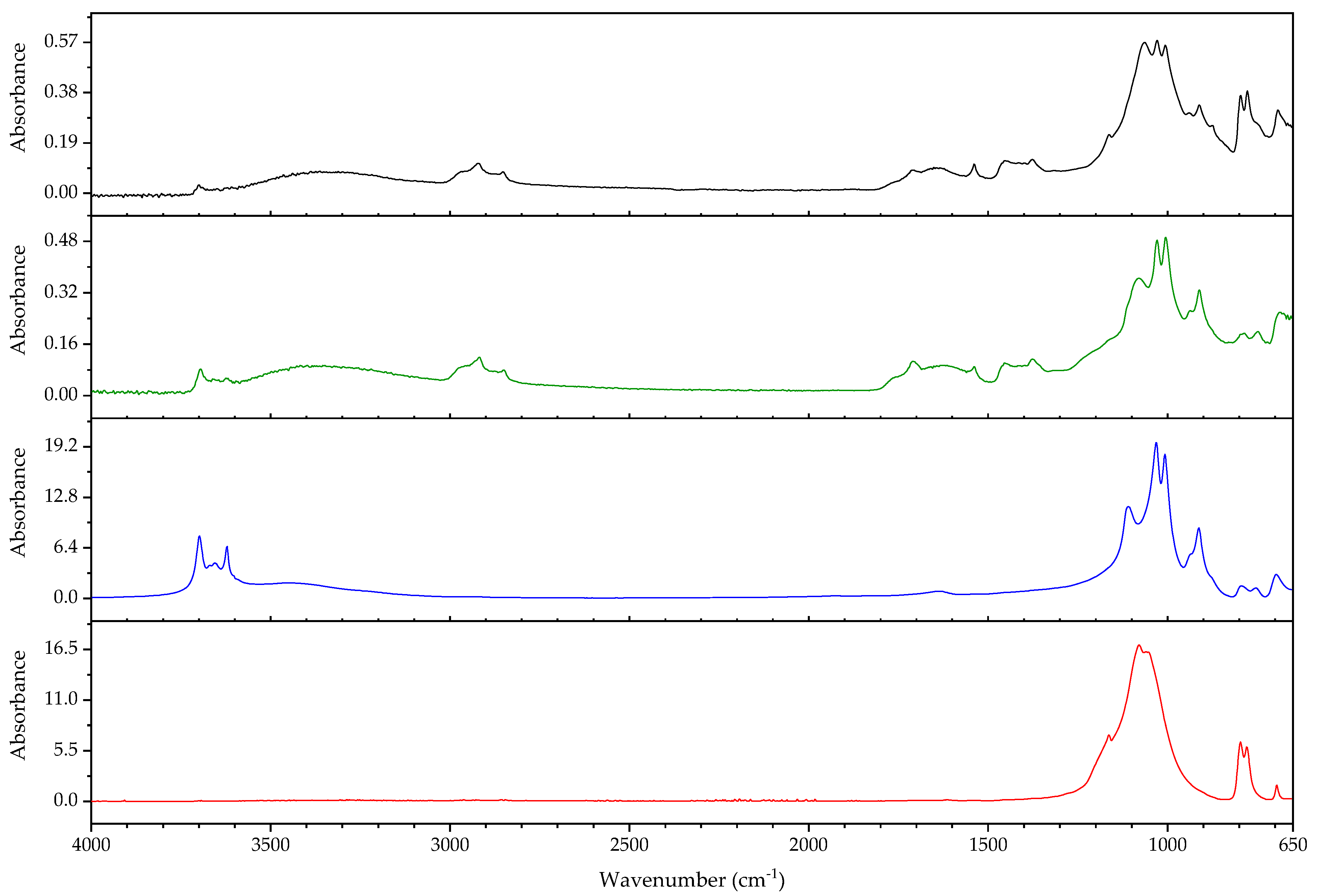
3.2.2. Case Study: Blooming
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waentig, F. Plastics in Art, 1st ed.; Michael Imhof Verlag: Petersberg, Germany, 2009; ISBN 978-3-86568-405-9. [Google Scholar]
- Williams, R.S. Care of plastics: Malignant plastics. Waac Newsl. 2002, 24, 11. [Google Scholar]
- Fenn, J.; Williams, S. Caring for Plastics and Rubbers—Preventive Conservation Guidelines for Collections. Available online: https://www.canada.ca/en/conservation-institute/services/preventive-conservation/guidelines-collections/caring-plastics-rubbers.html (accessed on 1 October 2021).
- Curran, K.; Možir, A.; Underhill, M.; Gibson, L.T.; Fearn, T.; Strlič, M. Cross-infection effect of polymers of historic and heritage significance on the degradation of a cellulose reference test material. Polym. Degrad. Stab. 2014, 107, 294–306. [Google Scholar] [CrossRef] [Green Version]
- Mossman, S. Plastics in the Science Museum, London: A Curator’s View. In Proceedings of the Saving the Twentieth Century: The Conservation of Modern Materials, Ottawa, ON, Canada, 15–20 September 1991; pp. 25–35. [Google Scholar]
- DIN EN 16095:2012-10, Erhaltung des Kulturellen Erbes—Zustandsaufnahme an Beweglichem Kulturerbe, Deutsche Fassung EN_16095:2012; Beuth Verlag GmbH: Berlin, Germany, 2012.
- Taylor, J. The impact of assessment guides on the reliability of collection condition surveys. In ICOM-CC 17th Triennial Conference Preprints, Melbourne, Australia, 15–19 September 2014; International Council of Museums: Paris, France, 2014; ISBN 978-92-9012-410-8. [Google Scholar]
- Keneghan, B. Conservation: A Survey of Synthetic Plastic and Rubber Objects in the Collections of the Victoria and Albert Museum. Mus. Manag. Curatorship 2001, 19, 321–331. [Google Scholar] [CrossRef]
- Michaels, J. The Condition Survey of Sound Recordings at the National Library of Canada: Implications for Conservation. In Proceedings of the Saving the Twentieth Century: The Conservation of Modern Materials, Ottawa, ON, Canada, 15–20 September 1991; pp. 13–20. [Google Scholar]
- Glossary. Available online: https://www.nfsa.gov.au/preservation/preservation-glossary (accessed on 1 October 2021).
- Visual Glossary—Australian Institute for the Conservation of Cultural Material. Available online: https://aiccm.org.au/conservation/visual-glossary/ (accessed on 1 October 2021).
- Shashoua, Y. Conservation of Plastics: Materials Science, Degradation and Preservation, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 978-0-7506-6495-0. [Google Scholar]
- What Is the Condition of the Collection?|Popart. Available online: https://popart-highlights.mnhn.fr/collection-survey/what-is-the-condition-of-the-collection/index.html (accessed on 1 October 2021).
- List of Degradation Terms, Popart. Available online: https://popart-highlights.mnhn.fr/wp-content/uploads/3_Collection_survey/1_What_is_the_condition_of_the_collection/List_of_terms.pdf (accessed on 1 October 2021).
- Damage Atlas|Popart. Available online: https://popart-highlights.mnhn.fr/collection-survey/damage-atlas/index.html (accessed on 1 October 2021).
- Lavédrine, B.; Fournier, A.; Martin, G. Preservation of Plastic Artefacts in Museum Collections; Comité des Travaux Historiques et Scientifiques—CTHS: Paris, France, 2012; ISBN 978-2-7355-0770-2. [Google Scholar]
- Bressan, F. A Framework for the Description of Age-related Symptoms in Audio Media: Definition and Implementation. Stud. Conserv. 2020, 65, 189–199. [Google Scholar] [CrossRef]
- Then, E.; Oakley, V. A Survey of Plastic Objects at the Victoria & Albert Museum. Conserv. J. 1993, 6, 11–14. [Google Scholar]
- Loadman, M.J.R. Analysis of Rubber and Rubber-Like Polymers, 4th ed.; Kluwer Academic: Dordrecht, The Netherlands, 1998; ISBN 978-0-412-81970-4. [Google Scholar]
- Domininghaus, H.; Elsner, P.; Eyerer, P.; Hirth, T. (Eds.) Kunststoffe—Eigenschaften und Anwendungen; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-72400-1. [Google Scholar]
- Gunasekaran, S.; Natarajan, R.K.; Kala, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ganzelewski, M. KUR-Projekt: Antiaging für Kulturgut mit Elastomeranteilen: Entwicklung und Praxiserprobung von Konservierungs- und Restaurierungsverfahren zur Erhaltung von Komposit-Objekten mit hohen Elastomeranteilen (natürliche und synthetische Gummimaterialien). 2012, 55. 2012; 55. [Google Scholar] [CrossRef]
- Blank, S.D. Rubber in Museums: A Conservation Problem. AICCM Bull. 1988, 14, 53–93. [Google Scholar] [CrossRef]
- Ward, C.; Shashoua, Y. Conservation Survey of Objects Containing Plastics and Rubber in the Department of Ethnography, Conservation Report of the British Museum Department of Conservation; British Museum: London, UK, 1994; p. 15. [Google Scholar]
- Forrest, M.J. Rubber Analysis: Characterisation, Failure Diagnosis and Reverse Engineering; De Gruyter: Berlin, Germany, 2019; ISBN 978-3-11-064028-1. [Google Scholar]
- Standard Practice for Rubber—Identification by Pyrolysis-Gas Chromatography; ASTM International: West Conshohocken, PA, USA, 2002; p. 5.
- Standard Test Methods for Rubber—Identification by Infrared Spectrophotometry; ASTM International: West Conshohocken, PA, USA, 1978; p. 21.
- Saviello, D.; Toniolo, L.; Goidanich, S.; Casadio, F. Non-invasive identification of plastic materials in museum collections with portable FTIR reflectance spectroscopy: Reference database and practical applications. Microchem. J. 2015. [Google Scholar] [CrossRef]
- Tsang, J.; Madden, O.; Coughlin, M.; Maiorana, A.; Watson, J.; Little, N.C.; Speakman, R.J. Degradation of “Lumarith” Cellulose Acetate: Examination and chemical analysis of a salesman’s sample kit. Stud. Conserv. 2009, 54, 90–105. [Google Scholar]
- Bell, J.; Nel, P.; Stuart, B. Non-invasive identification of polymers in cultural heritage collections: Evaluation, optimisation and application of portable FTIR (ATR and external reflectance) spectroscopy to three-dimensional polymer-based objects. Herit. Sci. 2019, 7, 95. [Google Scholar] [CrossRef]
- Picollo, M.; Bartolozzi, G.; Cucci, C.; Galeotti, M.; Marchiafava, V.; Pizzo, B. Comparative Study of Fourier Transform Infrared Spectroscopy in Transmission, Attenuated Total Reflection, and Total Reflection Modes for the Analysis of Plastics in the Cultural Heritage Field. Appl. Spectrosc. 2014, 68, 389–397. [Google Scholar] [CrossRef]
- Harms, D.L. Identification of Complex Organic Materials: By Infrared Spectra of Their Pyrolysis Products. Anal. Chem. 1953, 25, 1140–1155. [Google Scholar] [CrossRef]
- Fernández-Berridi, M.J.; González, N.; Mugica, A.; Bernicot, C. Pyrolysis-FTIR and TGA techniques as tools in the characterization of blends of natural rubber and SBR. Thermochim. Acta 2006, 444, 65–70. [Google Scholar] [CrossRef]
- González, N.; Fernández-Berridi, M.J. Fourier transform infrared spectroscopy in the study of the interaction between PVC and plasticizers: PVC/plasticizer compatibility. J. Appl. Polym. Sci. 2008, 107, 1294–1300. [Google Scholar] [CrossRef]
- Scheirs, J.; Camino, G.; Costa, L.; Tonelli, C.; Turri, S.; Scicchitano, M. Photooxidation of functionalized perfluorinated polyethers—I. Polym. Degrad. Stab. 1997, 56, 239–253. [Google Scholar] [CrossRef]
- Nouman, M.; Saunier, J.; Jubeli, E.; Yagoubi, N. Additive blooming in polymer materials: Consequences in the pharmaceutical and medical field. Polym. Degrad. Stab. 2017, 143, 239–252. [Google Scholar] [CrossRef]
- Pearlstein, E. Fatty bloom on wood sculpture from Mali. Stud. Conserv. 1986, 31, 83–91. [Google Scholar]
- Unger, A.; Schniewind, A.P.; Unger, W. Conservation of Wood Artifacts: A Handbook; Natural Science in Archaeology; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 978-3-540-41580-0. [Google Scholar]
- King, R.; Grau-Bové, J.; Curran, K. Plasticiser loss in heritage collections: Its prevalence, cause, effect, and methods for analysis. Herit. Sci. 2020, 8, 123. [Google Scholar] [CrossRef]
- Van Oosten, T.; Keune, P. Modern Art—Who Cares? An Interdisciplinary Research Project and an International Symposium on the Conservation of Modern and Contemporary Art. Hummelen, I.M.C., Sillé, D., Eds.; Archetype Publ.: London, UK, 2005; p. 51. [Google Scholar]
- Strlič, M.; Curran, K. Special issue “Polymers in Art and History”—Editorial. Polym. Degrad. Stab. 2014, 107, 189–190. [Google Scholar] [CrossRef]
- Taylor, J. Causes and extent of variation in collection condition survey data. Stud. Conserv. 2013, 58, 106–195. [Google Scholar] [CrossRef]
- U.S. Government Accountability Office. Content Analysis: A Methodology for Structuring and Analyzing Written Material. Available online: https://www.gao.gov/products/pemd-10.3.1 (accessed on 27 November 2021).
- Tsuge, S.; Ohtani, H.; Watanabe, C. Pyrolysis-GC/MS Data Book of Synthetic Polymers: Pyrograms, Thermograms and MS of Pyrolyzates, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-444-53892-5. [Google Scholar]
- Art & Architecture Thesaurus (Getty Research Institute). Available online: http://www.getty.edu/research/tools/vocabularies/aat/index.html (accessed on 1 October 2021).
- Materials Database—CAMEO. Available online: http://cameo.mfa.org/wiki/Category:Materials_database (accessed on 1 October 2021).
- Lexicon—Conservation Wiki. Available online: https://www.conservation-wiki.com/wiki/Lexicon (accessed on 1 October 2021).
- Lexicon Terms—Conservation Wiki. Available online: https://www.conservation-wiki.com/wiki/Lexicon_Terms (accessed on 1 October 2021).
- Work, W.J.; Horie, K.; Hess, M.; Stepto, R.F.T. Definitions of terms related to polymer blends, composites, and multiphase polymeric materials (IUPAC Recommendations 2004). Pure Appl. Chem. 2004, 76, 1985–2007. [Google Scholar] [CrossRef]
- VDI 3822 Blatt 2.1.4—Failure Analysis—Defects of Thermoplastic Products Made of Plastics Caused by Mechanical Stress; VDI-Gesellschaft Materials Engineering: Berlin, Germany, 2012.
- Köppen, J.; Brunner, S.; Gómez-Sánchez, E. Shoemaker’s Nightmare: Deterioration of Shoe Soles and Tests for the Conservation of Degraded Closed-Cell Polyester Urethane Museum Objects. In Proceedings of the Future Talks 019: Surfaces. Lectures and Workshops on Technology and Conservation of the Modern, Munich, Germany, 11–13 November 2019. [Google Scholar]
- Wei, X.-F.; Linde, E.; Hedenqvist, M.S. Plasticiser loss from plastic or rubber products through diffusion and evaporation. npj Mater. Degrad. 2019, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Cambridge Dictionary Online. Available online: https://dictionary.cambridge.org/us/dictionary/english/ (accessed on 7 October 2020).
- Ecomolding, Pitting Issue Plastic Injection Molding Issue-ecomolding.com. Available online: https://www.injectionmould.org/2019/06/17/pitting/ (accessed on 7 October 2020).
- Kurr, F. Praxishandbuch der Qualitäts- und Schadensanalyse für Kunststoffe, 2nd ed.; Hanser: München, Germany, 2014; ISBN 978-3-446-43775-3. [Google Scholar]
- ASTM D 883 Standard Terminology Relating to Plastics; ASTM International: West Conshohocken, PA, USA, 2012.
- Van Oosten, T.B. Crystals and crazes: Degradation in plastics due to microclimates. In Plastics in Art: History, Technology, Preservation; Siegel: Munich, Germany, 2002; pp. 80–89. [Google Scholar]
- Keßler, K. Celluloid-ein Werkstoff zersetzt sich. In Kunststoff als Werkstoff: Celluloid und Polyurethan-Weichschaum: Material—Eigenschaften—Erhaltung; Kölner Beiträge zur Restaurierung und Konservierung von Kunst- und Kulturgut; Siegl: München, Germany, 2001; Volume 13, pp. 9–98. ISBN 3-935643-01-2. [Google Scholar]
- Encyclopedia Britannica. Available online: https://www.britannica.com/science/elasticity-physics (accessed on 18 December 2020).
- Info|Plastic Identificatie Tool|Rijksdienst Cultureel Erfgoed. Available online: https://plastic-en.tool.cultureelerfgoed.nl/info (accessed on 1 October 2021).
- Plastic Identication Tool. Available online: https://www.vanaubelvanrooijen.com/plastics-project (accessed on 1 October 2021).
- Curran, K.; Underhill, M.; Gibson, L.T.; Strlic, M. The development of a SPME-GC/MS method for the analysis of VOC emissions from historic plastic and rubber materials. Microchem. J. 2015. [Google Scholar] [CrossRef]
- Curran, K.; Underhill, M.; Grau-Bové, J.; Fearn, T.; Gibson, L.T.; Strlič, M. Classifying Degraded Modern Polymeric Museum Artefacts by Their Smell. Angew. Chem. Int. Ed. Engl. 2018, 57, 7336–7340. [Google Scholar] [CrossRef] [Green Version]
- Hummel, D.O. Atlas of Plastics Additives: Analysis by Spectrometric Methods; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-540-42414-7. [Google Scholar]
- Danon, B.; van der Gryp, P.; Schwarz, C.E.; Görgens, J.F. A review of dipentene (dl-limonene) production from waste tire pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 1–13. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Analytical Pyrolysis of Synthetic Organic Polymers; Elsevier: Amsterdam, The Netherlands, 2005; Volume 25. [Google Scholar]
- Nickel, A.; Edgecombe, B.D. 4.30—Industrial Applications of ROMP. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 749–759. ISBN 978-0-08-087862-1. [Google Scholar]
- Kusch, P. Application of Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS). In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 75, pp. 169–207. ISBN 978-0-444-63898-4. [Google Scholar]
- Hewitt, N. CHAPTER 6—Compounding Precipitated Silica in Neoprene. In Compounding Precipitated Silica in Elastomers; Hewitt, N., Ed.; Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2007; pp. 387–415. ISBN 978-0-8155-1528-9. [Google Scholar]
- Engels, H.-W.; Weidenhaupt, H.-J.; Pieroth, M.; Hofmann, W.; Menting, K.-H.; Mergenhagen, T.; Schmoll, R.; Uhrlandt, S. Rubber, 4. Chemicals and Additives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; ISBN 978-3-527-30673-2. [Google Scholar]
- Brydson, J.A. Plastics Materials, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2000; ISBN 978-0-7506-4132-6. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2001; ISBN 978-0-471-85298-8. [Google Scholar]
- Hummel, D.O.; Scholl, F.K. Atlas der Polymer- und Kunststoffanalyse. Friedrich K. Scholl, Bd. 2 Teil b,2: Kunststoffe, Fasern, Kautschuk, Harze; Ausgangs- und Hilfsstoffe, Abbauprodukte = Plastics, Fibres, Rubbers, Resins; Starting and Auxiliary Materials, Degradation Products Bibliographie und Register, 2nd ed.; Hanser: München, Germany, 1988; ISBN 978-3-527-26092-8. [Google Scholar]
- Ploeger, R.; Chiantore, O. Characterization and Stability Issues of Artists Alkyd Paints. In Proceedings of the Cleaning 2010 International Conference, Valencia, Spain, 15 September 2010. [Google Scholar]
- Bolgar, M.; Hubball, J.; Groeger, J.; Meronek, S. Handbook for the Chemical Analysis of Plastic and Polymer Additives; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-4488-1. [Google Scholar]
- Dwivedi, A.P.; Ghosal, D.G.K.; Belkhode, D.P.N. Studies in Properties of Microcrystalline and Paraffin Waxes with the Help of Gas Chromatography (GC), DSC, FT-IR and by Conventional Methods. Int. J. Sci. Res. Sci. Technol. 2017, 3, 922–929. [Google Scholar] [CrossRef]
- Learner, T.J.S. Analysis of Modern Paints; Getty Conservation Institute: Los Angeles, CA, USA, 2004; ISBN 978-0-89236-779-5. [Google Scholar]
- Svečnjak, L.; Baranović, G.; Vinceković, M.; Prđun, S.; Bubalo, D.; Tlak Gajger, I. An Approach for Routine Analytical Detection of Beeswax Adulteration Using FTIR-ATR Spectroscopy. J. Apic. Sci. 2015, 59, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Li, G.-Y.; Koenig, J.L. A review of rubber oxidation. Rubber Chem. Technol. 2005, 78, 355–390. [Google Scholar] [CrossRef]
- Pellizzi, E.; Lattuati-Derieux, A.; Lavédrine, B.; Cheradame, H. Degradation of polyurethane ester foam artifacts: Chemical properties, mechanical properties and comparison between accelerated and natural degradation. Polym. Degrad. Stab. 2014, 107, 255–261. [Google Scholar] [CrossRef]
- Oosten, T.; van Shashoua, Y.; Waentig, F. Plastics in Art: History, Technology, Preservation; Siegl: München, Germany, 2002; ISBN 978-3-935643-05-4. [Google Scholar]
- Suzuki, M.; Shimanouchi, T. Infrared and Raman spectra of adipic acid crystal. J. Mol. Spectrosc. 1969, 29, 415–425. [Google Scholar] [CrossRef]




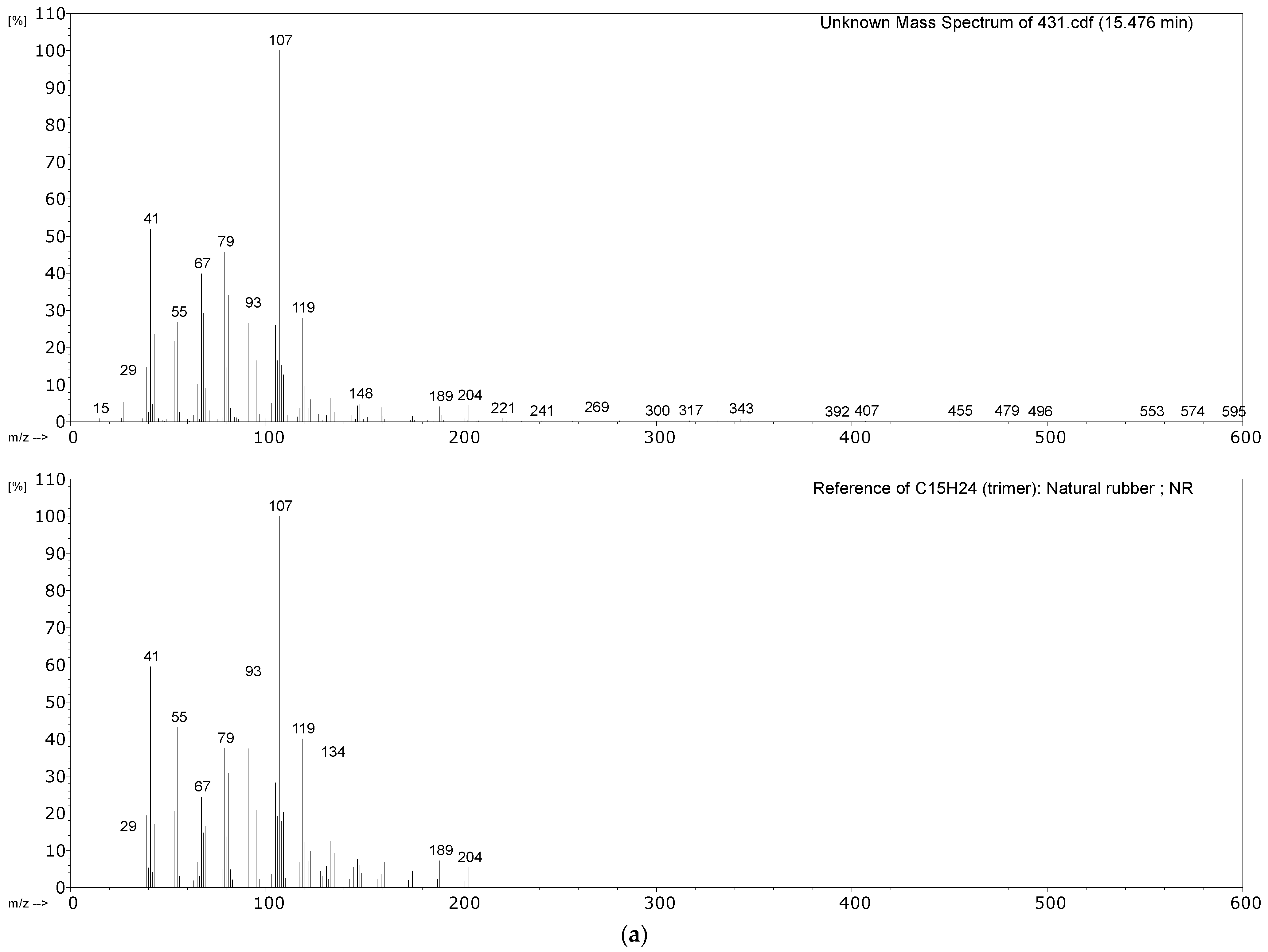
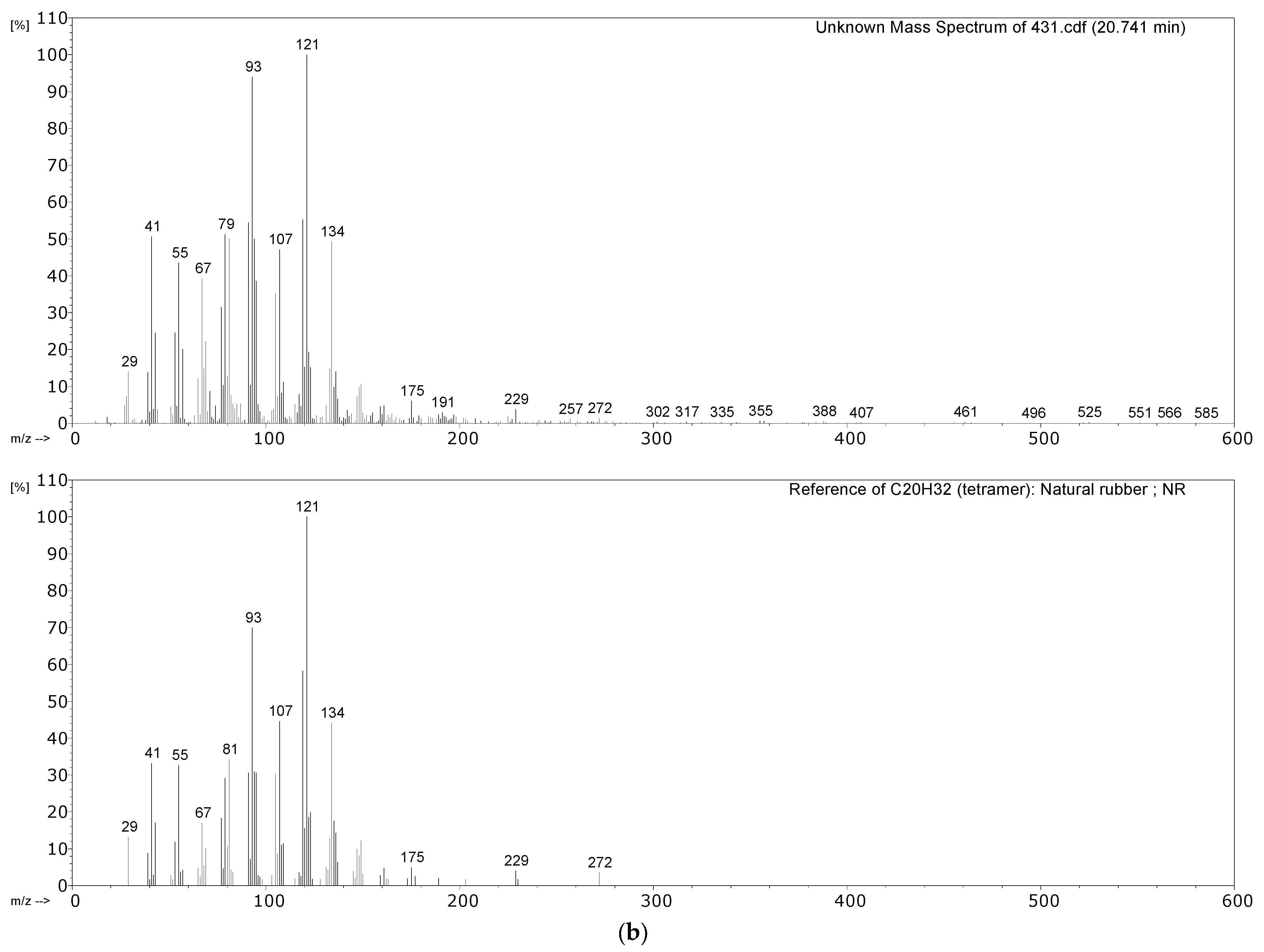


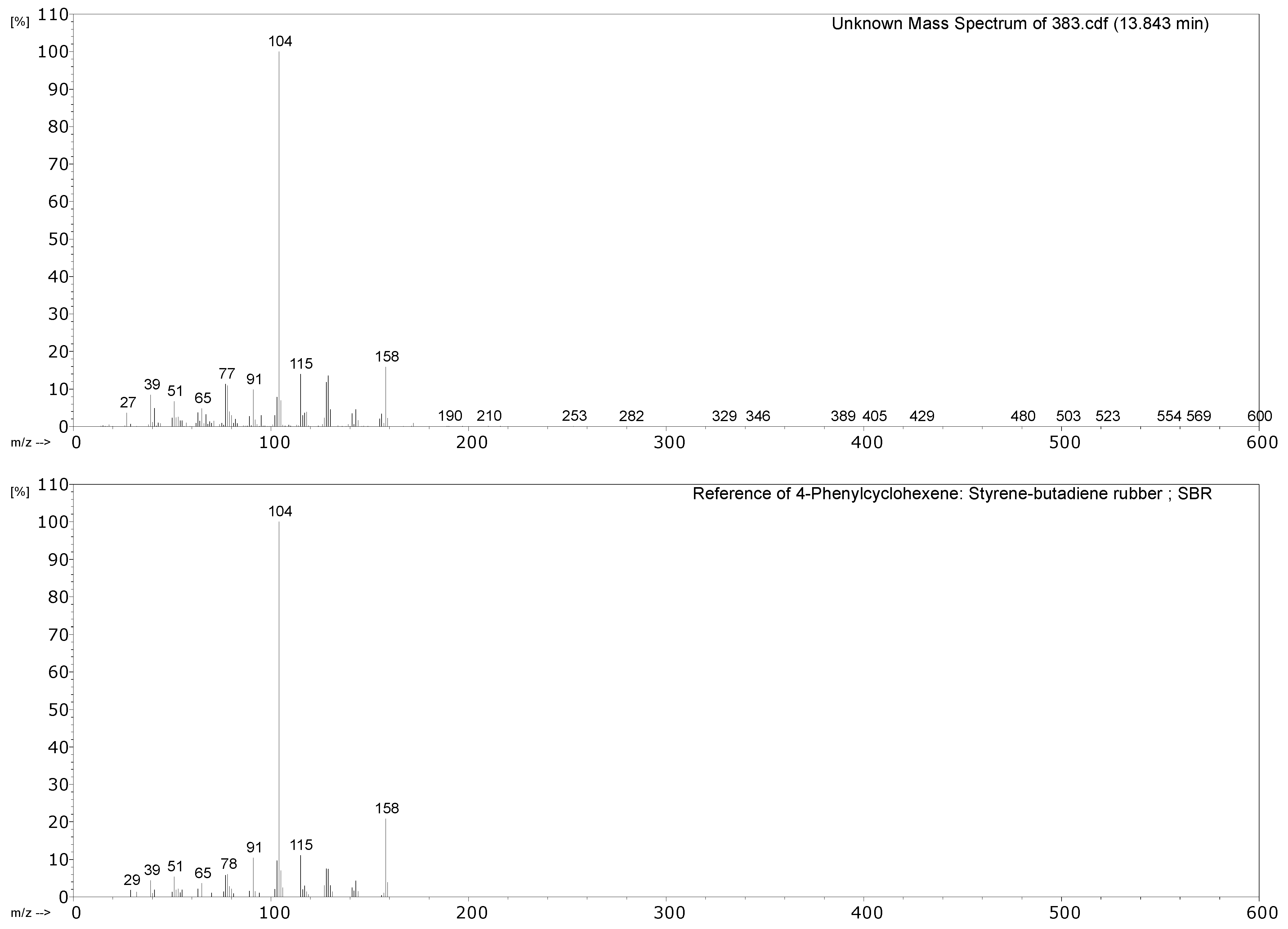






| Loss of Integrity Usually Involving Loss of Material | ||
|---|---|---|
| Abrasion | (a) Concentration of thin, shallow cuts or small chips and scratches, which may show up as a roughening of the surface with concomitant gloss reduction, appearing usually in (a) particular direction(s) |  |
| or (b) smoothening of a surface caused by the repeated friction against another surface, accompanied with gloss increase. |  | |
| Chip | Punctual loss of surface material, typically a spalled rim. In material with glass-like behaviour, a conchoidal fracture may appear (also: spalling) |  |
| Crumbling | Detachment of material due to loss of coherence, characterised by extensive friability that usually starts in the surface and advances into the depth of the material. It can appear, e.g., in the form of cube break, or, in the case of foams, lead to powder in its final stages. |  |
| Flaking | Usually applied to the detachment of material in the form of small flat platelets. |  |
| Loss of material (other) | Loss of a constituting part of an object, as caused by a different damage phenomenon than abrasion, chip, crumbling, flaking or scratch. |  |
| Scratch | Rather linear, occasional or isolated thin incision(s) or scrape(s) on the surface, regardless of its depth; extensive accumulation of scratches in a given direction is rather an <abrasion>. |  |
| Loss of Integrity not Necessarily Involving Loss of Material | ||
|---|---|---|
| Brittleness | Behaviour of materials through which they are likely to crack or break when subjected to pressure. |  |
| Break | Complete crack running through material, with separation of two or more pieces. For films and sheets, the term ‘tear’ is used; fracture is another descriptor. |  |
| Crack | Small fissure or opening which does not cause separation of an object part (cf. break). |  |
| Craquelure | Superficial network of cracks or fissures affecting the outmost layers of a material, such as is typically found in varnishes. |  |
| Crazing | Crazing may refer to (a) internal micro-crack region, macroscopically visible as stress whitening with possible loss of transparency. |  |
| or (b) network of fine cracks starting in the surface or within a material, which spreads throughout it. (Case b) typically affects cellulose nitrate and may be accompanied by concomitant whitening (loss of transparency). |  | |
| Hardening | Loss of elasticity and plasticity, (partial) loss of the ability to recover its form and of the ability to change it without break after being submitted to stress. |  |
| Loose | Partial detachment of a constituting part of an object, with risk of breakage or loss of material. |  |
| Peeling | Detachment of a relatively large coating, foil or other layer from the underlying surface, sometimes accompanied by curling of the detached part; delamination. |  |
| Smell | |
|---|---|
| Acrid smell | Acrid till pungent smell of certain substances (e.g., acid, ammonia, formaldehyde) and which cannot be described otherwise (e.g., as vinegar smell). |
| Camphor smell | Aromatic and fresh, menthol-like smell. |
| Naphthalene smell | Aromatic odour of the organic substance naphthalene, reminding of old, 20th century mothballs. |
| Rubber smell | A typical rubber smell can be noticed in tyres or cheap rubber products. |
| Vinegar smell | Typical smell of vinegar coming from acetic acid appearing as a degradation product. |
| Other/Miscellaneous Features | ||
|---|---|---|
| Adhesive tape | Repairs or fixations with self-adhesive or pressure-sensitive (‘sticky’) tape or similar products. Also meant by this term are residues or damage (e.g., stains, sticky areas) left and caused by these, and which are easily recognisable as such. |  |
| Biological attack | Any sign of mould or moss growth, or insect, bird or mammal attack, as evidenced by certain discolouration signs, fruiting bodies, excrements, feeding/scuff marks and galleries. |  |
| Corrosion (metal part) | Products of the degradation of metal elements near plastic parts. |  |
| Overpaint | Colour layer that has subsequently been applied over an original surface. |  |
| Sticky | Adhesive or gluey character of a surface; behaviour of a surface through which it stays attached to other surfaces that it touches. | |
| Sample Number, Object, Part, Approximate Production Date | Photo of Object and Object Part | |
|---|---|---|
| 194 Object: miner‘s bathing slippers Part: belt 1950s |  |  |
| 383 Object: Dosco Roadway Cutter Loader (roadheader) Part: supply hose for water nozzle 1960s |  |  |
| 431 Object: diesel-hydraulic Trolley of a monorail system Part: hose sheath of centrifugal governor 1970s |  |  |
| 530 Object: air-powered monorail trolley Part: hoses around 1980 |  |  |
| 598 Object: methanometer Part: pillow-shaped air bulb/rubber bulb probably between 1950s–1980s |  |  |
| Object/Part | Main py-GCMS Peaks (Major Peaks are Underlined) | Main Polymeric Component |
|---|---|---|
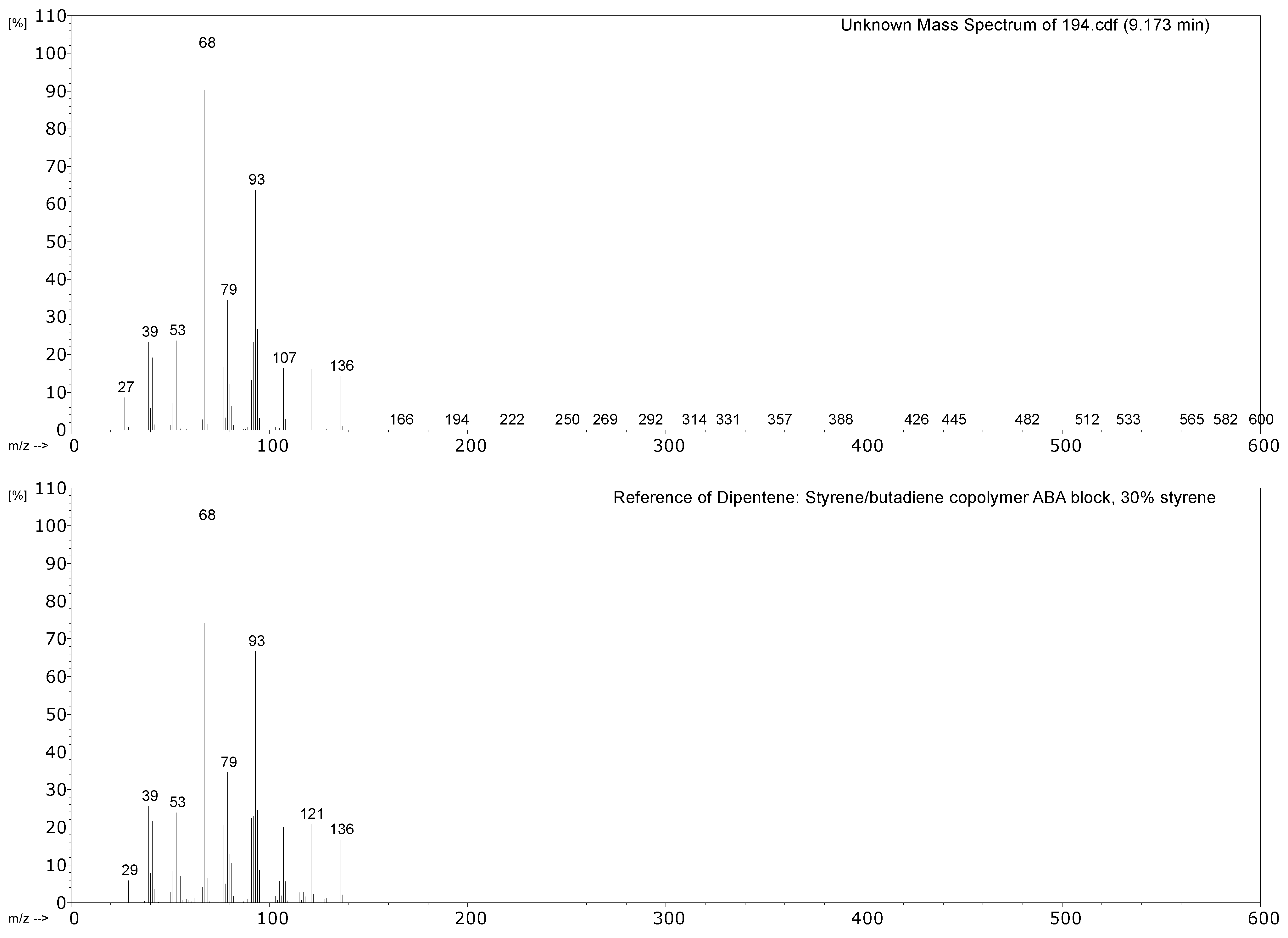
| 194 Drive belt from the model of a coal cutting machine | CO2, propene, SO2, isoprene, toluene, xylene, dipentene, 2,4-dimethyl-4-vinylcyclohexene. | NR, vulcanised with sulphur. |
| 383 Hose for water nozzle | CO2, propene, SO2, 1,3-butadiene, benzene, toluene, xylene, 4-vinylcyclohexene, styrene, α-methylstyrene, benzothiazole. | Styrene containing elastomer (probably SBR), vulcanised with sulphur. |
| 431 Hose sheath | Propene, SO2, isoprene, xylene, dipentene, 2,4-dimethyl-4-vinylcyclohexene, long chain alkenes, bis(2-ethylhexyl) phthalate (plasticiser). | NR, vulcanised with sulphur. Minor EPDM component? |
| 530 Hose of air-powered monorail trolley | 1,3-butadiene, HCl, 2-chloro-1,3-butadiene (chloroprene), 4-vinylcyclohexene, indane, indene, 1-chloro-4-(1-chlorovinyl)cyclohexene. | Chloroprene as main component; markers of poly(1,2-butadiene), CM and CSM also present. |
| Sample Number, Object, Part, Approximate Production Date and Sample Name | Photos of Object and Object Part | |
|---|---|---|
| 63 Object: calculator Part: coating of casing 1920s Sample: LackGehäuseteile 63 B B(c) |  |  |
| 579 Object: smoke helmet Part: inner sealing band 1930s Samples: Innendichtband 579 A B(o) |  |  |
| 594 Object: inclined tube manometer Part: stopper 2nd half 20th century Sample: Stopfen 594 B B(c) |  |  |
| 610 Object: mining hammer Part: pressure handle 1970s Sample: Ballendrücker 610 B B(o) |  |  |
| 2223-2 Object: gas mask Part: headband 1980s Sample: Atemschutzmaske 2223-2 B(o) |  |  |
| Object/Part | Chemical Nature of the Plastic on Which It Was Found | Nature of the Blooming |
|---|---|---|
| 63 Coating of a calculator casing | Unknown | Fatty acid(s) |
| 579 Inner sealing band of a smoke helmet | Rubber (butene-based?) | Talc |
| 594 Stopper | Polyisoprene (main component), contains styrene as a secondary monomer | Mineral wax |
| 610 Pressure handle of a mining hammer | Polyester urethane | Adipic acid |
| 2223-2 Gas mask | Chloroprene-containing | Mineral wax |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krieg, T.; Mazzon, C.; Gómez-Sánchez, E. Material Analysis and a Visual Guide of Degradation Phenomena in Historical Synthetic Polymers as Tools to Follow Ageing Processes in Industrial Heritage Collections. Polymers 2022, 14, 121. https://doi.org/10.3390/polym14010121
Krieg T, Mazzon C, Gómez-Sánchez E. Material Analysis and a Visual Guide of Degradation Phenomena in Historical Synthetic Polymers as Tools to Follow Ageing Processes in Industrial Heritage Collections. Polymers. 2022; 14(1):121. https://doi.org/10.3390/polym14010121
Chicago/Turabian StyleKrieg, Till, Cristian Mazzon, and Elena Gómez-Sánchez. 2022. "Material Analysis and a Visual Guide of Degradation Phenomena in Historical Synthetic Polymers as Tools to Follow Ageing Processes in Industrial Heritage Collections" Polymers 14, no. 1: 121. https://doi.org/10.3390/polym14010121
APA StyleKrieg, T., Mazzon, C., & Gómez-Sánchez, E. (2022). Material Analysis and a Visual Guide of Degradation Phenomena in Historical Synthetic Polymers as Tools to Follow Ageing Processes in Industrial Heritage Collections. Polymers, 14(1), 121. https://doi.org/10.3390/polym14010121







