Synthesis, Characterization of Chitosan-Aluminum Oxide Nanocomposite for Green Synthesis of Annulated Imidazopyrazol Thione Derivatives
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Preparation of Heterogeneous Catalyst (Chitosan-Al2O3 Hybrid Nanocomposite)
2.3. Recyclability of the Catalyst
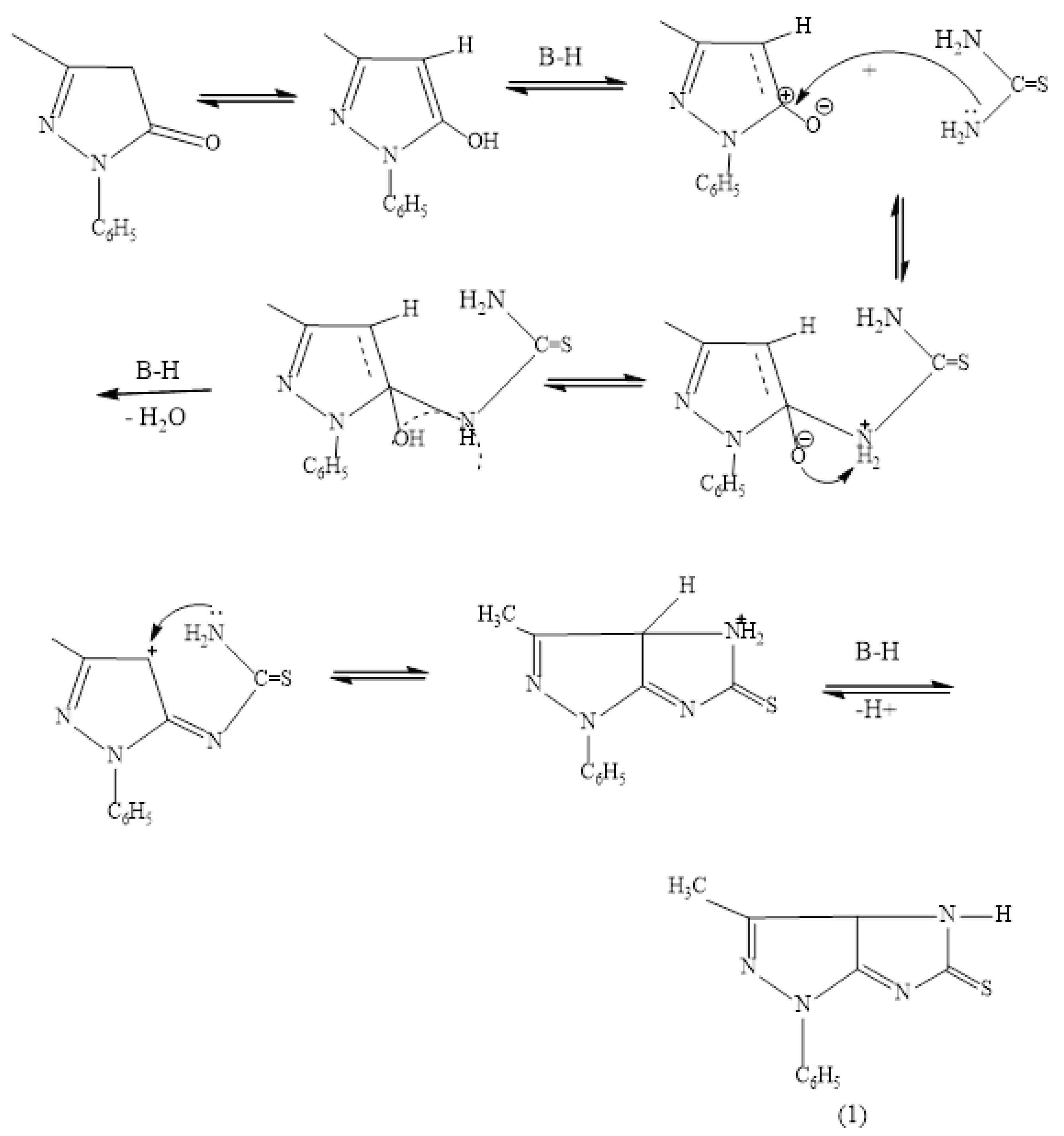
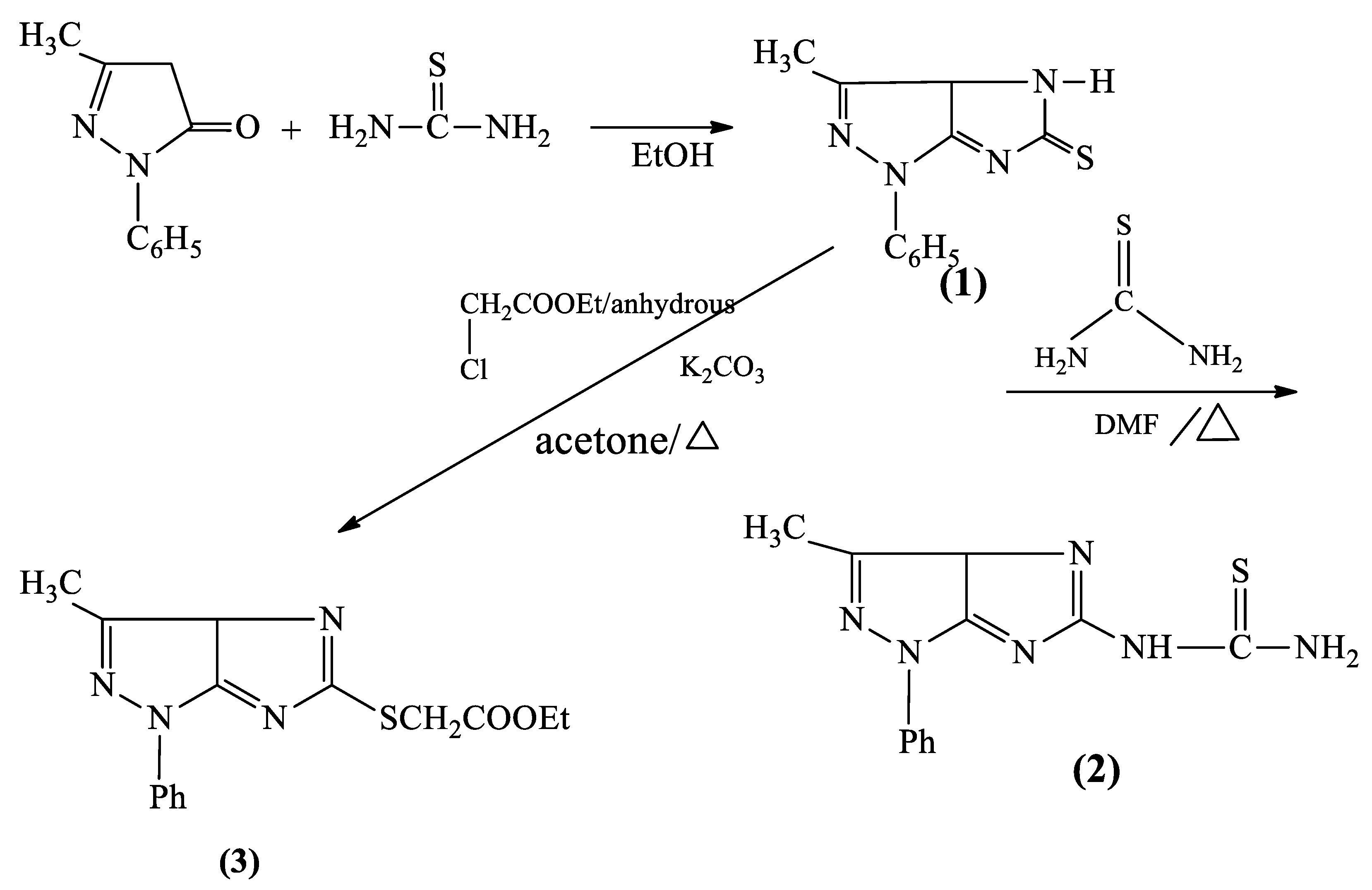
2.4. Synthesis of 3-Methyl-1-Phenyl-3a,4-Dihydro Imidazo (4,5-c) Pyrazole-5(1H)-Thione (1)
2.5. Synthesis of 1-(3-Methyl-1-Phenyl-1,3a-Dihydroimidazo [4,5-c] Pyrazol-5-yl) Thiourea (2)
2.6. Synthesis of Ethyl 2-((3-Methyl-1-Phenyl-1,3adihydroimidazo [4,5-c] Pyrazol-5-yl) thio) Acetate (3)
2.7. Biological Evaluation
2.7.1. In Vitro Antibacterial Evaluation
2.7.2. In Silico Protein Preparation and Active Site Prediction
2.7.3. In Silico Ligand Preparation
2.7.4. Molecular Docking Study
3. Results and Discussion
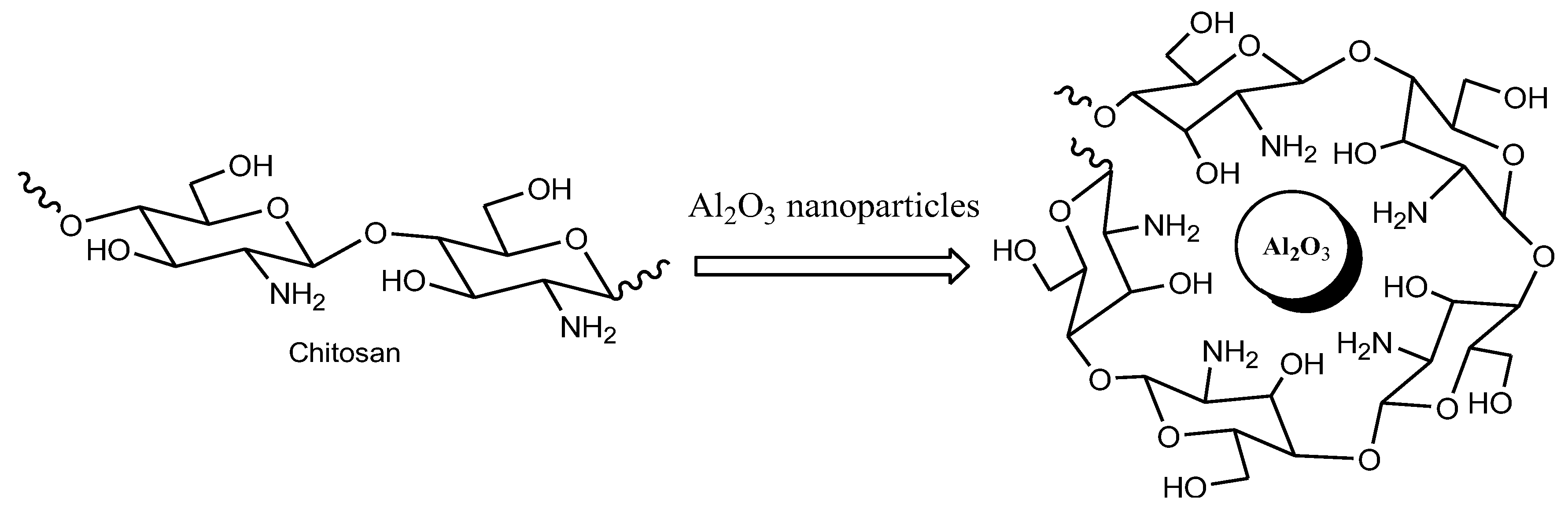
3.1. Preparation of Chitosan-Al2O3 Hybrid Nanocomposite
3.2. Characterization of Chitosan Based Al2O3 Nanocomposite
3.2.1. FTIR Characterization
3.2.2. XRD Characterization
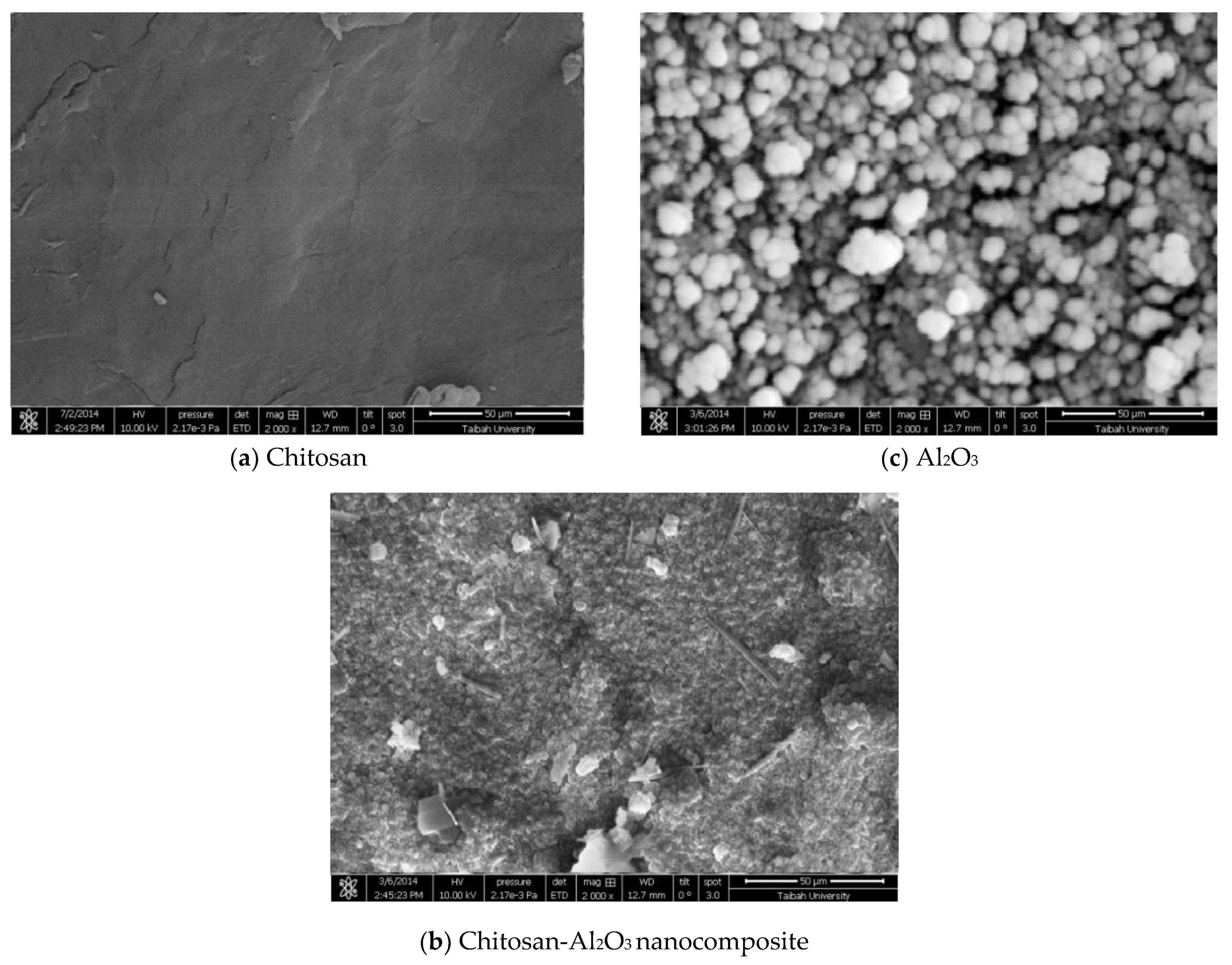
3.2.3. Emission Scanning Electron Microscopy (ESEM) and Morphological Changes
3.2.4. Energy Dispersive X-ray Measurements and Estimation of Aluminum
3.3. CS-Al2O3 Nanocomposites as Base Heterogeneous Catalyst for the Synthesis of Imidazo Pyrazolyl Thione Derivatives
3.4. Reusability of the Heterogeneous Chitosan-Aluminum Oxide Nanocomposite for Synthesis of Various Imidazo Pyrazolyl Thione Derivatives
3.5. Antibacterial Activities
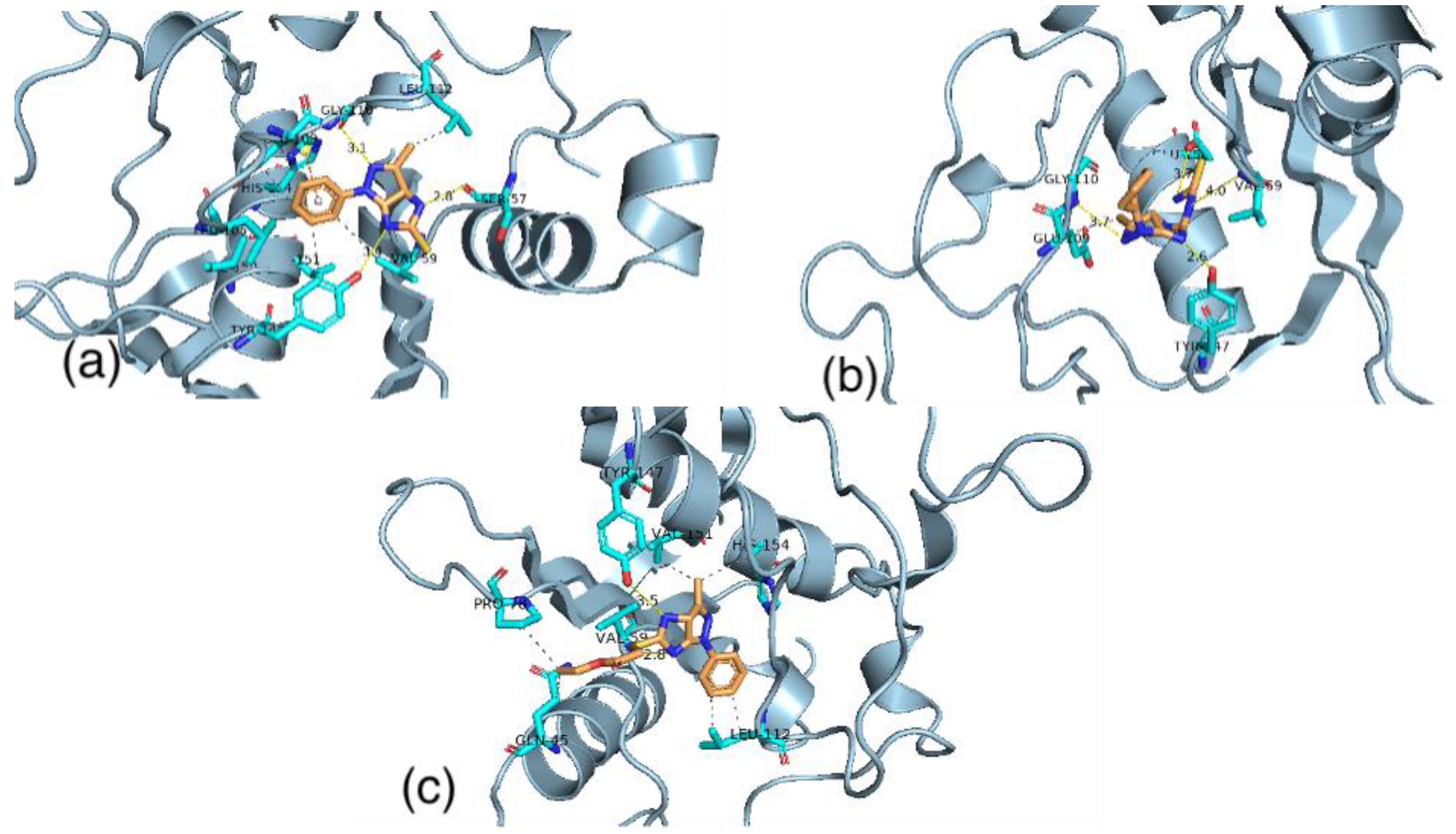
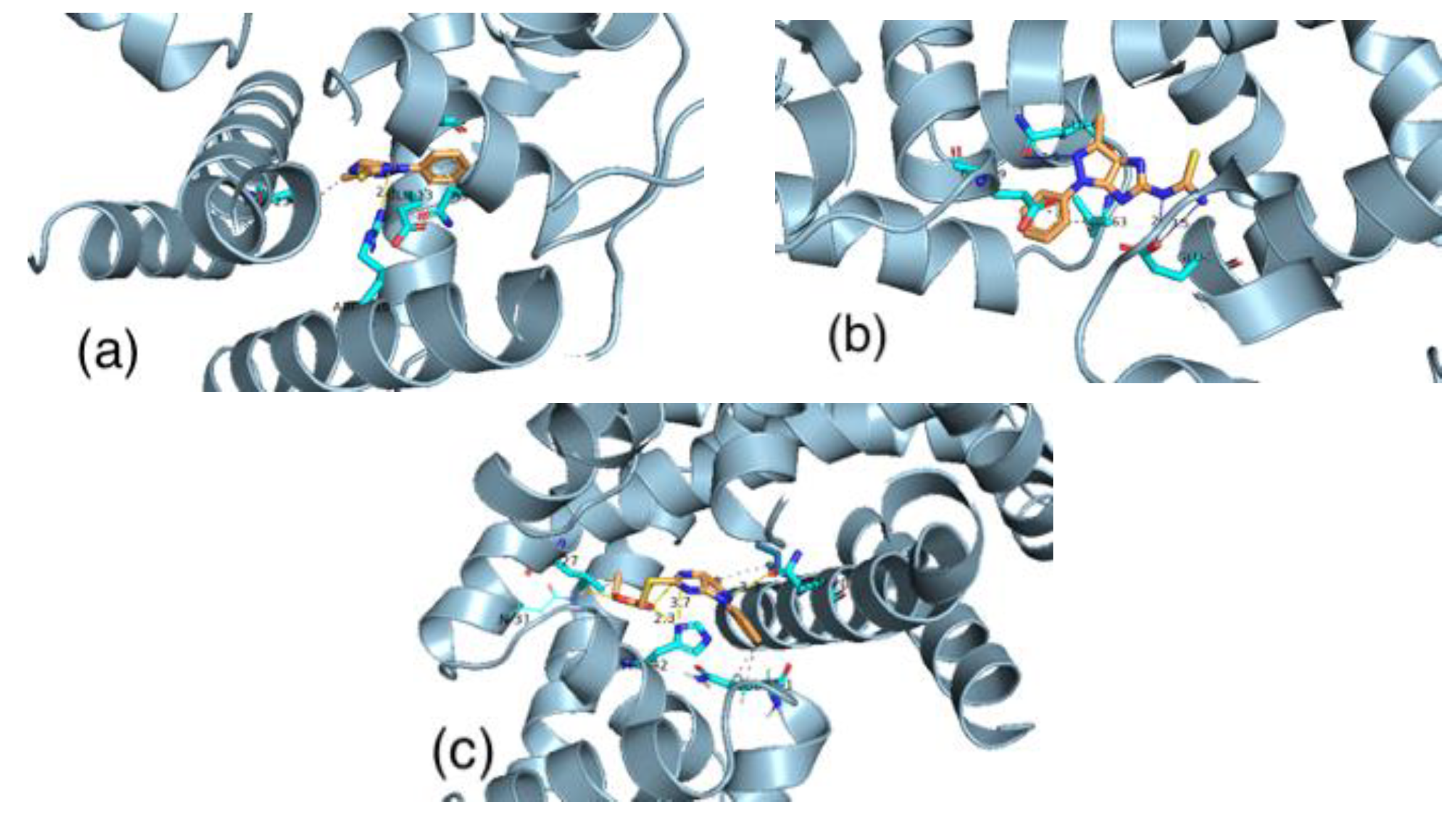
3.6. Molecular Docking
3.6.1. Pseudomonas Aeruginosa
3.6.2. Staphylococcus Aureus
3.6.3. Staphylococcus Epidermidis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Sareen, V.; Khatri, V.; Sareen, S. Recent Applications of Pyrazole and its Substituted Analogs. Int. J. Appl. Res. 2016, 2, 461–469. [Google Scholar]
- Parajuli, R.R.; Pokhrel, P.; Tiwari, A.K.; Banerjee, J. Pharmacological activities of pyrazolone derivatives. Int. J. Appl. Pharm. Res. 2013, 10, 5–13. [Google Scholar]
- Bhatt, H.B.; Sharma, S. Synthesis and antimicrobial activity of pyrazole nucleus containing 2-thioxothiazolidin-4-one derivatives. Arab. J. Chem. 2017, 10, S1590–S1596. [Google Scholar] [CrossRef] [Green Version]
- Patra, P.K.; Patra, C.N.; Pattnaik, S. Synthesis and antibacterial activity screening of some novel pyrazole derivatives. Int. J. Pharm. Pharm. Sci. 2014, 6, 801–805. [Google Scholar]
- Kumar, P.; Chandak, N.; Kaushik, P.; Sharma, C.; Kaushik, D.; Aneja, K.R.; Sharma, P.K. Synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory–antibacterial agents. Med. Chem. Res. 2012, 21, 3396–3405. [Google Scholar] [CrossRef]
- Burnett, M.L. The pollution prevention act of 1990: A policy whose time has come or symbolic legislation. Environ. Manag. 1998, 22, 213–224. [Google Scholar] [CrossRef]
- Zhu, J.; Bienaymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar] [CrossRef] [Green Version]
- Candeias, N.R.; Cal, P.M.S.D.; Andre, V.; Duarte, M.T.; Veiros, L.F.; Gois, P.M.P. Water as the reaction mediumfor multicomponent reactions based on boronic acid. Tetrahedron 2010, 66, 2736–2745. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Khalil, K.D.; Adam, A.Y.; Elnagdi, M.H. Green one pot solvent-free synthesis of pyrano[2,3-c]-pyrazoles and pyrazolo[1,5-a]pyrimidines. Molecules 2010, 15, 6619–6629. [Google Scholar] [CrossRef] [Green Version]
- Gou, S.B.; Wang, S.X.; Li, J.D. d,l-proline catalyzed one-pot synthesis of pyrans and pyrano[2,3-c]pyrazole derivatives by a grinding method under solvent-free conditions. Synth. Commun. 2007, 37, 2111–2120. [Google Scholar] [CrossRef]
- Elnagdi, N.M.H.; Al-Hokbany, N.S. Organocatalysis in synthesis: l-proline as an enantioselective catalyst in the synthesis of pyrans and thiopyrans. Molecules 2012, 17, 4300–4312. [Google Scholar] [CrossRef] [Green Version]
- Al-Zaydi, K.M. Microwave assisted synthesis, part 1: Rapid solventless synthesis of 3- substituted coumarins and benzocoumarins by microwave irradiation of the corresponding enaminones. Molecules 2003, 8, 541–555. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y. Speciation of arsenic and chromium metal ions by reversed phase high performance liquid chromatography. Chemosphere 2002, 48, 275–278. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Khalil, K.D.; Meier, H.; Kolshorn, H.; Elnagdi, M.H. Chitosan as heterogeneous catalyst in Michael additions: The reaction of cinnamonitriles with active methylene Moieties and phenols. Arkivoc 2008, 16, 288–301. [Google Scholar] [CrossRef] [Green Version]
- Khalil, K.D.; Al-Matar, H.M. Chitosan based heterogeneous catalyses: 4-Vinylpyridine grafted chitosan as catalyses for Michael additions and alkylpyridazinyl carbonitrile oxidation. Molecules 2013, 18, 5288–5305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramod, K.S.; Praveen, K.S.; Sushil, K.G.; Dau, D.A. Chitosan: An efficient, reusable, and biodegradable catalyst for green synthesis of heterocycles. Ind. Eng. Chem. Res. 2014, 53, 2085–2091. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3] triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef]
- Cartwright, A.; Jackson, K.; Morgan, C.; Anderson, A.; Britt, D.W.A. Review of Metal and Metal-Oxide Nanoparticle Coating Technologies to Inhibit Agglomeration and Increase Bioactivity for Agricultural Applications. Agronomy 2020, 10, 1018. [Google Scholar] [CrossRef]
- Nikoofar, K.; Shahedi, Y.; Chenarboo, F.J. Nano Alumina Catalytic Applications in Organic Transformations. Mini Rev. Org. Chem. 2019, 16, 102–110. [Google Scholar] [CrossRef]
- Shoichet, B.K.; McGovern, S.L.; Wei, B.; Irwin, J.J. Lead discovery using molecular docking. Curr. Opin. Chem. Biol. 2002, 6, 439–446. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Santos, R.N.D.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Sarojini, B.K.; Krishna, B.G.; Darshanraj, C.G.; Bharath, B.R.; Manjunatha, H. Synthesis, characterization, in vitro and molecular docking studies of new 2,5-dichloro thienyl substituted thiazole derivatives for antimicrobial properties. Eur. J. Med. Chem. 2010, 45, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard, 11th ed.; CLSI document M02-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Kreusch, A.; Spraggon, G.; Lee, C.C.; Klock, H.; McMullan, D.; Ng, K.; Shin, T.; Vincent, J.; Warner, I.; Ericson, C.; et al. Structure analysis of peptide deformylases from Streptococcus pneumoniae, Staphylococcus aureus, Thermotoga maritima and Pseudomonas aeruginosa: Snapshots of the oxygen sensitivity of peptide deformylase. J. Mol. Biol. 2003, 330, 309–321. [Google Scholar] [CrossRef]
- Guilloteau, J.P.; Mathieu, M.; Giglione, C.; Blanc, V.; Dupuy, A.; Chevrier, M.; Gil, P.; Famechon, A.; Meinnel, T.; Mikol, V. The crystal structures of four peptide deformylases bound to the antibiotic actinonin reveal two distinct types: A platform for the structure-based design of antibacterial agents. J. Mol. Biol. 2002, 320, 951–962. [Google Scholar] [CrossRef]
- Chang, Y.M.; Jeng, W.Y.; Ko, T.P.; Yeh, Y.J.; Chen, C.K.; Wang, A.H. Structural study of TcaR and its complexes with multiple antibiotics from Staphylococcus epidermidis. Proc. Natl. Acad. Sci. USA 2010, 107, 8617–8622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apfel, C.M.; Locher, H.; Evers, S.; Takács, B.; Hubschwerlen, C.; Pirson, W.; Page, M.G.; Keck, W. Peptide deformylase as an antibacterial drug target: Target validation and resistance development. Antimicrob. Agents Chemother. 2001, 45, 1058–1064. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, T.A.; Gupta, P.R.; Asthana, S.O.; Khursheed, A.S. In silico analysis to access the antibacterial effect of thiazides on pdfs: Molecular docking approach. Int. J. Pharm. Pharm. Sci. 2014, 6, 387–391. [Google Scholar]
- Lin, P.; Hu, T.; Hu, J.; Yu, W.; Han, C.; Zhang, J.; Qin, G.; Yu, K.; Goetz, F.; Shen, X.; et al. Characterization of peptide deformylase homologues from Staphylococcus epidermidis. Microbiology 2010, 156 Pt 10, 3194–3202. [Google Scholar] [CrossRef] [Green Version]
- Mazel, D.; Pochet, S.; Marlière, P. Genetic characterization of polypeptide deformylase, a distinctive enzyme of eubacterial translation. EMBO J. 1994, 13, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef] [Green Version]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- ACD/ChemSketch; Advanced Chemistry Development, Inc: Toronto, ON, Canada. 2021. Available online: www.acdlabs.com (accessed on 6 January 2021).
- The PyMOL Molecular Graphics System; Schrodinger, LLC: New York, NY, USA. 2010. Available online: https://pymol.org/ (accessed on 8 January 2021).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, K.D.; Ibrahim, E.I.; Al-Sagheer, F.A. A novel, efficient, and recyclable biocatalyst for Michael addition reactions and its iron(iii) complex as promoter for alkyl oxidation reactions. Catal. Sci. Technol. 2016, 6, 1410–1416. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical, Optical and Biological Activity of Chitosan-Chromone Derivative for Biomedical Applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Scheme 1 | Fresh Catalyst | Recycled (1) | Recycled (2) | Recycled (3) | Recycled (4) |
|---|---|---|---|---|---|
| Product 2 (%Yield) | 95 | 94 | 93 | 92 | 92 |
| Bacterial Test | (1) | (2) | (3) | Positive Control Gentamicin (CN10 µg) | |||
|---|---|---|---|---|---|---|---|
| 0.05 g/mL | 0.1 g/mL | 0.05 g/mL | 0.1 g/mL | 0.05 g/mL | 0.1 g/mL | ||
| P. aeruginosa ATCC 27853 | 10.0 ± 1.0 | 11.0 ± 1.0 | 7.7 ± 0.6 | 8.3 ± 0.6 | 7.3 ± 0.6 | 7.3 ± 0.6 | 20.7 ± 1.2 |
| Staph. epidermidis ATCC 12228 | 8.3 ± 0.6 | 8.3 ± 0.6 | 8.3 ± 0.6 | 9.3 ± 0.6 | 7.3 ± 0.6 | 8.7 ± 0.6 | 21.3 ± 1.2 |
| Staph. aureus ATCC 29213 | 7.7 ± 0.6 | 9.0 ± 1.0 | 18.0 ± 0.0 | 18.7 ± 0.6 | 7.3 ± 0.6 | 8.7 ± 0.6 | 18.7 ± 1.5 |
| Bacteria | Ligands | ||
|---|---|---|---|
| (1) | (2) | (3) | |
| Pseudomonas aeruginosa | −7.73 | −8.17 | −8.68 |
| Staphylococcus aureus | −7.11 | −8.12 | −7.98 |
| Staphylococcus epidermidis | −7.06 | −7.89 | −7.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Naby, A.S.; Nabil, S.; Aldulaijan, S.; Ababutain, I.M.; Alghamdi, A.I.; Almubayedh, S.; Khalil, K.D. Synthesis, Characterization of Chitosan-Aluminum Oxide Nanocomposite for Green Synthesis of Annulated Imidazopyrazol Thione Derivatives. Polymers 2021, 13, 1160. https://doi.org/10.3390/polym13071160
Abdel-Naby AS, Nabil S, Aldulaijan S, Ababutain IM, Alghamdi AI, Almubayedh S, Khalil KD. Synthesis, Characterization of Chitosan-Aluminum Oxide Nanocomposite for Green Synthesis of Annulated Imidazopyrazol Thione Derivatives. Polymers. 2021; 13(7):1160. https://doi.org/10.3390/polym13071160
Chicago/Turabian StyleAbdel-Naby, Abir S., Sara Nabil, Sarah Aldulaijan, Ibtisam M. Ababutain, Azzah I. Alghamdi, Somaiah Almubayedh, and Khaled D. Khalil. 2021. "Synthesis, Characterization of Chitosan-Aluminum Oxide Nanocomposite for Green Synthesis of Annulated Imidazopyrazol Thione Derivatives" Polymers 13, no. 7: 1160. https://doi.org/10.3390/polym13071160
APA StyleAbdel-Naby, A. S., Nabil, S., Aldulaijan, S., Ababutain, I. M., Alghamdi, A. I., Almubayedh, S., & Khalil, K. D. (2021). Synthesis, Characterization of Chitosan-Aluminum Oxide Nanocomposite for Green Synthesis of Annulated Imidazopyrazol Thione Derivatives. Polymers, 13(7), 1160. https://doi.org/10.3390/polym13071160







