Effects of Dentine Pretreatment Solutions Containing Flavonoids on the Resin Polymer-Dentine Interface Created Using a Modern Universal Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of the Experimental Pretreatment Solutions
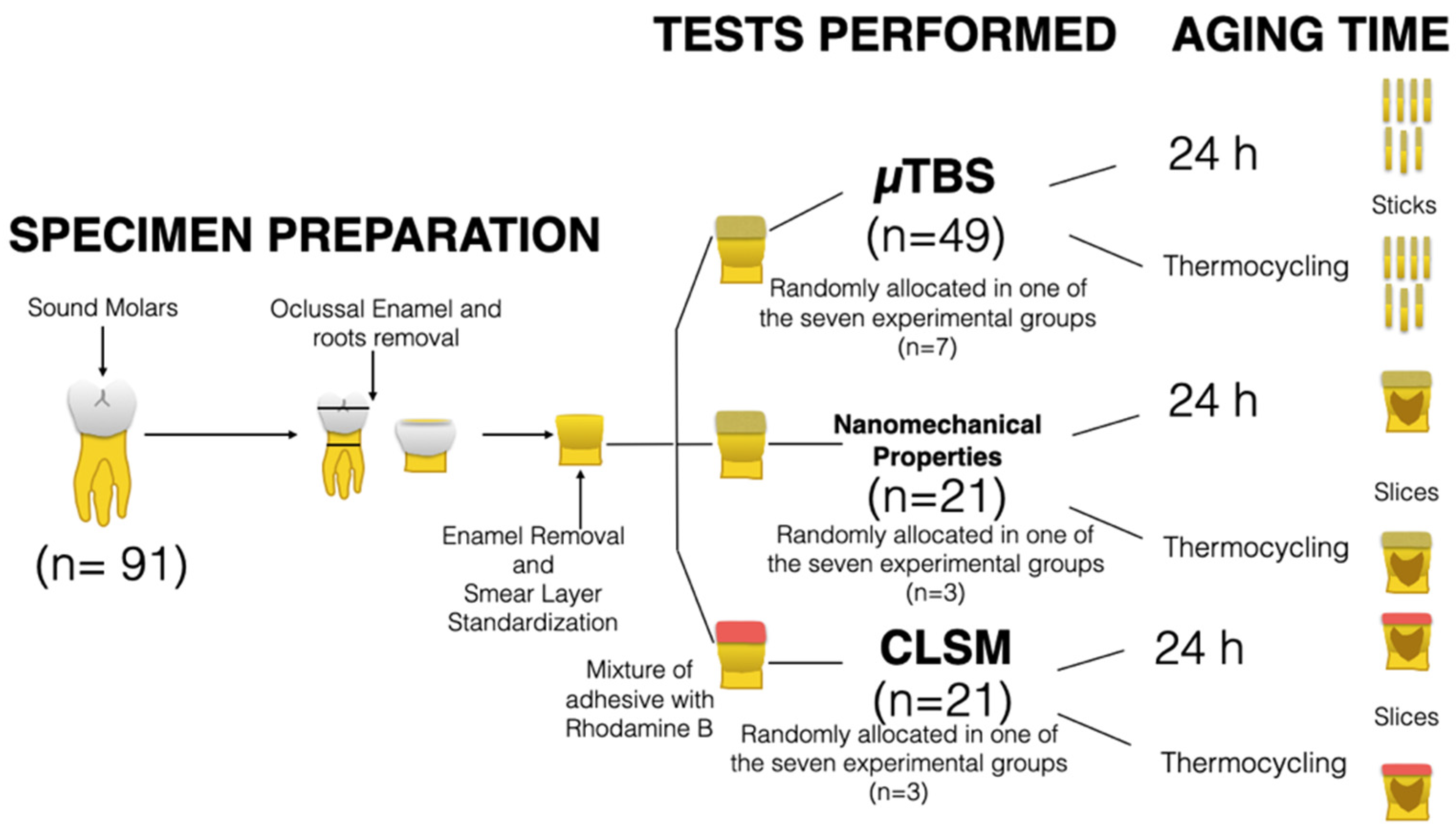
2.2. Preparation of Specimens and Application of the Adhesive System
2.3. Evaluation of Microtensile Bond Strength (µTBS)
2.4. Nanoindentation: Hardness and Modulus of Elasticity across the Interface
2.5. Confocal Laser Scanning Microscopy Analysis of the Adhesive Interface
2.6. Statistical Analysis
3. Results
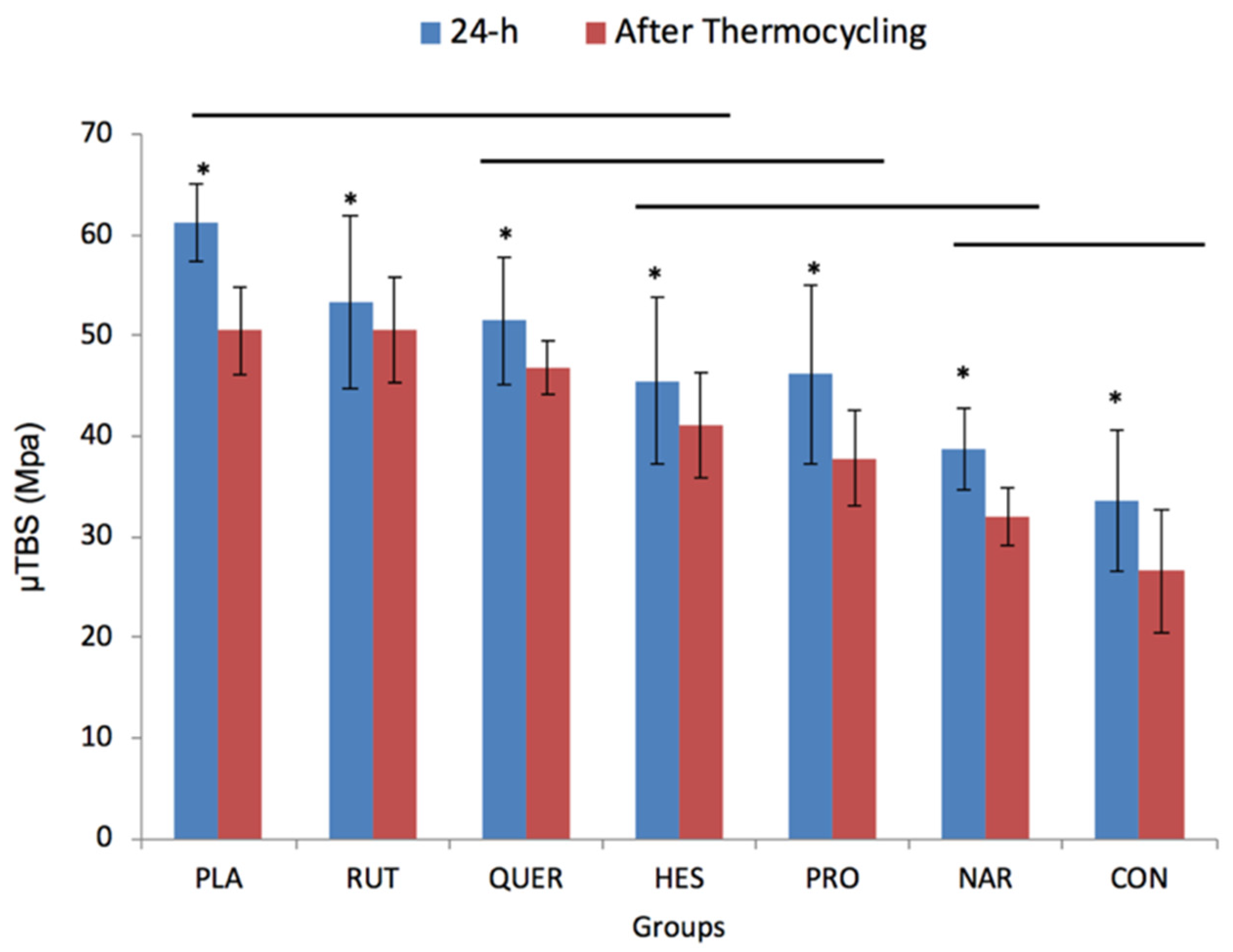
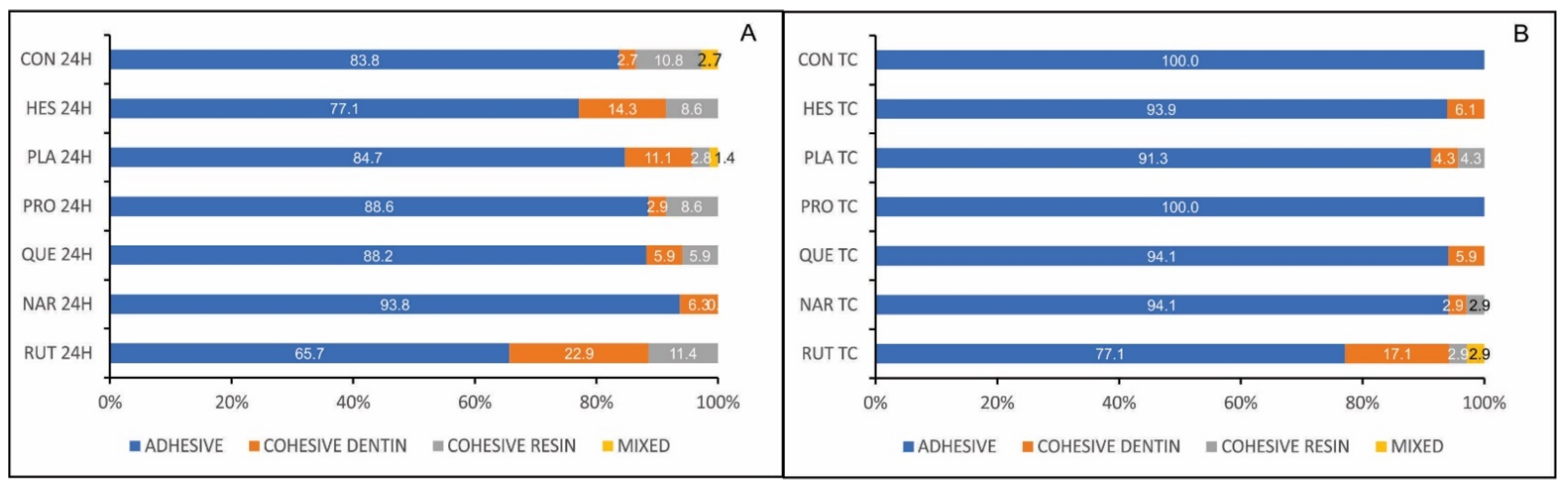
3.1. Microtensile Bond Strength Testing and Failure Mode Analysis
3.2. Nanoindentation: Hardness and Modulus of Elasticity across the Interface
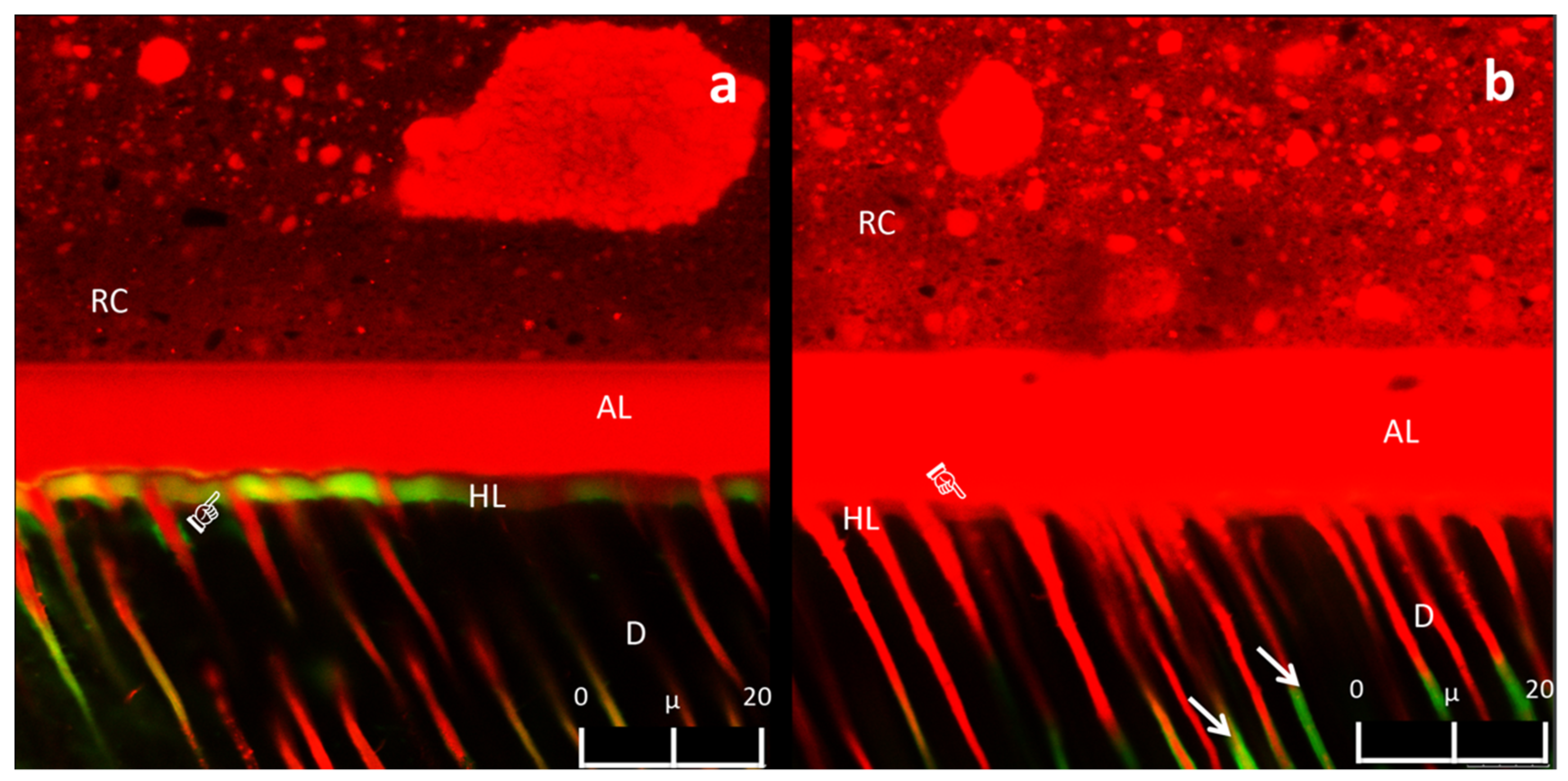
3.3. Confocal Laser Scanning Microscopy Analysis of the Adhesive Interface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability. A literature review. Dent. Mater. 2016, 32, 41–53. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin interface: The weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef]
- Liu, Y.; Tjaderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef]
- Carvalho, R.M.; Manso, A.P.; Geraldeli, S.; Tay, F.R.; Pashley, D.H. Durability of bonds and clinical success of adhesive restorations. Dent. Mater. 2012, 28, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Tjaderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Hannas, A.R.; Pereira, J.C.; Granjeiro, J.M.; Tjaderhane, L. The role of matrix metalloproteinases in the oral environment. Acta Odontol. Scand. 2007, 65, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tjaderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef]
- Gomes, G.M.; Gomes, O.M.; Reis, A.; Gomes, J.C.; Loguercio, A.D.; Calixto, A.L. Effect of operator experience on the outcome of fiber post cementation with different resin cements. Oper. Dent. 2013, 38, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Sono, R.; Sofiqul, I.; Yiu, C.; Nakamura, H.; Otsuki, M.; Takatsuka, T.; Tagami, J. In vitro evaluation of plant-derived agents to preserve dentin collagen. Dent. Mater. 2013, 29, 1048–1054. [Google Scholar] [CrossRef]
- Abuelsaad, A.S.; Mohamed, I.; Allam, G.; Al-Solumani, A.A. Antimicrobial and immunomodulating activities of hesperidin and ellagic acid against diarrheic Aeromonas hydrophila in a murine model. Life Sci. 2013, 93, 714–722. [Google Scholar] [CrossRef]
- Bakar, N.S.; Zin, N.M.; Basri, D.F. Synergy of flavone with vancomycin and oxacillin against vancomycin-intermediate Staphyloccus aureus. Pak. J. Pharm. Sci. 2012, 25, 633–638. [Google Scholar]
- Basile, A.; Sorbo, S.; Giordano, S.; Ricciardi, L.; Ferrara, S.; Montesano, D.; Castaldo Cobianchi, R.; Vuotto, M.L.; Ferrara, L. Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia 2000, 71, 110–116. [Google Scholar] [CrossRef]
- Leme-Kraus, A.A.; Aydin, B.; Vidal, C.M.; Phansalkar, R.M.; Nam, J.W.; McAlpine, J.; Pauli, G.F.; Chen, S.; Bedran-Russo, A.K. Biostability of the proanthocyanidins-dentin complex and adhesion studies. J. Dent. Res. 2017, 96, 406–412. [Google Scholar] [CrossRef]
- Yang, H.; Li, K.; Yan, H.; Liu, S.; Wang, Y.; Huang, C. High-performance therapeutic quercetin-doped adhesive for adhesive-dentin interfaces. Sci. Rep. 2017, 7, 8189. [Google Scholar] [CrossRef]
- Islam, M.S.; Hiraishi, N.; Nassar, M.; Yiu, C.; Otsuki, M.; Tagami, J. Effect of hesperidin incorporation into a self-etching primer on durability of dentin bond. Dent. Mater. 2014, 30, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Hiraishi, N.; Nassar, M.; Yiu, C.; Otsuki, M.; Tagami, J. Effect of natural cross-linkers incorporation in a self-etching primer on dentine bond strength. J. Dent. 2012, 40, 1052–1059. [Google Scholar] [CrossRef]
- Epasinghe, D.J.; Yiu, C.K.; Burrow, M.F.; Tsoi, J.K.; Tay, F.R. Effect of flavonoids on the mechanical properties of demineralised dentine. J. Dent. 2014, 42, 1178–1184. [Google Scholar] [CrossRef]
- Castellan, C.S.; Bedran-Russo, A.K.; Karol, S.; Pereira, P.N. Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. J. Mech. Behav. Biomed. Mater. 2011, 4, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Yao, X.; Xu, C.; Zhang, Y.; Wang, Y. Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dent. Mater. 2013, 29, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Haibao, L.; Yanju, L.; Jinsong, L.; Shanyi, D. Qualitative separation of the physical swelling effect on the recovery behavior of shape memory polymer. Eur. Polym. J. 2010, 46, 1908–1914. [Google Scholar] [CrossRef]
- Haibao, L.; Shanyi, D. A phenomenological thermodynamic model for the chemo-responsive shape memory effect in polymers based on Flory-Huggins solution theory. Polym. Chem. 2014, 5, 1155–1162. [Google Scholar] [CrossRef]
- Fang, M.; Liu, R.; Xiao, Y.; Li, F.; Wang, D.; Hou, R.; Chen, J. Biomodification to dentin by a natural crosslinker improved the resin-dentin bonds. J. Dent. 2012, 40, 458–466. [Google Scholar] [CrossRef]
- Davila-Sanchez, A.; Gutierrez, M.F.; Bermudez, J.P.; Mendez-Bauer, M.L.; Hilgemberg, B.; Sauro, S.; Loguercio, A.D.; Arrais, C.A.G. Influence of flavonoids on long-term bonding stability on caries-affected dentin. Dent. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, D.Y.; Fan, J.; Li, F.; Chen, Y.J.; Chen, J.H. Stability of bonds made to superficial vs. deep dentin, before and after thermocycling. Dent. Mater. 2014, 30, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface. J. Mater. Sci. 2012, 23, 1521–1532. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar] [CrossRef]
- Han, B.; Jaurequi, J.; Tang, B.W.; Nimni, M.E. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. J. Biomed. Mater. Res. A 2003, 65, 118–124. [Google Scholar] [CrossRef]
- Aguiar, T.R.; Vidal, C.M.; Phansalkar, R.S.; Todorova, I.; Napolitano, J.G.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F.; Bedran-Russo, A.K. Dentin biomodification potential depends on polyphenol source. J. Dent. Res. 2014, 93, 417–422. [Google Scholar] [CrossRef]
- De Souza, L.C.; Rodrigues, N.S.; Cunha, D.A.; Feitosa, V.P.; Santiago, S.L.; Reis, A.; Loguercio, A.D.; Perdigao, J.; Saboia, V.P.A. Two-year clinical evaluation of a proanthocyanidins-based primer in non-carious cervical lesions: A double-blind randomized clinical trial. J. Dent. 2020, 96, 103325. [Google Scholar] [CrossRef]
- De Souza, L.C.; Rodrigues, N.S.; Cunha, D.A.; Feitosa, V.P.; Santiago, S.L.; Reis, A.; Loguercio, A.D.; Matos, T.P.; Saboia, V.P.A.; Perdigao, J. Two-year clinical evaluation of proanthocyanidins added to a two-step etch-and-rinse adhesive. J. Dent. 2019, 81, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, X.; Li, S.; Liu, Y.; Keightley, A.; Wang, Y. Molecular weight and galloylation affect grape seed extract constituents’ ability to cross-link dentin collagen in clinically relevant time. Dent. Mater. 2015, 31, 814–821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, L.; Zhang, Z.H.; Sun, E.; Jia, X. Effect of β-Cyclodextrin Complexation on Solubility and Enzymatic Conversion of Naringin. Int. J. Mol. Sci. 2012, 13, 14251–14261. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.; Juhler, R.K.; Svensmark, B.; Cech, N.B. The relative influences of acidity and polarity on responsiveness of small organic molecules to analysis with negative ion electrospray ionization mass spectrometry (ESI-MS). J. Am. Soc. Mass Spectrom. 2005, 16, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Meredith, H.J.; Wilker, J.J. Molecular weight effects upon the adhesive bonding of a mussel mimetic polymer. ACS Appl. Mater. Interfaces 2013, 5, 5091–5096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bre, L.P.; Zhao, T.; Zheng, Y.; Newland, B.; Wang, W. Mussel-inspired hyperbranched poly(amino ester) polymer as strong wet tissue adhesive. Biomaterials 2014, 35, 711–719. [Google Scholar] [CrossRef]
- Maier, G.P.; Rapp, M.V.; Waite, J.H.; Israelachvili, J.N.; Butler, A. Biological Adhesives. Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science 2015, 349, 628–632. [Google Scholar] [CrossRef]
- Cho, J.H.; Shanmuganathan, K.; Ellison, C.J. Bioinspired catecholic copolymers for antifouling surface coatings. ACS Appl. Mater. Interfaces 2013, 5, 3794–3802. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; Garcia, P.C.; Lopez-Lefebre, L.R.; Sanchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant. Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Gao, B.T.; Lin, H.; Zheng, G.; Xu, Y.X.; Yang, J.L. Comparison between a silorane-based composite and methacrylate-based composites: Shrinkage characteristics, thermal properties, gel point and vitrification point. Dent. Mater. J. 2012, 31, 76–85. [Google Scholar] [CrossRef]
- Fathima, N.N.; Baias, M.; Blumich, B.; Ramasami, T. Structure and dynamics of water in native and tanned collagen fibers: Effect of crosslinking. Int. J. Biol. Macromol. 2010, 47, 590–596. [Google Scholar] [CrossRef]
- Guneser, M.B.; Arslan, D.; Dincer, A.N.; Er, G. Effect of sodium hypochlorite irrigation with or without surfactants on the bond strength of an epoxy-based sealer to dentin. Clin. Oral Investig. 2017, 21, 1259–1265. [Google Scholar] [CrossRef]
- Zanchi, C.H.; Munchow, E.A.; Ogliari, F.A.; de Carvalho, R.V.; Chersoni, S.; Prati, C.; Demarco, F.F.; Piva, E. Effects of long-term water storage on the microtensile bond strength of five experimental self-etching adhesives based on surfactants rather than HEMA. Clin. Oral. Investig. 2013, 17, 833–839. [Google Scholar] [CrossRef]
- Somasundaran, P.; Krishnakumar, S. Adsorption of surfactants and polymers at the solid-liquid interface. Colloids Surf. A 1997, 123, 491–513. [Google Scholar] [CrossRef]
- Greco, K.V.; Francis, L.; Huang, H.; Ploeg, R.; Boccaccini, A.R.; Ansari, T. Is quercetin an alternative natural crosslinking agent to genipin for long-term dermal scaffolds implantation? J. Tissue Eng. Regen. Med. 2018, 12, 1716–1724. [Google Scholar] [CrossRef]
- Greco, K.V.; Francis, L.; Somasundaram, M.; Greco, G.; English, N.R.; Roether, J.A.; Boccaccini, A.R.; Sibbons, P.; Ansari, T. Characterisation of porcine dermis scaffolds decellularised using a novel non-enzymatic method for biomedical applications. J. Biomater. Appl. 2015, 30, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.; Rao, V.H.; Steinmann, B. Influence of bioflavonoids on the metabolism and crosslinking of collagen. Ital. J. Biochem. 1981, 30, 259–270. [Google Scholar] [PubMed]
- Rao, C.N.; Rao, V.H.; Steinmann, B. Influence of bioflavonoids on the collagen metabolism in rats with adjuvant induced arthritis. Ital. J. Biochem. 1981, 30, 54–62. [Google Scholar] [PubMed]
- Samadian, H.; Vaez, A.; Ehterami, A.; Salehi, M.; Farzamfar, S.; Sahrapeyma, H.; Norouzi, P. Sciatic nerve regeneration by using collagen type I hydrogel containing naringin. J. Mater. Sci. Mater. Med. 2019, 30, 107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 162750. [Google Scholar] [CrossRef] [PubMed]


| Component | Compound | Quantity % |
|---|---|---|
| Active Compound | Flavonoid | 6.5% mass |
| Vehicle (Pure Ethanol) | Pure Ethanol | 30% (3 mL) |
| Surfactant (Polysorbate 20) | SPAN 20 | 1% (0.1 g) |
| Aqueous Medium | Distilled Water | QS 10 mL |
| Substance | Molecular Mass | Number of Hydroxyphenyl Radicals | Number of Alcoholic Radicals | Number of Mols (6.5% Mass) | Solubility in Water |
|---|---|---|---|---|---|
| Hesperidin | 610.56 g/mol | 2 | 6 | 1.06 mm | 0.02 mg/mL |
| Naringin | 580.53 g/mol | 2 | 6 | 1.12 mm | 1 mg/mL at 40 °C |
| Proanthocianydin | 595.55 g/mol | 7 * | 2 * | 1.09 mm * | 0.130 mg/mL * |
| Quercetin | 302.24 g/mol | 5 | - | 2.15 mm | 0.06 mg/ml |
| Rutin | 610.52 g/mol | 4 | 6 | 1.06 mm | 0.125 mg/ml |
| Indentation Site | Flavonoid | NH 24 h | NH Thermocycling | E 24 h | E Thermocycling |
|---|---|---|---|---|---|
| Adhesive Layer | PLA | 0.265 (0.051) Ac | 0.256 (0.018) Aa | 6.55 (0.86) Ac | 5.63 (0.89) Aa |
| RUT | 0.282 (0.002) Abc | 0.249 (0.017) Aa | 7.10 (0.88) Ac | 6.29 (0.56) Aa | |
| QUE | 0.274 (0.022) Ac | 0.250 (0.011) Aa | 7.14 (1.44) Ac | 6.16 (0.31) Aa | |
| HES | 0.444 (0.076) Aa | 0.285 (0.039) Ba | 12.90 (2.77) Aab | 7.40 (0.76) Ba | |
| PRO | 0.290 (0.051) Aabc | 0.244 (0.024) Aa | 7.81 (1.59) Abc | 7.20 (0.86) Aa | |
| NAR | 0.440 (0.061) Aab | 0.306 (0.018) Ba | 13.60 (1.82) Aa | 8.89 (3.27) Ba | |
| CON | 0.275 (0.07) Ac | 0.286 (0.040) Aa | 7.48 (1.96) Ac | 7.66 (0.86) Aa | |
| Hybrid Layer | PLA | 0.330 (0.04) Ad | 0.350 (0.060) Aa | 10.79 (0.88) Ad | 10.86 (1.19) Aa |
| RUT | 0.460 (0.04) Abcd | 0.310 (0.010) Ba | 13.64 (0.29) Acd | 9.58 (0.56) Ba | |
| QUE | 0.640 (0.11) Aab | 0.320 (0.010) Ba | 18.14 (3.28) Aab | 9.31 (0.42) Ba | |
| HES | 0.740 (0.01) Aa | 0.340 (0.010) Ba | 22.51 (0.18) Aa | 8.30 (2.10) Ba | |
| PRO | 0.520 (0.03) Abc | 0.380 (0.040) Ba | 14.90 (0.85) Abc | 12.36 (3.31) Aa | |
| NAR | 0.580 (0.07) Aab | 0.320 (0.090) Ba | 18.97 (0.77) Aa | 9.05 (2.57) Ba | |
| CON | 0.367 (0.08) Acd | 0.384 (0.030) Aa | 12.44 (0.61) Acd | 11.43 (0.98) Aa |
| 10 μm Dentin | PLA | RUT | QUE | HES | PRO | NAR | CON | Average | ||
| NH | 24 h | 0.649 (0.096) | 0.767 (0.055) | 0.757 (0.024) | 0.788 (0.053) | 0.698 (0.042) | 0.637(0.078) | 0.677 (0.141) | 0.711 A | |
| Thermocycling | 0.574(0.055) | 0.633 (0.105) | 0.560 (0.047) | 0.690 (0.072) | 0.609 (0.067) | 0.464 (0.028) | 0.663 (0.042) | 0.599 B | ||
| Average | 0.612 ab | 0.700 a | 0.659 ab | 0.739 a | 0.654 ab | 0.550 b | 0.670 ab | |||
| E | 24 h | 20.27 (1.22) | 23.20 (0.32) | 22.86 (1.12) | 24.52 (0.70) | 21.20 (0.87) | 20.93 (1.02) | 21.94 (0.99) | 22.13 A | |
| Thermocycling | 16.44 (4.35) | 18.16 (1.52) | 17.47 (1.21) | 20.25 (3.30) | 19.06 (1.50) | 13.24 (4.31) | 20.52(0.67) | 17.88 B | ||
| Average | 18.35 ab | 20.67 ab | 20.16 ab | 22.38 a | 20.13 ab | 17.08 b | 21.23 ab | |||
| 20 μm Dentin | NH | 24 h | 0.660 (0.074) | 0.819 (0.079) | 0.745 (0.028) | 0.786 (0.053) | 0.720 (0.054) | 0.633 (0.10) | 0.738 (0.069) | 0.730 A |
| Thermocycling | 0.578 (0.073) | 0.762 (0.048) | 0.680 (0.079) | 0.743 (0.032) | 0.712 (0.083) | 0.547 (0.094) | 0.685 (0.127) | 0.670 B | ||
| Average | 0.619 b | 0.791 a | 0.713 ab | 0.764 a | 0.716 ab | 0.590 b | 0.712 ab | |||
| E | 24 h | 21.13 (1.55) | 25.09 (0.86) | 23.12 (1.15) | 24.15 (0.40) | 21.96 (0.16) | 20.98 (0.68) | 23.16 (0.19) | 22.80 A | |
| Thermocycling | 19.61 (0.51) | 21.832 (0.86) | 21.9 (1.15) | 22.85 (0.54) | 21.75 (1.87) | 18.74 (0.44) | 21.17 (1.66) | 21.13 B | ||
| Average | 20.37 c | 23.46 ab | 22.53 ab | 23.50 a | 21.86 abc | 19.86 c | 22.16 abc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dávila-Sánchez, A.; Gutierrez, M.F.; Bermudez, J.P.; Méndez-Bauer, L.; Pulido, C.; Kiratzc, F.; Alegria-Acevedo, L.F.; Farago, P.V.; Loguercio, A.D.; Sauro, S.; et al. Effects of Dentine Pretreatment Solutions Containing Flavonoids on the Resin Polymer-Dentine Interface Created Using a Modern Universal Adhesive. Polymers 2021, 13, 1145. https://doi.org/10.3390/polym13071145
Dávila-Sánchez A, Gutierrez MF, Bermudez JP, Méndez-Bauer L, Pulido C, Kiratzc F, Alegria-Acevedo LF, Farago PV, Loguercio AD, Sauro S, et al. Effects of Dentine Pretreatment Solutions Containing Flavonoids on the Resin Polymer-Dentine Interface Created Using a Modern Universal Adhesive. Polymers. 2021; 13(7):1145. https://doi.org/10.3390/polym13071145
Chicago/Turabian StyleDávila-Sánchez, Andrés, Mario Felipe Gutierrez, Jorge Pailover Bermudez, Luján Méndez-Bauer, Camilo Pulido, Fagner Kiratzc, Luisa Fernanda Alegria-Acevedo, Paulo Vitor Farago, Alessandro Dourado Loguercio, Salvatore Sauro, and et al. 2021. "Effects of Dentine Pretreatment Solutions Containing Flavonoids on the Resin Polymer-Dentine Interface Created Using a Modern Universal Adhesive" Polymers 13, no. 7: 1145. https://doi.org/10.3390/polym13071145
APA StyleDávila-Sánchez, A., Gutierrez, M. F., Bermudez, J. P., Méndez-Bauer, L., Pulido, C., Kiratzc, F., Alegria-Acevedo, L. F., Farago, P. V., Loguercio, A. D., Sauro, S., & Arrais, C. A. G. (2021). Effects of Dentine Pretreatment Solutions Containing Flavonoids on the Resin Polymer-Dentine Interface Created Using a Modern Universal Adhesive. Polymers, 13(7), 1145. https://doi.org/10.3390/polym13071145










