Development of a New Polymeric Nanocarrier Dedicated to Controlled Clozapine Delivery at the Dopamine D2-Serotonin 5-HT1A Heteromers
Abstract
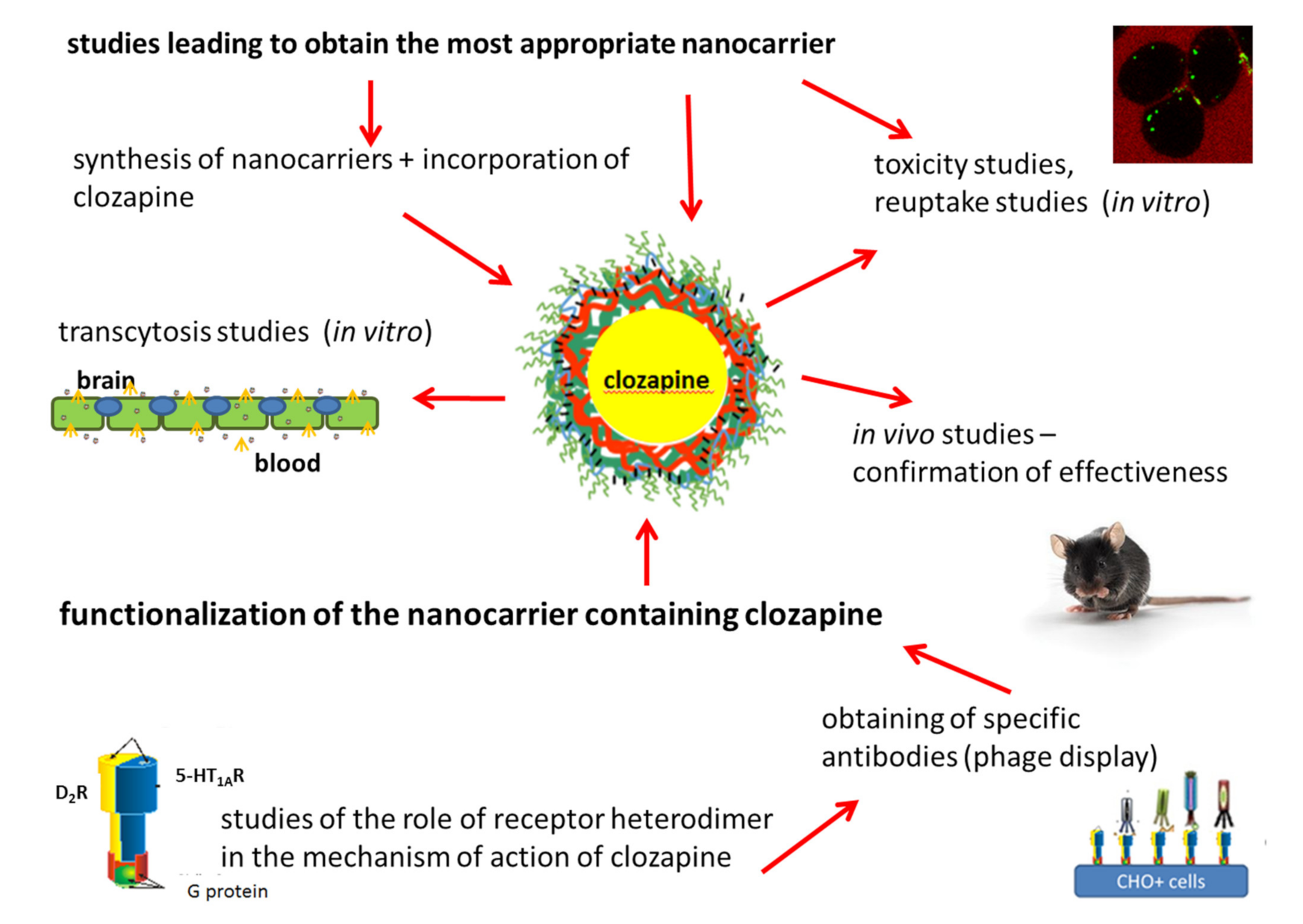
1. Introduction
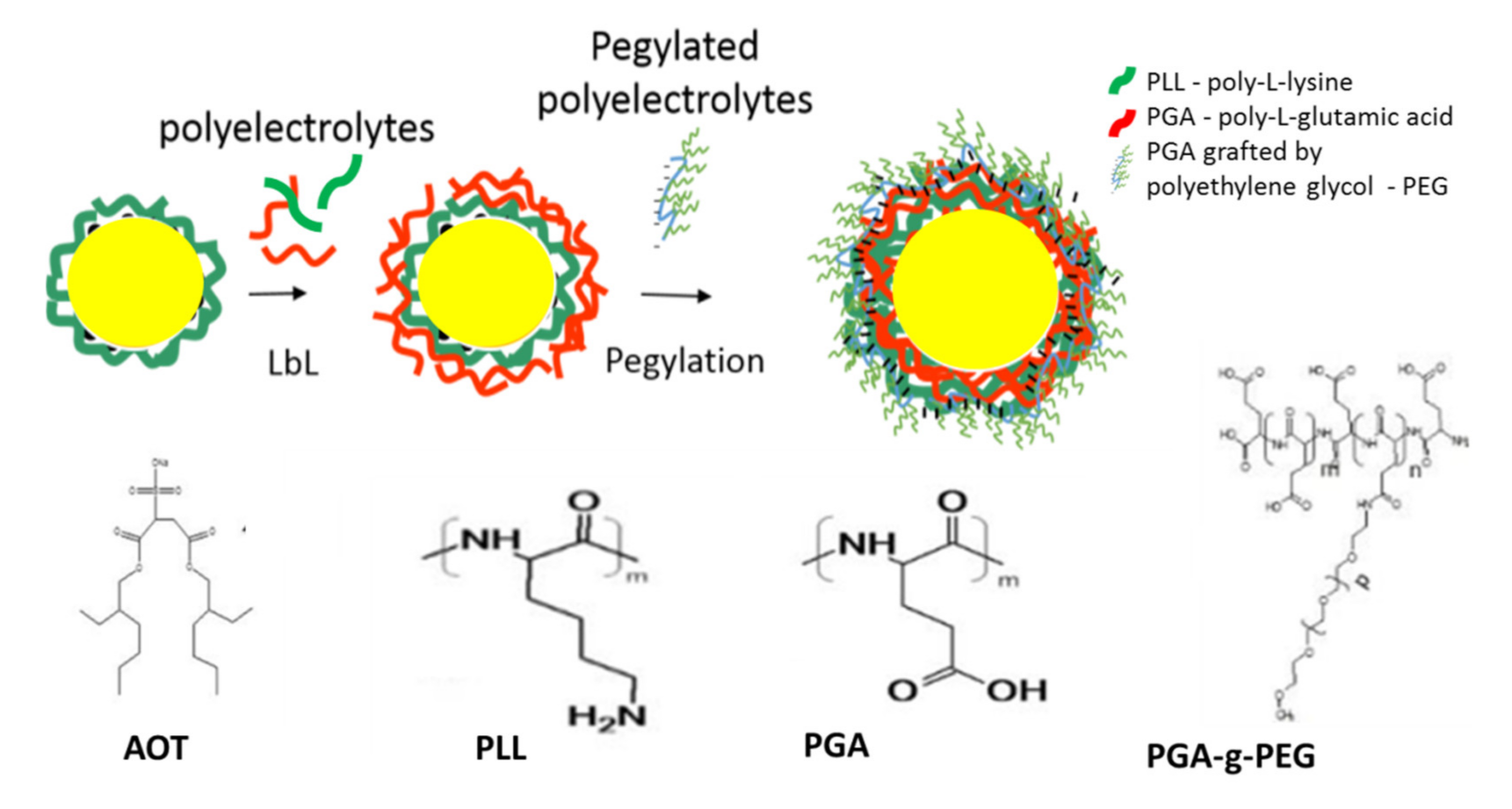
1.1. Preparation of Polymeric Nanocapsules (NCs)
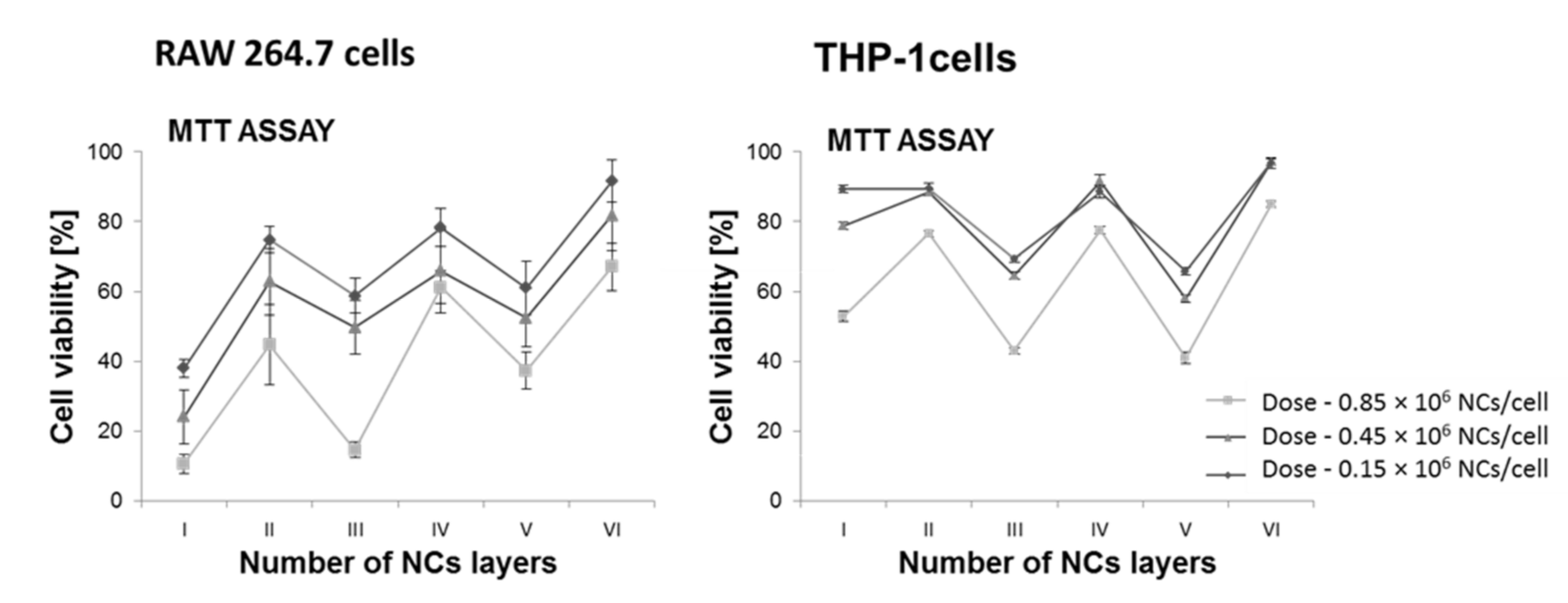
1.2. Interaction of the Nanocapsule/Target Cell—Cytotoxicity Studies of the Obtained Nanoformulations
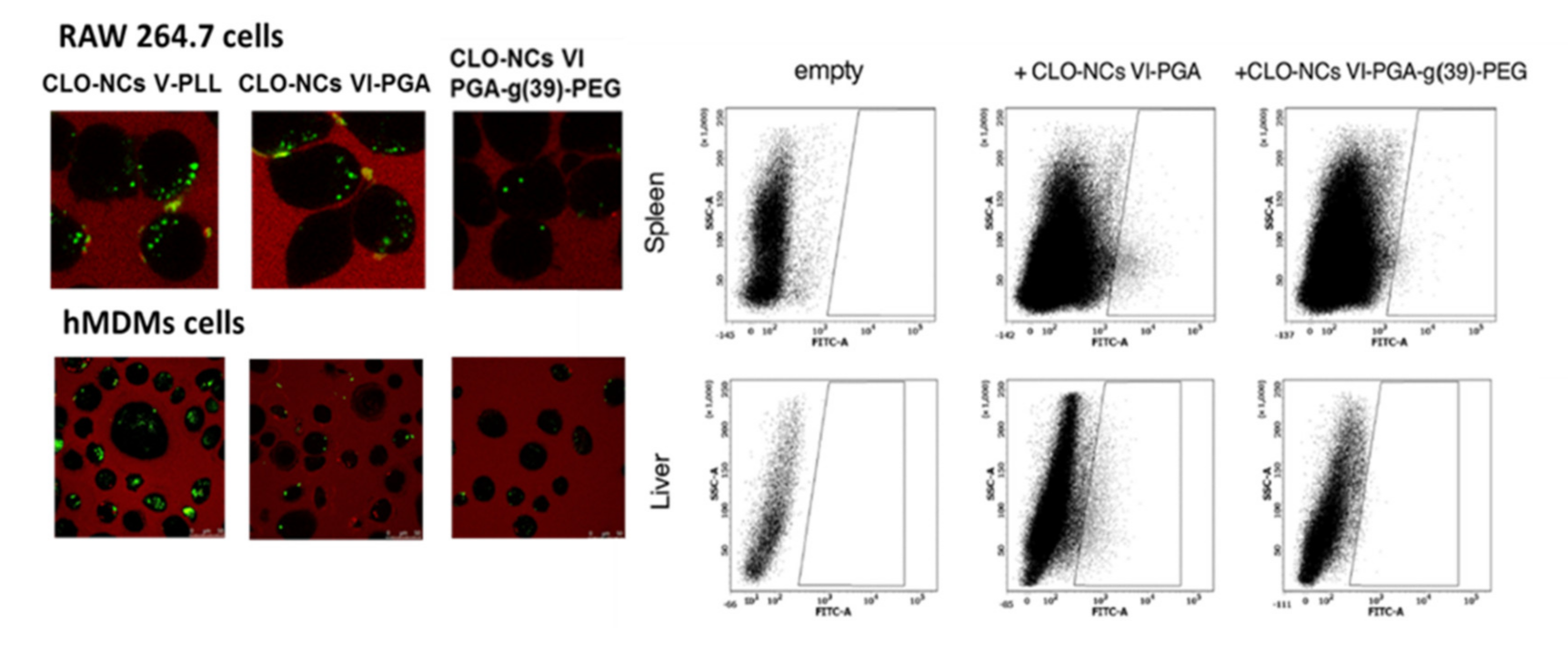
1.3. Interactions of the Obtained NCs with Cells of the Immune System
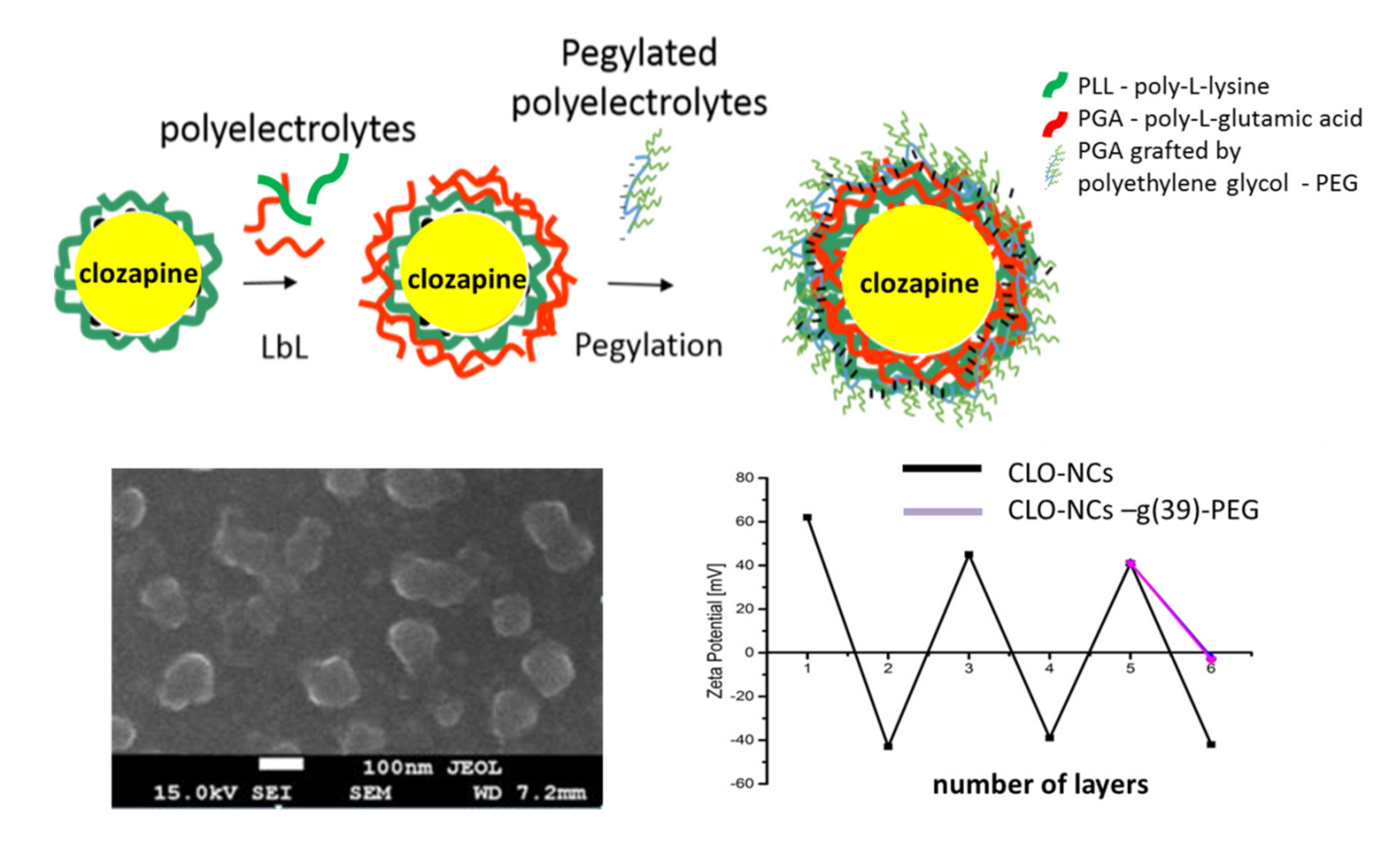
1.4. Obtaining and Characterization of the Encapsulated Form of Clozapine
1.5. NCs Interactions with hCMEC/D3 Cells (Immortalized Human Cerebral Endocrine Cells, D3 Clone) Constituting the In Vitro Model of the Human Blood-Brain Barrier
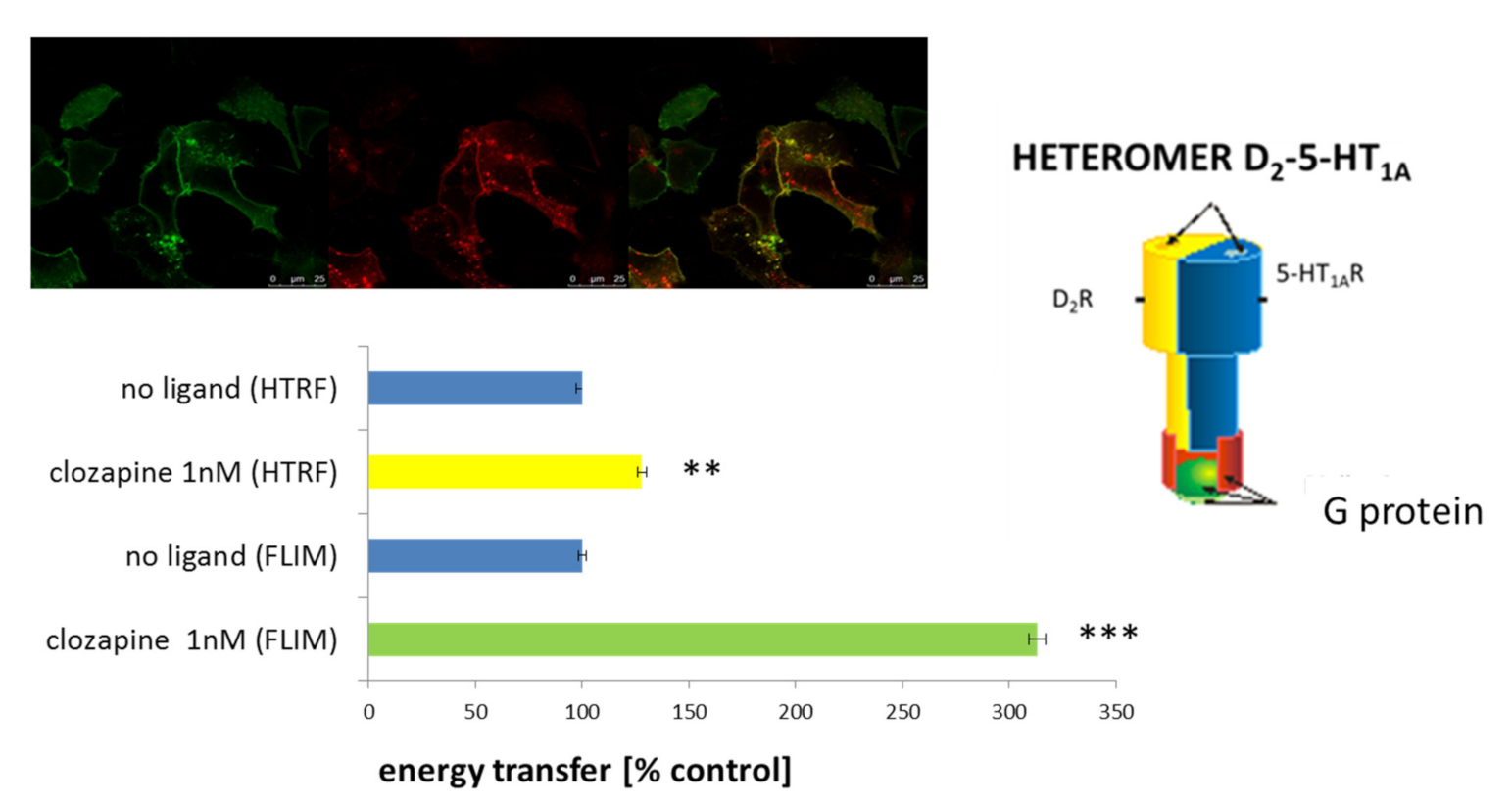
1.6. Heteromer of the D2-5-HT1A Receptors as an Important Target for Clozapine
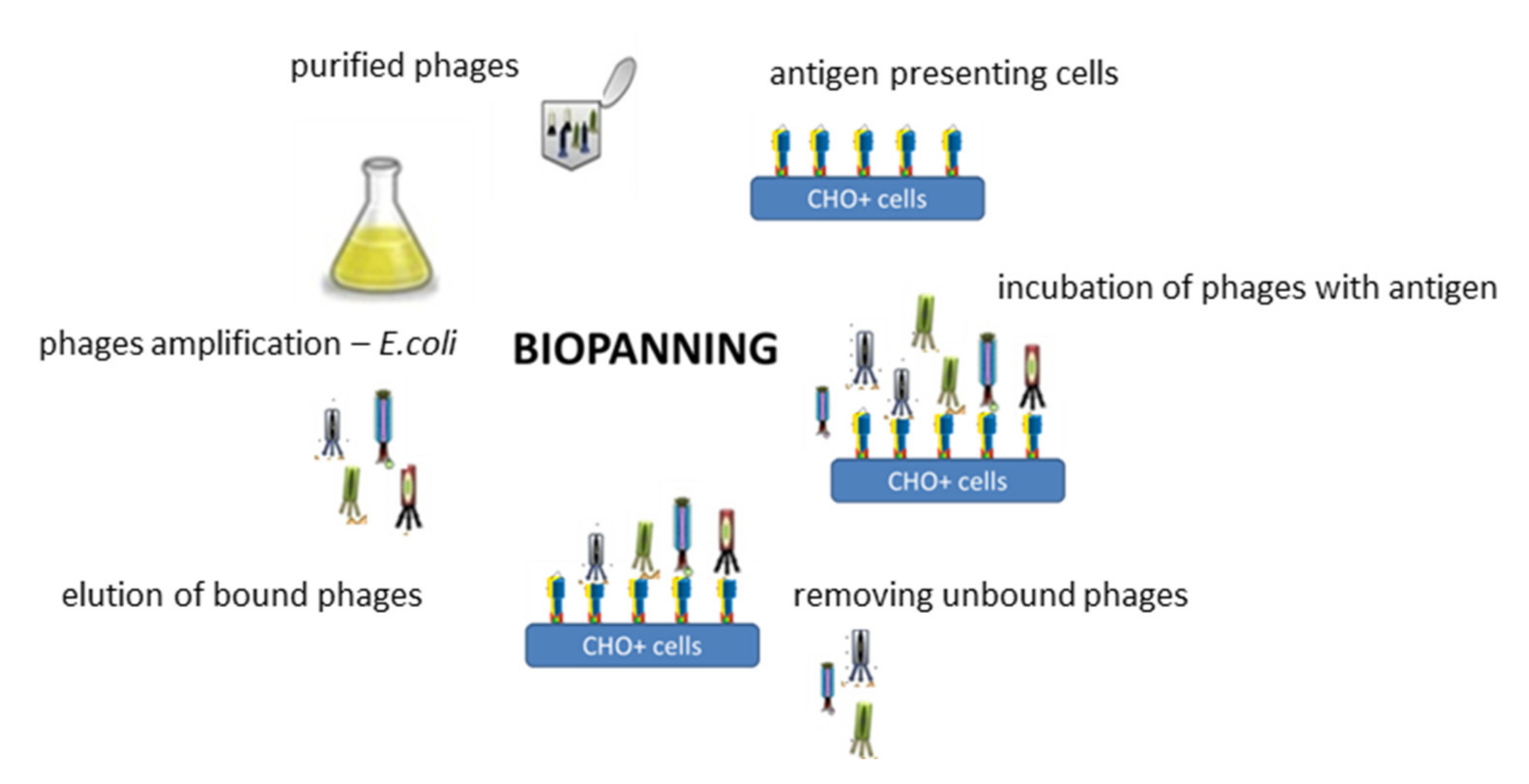
1.7. Synthesis of a Targeting Ligand Specifically Recognizing the D2-5-HT1A Heteromer for Functionalization of the Obtained CLO-NCs VI-PGA-g(39)-PEG
2. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Fond, G.; Macgregor, A.; Miot, S. Nanopsychiatry—The potential role of nanotechnologies in the future of psychiatry: A systematic review. Eur. Neuropsychopharmacol. 2013, 23, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; De La Fuente, J.M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7, 3. [Google Scholar] [CrossRef]
- Reinholz, J.; Landfester, K.; Mailänder, V. The challenges of oral drug delivery via nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil—An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111591. [Google Scholar] [CrossRef] [PubMed]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.-M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109828. [Google Scholar] [CrossRef]
- Kadam, R.S.; Bourne, D.W.; Kompella, U.B. Nano-advantage in enhanced drug delivery with biodegradable nanoparticles: Contribution of reduced clearance. Drug Metab. Dispos. 2012, 40, 1380–1388. [Google Scholar] [CrossRef]
- Bennewitz, S. Nanotechnology for delivery of drugs to the brain for epilepsy. Neurotherapeutics 2009, 6, 323–336. [Google Scholar] [CrossRef]
- Mansor, N.I.; Nordin, N.; Mohamed, F.; Ling, K.H.; Rosli, R.; Hassan, Z. Crossing the Blood-Brain Barrier: A Review on Drug Delivery Strategies for Treatment of the Central Nervous System Diseases. Curr. Drug Deliv. 2019, 16, 698–711. [Google Scholar] [CrossRef]
- Sánchez, A.; Mejía, S.P.; Orozco, J. Recent Advances in Polymeric Nanoparticle-Encapsulated Drugs against Intracellular Infections. Molecules 2020, 25, 3760. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.; Salahpour, A.; Didriksen, M.; Ghisi, V.; Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. USA 2008, 105, 13656–13661. [Google Scholar] [CrossRef]
- De Berardis, D.; Rapini, G.; Olivieri, L.; Di Nicola, D.; Tomasetti, C.; Valchera, A.; Fornaro, M.; Di Fabio, F.; Perna, G.; Di Nicola, M.; et al. Safety of antipsychotics for the treatment of schizophrenia: A focus on the adverse effects of clozapine. Ther. Adv. Drug Saf. 2018, 9, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Berardis, D.; Serroni, N.; Campanella, D.; Olivieri, L.; Ferri, F.; Carano, A.; Cavuto, M.; Martinotti, G.; Cicconetti, A.; Piersanti, M.; et al. Update on the Adverse Effects of Clozapine: Focus on Myocarditis. Curr. Drug Saf. 2012, 7, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, X.; Li, M.; Li, Y.; Sun, M. Use of Solid Lipid Nanoparticles for the Treatment of Acute Acoustic Stress-Induced Cochlea Damage. J. Nanosci. Nanotechnol. 2020, 20, 7412–7418. [Google Scholar] [CrossRef] [PubMed]
- Ishak, R.A.; Awad, G.A.; Zaki, N.M.; El-Shamy, A.E.-H.A.; Mortada, N.D. A comparative study of chitosan shielding effect on nano-carriers hydrophilicity and biodistribution. Carbohydr. Polym. 2013, 94, 669–676. [Google Scholar] [CrossRef]
- Pinkerton, N.M.; Grandeury, A.; Fisch, A.; Brozio, J.; Riebesehl, B.U.; Prud’Homme, R.K. Formation of Stable Nanocarriers by in Situ Ion Pairing during Block-Copolymer-Directed Rapid Precipitation. Mol. Pharm. 2013, 10, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kang, C.; Liu, F.; Song, L. Delivery of Antipsychotics with Nanoparticles. Drug Dev. Res. 2016, 77, 393–399. [Google Scholar] [CrossRef]
- Masoumi, H.; Basri, M.; Samiun, S.; Izadiyan, Z.; Lim, C.J. Enhancement of encapsulation efficiency of nanoemulsion-containing aripiprazole for the treatment of schizophrenia using mixture experimental design. Int. J. Nanomed. 2015, 13, 6469–6476. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.; Reddi, S.; Rinwa, V.; Balwani, G.; Saha, R. DoE based Olanzapine loaded poly-caprolactone nanoparticles decreases extrapyramidal effects in rodent model. Int. J. Pharm. 2018, 25, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sherje, A.P.; Surve, A.; Shende, P. CDI cross-linked β-cyclodextrin nanosponges of paliperidone: Synthesis and physicochemical characterization. J. Mater. Sci. Mater. Med. 2019, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Mikołajczyk, A.; Szczęch, M.; Szczepanowicz, K.; Warszyński, P.; Dziedzicka-Wasylewska, M. Encapsulation of clozapine into polycaprolactone nanoparticles as a promising strategy of the novel nanoformulation of the active compound. J. Nanoparticle Res. 2019, 21, 149. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Szczepanowicz, K. In vitro interaction of polyelectrolyte nanocapsules with model cells. Langmuir 2014, 30, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Szczepanowicz, K.; Błasiak, E.; Dziedzicka-Wasylewska, M. Biocompatible Polymeric Nanoparticles as Promising Candidates for Drug Delivery. Langmuir 2015, 31, 6415–6425. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Szczepanowicz, K.; Podgórna, K.; Błasiak, E.; Majeed, N.; Ogren, S.O.Ö.; Nowak, W.; Warszyński, P.; Dziedzicka-Wasylewska, M. Encapsulation of clozapine in polymeric nanocapsules and its biological effects. Colloids Surf. B Biointerfaces 2016, 140, 342–352. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.-M.; Islam, M.T.; Orr, B.G.; Baker, J.J.R.; Holl, M.M.B. Interaction of Polycationic Polymers with Supported Lipid Bilayers and Cells: Nanoscale Hole Formation and Enhanced Membrane Permeability. Bioconjugate Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Larson, R.G. Lipid Bilayer Curvature and Pore Formation Induced by Charged Linear Polymers and Dendrimers: The Effect of Molecular Shape. J. Phys. Chem. B 2008, 112, 12279–12285. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.M.; McNerny, D.Q.; Baker, J.R., Jr.; Orr, B.G.; Holl, M.M.B. Wide Varieties of Cationic Nanoparticles Induce Defects in Supported Lipid Bilayers. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle? Cell Interact. Small 2010, 6, 12–21. [Google Scholar]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.; Braguer, D.; Esteve, M.A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef]
- Mailander, V.; Landfester, K. Interaction of Nanoparticles with Cells. Biomacromolecules 2009, 10, 2379–2400. [Google Scholar] [CrossRef]
- Stark, W.J. Nanoparticles in Biological Systems. Angew. Chem. Int. Ed. 2011, 50, 1242–1258. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Gaucher, G.; Asahina, K.; Wang, J.; Leroux, J. Effect of Poly(NVinyl-Pyrrolidone)-Block-Poly(d,l-Lactide) as Coating Agent on the Opsonization, Phagocytosis, and Pharmacokinetics of Biodegradable Nanoparticles. Biomacromolecules 2009, 10, 408–416. [Google Scholar] [CrossRef]
- Tadros, T.F.; Warszyński, P.; Zembala, M. The Influence of Polymer Adsorption on Deposition Kinetics of Colloid Particles II. Experimental Studies. Colloids Surf. 1989, 39, 93–105. [Google Scholar] [CrossRef]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int. J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Kohler, N.; Zhang, M. Surface Modification of Superparamagnetic Magnetite Nanoparticles and Their Intracellular Uptake. Biomaterials 2002, 23, 1553–1561. [Google Scholar] [CrossRef]
- Chaudhari, K.R.; Ukawala, M.; Manjappa, A.S.; Kumar, A.; Mundada, P.K.; Mishra, A.K.; Mathur, R.; Mönkkönen, J.; Murthy, R.S. Opsonization, biodistribution, cellular uptake and apoptosis study of PEGylated PBCA nanoparticle as potential drug delivery carrier. Pharm. Res. 2012, 29, 53–68. [Google Scholar] [CrossRef]
- Poon, Z.; Lee, J.B.; Morton, S.W.; Hammond, P.T. Controlling in vivo stability and biodistribution in electrostatically assembled nanoparticles for systemic delivery. Nano Lett. 2011, 11, 2096–2103. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Weksler, B.; Romero, I.A.; Couraud, P.-O.; Reis, S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: Two new strategies of functionalization with apolipoprotein E. Nanotechnology 2015, 26, 495103. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Błasiak, E.; Szczepanowicz, K.; Guzik, K.; Bzowska, M.; Warszyński, P.; Dziedzicka-Wasylewska, M. The interaction of clozapine loaded nanocapsules with the hCMEC/D3 cells—In vitro model of blood brain barrier. Colloids Surf. B Biointerfaces 2017, 159, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Markoutsa, E.; Papadia, K.; Clemente, C.; Flores, O.; Antimisiaris, S.G. Anti-Abeta-MAb and dually decorated nanoliposomes: Effect of Abeta1-42 peptides on interaction with hCMEC/D3 cells. Eur. J. Pharm. Biopharm. 2012, 81, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.H.; Kalicharan, D.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Hoekstra, D.; Zuhorn, I.S. Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitro. Mol. Ther. 2011, 19, 318–325. [Google Scholar] [CrossRef]
- Tosi, G.; Duskey, J.T.; Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 2019, 17, 23–32. [Google Scholar] [CrossRef]
- Xiao, G.; Gan, L.-S. Receptor-Mediated Endocytosis and Brain Delivery of Therapeutic Biologics. Int. J. Cell Biol. 2013, 2013, 703545. [Google Scholar] [CrossRef]
- Brzezińska, K.; Ziaja, M. Struktura i funkcje bariery krew-mózg. Postępy Biologii Komórki 2012, 1, 84–99. [Google Scholar]
- Smith, M.W.; Gumbleton, M. Endocytosis at the blood-brain barrier: From basic understanding to drug delivery strategies. J. Drug Target. 2006, 14, 191–214. [Google Scholar] [CrossRef]
- Gomes, I.; Ayoub, M.A.; Fujita, W.; Jaeger, W.C.; Pfleger, K.D.; Devi, L.A. G Protein–Coupled Receptor Heteromers. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 403–425. [Google Scholar] [CrossRef]
- Rozenfeld, R.; Devi, L.A. Exploring a role for heteromerization in GPCR signalling specificity. Biochem. J. 2010, 433, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Albizu, L.; Moreno, J.L.; González-Maeso, J.; Sealfon, S.C. Heteromerization of G protein-coupled receptors: Relevance to neurological disorders and neurotherapeutics. CNS Neurol. Disord. Drug Targets 2010, 9, 636–650. [Google Scholar] [CrossRef]
- Fujita, W.; Gomes, I.; Devi, L.A. Revolution in GPCR signalling: Opioid receptor heteromers as novel therapeutic targets: IUPHAR Review 10. Br. J. Pharmacol. 2014, 171, 4155–4176. [Google Scholar] [CrossRef] [PubMed]
- Derouiche, L.; Massotte, D. G protein-coupled receptor heteromers are key players in substance use disorder. Neurosci. Biobehav. Rev. 2019, 106, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Jockers, R. Biological Significance of GPCR Heteromerization in the Neuro-Endocrine System. Front. Endocrinol. (Lausanne) 2011, 1, 2. [Google Scholar] [CrossRef]
- Carriba, P.; Ortiz, O.; Patkar, K.; Justinova, Z.; Stroik, J.; Themann, A.; Muller, C.; Woods, A.S.; Hope, B.T.; Ciruela, F.; et al. Striatal Adenosine A2A and Cannabinoid CB1 Receptors Form Functional Heteromeric Complexes that Mediate the Motor Effects of Cannabinoids. Neuropsychopharmacology 2007, 32, 2249–2259. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Kleven, M.S. Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology 2011, 216, 451–473. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Błasiak, E.; Szafran-Pilch, K.; Dziedzicka-Wasylewska, M. Dopamine D2 and serotonin 5-HT1A receptor interaction in the context of the effects of antipsychotics-in vitro studies. J. Neurochem. 2016, 137, 549–560. [Google Scholar] [CrossRef]
- Faron-Górecka, A.; Górecki, A.; Kuśmider, M.; Wasylewski, Z.; Dziedzicka-Wasylewska, M. The role of D1-D2 receptor hetero-dimerization in the mechanism of action of clozapine. Eur. Neuropsychopharmacol. 2008, 18, 682–691. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Faron-Górecka, A.; Kędracka-Krok, S.; Dziedzicka-Wasylewska, M. Effect of clozapine on the dimerization of serotonin 5-HT(2A) receptor and its genetic variant 5-HT(2A)H425Y with dopamine D(2) receptor. Eur. J. Pharmacol. 2011, 659, 114–123. [Google Scholar] [CrossRef]
- Bird, R.E.; Hardman, K.D.; Jacobson, J.W.; Johnson, S.; Kaufman, B.M.; Lee, S.M.; Pope, S.H.; Riordan, G.S.; Whitlow, M. Single-chain antigen-binding proteins. Science 1988, 242, 423–426. [Google Scholar] [CrossRef]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of Recombinant Antibodies. Front. Immunol. 2013, 4, 217. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Fic, E.; Bzowska, M.; Dziedzicka-Wasylewska, M. Isolation of Human Monoclonal scfv Antibody Specifically Recognizing the D2-5-HT1A Heteromer. J. New Dev. Chem. 2019, 2, 18–25. [Google Scholar] [CrossRef]
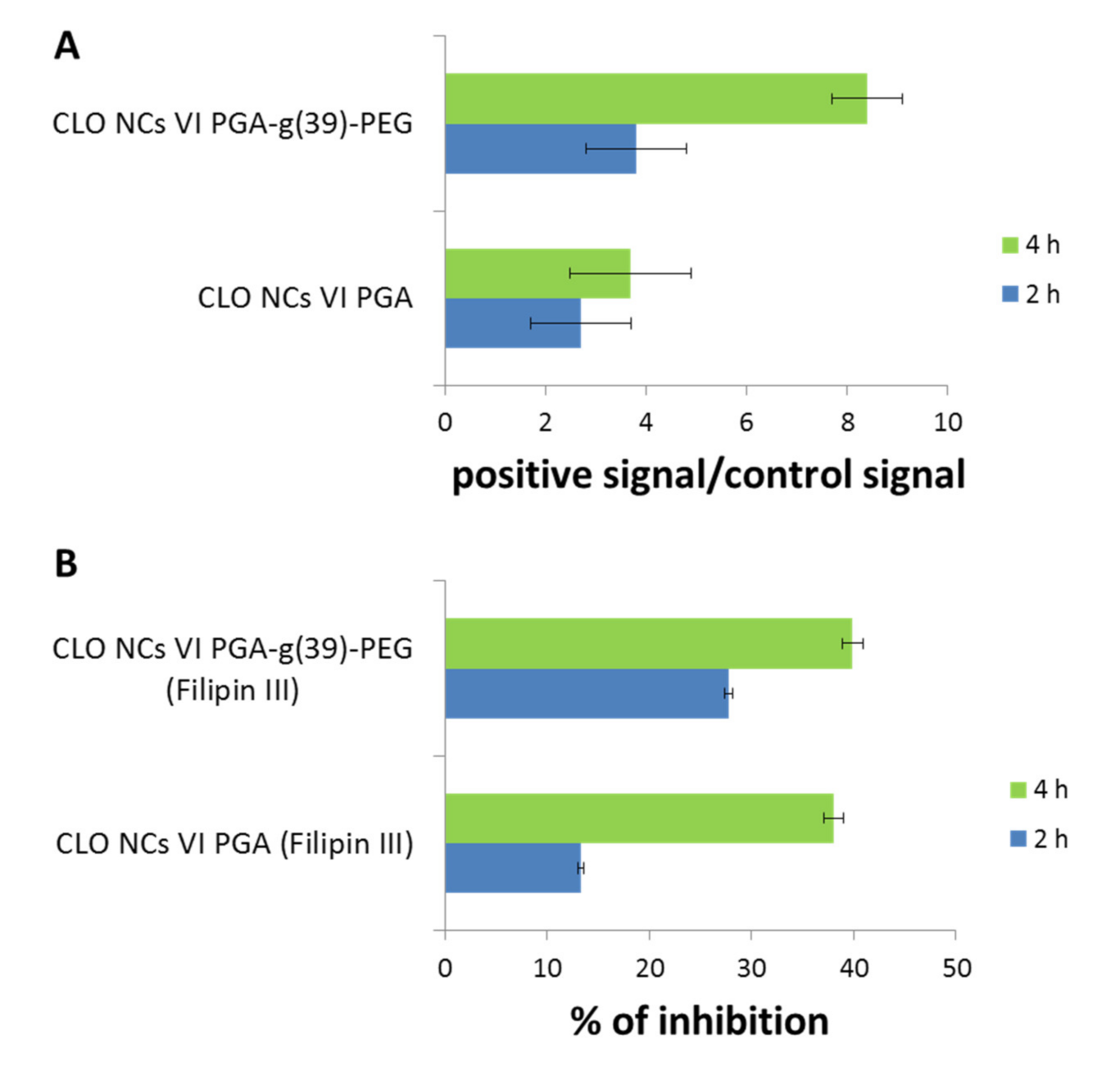
| NCs I/III-PLL b | NCs II/IV-PGA c | NCs V-PLL d | NCs VI-PGA e | NCs VI-PGA-g(39)-PEG f | CLO-NCs V-PLL g | CLO-NCs VI-PGA h | CLO-NCs VI-PGA-g(39)-PEG i | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Charge of outer layer of nanocarrier | Positive | Negative | Positive | Negative | Neutral | Positive | Negative | Neutral | [23,24,25] |
| Toxicity a | High | Midium | Medium | Low | Low | Medium | Low | Very low | [23,24,25,43] |
| RAW and THP-1 cellls uptake | - | - | High +++ Clatrine path | High ++ Clatrine path | Low Clatrine path and passive transport | High +++ Clatrine path | High ++ Clatrine path | Low Clatrine path and passive transport | [24,25] |
| hMDMs cells Uptake | - | - | High +++ | High ++ | Low | High +++ | High ++ | Low | [43] |
| Phagocytic cells | Visible +++ | Visible ++ | Visible +++ | Visible ++ | Invisible | Visible +++ | Visible ++ | Invisible | [23,24,25,43] |
| hCMEC/D3 cells (in vitro BBB model) Uptake | - | - | High +++ Clatrine path | High ++ Clatrine path | High ++ Clatrine path and passive transport | High +++ Clatrine path | High ++ Clatrine path | High ++ Clatrine path and passive transport | [43] |
| Stimulation of phagocytic potential | - | - | - | - | - | High +++ | High ++ | Medium | [23,24,43] |
| Biodistribution In vivo | - | - | - | - | - | - | Lungs ++ Liver +++ Spleen +++ Kidney ++ | Spleen + kidney + | [25] |
| Behavioral studies In vivo | - | - | - | - | - | - | Mice locomotor activity reductio, similarity to clozapine + | Mice locomotor activity reductio, similarity to clozapine ++ | [25] |
| Transcytosis (hCMEC/D3 cells) In vitro BBB model) | - | - | High ++ | High + | High +++ | High ++ | High + Caveole path | High +++ Caveole path | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukasiewicz, S. Development of a New Polymeric Nanocarrier Dedicated to Controlled Clozapine Delivery at the Dopamine D2-Serotonin 5-HT1A Heteromers. Polymers 2021, 13, 1000. https://doi.org/10.3390/polym13071000
Łukasiewicz S. Development of a New Polymeric Nanocarrier Dedicated to Controlled Clozapine Delivery at the Dopamine D2-Serotonin 5-HT1A Heteromers. Polymers. 2021; 13(7):1000. https://doi.org/10.3390/polym13071000
Chicago/Turabian StyleŁukasiewicz, Sylwia. 2021. "Development of a New Polymeric Nanocarrier Dedicated to Controlled Clozapine Delivery at the Dopamine D2-Serotonin 5-HT1A Heteromers" Polymers 13, no. 7: 1000. https://doi.org/10.3390/polym13071000
APA StyleŁukasiewicz, S. (2021). Development of a New Polymeric Nanocarrier Dedicated to Controlled Clozapine Delivery at the Dopamine D2-Serotonin 5-HT1A Heteromers. Polymers, 13(7), 1000. https://doi.org/10.3390/polym13071000





