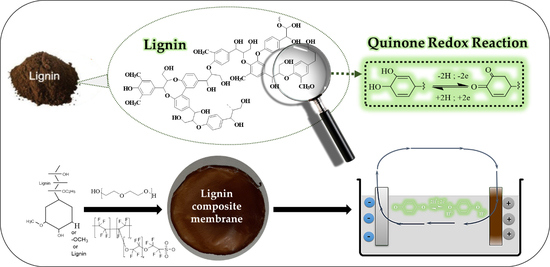
Electrochemical Activity of Lignin Based Composite Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Lignin
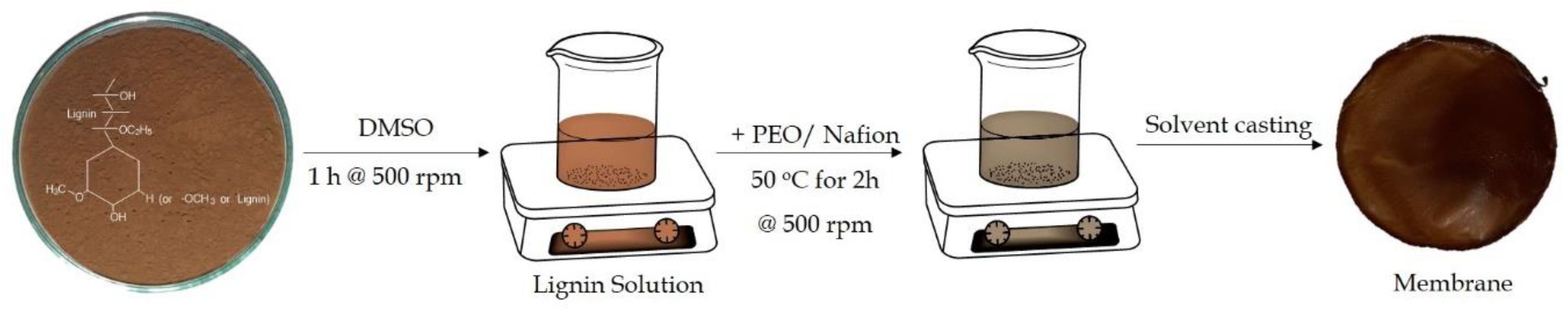
2.2. Preparation of Lignin Based Composite Membranes
2.3. Characterization
3. Results
3.1. Water Uptake (WU), Swelling Ratio (SR) and Gel Content (GC) % Tests
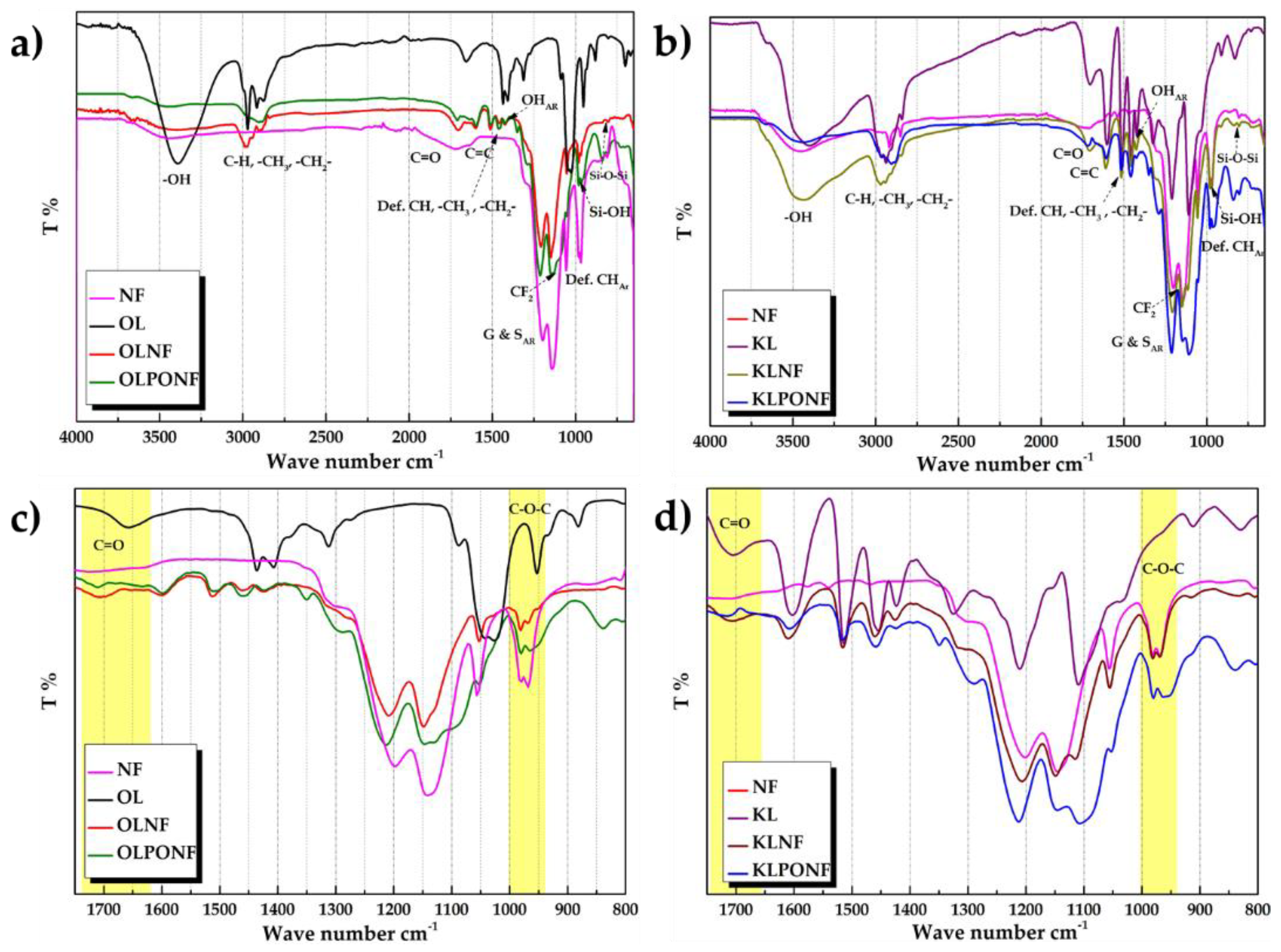
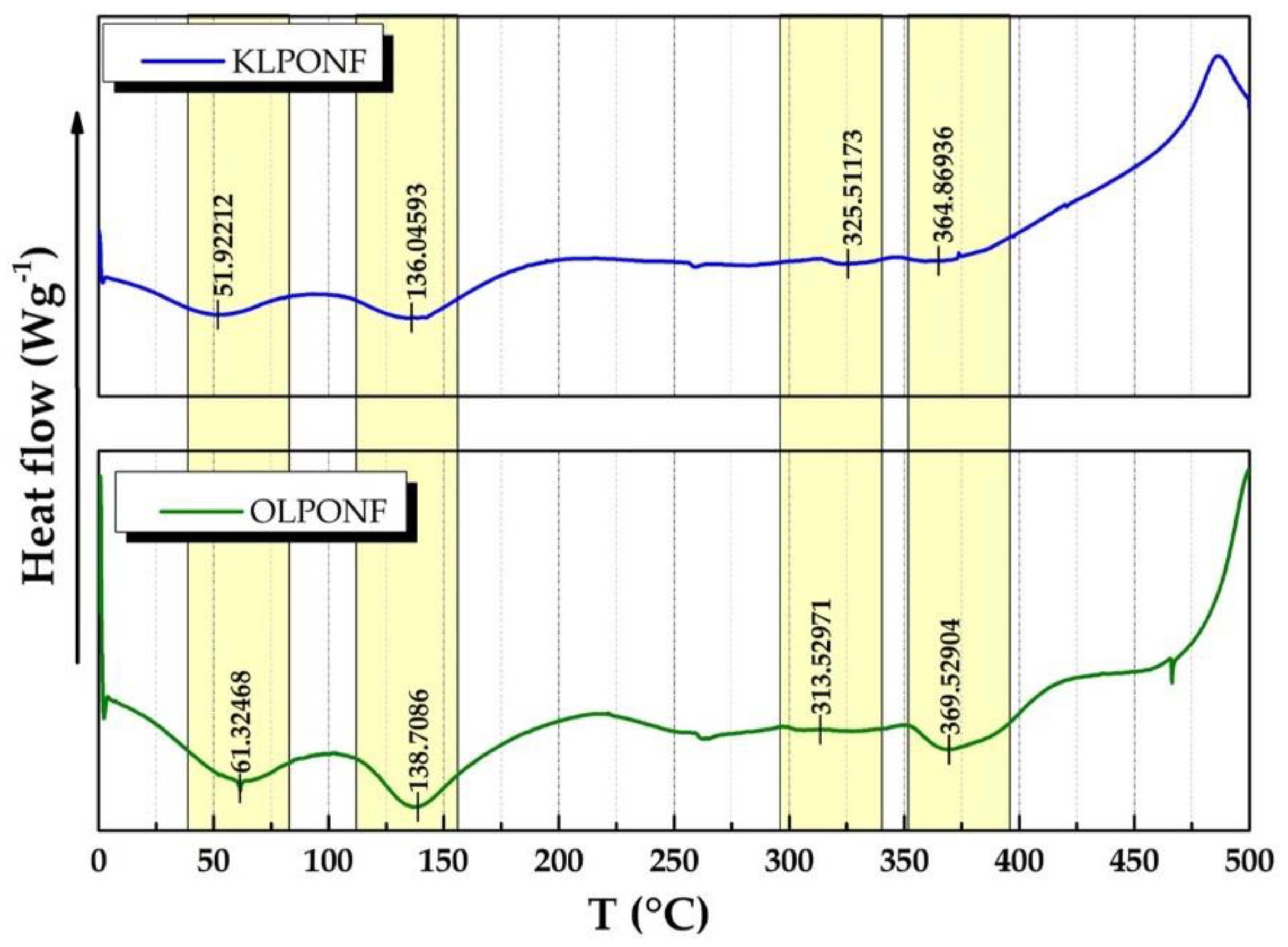
3.2. Fourier Transform Infrared (FTIR) Spectroscopy and Differential Scanning Calorimetry (DSC)
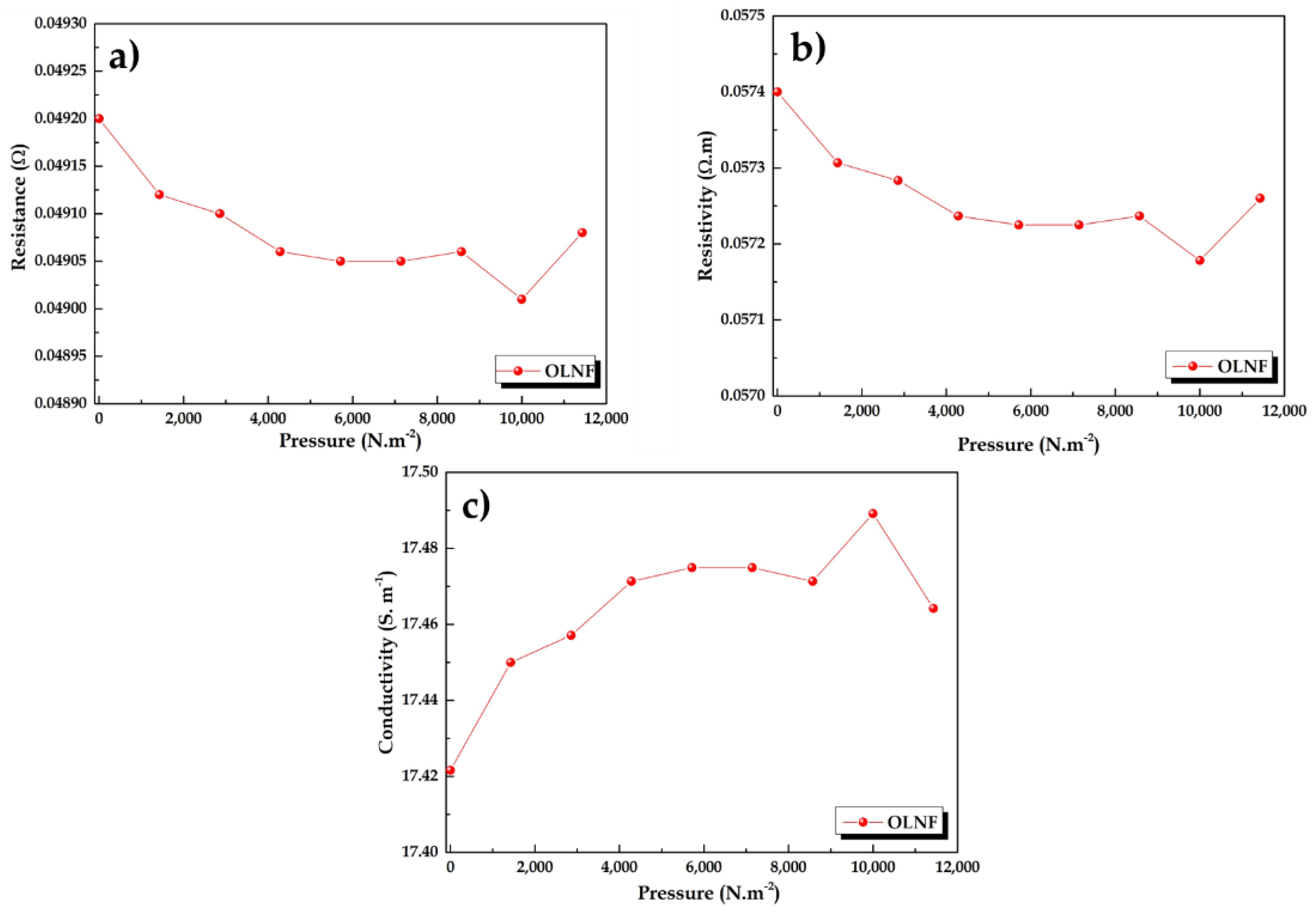
3.3. Conductivity Measurements
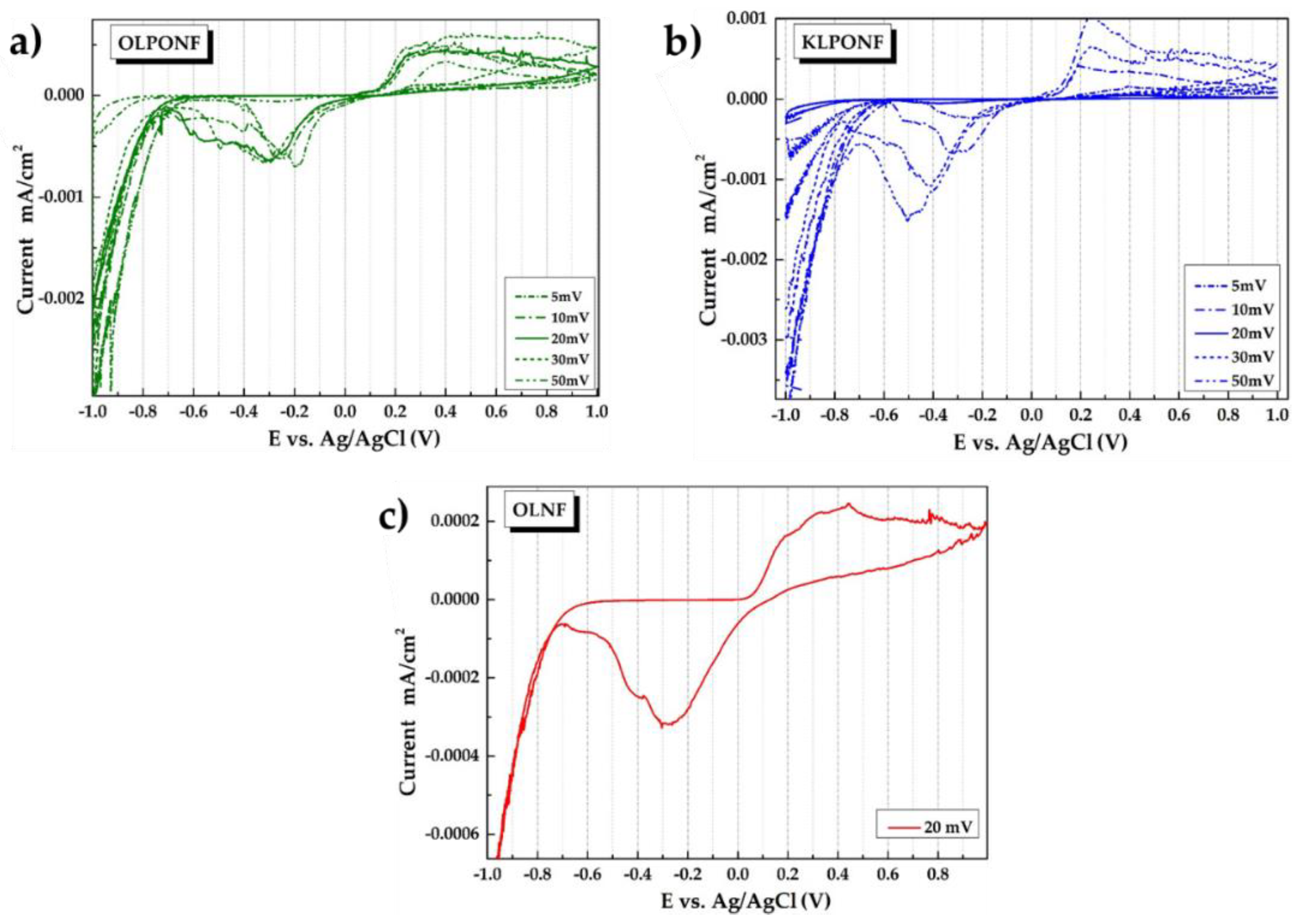
3.4. Cyclic Voltammetry (CV) Measurements
3.5. Electrochemical Impedance Spectroscopy (EIS)
3.6. Perspectives and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Tong, Y.; Liang, J.; Liu, H.K.; Dou, S.X. Energy storage in Oceania. Energy Storage Mater. 2019. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.R.; Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Ni, D.; Song, H.; Chen, Y.; Cai, K. Free-standing highly conducting PEDOT films for flexible thermoelectric generator. Energy 2019, 170, 53–61. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Mikoliunaite, L.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. Comparative study of polyaniline (PANI), poly(3,4-ethylenedioxythiophene) (PEDOT) and PANI-PEDOT films electrochemically deposited on transparent indium thin oxide based electrodes. Polymer 2019, 172, 133–141. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Chen, F.; Zhang, N.; Ma, M. Extremely strong and tough polythiophene composite for flexible electronics. Chem. Eng. J. 2019, 368, 933–940. [Google Scholar] [CrossRef]
- Dong, J.; Lin, Y.; Zong, H.; Yang, H. Hierarchical LiFe5O8@PPy core-shell nanocomposites as electrode materials for supercapacitors. Appl. Surf. Sci. 2019, 470, 1043–1052. [Google Scholar] [CrossRef]
- Muniz, C.R.; Cunha, M.S. Effects of strong electric fields in a polyacetylene chain. J. Phys. Chem. Solids 2015, 82, 17–20. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, Q.; Dong, A.; Gao, Y.; Qian, Y.; Wang, D.; Dong, M.; Shao, Q.; Liu, H.; Han, B.H.; et al. Metal complex hybrid composites based on fullerene-bearing porous polycarbazole for H2, CO2 and CH4 uptake and heterogeneous hydrogenation catalysis. Polymer 2019, 169, 255–262. [Google Scholar] [CrossRef]
- Gao, C.; Chen, G. Conducting polymer/carbon particle thermoelectric composites: Emerging green energy materials. Compos. Sci. Technol. 2016, 124, 52–70. [Google Scholar] [CrossRef]
- Bae, J.; Park, J.Y.; Kwon, O.S.; Lee, C.S. Energy efficient capacitors based on graphene/conducting polymer hybrids. J. Ind. Eng. Chem. 2017, 51, 1–11. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Silva, F.T.M.; Ataíde, C.H. Valorization of eucalyptus urograndis wood via carbonization: Product yields and characterization. Energy 2019, 172, 509–516. [Google Scholar] [CrossRef]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; De Peinder, P.; Boelens, R.; Van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Hirai, N.; Tanaka, T.; Kubo, S.; Ikeda, T.; Magara, K.; Ban, I.; Shiota, M. Density and hardness of negative pastes of lead-acid batteries containing organic additives with or without quinone structure. J. Power Sources 2006, 158, 1106–1109. [Google Scholar] [CrossRef]
- Jablonsky, M.; Andrea, S.; Haz, A.; Ľudmila, H.; Michal, J.; Andrea, Š.; Aleš, H. Lignin, Potential Products and Their Market Value. Wood Res. 2015, 60, 973–986. [Google Scholar]
- Amezcua-Allieri, M.A.; Aburto, J. Conversion of Lignin to Heat and Power, Chemicals or Fuels into the Transition Energy Strategy. Lignin Trends Appl. 2018. [Google Scholar] [CrossRef]
- Xia, K.; Ouyang, Q.; Chen, Y.; Wang, X.; Qian, X.; Wang, L. Preparation and Characterization of Lignosulfonate-Acrylonitrile Copolymer as a Novel Carbon Fiber Precursor. ACS Sustain. Chem. Eng. 2016, 4, 159–168. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.S. Biomass-derived carbon electrode materials for supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281. [Google Scholar] [CrossRef]
- Fang, Z., Jr.; Smith, R.L. Production of Biofuels and Chemicals from Lignin; Springer Science+Business Media: Singapore, 2016; Volume 6. [Google Scholar]
- Tenhaeff, W.E.; Rios, O.; More, K.; McGuire, M.A. Highly robust lithium ion battery anodes from lignin: An abundant, renewable, and low-cost material. Adv. Funct. Mater. 2014, 24, 86–94. [Google Scholar] [CrossRef]
- Lu, H.; Cornell, A.; Alvarado, F.; Behm, M.; Leijonmarck, S.; Li, J.; Tomani, P.; Lindbergh, G. Lignin as a binder material for eco-friendly Li-ion batteries. Materials 2016, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Nirmale, T.C.; Kale, B.B.; Varma, A.J. A review on cellulose and lignin based binders and electrodes: Small steps towards a sustainable lithium ion battery. Int. J. Biol. Macromol. 2017, 103, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Hamel, J.; Katahira, R.; Zhu, H. Metal-Free Aqueous Flow Battery with Novel Ultrafiltered Lignin as Electrolyte. ACS Sustain. Chem. Eng. 2018, 6, 5394–5400. [Google Scholar] [CrossRef]
- Hill, C.A.S. The Use of Timber in the Twenty-first Century. Wood Modif. 2006, 1–18. [Google Scholar] [CrossRef]
- Matrakova, M.; Rogachev, T.; Pavlov, D.; Myrvold, B.O. Influence of phenolic group content in lignin expanders on the performance of negative lead-acid battery plates. J. Power Sources 2003, 113, 345–354. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Opra, D.P.; Zemnukhova, L.A.; Sinebryukhov, S.L.; Kedrinskii, I.A.; Patrusheva, O.V.; Sergienko, V.I. Electrochemical performance of Klason lignin as a low-cost cathode-active material for primary lithium battery. J. Energy Chem. 2015, 24, 346–352. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Wang, C.; Liu, J.; Pu, Y.; Ragauskas, A.; Li, S.; Yang, B. Lignin-derived electrochemical energy materials and systems. Biofuels Bioprod. Biorefining 2020, 14, 650–672. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Cao, H.; Song, A.; Lin, Y.; Wang, M.; Li, X. A high-performance and environment-friendly gel polymer electrolyte for lithium ion battery based on composited lignin membrane. J. Solid State Electrochem. 2018, 22, 807–816. [Google Scholar] [CrossRef]
- Chaleawlert-umpon, S.; Berthold, T.; Wang, X.; Antonietti, M.; Liedel, C. Kraft Lignin as Electrode Material for Sustainable Electrochemical Energy Storage. Adv. Mater. Interfaces 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Casado, N.; Hilder, M.; Pozo-Gonzalo, C.; Forsyth, M.; Mecerreyes, D. Electrochemical Behavior of PEDOT/Lignin in Ionic Liquid Electrolytes: Suitable Cathode/Electrolyte System for Sodium Batteries. ChemSusChem 2017, 10, 1783–1791. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.L.; Wang, A.; Liu, X.; Chen, J.; Wang, Z.; Zeng, Q.; Zhou, H.H.; Jiang, X.; Zhang, L.L. Polymer-Laden Composite Lignin-Based Electrolyte Membrane for High-Performance Lithium Batteries. ACS Sustain. Chem. Eng. 2018, 6, 14460–14469. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.V.V.; Sinebryukhov, S.L.L.L.; Opra, D.P.P.P.; Zemnukhova, L.A.A.A.; Tsvetnikov, A.K.K.; Minaev, A.N.N.; Sokolov, A.A.A.; Sergienko, V.I.I. Electrochemistry of Klason Lignin. Procedia Chem. 2014, 11, 96–100. [Google Scholar] [CrossRef][Green Version]
- Gnedenkov, S.V.; Opra, D.P.; Sinebryukhov, S.L.; Tsvetnikov, A.K.; Ustinov, A.Y.; Sergienko, V.I. Hydrolysis lignin: Electrochemical properties of the organic cathode material for primary lithium battery. J. Ind. Eng. Chem. 2014, 20, 903–910. [Google Scholar] [CrossRef]
- Ye, J.; Lou, X.; Wu, C.; Wu, S.; Ding, M.; Sun, L.; Jia, C.; Ding, M. Ion Selectivity and Stability Enhancement of SPEEK / Lignin Membrane for Vanadium Redox Flow Battery: The Degree of Sulfonation Effect. Front. Chem. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Ye, J.; Cheng, Y.; Sun, L.; Ding, M.; Wu, C.; Yuan, D.; Zhao, X.; Xiang, C.; Jia, C. A green SPEEK/lignin composite membrane with high ion selectivity for vanadium redox flow battery. J. Memb. Sci. 2019, 572, 110–118. [Google Scholar] [CrossRef]
- Admassie, S.; Ajjan, F.N.; Elfwing, A.; Inganäs, O. Biopolymer hybrid electrodes for scalable electricity storage. Mater. Horizons 2016, 3, 174–185. [Google Scholar] [CrossRef]
- Rębiś, T.; Nilsson, T.Y.; Inganäs, O. Hybrid materials from organic electronic conductors and synthetic-lignin models for charge storage applications. J. Mater. Chem. A 2016, 4, 1931–1940. [Google Scholar] [CrossRef][Green Version]
- Milczarek, G. Lignosulfonate-modified electrodes: Electrochemical properties and electrocatalysis of NADH oxidation. Langmuir 2009, 25, 10345–10353. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, G. Preparation and characterization of a lignin modified electrode. Electroanalysis 2007, 19, 1411–1414. [Google Scholar] [CrossRef]
- Lota, G.; Milczarek, G. The effect of lignosulfonates as electrolyte additives on the electrochemical performance of supercapacitors. Electrochem. Commun. 2011, 13, 470–473. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, C.; Zhang, X.; Yang, C.; Jiang, M.; Zhang, X. A sustainable platform of lignin: From bioresources to materials and their applications in rechargeable batteries and supercapacitors. Prog. Energy Combust. Sci. 2020, 76, 100788. [Google Scholar] [CrossRef]
- Becker, J.; Wittmann, C. A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef]
- Imadi, S.R.; Kazi, A.G. Extraction of lignin from biomass for biofuel production. In Agricultural Biomass Based Potential Materials; Springer Nature: Basingstoke, UK, 2015; pp. 155–180. ISBN 9783319138473. [Google Scholar]
- Obydenkova, S.V.; Kouris, P.D.; Hensen, E.J.M.M.; Heeres, H.J.; Boot, M.D. Environmental economics of lignin derived transport fuels. Bioresour. Technol. 2017, 243, 589–599. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Ding, R.; Wu, H.; Thunga, M.; Bowler, N.; Kessler, M.R. Processing and characterization of low-cost electrospun carbon fibers from organosolv lignin/polyacrylonitrile blends. Carbon N. Y. 2016, 100, 126–136. [Google Scholar] [CrossRef]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the Physicochemical Properties and Thermal Stability of Organosolv and Kraft Lignins from Hardwood and Softwood Biomass for Their Potential Valorization. Waste Biomass Valorization 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Liu, G.; Shi, H.; Ping, Q.; Zhou, J.; Zhang, J.; Li, N.; Niu, M.; Fatehi, P.; Xiao, H.; Ni, Y. Complex Formation of PEO and Lignin in Prehydrolysis Liquor and its Enhancing Effect on Lignin Removal. BioResources 2013, 8, 4004–4015. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Kraft lignin/poly(ethylene oxide) blends: Effect of lignin structure on miscibility and hydrogen bonding. J. Appl. Polym. Sci. 2005, 98, 1437–1444. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Q.; Xu, J.; Pan, J.; Cheng, Y.T. Binder-free lithium ion battery electrodes made of silicon and pyrolized lignin†. RSC Adv. 2016, 6, 29308–29313. [Google Scholar] [CrossRef]
- Stergiou, D.V.; Veltsistas, P.G.; Prodromidis, M.I. An electrochemical study of lignin films degradation: Proof-of-concept for an impedimetric ozone sensor. Sens. Actuators B Chem. 2008, 129, 903–908. [Google Scholar] [CrossRef]
- Illig, J.; Chrobak, T.; Klotz, D.; Ivers-Tiffée, E. Evaluation of the Rate Determining Processes for LiFePO 4 as Cathode Material in Lithium-Ion-Batteries. ECS Trans. 2019, 33, 3–15. [Google Scholar] [CrossRef]
- Available online: https://sites.google.com/site/drttools/ (accessed on 8 December 2020).
- Ciucci, F.; Chen, C. Analysis of electrochemical impedance spectroscopy data using the distribution of relaxation times: A Bayesian and hierarchical Bayesian approach. Electrochim. Acta 2015, 167, 439–454. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Chrobak, T.; Ender, M.; Illig, J.; Klotz, D.; Ivers-Tiffée, E. Studies on LiFePO4 as cathode material using impedance spectroscopy. J. Power Sources 2011, 196, 5342–5348. [Google Scholar] [CrossRef]
- Li, X.; Ahmadi, M.; Collins, L.; Kalinin, S.V. Deconvolving distribution of relaxation times, resistances and inductance from electrochemical impedance spectroscopy via statistical model selection: Exploiting structural-sparsity regularization and data-driven parameter tuning. Electrochim. Acta 2019, 313, 570–583. [Google Scholar] [CrossRef]
- Li, Q.; Thangadurai, V. Synthesis, Structure and Electrical Properties of Mo-doped CeO2–Materials for SOFCs. Fuel Cells 2009, 9, 684–698. [Google Scholar] [CrossRef]
- Li, Q.; Xia, T.; Liu, X.D.; Ma, X.F.; Meng, J.; Cao, X.Q. Fast densification and electrical conductivity of yttria-stabilized zirconia nanoceramics. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2007, 138, 78–83. [Google Scholar] [CrossRef]
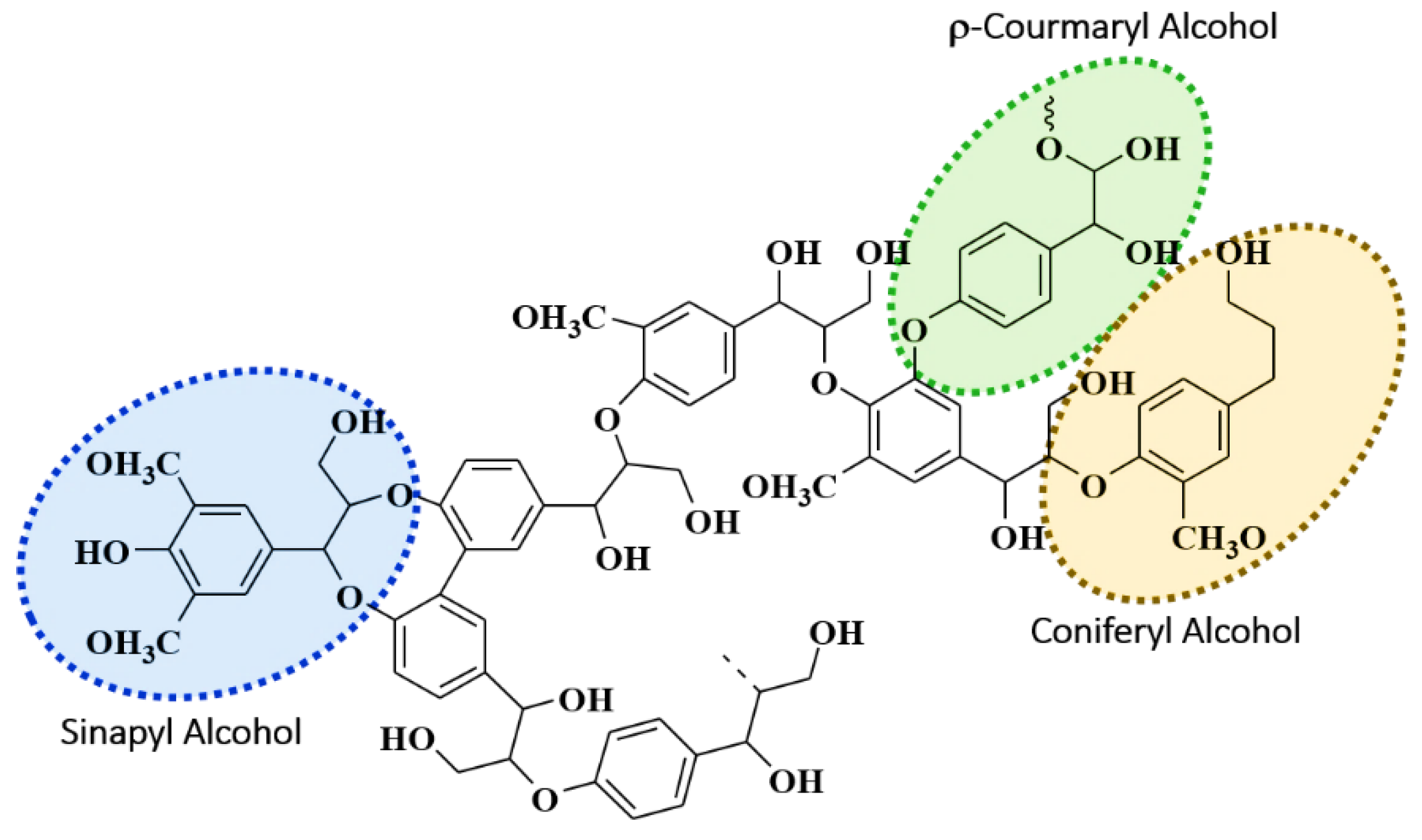



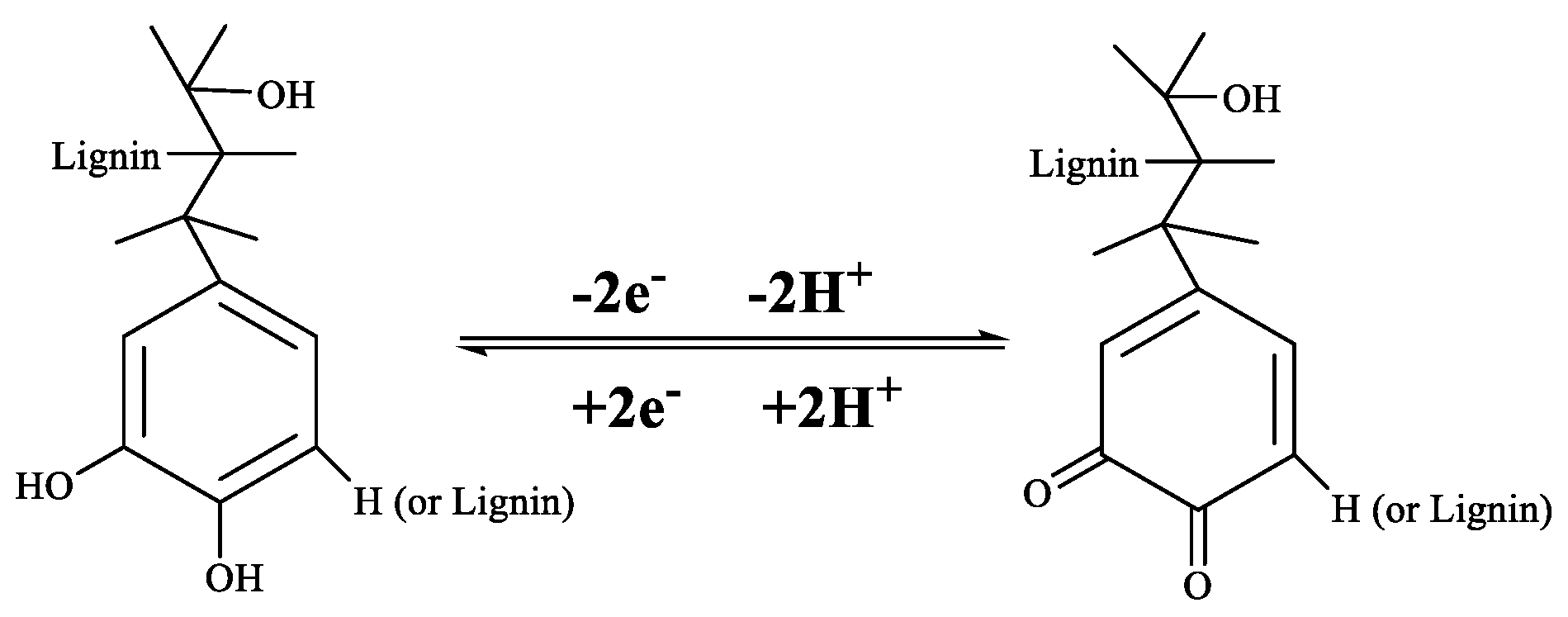


| Type | Source | Origin | Ratios | |||
|---|---|---|---|---|---|---|
| S | G | H | S/G | |||
| Organosolv | Commercial | Softwood | 15.57 | 42.77 | 9.28 | 0.36 |
| Kraft | Industry | Hardwood | 64.88 | 32.20 | 1.42 | 2.01 |
| Solid | Liquid |
|---|---|
| 1.742 × 10−1 S.cm−2 | 1.79 × 10−4 S.cm−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baloch, M.; Alberro, M.; Labidi, J. Electrochemical Activity of Lignin Based Composite Membranes. Polymers 2021, 13, 643. https://doi.org/10.3390/polym13040643
Baloch M, Alberro M, Labidi J. Electrochemical Activity of Lignin Based Composite Membranes. Polymers. 2021; 13(4):643. https://doi.org/10.3390/polym13040643
Chicago/Turabian StyleBaloch, Marya, Mikel Alberro, and Jalel Labidi. 2021. "Electrochemical Activity of Lignin Based Composite Membranes" Polymers 13, no. 4: 643. https://doi.org/10.3390/polym13040643
APA StyleBaloch, M., Alberro, M., & Labidi, J. (2021). Electrochemical Activity of Lignin Based Composite Membranes. Polymers, 13(4), 643. https://doi.org/10.3390/polym13040643