Preparing, Characterization and Anti-Biofilm Activity of Polymer Fibers Doped by Green Synthesized AgNPs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparing of AgNPs and PMCs
- 1.
- Ex Situ prepared PVA-5AgNPs composite—to prepare the PVA-5AgNPs composite 8 wt.% solution of PVA and AgNPs were mixed together:
- (a)
- Preparing of 8 wt.% PVA solution—PVA powder and deionized water were mixed in a beaker, in a water bath at 70–80 °C for 2 h,
- (b)
- Synthesis of AgNPs—chemicals for experiment included the stock silver solution and leaves extract of R. officinalis:
- (i)
- the stock silver solution was prepared by dissolving of AgNO3 in deionized water (concentration of solution 50 mg/L Ag),
- (ii)
- the leaf extract was prepared using 10 g of fresh leaves of R. officinalis. Cleaned leaves were ground and mixed with 125 mL of deionized water, heated at 70 °C for 10 min, filtered, and centrifuged to remove solid residue; 100 mL of pure leaf extract was obtained,
- (iii)
- for the synthesis of AgNPs 20 mL of extract was added dropwise to 80 mL of stock silver solution heated to 70–80 °C. The pre-prepared 100 mL of AgNPs colloidal solution was thickened by centrifuge (3 mL of colloidal AgNPs was received).
- 2.
- In Situ prepared PVA-5AgNPs composite-PVA powder, AgNO3 water solution, and R. officinalis leaf extract were mixed simultaneously in a beaker by magnetic stirrer at 80 °C in a water bath for 3 h. The amounts of the individual components were adjusted so that the concentration of PVA and Ag was the same as in the ex situ preparation process.
2.3. Methods of Measurements
2.4. Antibiofilm Activity
3. Results and Discussion
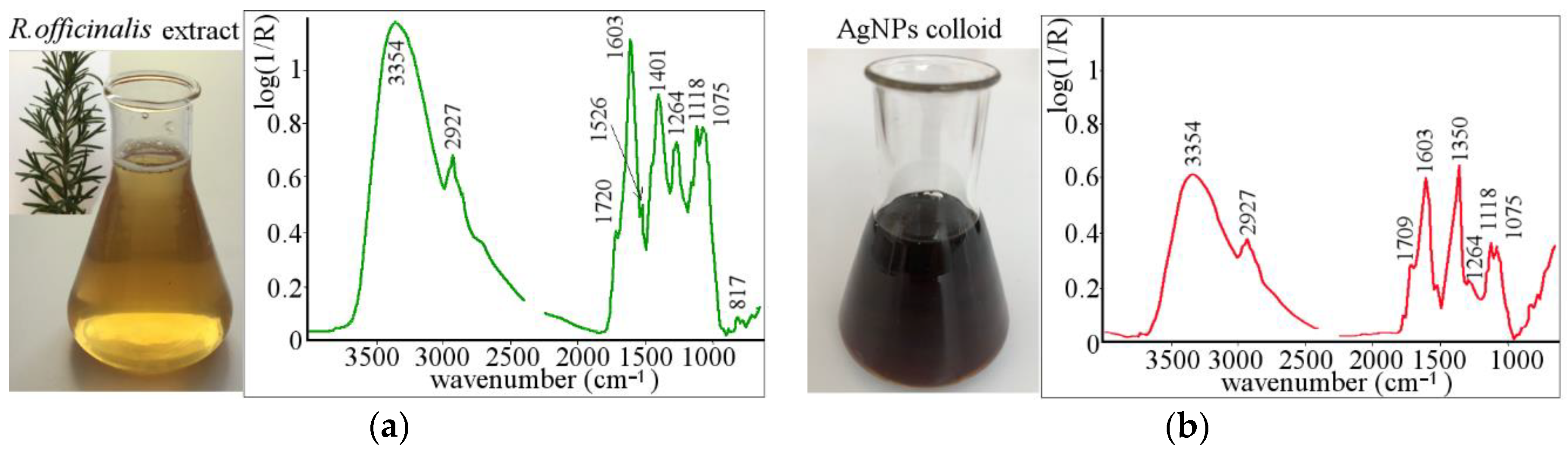
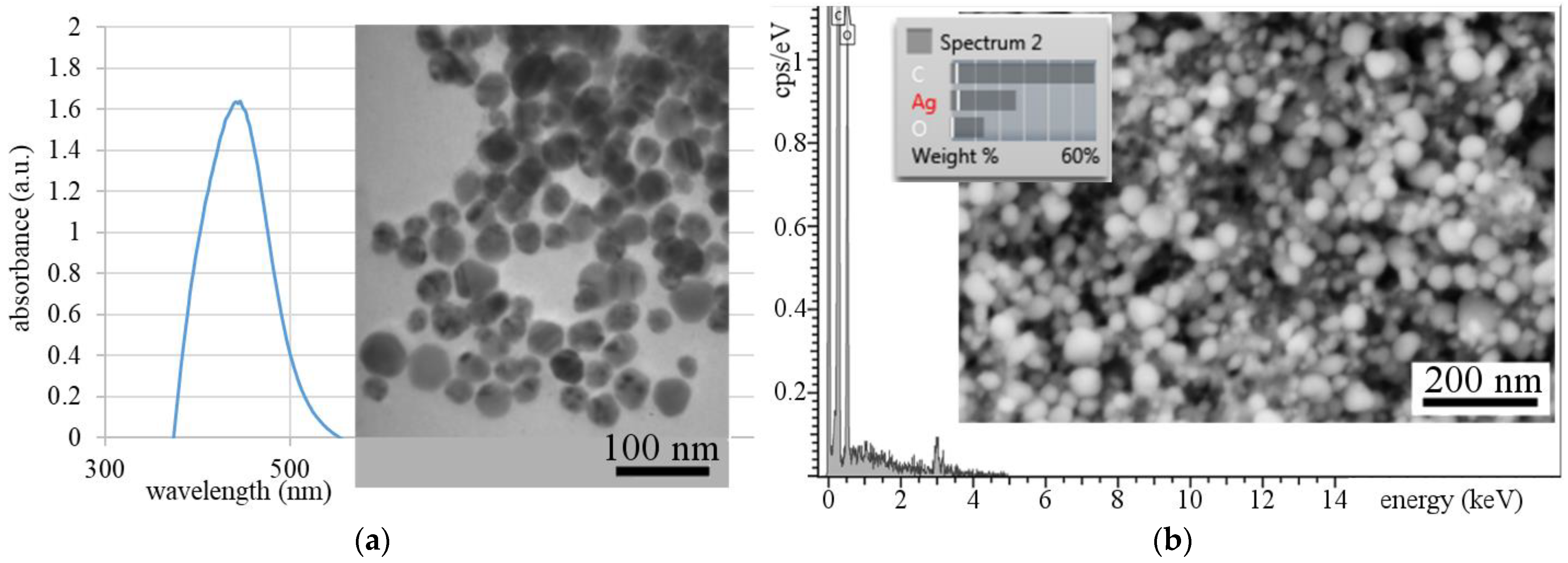
3.1. Synthesis and Characterization of Pre-Prepared Silver Nanoparticles
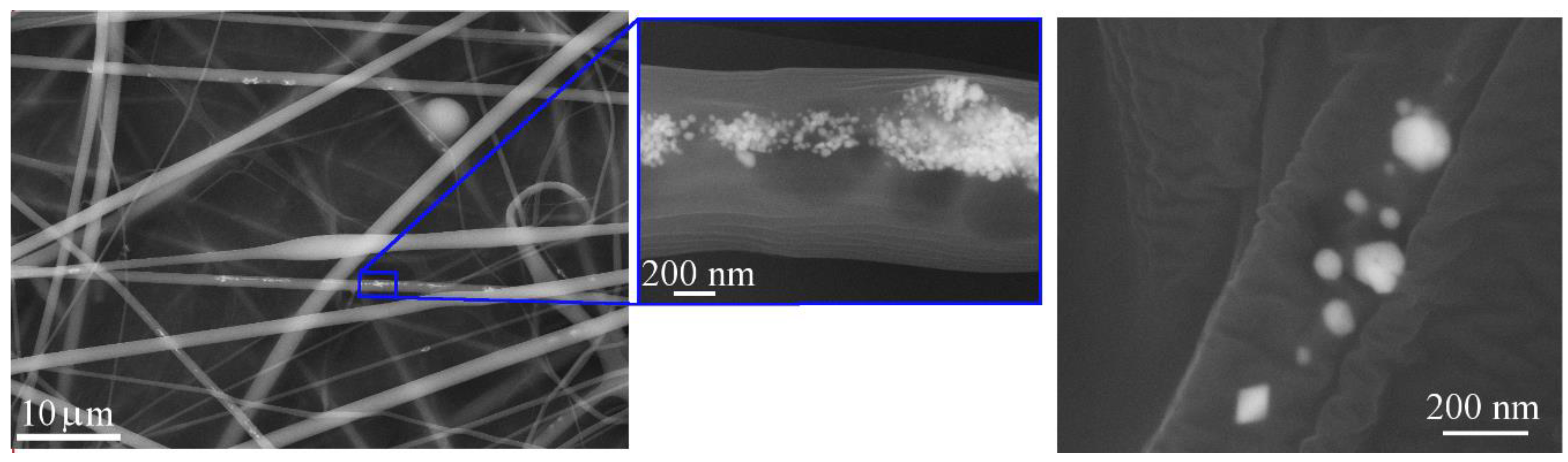
3.2. Characterization of Polymer Matrix Composite
3.3. Antibiofilm Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Khan, R.M.R.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Ahila, N.; Ramkumar, V.S.; Prakash, S.; Manikandan, B.; Ravindran, J.; Dhanalakshmi, P.; Kannapiran, E. Synthesis of stable nanosilver particles (AgNPs) by the proteins of seagrass Syringodium isoetifolium and its biomedicinal properties. Biomed. Pharmacother. 2016, 84, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Nikaeen, G.; Yousefinejad, S.; Rahmdel, S.; Samari, F.; Mahdavinia, S. Central Composite Design for Optimizing the Biosynthesis of Silver Nanoparticles using Plantago major Extract and Investigating Antibacterial, Antifungal and Antioxidant Activity. Sci. Rep. 2020, 10, 9642. [Google Scholar]
- Ebrahimzadeh, M.A.; Naghizadeh, A.; Amiri, O.; Shirzadi-Ahodashti, M.; Mortazavi-Derazkola, S. Green and facile synthesis of Ag nanoparticles using Crataegus pentagynafruit extract (CP-AgNPs) for organic pollution dyes degradation andantibacterial application. Bioorg. Chem. 2020, 94, 103425. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.-S.; Kumar, G.; Benelli, G.; Ghodake, G.S.; Jiang, Y.Y.; Kim, D.S.; Saratale, G.D. Exploiting fruit byproducts for eco-friendly nanosynthesis: Citrus × clementina peel extract mediated fabrication of silver nanoparticles with high efficacy against microbial pathogens and rat glial tumor C6 cells. Environ. Sci. Pollut. Res. 2017, 25, 10250–10263. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Kim, D.-S.; Kim, D.-Y.; Shin, H.S. Exploiting Fruit Waste Grape Pomace for Silver Nanoparticles Synthesis, Assessing Their Antioxidant, Antidiabetic Potential and Antibacterial Activity Against Human Pathogens: A Novel Approach. Nanomaterials 2020, 10, 1457. [Google Scholar] [CrossRef]
- Shakeel, A.; Mudasir, A.; Babu, L.S.; Saiqa, I. A review on plants extracts mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar]
- Sadasivuni, K.K.; Ponnamma, D.; Rajan, M.; Ahamed, M.B.; Al-Maadeed, M.A.S.A. Polymer Nanocomposites in Biomedical Engineering; Springer Nature: Cham, Switzerland, 2019; Correction to: Polymer Nanocomposites in Biomedical Engineering; Sadasivuni, K.K., Ponnamma, D., Rajan, M., Ahmed, B., Al-Maadeed, M., Eds.; Lecture Notes in Bioengineering; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Zhu, M.; Hua, D.; Zhong, M.; Zhang, L.; Wang, F.; Gao, B.; Xiong, R.; Huang, C. Antibacterial and Effective Air Filtration Membranes by “Green” Electrospinning and Citric Acid Crosslinking. Colloid Interface Sci. Commun. 2018, 23, 52–58. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Román, J.S. (Eds.) Chapter 1—Introduction to Smart Polymers and Their Applications. In Smart Polymers and their Applications, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–11. [Google Scholar]
- Zhu, M.; Xiong, R.; Huang, C. Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration. Carbohydr. Polym. 2019, 205, 55–62. [Google Scholar] [CrossRef]
- Mahmoud, K. Synthesis, characterization, optical and antimicrobial studies of polyvinyl alcohol–silver nanocomposites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 434–440. [Google Scholar] [CrossRef]
- Ma, H.; Li, A.; Xu, Y.; Zhang, W.; Liu, J. Preparation of pH-responsive AgNPs/polymer nanohybrids with controllable metal-enhanced fluorescence behavior. Eur. Polym. J. 2015, 72, 212–221. [Google Scholar] [CrossRef]
- Paridaa, D.; Simonettia, P.; Frisonb, R.; Bulbula, E.; Altenriedc, S.; Arroyod, Y.; Balogh-Michelsb, Z.; Caserie, W.; Renc, Q.; Hufenusa, R.; et al. Polymer-assisted in-situ thermal reduction of silver precursors: A solventless route for silver nanoparticles-polymer composites. Chem. Eng. J. 2020, 389, 123983. [Google Scholar] [CrossRef]
- Mansour, A.; Poncin-Epaillard, F.; Debarnot, D. Distribution of metal nanoparticles in a plasma polymer matrix according to the structure of the polymer and the nature of the metal. Thin Solid Films 2020, 699, 137261. [Google Scholar] [CrossRef]
- Das, J.; Velusamy, P. Antibacterial effects of biosynthesized silver nanoparticles using aqueous leaf extract of Rosmarinus officinalis L. Mater. Res. Bull. 2013, 48, 4531–4537. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Botero, L.E.; Zárate-Triviño, D.; Acevedo-Yepes, N.; Escobar, J.S.; Pérez, V.Z.; Riano, L.J.C. Synthesis and characterization of a silver nanoparticle-containing polymer composite with antimicrobial abilities for application in prosthetic and orthotic devices. Biomater. Res. 2020, 24, 1–17. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vázquez, B.; Román, J.S. Smart Polymers and Their Applications as Biomaterials. In Topics in Tissue Engineering; Ashammakhi, N., Reis, R., Chiellini, E., Eds.; Oulu University: Oulu, Finland, 2007; pp. 1–27. [Google Scholar]
- Ghaedi, M.; Yousefinejad, M.; Safarpoor, M.; Khafri, H.Z.; Purkait, M. Rosmarinus officinalis leaf extract mediated green synthesis of silver nanoparticles and investigation of its antimicrobial properties. J. Ind. Eng. Chem. 2015, 31, 167–172. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Berent, S.; Motyka, A.; Jamroz, P.; Kurcbach, K.; Sledz, W.; Pohl, P. Comparison of the characteristics of gold nanoparticles synthesized using aqueous plant extracts and natural plant essential oils of Eucalyptus globulus and Rosmarinus officinalis. Arab. J. Chem. 2019, 12, 4795–4805. [Google Scholar] [CrossRef]
- Vaquero, M.R.; Yáñez-Gascón, M.-J.; Villalba, R.G.; Larrosa, M.; Fromentin, E.; Ibarra, A.; Roller, M.; Tomás-Barberán, F.; De Gea, J.C.E.; Garcia-Conesa, M.-T. Inhibition of Gastric Lipase as a Mechanism for Body Weight and Plasma Lipids Reduction in Zucker Rats Fed a Rosemary Extract Rich in Carnosic Acid. PLoS ONE 2012, 7, e39773. [Google Scholar] [CrossRef] [PubMed]
- Akbari, J.; Saeedi, M.; Farzin, D.; Morteza-Semnani, K.; Esmaili, Z. Transdermal absorption enhancing effect of the essential oil of Rosmarinus officinalis on percutaneous absorption of Na diclofenac from topical gel. Pharm. Biol. 2015, 53, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.; Fu, Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Imad, H.H.; Israa, A.I.; Hawraa, J.K.; Hameed, I.H.; Ibraheam, I.A.; Kadhim, H.J. Gas chromatography mass spectrum and fourier-transform infrared spectroscopy analysis of methanolic extract of Rosmarinus oficinalis leaves. J. Pharmacogn. Phytother. 2015, 7, 90–106. [Google Scholar] [CrossRef]
- Saber, M.M.; Mirtajani, S.B.; Karimzadeh, K. Green synthesis of silver nanoparticles using Trapa natansextract and theiranticancer activity against A431 human skin cancer cells. J. Drug Deliv. Sci. Technol. 2018, 47, 375–379. [Google Scholar] [CrossRef]
- Li, H.-J.; Zhang, A.-Q.; Hu, Y.; Sui, L.; Qian, D.-J.; Chen, M. Large-scale synthesis and self-organization of silver nanoparticles with Tween 80 as a reductant and stabilizer. Nanoscale Res. Lett. 2012, 7, 612. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Xin, Z.; Deng, M.; Wen, Y.; Song, Y. Synthesis of monodisperse silver nanoparticles for ink-jet printed flexible electronics. Nanotechnology 2011, 22, 425601. [Google Scholar] [CrossRef]
- Hernández-Morales, L.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Núñez, G.; Espinoza, K.A. Study of the green synthesis of silver nanoparticles using a natural extract of dark or white Salvia hispanica L. seeds and their antibacterial application. Appl. Surf. Sci. 2019, 489, 952–961. [Google Scholar]
- Sarkar, S.; Guibal, E.; Quignard, F.; Sengupta, A.K. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanopart. Res. 2012, 14, 1–24. [Google Scholar] [CrossRef]
- Kickelbick, G. (Ed.) Hybrid Materials: Synthesis, Characterization, and Applications; Wiley-VCH: Weinheim, Germany, 2007; p. 516. [Google Scholar]
- Oliveira, M.; Machado, A.V. Chapter 4: Preparation of Polymer-Based Nanocomposites by Different Routes. In Nanocomposites: Synthesis, Characterization and Applications; Wang, X., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2013. [Google Scholar]
- Jatoi, A.W.; Kim, I.S.; Ogasawara, H.; Ni, Q.-Q. Characterizations and application of CA/ZnO/AgNP composite nanofibers for sustained antibacterial properties. Mater. Sci. Eng. C 2019, 105, 110077. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, J.; Lin, L.; Wang, R.; Cheng, B.; Chen, Y.; Tang, L.; Chen, J.; Ma, X. In situ synthesis of poly (γ-glutamic acid)/alginate/AgNP composite microspheres with antibacterial and hemostatic properties. Carbohydr. Polym. 2019, 221, 21–28. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velgosova, O.; Mudra, E.; Vojtko, M. Preparing, Characterization and Anti-Biofilm Activity of Polymer Fibers Doped by Green Synthesized AgNPs. Polymers 2021, 13, 605. https://doi.org/10.3390/polym13040605
Velgosova O, Mudra E, Vojtko M. Preparing, Characterization and Anti-Biofilm Activity of Polymer Fibers Doped by Green Synthesized AgNPs. Polymers. 2021; 13(4):605. https://doi.org/10.3390/polym13040605
Chicago/Turabian StyleVelgosova, Oksana, Erika Mudra, and Marek Vojtko. 2021. "Preparing, Characterization and Anti-Biofilm Activity of Polymer Fibers Doped by Green Synthesized AgNPs" Polymers 13, no. 4: 605. https://doi.org/10.3390/polym13040605
APA StyleVelgosova, O., Mudra, E., & Vojtko, M. (2021). Preparing, Characterization and Anti-Biofilm Activity of Polymer Fibers Doped by Green Synthesized AgNPs. Polymers, 13(4), 605. https://doi.org/10.3390/polym13040605







