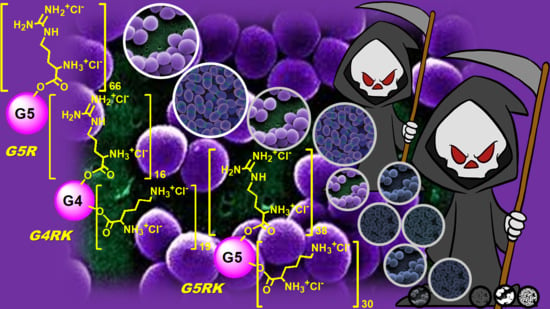
Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry: Materials and Measurements
2.2. Synthesis of the Amino Acid-Conjugated Cationic Empty Dendrimers G4R(16)K(19), G5R(66) and G5R(38)K(30): General Procedure
2.3. Preparation of the Physical Mixture of UA and OA (1:1) (UOA)
2.4. Preparation of UOA-Loaded Dendrimers (UOACDs): General Procedure
2.5. Microorganisms
2.6. Antimicrobial Assays
2.7. Statistical Analysis
3. Results and Discussion
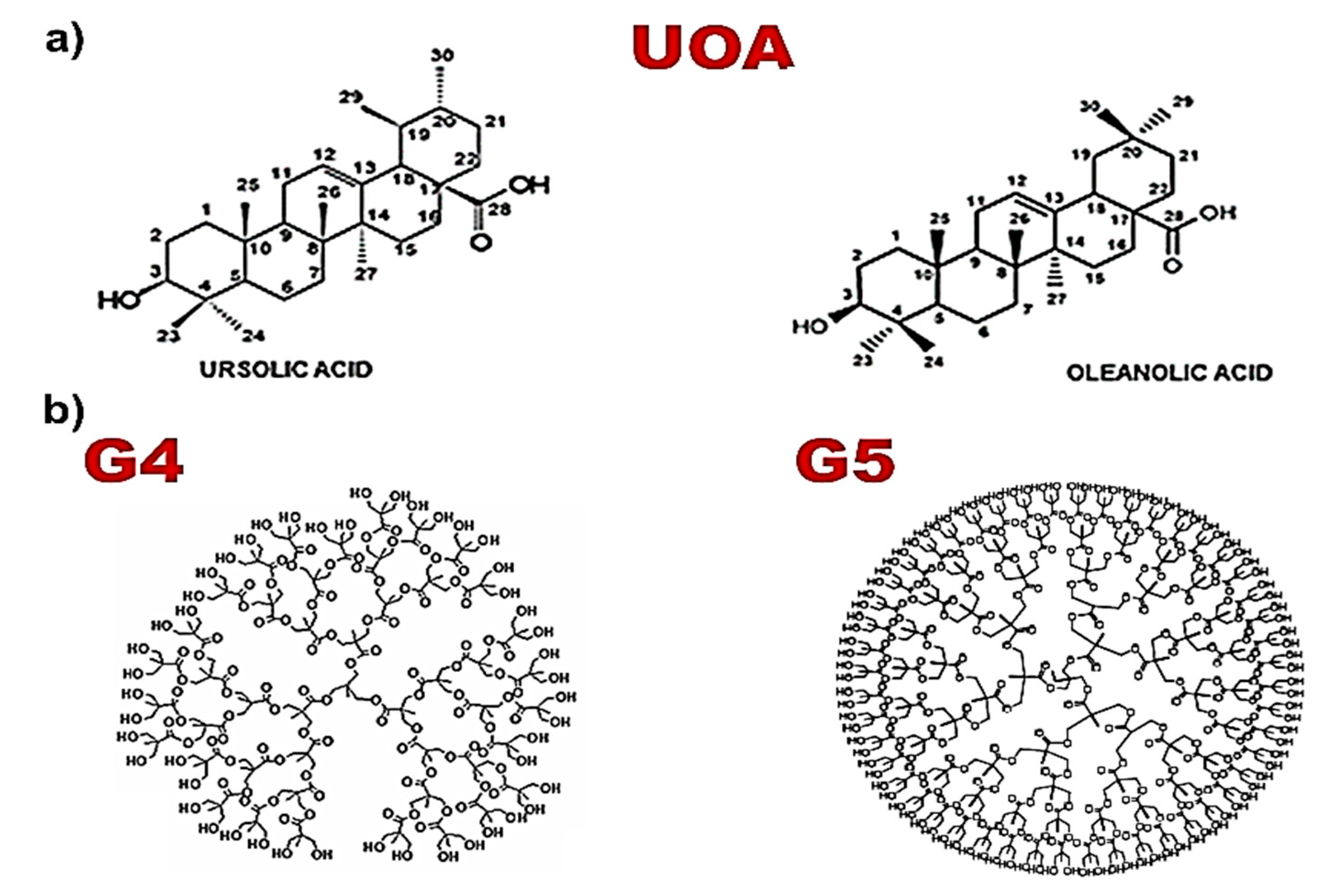
3.1. Positively Charged Amino Acid-Conjugated Dendrimer-Drug Compounds Designed for This Study
3.2. Positively Charged Amino Acid-Conjugated Empty Dendrimers
3.3. Physical Mixture of Commercial UA and OA, 1:1 (UOA)
3.4. Preparation of Dendrimers Loaded with the Physical Mixture of UA and OA, 1:1 (UOA)
3.5. Antimicrobial Activities of G4R(16)K(19), G5R(38)K(30) and G5R(66)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug Resistant Bacterial Infections, Including Tuberculosis; WHO: Geneva, Switzerland, 2017; WHO/EMP/IAU/2017.12; Available online: https://www.who.int/medicines/areas/rational_use/PPLreport_2017_09_19.pdf?ua=1 (accessed on 18 January 2021).
- World Health Organization (WHO). No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations. Interagency Coordination Group on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1 (accessed on 18 January 2021).
- Koulenti, D.; Xu, E.; Mok, I.Y.S.; Song, A.; Karageorgopoulos, D.E.; Armaganidis, A.; Lipman, J.; Tsiodras, S. Novel Antibiotics for Multidrug-Resistant Gram-Positive Microorganisms. Microorganisms 2019, 7, 270. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Seo, M.D.; Won, H.S.; Kim, J.H.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides for therapeutic applications: A review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef]
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins Revisited. Clinic. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A. Positively charged polymers as promising devices against multidrug resistant Gram-Negative bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gordon, V.D.; Trinkle, D.R.; Schmidt, N.W.; Davis, M.A.; DeVries, C.; Som, A.; Cronan, J.E., Jr.; Tew, G.N.; Wong, G.C.L. Mechanism of a prototypical synthetic membrane-active antimicrobial: Efficient hole-punching via interaction with negative intrinsic curvature lipids. Proc. Natl. Acad. Sci. USA 2008, 105, 20595–20600. [Google Scholar] [CrossRef] [PubMed]
- Spohn, R.; Daruka, L.; Lázár, V.; Martins, A.; Vidovics, F.; Grézal, G.; Méhi, O.; Kintses, B.; Számel, M.; Jangir, P.K.; et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 2019, 10, e4538. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. From nanobiotechnology, positively charged biomimetic dendrimers as novel antibacterial agents: A review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef]
- Alfei, S.; Signorello, M.G.; Schito, A.; Catena, S.; Turrini, F. Reshaped as polyester-based nanoparticles, gallic acid inhibits platelet aggregation, reactive oxygen species production and multi-resistant Gram-positive bacteria with an efficiency never obtained. Nanoscale Adv. 2019, 1, 4148–4157. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from "oxidative stress". Drug Deliv. Transl. Res. 2020, 10, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-based dendrimer nanoparticles combined with etoposide have an improved cytotoxic and pro-oxidant effect on human neuroblastoma cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer nanodevices and gallic acid as novel strategies to fight chemoresistance in neuroblastoma cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Mohammadi, R.; Arzanlou, M.; Akbari Dourbash, F.; Kouhsari, E.; Majidi, G.; Mohseni, S.M.; Nazari, S. In vitro antibacterial activity of poly (amidoamine)-G7 dendrimer. BMC Infect. Dis. 2017, 17, 395. [Google Scholar] [CrossRef]
- Stenström, P.; Hjorth, E.; Zhang, Y.; Andrén, O.C.J.; Guette-Marquet, S.; Schultzberg, M.; Malkoch, M. Synthesis and in Vitro Evaluation of Monodisperse Amino-Functional Polyester Dendrimers with Rapid Degradability and Antibacterial Properties. Biomacromolecules 2017, 18, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Karanastasis, A.; Casey, K.R.; Necelis, M.; Carone, B.R.; Caputo, G.A.; Palermo, E.F. Cationic Molecular Umbrellas as Antibacterial Agents with Remarkable Cell-Type Selectivity. ACS Appl. Mater. Interfaces 2020, 12, 21270–21282. [Google Scholar] [CrossRef]
- Schito, A.M.; Alfei, S. Antibacterial activity of non-cytotoxic, amino acid-modified polycationic dendrimers against Pseudomonas aeruginosa and other non-fermenting Gram-negative bacteria. Polymers 2020, 12, 1818. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S. Synthesis and characterization of polyester-based dendrimers containing peripheral arginine or mixed amino acids as potential vectors for gene and drug delivery. Macromol. Res. 2017, 25, 1172–1186. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and characterization of versatile amphiphilic dendrimers peripherally decorated with positive charged amino acids. Polym. Int. 2018, 67, 1572–1584. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and characterization of fourth generation polyester-based dendrimers with cationic amino acids-modified crown as promising water soluble biomedical devices. Polym. Adv. Technol. 2018, 29, 2735–2749. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S.; Taptue, G.B. Synthesis and NMR characterization of dendrimers based on 2, 2-bis-(hydroxymethyl)-propanoic acid (bis-HMPA) containing peripheral amino acid residues for gene transfection. Org. Commun. 2017, 10, 144–177. [Google Scholar] [CrossRef]
- Alfei, S.; Taptue, G.B.; Catena, S.; Bisio, A. Synthesis of Water-soluble, Polyester-based Dendrimer Prodrugs for Exploiting Therapeutic Properties of Two Triterpenoid Acids. Chin. J. Polym. Sci. 2018, 36, 999–1010. [Google Scholar] [CrossRef]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/ast_of_bacteria/ (accessed on 18 January 2021).
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Do Nascimento, P.G.; Lemos, T.L.; Bizerra, A.M.; Arriaga, Â.M.; Ferreira, D.A.; Santiago, G.M.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mat. 2014, 3, 1969. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Gonzalez, M.; Cerecetto, H.; Santo, M.; Silber, J.J. Solubilization and Release Properties of Dendrimers. Evaluation as Prospective Drug Delivery Systems. J. Supramol. Chem. 2006, 18, 633–643. [Google Scholar] [CrossRef]
- Santo, M.; Fox, M.A. Hydrogen bonding interactions between starburst dendrimers and several molecules of biological interest. Phys. Org. Chem. 1999, 12, 293–307. [Google Scholar]
- Cheng, Y.; Xu, Z.; Ma, M.; Xu, T. Dendrimers as Drug Carriers: Applications in Different Routes of Drug Administration. J. Pharm. Sci. 2008, 97, 123–143. [Google Scholar] [CrossRef]
- Milhem, O.M.; Myles, C.; McKeown, N.B.; Attwood, D.; D’Emanuele, A. Polyamidoamine starburst dendrimers as solubility enhancers. Int. J. Pharm. 2000, 197, 239–241. [Google Scholar] [CrossRef]
- Kolhe, P.; Misra, E.; Kannan, R.M.; Kannan, S.; Lieh-Lai, M. Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int. J. Pharm. 2003, 259, 143–160. [Google Scholar] [CrossRef]
- Twyman, L.J.; Beezer, A.E.; Esfand, R.; Hardy, M.J.; Mitchell, J.C. The synthesis of water soluble dendrimers, and their application as possible drug delivery systems. Tetrahedron Lett. 1999, 40, 1743–1746. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Spectral Assignments and Reference Data. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Wang, J.; Mao, W.; Lock, L.L.; Tang, J.; Sui, M.; Sun, W.; Cui, H.; Xu, D.; Shen, Y. The Role of Micelle Size in Tumor Accumulation, Penetration, and Treatment. ACS Nano 2015, 9, 7195–7206. [Google Scholar] [CrossRef]
- Yu, H.; Cui, Z.; Yu, P.; Guo, C.; Feng, B.; Jiang, T.; Wang, S.; Yin, Q.; Zhong, D.; Yang, X.; et al. PH- and NIR Light-Responsive Micelles with Hyperthermia-Triggered Tumor Penetration and Cytoplasm Drug Release to Reverse Doxorubicin Resistance in Breast Cancer. Adv. Funct. Mater. 2015, 25, 2489–2500. [Google Scholar] [CrossRef]


| Compound | mg, mmol Yield % | Physical State | FTIR (KBr, cm−1) | 1H NMR (300 MHz, CD3OD, δ, ppm) |
|---|---|---|---|---|
| G4[R(16)K(19)OH(13)UOA(4)] | 27.0, 0.0018 90 | Slightly hygroscopic off-white glassy solid | 3431 (OH and NH3+) 1747 (C=O ester) 1631 (NH) | 0.78–0.99 (several s, 88H, H of triterpenoids) 1.00–2.40 (m, 404H, H of dendrimer + H of Arg + H of Lys + H of triterpenoids) 2.94–3.17 (m, 46H, H of Lys + H of triterpenoids) 3.30–3.50 (m, 32H, H of Arg) 3.54–3.82 (m, 26H, H of dendrimer) 4.10–4.50 (m, 195H, H of dendrimer + H of Lys + H of Arg) 4.59–4.71 (dd, 4H, H of triterpenoids) 5.22 (q, 4H, H of triterpenoids) |
| G5[R(66)OH(30)UOA(3)] | 44.2, 0.0016 100 | Slightly hygroscopic off-white fluffy solid | 3392 (OH and NH3+) 1743 (C=O ester) 1663 (NH) | 0.78–0.97 (several s, 66H, H of triterpenoids) 1.00–2.30 (m, 612H, H of dendrimer + H of Arg + H of triterpenoids) 3.27 (m, 132H, H of Arg) 3.68–3.74 (br, 60H, H of dendrimer) 4.04–4.63 (m, 384H, H of dendrimer + H of Arg) 5.22 (q, 3H, H of triterpenoids) |
| G5[R(38)K(30)OH(28)UOA(8)] | 20.5, 0.00070 64 | Slightly hygroscopic pale yellow fluffy solid | 3431 (OH and NH3+) 1747 (C=O ester) 1635 (NH) | 0.75–0.98 (several s, 176 H, H of triterpenoids) 1.00–2.40 (m, 790 H, H of dendrimer + H of Arg + H of Lys + H of triterpenoids) 2.95–3.16 (m, 76H, H of Lys + H of triterpenoids) 3.38 (m, 76H, H of Arg) 3.68 (m, 56H, H of dendrimer) 4.10–4.50 (m, 390 H, H of dendrimer + H of Lys + H of Arg) 4.58–4.70 (dd, 8H, H of triterpenoids) 5.22 (q, 8H, H of triterpenoids) |
| Features | G4R(16)K(19)UOA(4) | G5R(38)K(30)UOA(8) | G5R(66)UOA(3) |
|---|---|---|---|
| Arginine, lysine, residual hydroxyl units | 16, 19, 13 | 38, 30, 28 | 66, 0, 30 |
| UOA moles per dendrimer mole | 4 | 8 | 3 |
| UOA loading % (wt/wt) | 12.6 | 12.7 | 5.0 |
| Dendrimer surface covered (%) | 63 | 69 | 71 |
| Cationic groups | 70 | 136 | 132 |
| Molecular weight | 14,600 | 29,300 | 27,400 |
| Z-potential (mV) | 24.8 | 31.8 | 34.0 |
| Z-Ave size (nm) | 24.9 | 20.3 | 16.1 |
| UOA released by complexes after 24 h (µg/10 mg) | 75.5 | 65.9 | 65.2 |
| UOA MW 456.7 | G5R(66)UOA(3) MW 27,400 | Max UOA Released 1 | G4R(16)K(19)UOA(4) MW 14,600 | Max UOA Released 2 | G5R(38)K(30)UOA(8) MW 29,300 | Max UOA Released 3 | |
|---|---|---|---|---|---|---|---|
| MIC values 4 | µg/mL, µM | µg/mL, µM | µg/mL, µM | µg/mL, µM | µg/mL, µM | µg/mL, µM | µg/mL, µM |
| S. aureus 118 * | 32, 70.1 | 256, 9.3 | 1.7, 3.7 | 256, 17.5 | 1.9, 4.2 | 128, 4.4 | 0.8, 1.8 |
| S. aureus 120 * | 16, 35.0 | 512, 18.7 | 3.4, 7.4 | 512, 35.1 | 3.9, 8.5 | 256, 8.7 | 1.6, 3.6 |
| S. aureus 119 | 32, 70.1 | 512, 18.7 | 3.4, 7.4 | 512, 35.1 | 3.9, 8.5 | 256, 8.7 | 1.6, 3.6 |
| S. epidermidis 127 * | 16, 35.0 | 128, 4.7 | 0.9, 1.9 | 128, 8.8 | 1.0, 2.1 | 64, 2.2 | 0.4, 0.9 |
| S. epidermidis 201 * | 32, 70.1 | 256, 9.3 | 1.7, 3.7 | 256, 17.5 | 1.9, 4.2 | 128, 4.4 | 0.8, 1.8 |
| S. epidermidis 119 | 16, 35.0 | 512, 18.7 | 3.4, 7.4 | 256, 17.5 | 1.9, 4.2 | 64, 2.2 | 0.4, 0.9 |
| E. faecalis 110 # | 8, 17.5 | 512, 18.7 | 3.4, 7.4 | 32, 2.2 | 0.2, 0.5 | 32, 1.1 | 0.2, 0.5 |
| E. faecalis 124 # | 4, 8.8 | 256, 9.3 | 1.7, 3.7 | 64, 4.4 | 0.4, 1.0 | 16, 0.5 | 0.1, 0.3 |
| E. faecalis 127 | 8, 17.5 | 256, 9.3 | 1.7, 3.7 | 64, 4.4 | 0.4, 1.0 | 32, 1.1 | 0.2, 0.5 |
| E. faecalis 19 †,# | 8, 17.5 | 256, 9.3 | 1.7, 3.7 | 32, 2.2 | 0.2, 0.5 | 16, 0.5 | 0.1, 0.3 |
| E. faecalis 51 †,# | 4, 8.8 | 256, 9.3 | 1.7, 3.7 | 32, 2.2 | 0.2, 0.5 | 16, 0.5 | 0.1, 0.3 |
| E. faecium 118 # | 4, 8.8 | 256, 9.3 | 1.7, 3.7 | 64, 4.4 | 0.4, 1.0 | 32, 1.1 | 0.2, 0.5 |
| E. faecium 123 # | 2, 4.4 | 512, 18.7 | 3.4, 7.4 | 32, 2.2 | 0.2, 0.5 | 16, 0.5 | 0.1, 0.3 |
| E. faecium 127 | 4, 8.8 | 512, 18.7 | 3.4, 7.4 | 64, 4.4 | 0.4, 1.0 | 32, 1.1 | 0.2, 0.5 |
| E. faecium 3 †,# | 4, 8.8 | 512, 18.7 | 3.4, 7.4 | 32, 2.2 | 0.2, 0.5 | 16, 0.5 | 0.1, 0.3 |
| G5R(66)OH(30)MW 26,000 | G4R(16)K(19)OH(13) MW 12,800 | G5R(38)K(30)OH(28) MW 25,700 | Commercial Antibiotics | |
|---|---|---|---|---|
| MIC values 1 | µg/mL, µM | µg/mL, µM | µg/mL, µM | µg/mL, µM 2 |
| S. aureus 118 * | 256, 9.8 | 256, 20.0 | 128, 4.98 | 256, 637.7 |
| S. aureus 120 * | 512, 19.6 | 512, 40.0 | 256, 9.96 | 512, 1275 |
| S. aureus 119 | 512, 19.6 | 512, 40.0 | 256, 9.96 | 1, 2.5 |
| S. epidermidis 127 * | 128, 4.9 | 128, 40.0 | 64, 2.5 | 256, 637.7 |
| S. epidermidis 201 * | 256, 9.8 | 256, 20.0 | 128, 4.98 | 256, 637.7 |
| S. epidermidis 119 | 512, 19.6 | 256, 20.0 | 64, 2.5 | 0.5, 1.25 |
| µg/mL, µM 3 | ||||
| E. faecalis 110 # | 512, 19.6 | 32, 2.5 | 32, 1.2 | 128, 88.3 |
| E. faecalis 124 # | 256, 9.8 | 64, 10.0 | 16, 0.6 | 32, 22.1 |
| E. faecalis 127 | 256, 9.8 | 64, 10.0 | 32, 1.2 | 1, 0.7 |
| E. faecalis 19 †,# | 256, 9.8 | 32, 2.5 | 16, 0.6 | 32, 22.1 |
| E. faecalis 51 †,# | 256, 9.8 | 32, 2.5 | 16, 0.6 | 32, 22.1 |
| E. faecium 118 # | 256, 9.8 | 64, 10.0 | 32, 1.2 | 256, 176.6 |
| E. faecium 123 # | 512, 19.6 | 32, 2.5 | 16, 0.6 | 128, 88.3 |
| E. faecium 127 | 512, 19.6 | 64, 10.0 | 32, 1.2 | 2, 1.4 |
| E. faecium 3 †,# | 512, 19.6 | 32, 2.5 | 16, 0.6 | 128, 88.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schito, A.M.; Schito, G.C.; Alfei, S. Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers 2021, 13, 521. https://doi.org/10.3390/polym13040521
Schito AM, Schito GC, Alfei S. Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers. 2021; 13(4):521. https://doi.org/10.3390/polym13040521
Chicago/Turabian StyleSchito, Anna Maria, Gian Carlo Schito, and Silvana Alfei. 2021. "Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids" Polymers 13, no. 4: 521. https://doi.org/10.3390/polym13040521
APA StyleSchito, A. M., Schito, G. C., & Alfei, S. (2021). Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers, 13(4), 521. https://doi.org/10.3390/polym13040521