Supramolecular Polymer Nanocomposites for Biomedical Applications
Abstract
1. Introduction
2. Classification of Supramolecular Polymer Nanocomposites
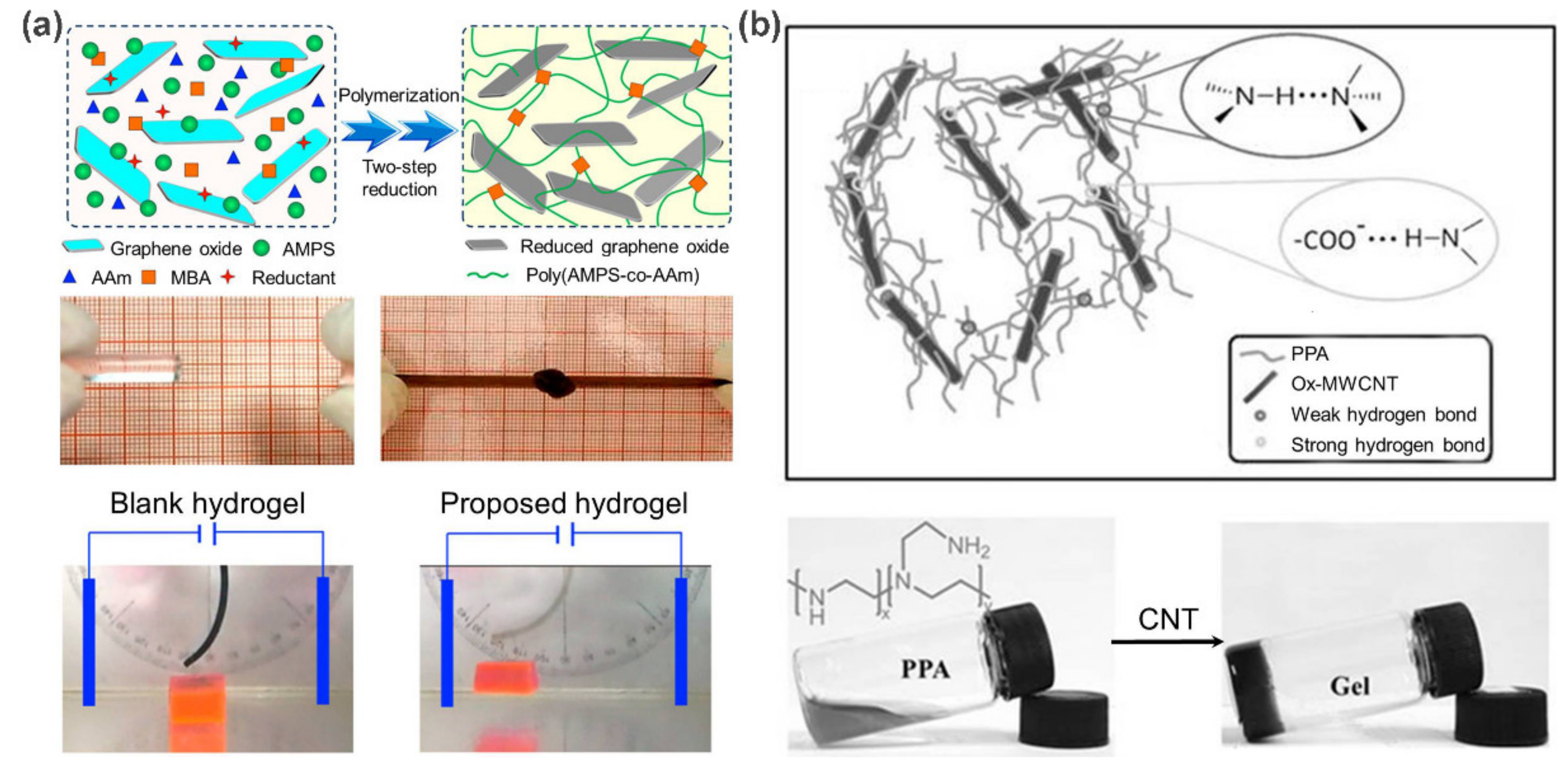
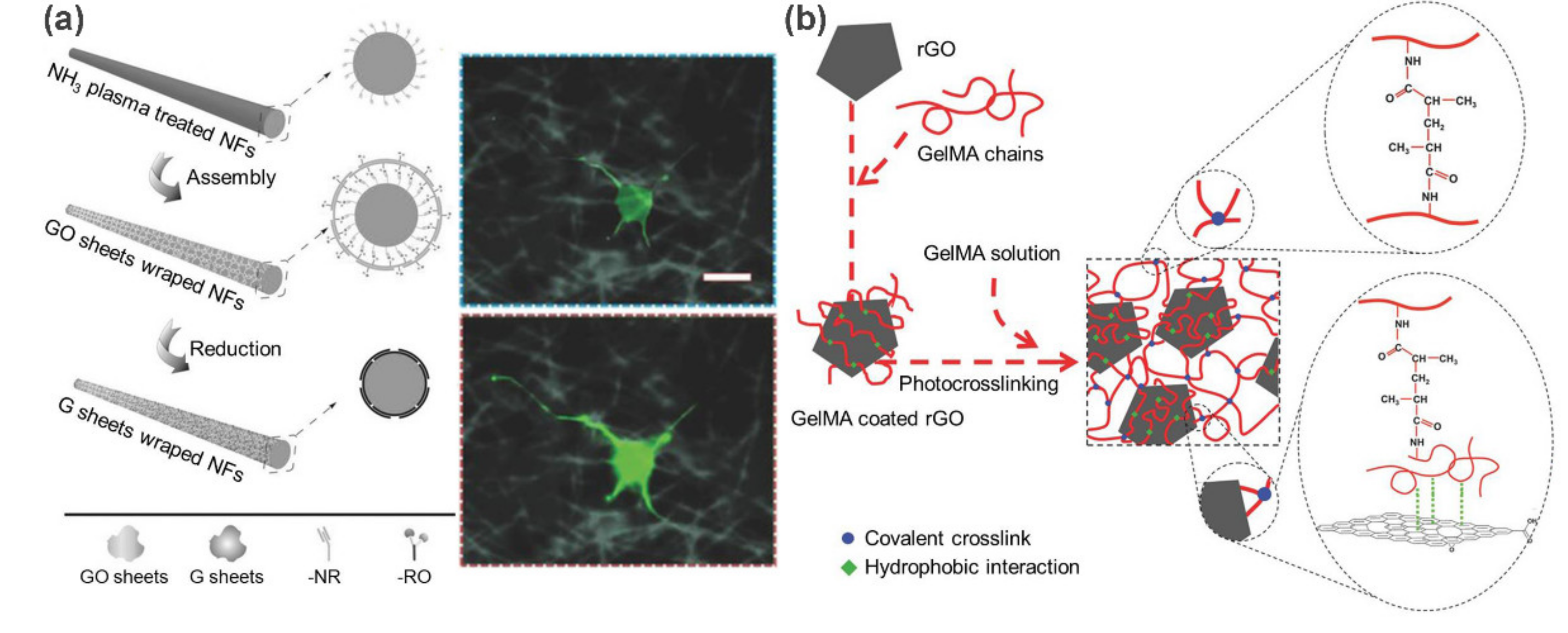
2.1. Carbon-Based Supramolecular Polymer Nanocomposites
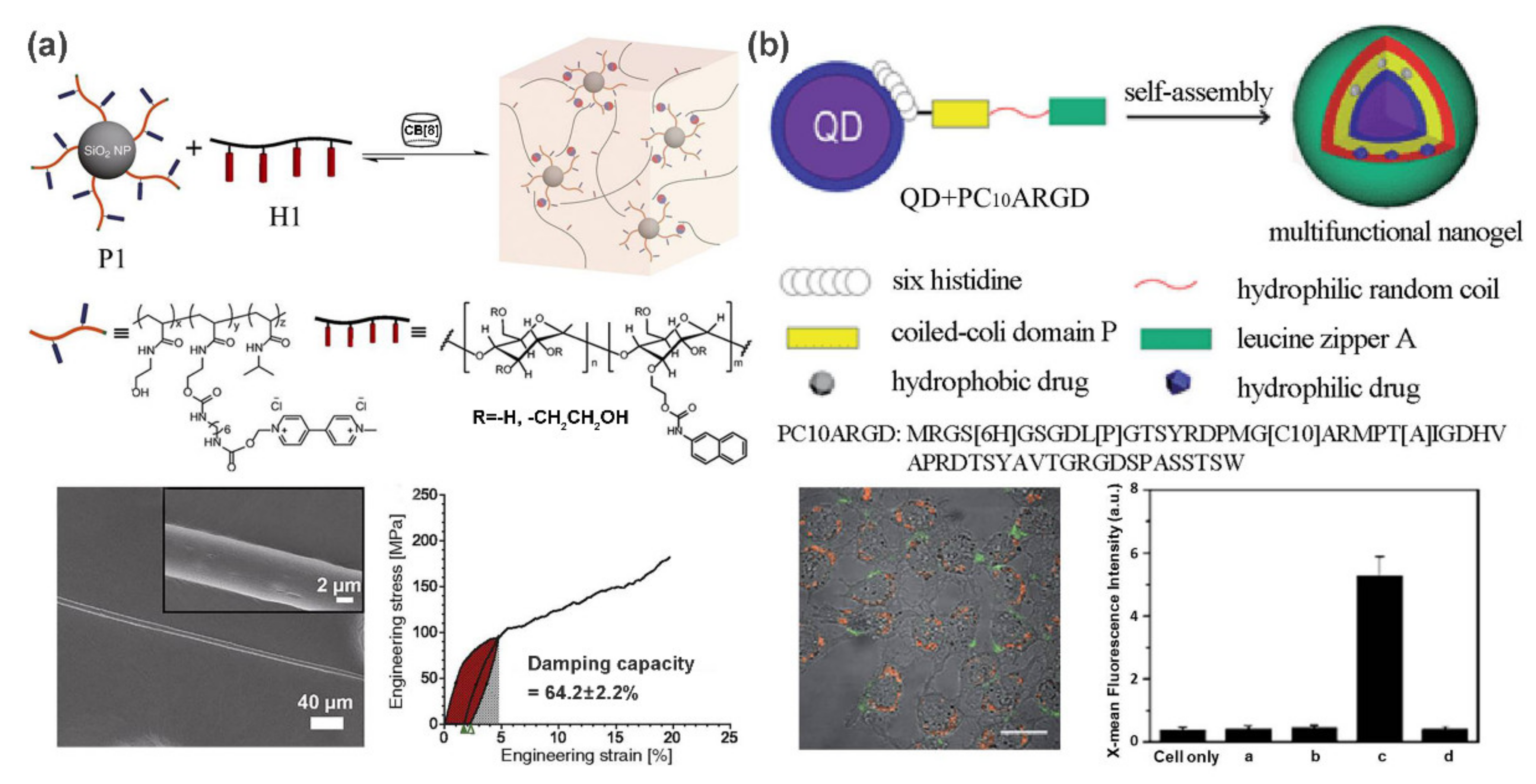
2.2. Inorganic/Semiconductor Nanoparticle-Based Supramolecular Polymer Nanocomposites
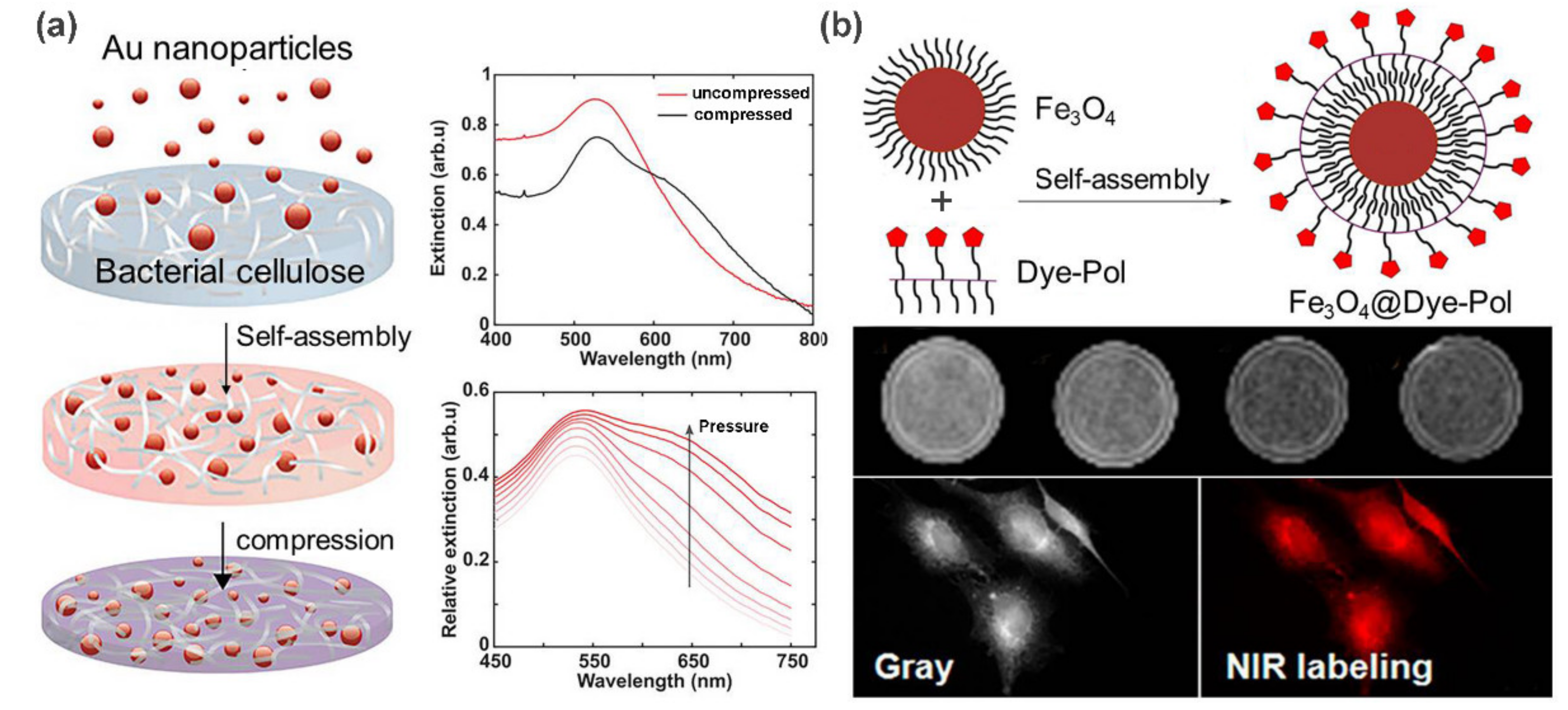
2.3. Metal/Metal-Oxide Nanoparticle-Based Supramolecular Polymer Nanocomposites
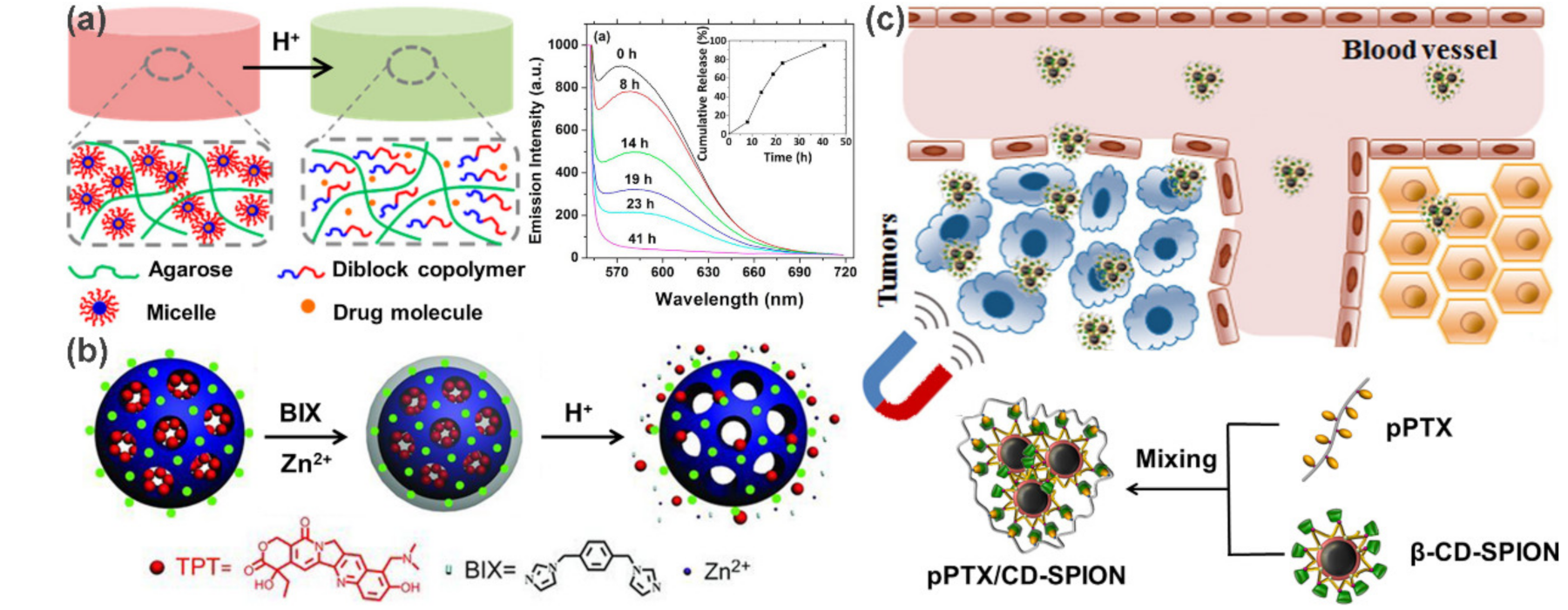
2.4. Other Nanoparticle/Nanostructure-Based Supramolecular Polymer Nanocomposites
3. Biomedical Applications
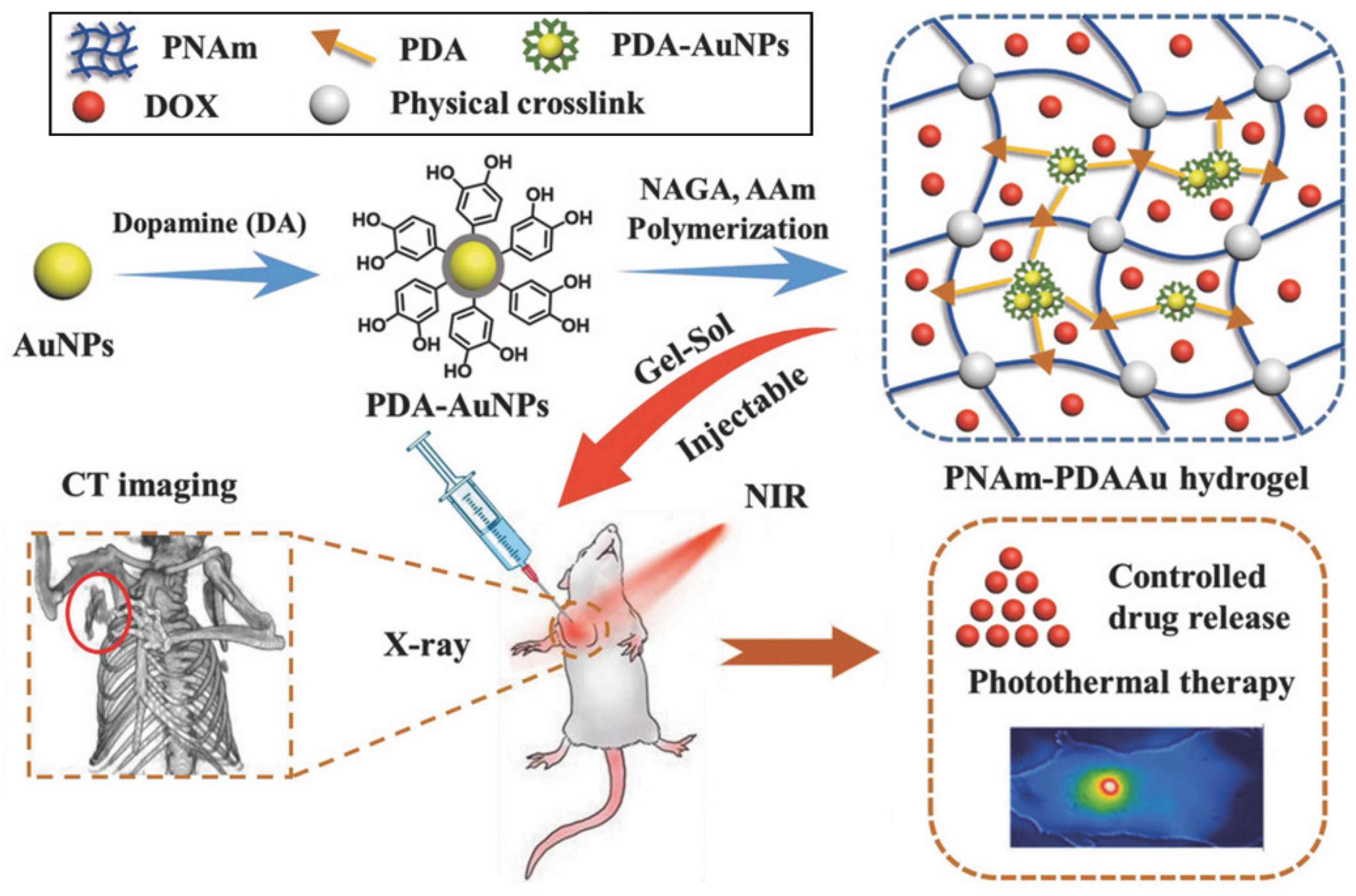
3.1. Therapeutic Delivery
3.2. Bioimaging
3.3. Tissue Engineering
4. Conclusion and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Kumar, S.; Sarita Nehra, M.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Recent advances and remaining challenges for polymeric nanocomposites in healthcare applications. Prog. Polym. Sci. 2018, 80, 1–38. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Li, S.; Meng Lin, M.; Toprak, M.S.; Kim, D.K.; Muhammed, M. Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 2010, 1, 5214. [Google Scholar] [CrossRef]
- Dziadek, M.; Stodolak-Zych, E.; Cholewa-Kowalska, K. Biodegradable ceramic-polymer composites for biomedical applications: A review. Mater. Sci. Eng. C 2017, 71, 1175–1191. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, X.; Wang, H. Self-assembled polymer nanocomposites for biomedical application. Curr. Opin. Colloid Interface Sci. 2018, 35, 36–41. [Google Scholar] [CrossRef]
- Wang, D.; Tong, G.; Dong, R.; Zhou, Y.; Shen, J.; Zhu, X. Self-assembly of supramolecularly engineered polymers and their biomedical applications. Chem. Commun. 2014, 50, 11994–12017. [Google Scholar] [CrossRef]
- Li, M.; Luo, Z.; Zhao, Y. Self-assembled hybrid nanostructures: Versatile multifunctional nanoplatforms for cancer diagnosis and therapy. Chem. Mater. 2018, 30, 25–53. [Google Scholar] [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderon, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef]
- Strong, L.E.; West, J.L. Thermally responsive polymer-nanoparticle composites for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 307–317. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Du, F.-P.; Chen, L.; Yeung, K.-W.; Dong, Y.; Law, W.-C.; Tsui, G.C.-P.; Tang, C.-Y. Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance. Nanotechnol. Rev. 2020, 9, 478–488. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Z.; Chen, C.; Shi, K.; Zhang, L.; Ju, X.-J.; Wang, W.; Xie, R.; Chu, L.-Y. Reduced graphene oxide-containing smart hydrogels with excellent electro-response and mechanical properties for soft actuators. ACS Appl. Mater. Interfaces 2017, 9, 15758–15767. [Google Scholar] [CrossRef]
- Du, R.; Wu, J.; Chen, L.; Huang, H.; Zhang, X.; Zhang, J. Hierarchical hydrogen bonds directed multi-functional carbon nanotube-based supramolecular hydrogels. Small 2014, 10, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yan, L. Supramolecular hydrogel of chitosan in the presence of graphene oxide nanosheets as 2D cross-linkers. ACS Sustain. Chem. Eng. 2014, 2, 296–300. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. Supercritical CO2 processing to improve the electrochemical properties of graphene oxide. J. Supercrit. Fluids 2016, 118, 119–127. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. SC-CO2-assisted process for a high energy density aerogel supercapacitor: The effect of GO loading. Nanotechnology 2017, 28, 204001–204013. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shah, D.U.; Liu, C.; Yu, Z.; Liu, J.; Ren, X.; Rowland, M.J.; Abell, C.; Ramage, M.H.; Scherman, O.A. Bioinspired supramolecular fibers drawn from a multiphase self-assembled hydrogel. Proc. Natl. Acad. Sci. USA. 2017, 114, 8163–8168. [Google Scholar] [CrossRef]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Li, Z.; Xie, X.; Zhang, Z.-Y.; Bian, L. Bisphosphonate-based nanocomposite hydrogels for biomedical applications. Bioact. Mater. 2020, 5, 819–831. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Kairdolf, B.A.; Smith, A.M.; Stokes, T.H.; Wang, M.D.; Young, A.N.; Nie, S. Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu. Rev. Anal. Chem. 2013, 6, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yao, M.-H.; Wen, L.; Song, J.-T.; Zhang, M.-Z.; Zhao, Y.-D.; Liu, B. Multifunctional quantum dot-polypeptide hybrid nanogel for targeted imaging and drug delivery. Nanoscale 2014, 6, 11282–11292. [Google Scholar] [CrossRef] [PubMed]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Elbahri, M. Metal-polymer nanocomposites for functional applications. Adv. Eng. Mater. 2010, 12, 1177–1190. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Nenadovic, M.; Potocnik, J.; Mitric, M.; Strbac, S.; Rakocevic, Z. Modification of high density polyethylene by gold implantation using different ion energies. Mater. Chem. Phys. 2013, 142, 633–639. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabo, D.V. Polymer-nanoparticle composites: From synthesis to modern applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Guerrero, A.R.; Hassan, N.; Escobar, C.A.; Albericio, F.; Kogan, M.J.; Araya, E. Gold nanoparticles for photothermally controlled drug release. Nanomedicine 2014, 9, 2023–2039. [Google Scholar] [CrossRef]
- Eskilson, O.; Lindström, S.B.; Sepulveda, B.; Shahjamali, M.M.; Güell-Grau, P.; Sivlér, P.; Skog, M.; Aronsson, C.; Björk, E.M.; Nyberg, N. Self-assembly of mechanoplasmonic bacterial cellulose-metal nanoparticle composites. Adv. Funct. Mater. 2020, 30, 2004766. [Google Scholar] [CrossRef]
- Kalia, S.; Kango, S.; Kumar, A.; Haldorai, Y.; Kumari, B.; Kumar, R. Magnetic polymer nanocomposites for environmental and biomedical applications. Coll. Polym. Sci. 2014, 292, 2025–2052. [Google Scholar] [CrossRef]
- Yen, S.K.; Janczewski, D.; Lakshmi, J.L.; Dolmanan, S.B.; Tripathy, S.; Ho, V.H.; Vijayaragavan, V.; Hariharan, A.; Padmanabhan, P.; Bhakoo, K.K. Design and synthesis of polymer-functionalized NIR fluorescent dyes-magnetic nanoparticles for bioimaging. ACS Nano 2013, 7, 6796–6805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, J.; Tian, W. Shape memory polymer composites of poly (styrene-b-butadiene-b-styrene) copolymer/liner low density polyethylene/Fe3O4 nanoparticles for remote activation. Appl. Sci. 2016, 6, 333. [Google Scholar] [CrossRef]
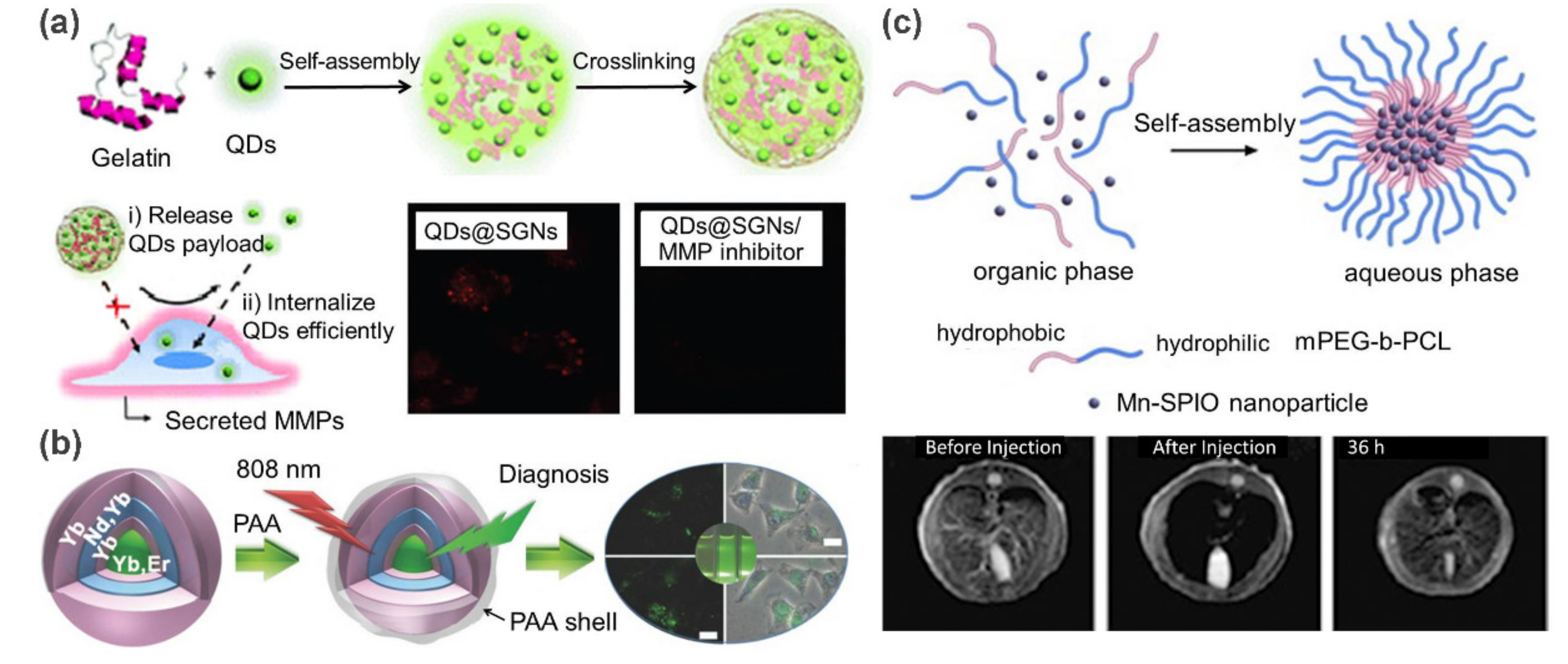
- Cheng, Z.; Lin, J. Synthesis and application of nanohybrids based on upconverting nanoparticles and polymers. Macromol. Rapid Commun. 2015, 36, 790–827. [Google Scholar] [CrossRef]
- Ma, P.a.; Xiao, H.; Li, X.; Li, C.; Dai, Y.; Cheng, Z.; Jing, X.; Lin, J. Rational design of multifunctional upconversion nanocrystals/polymer nanocomposites for cisplatin (IV) delivery and biomedical imaging. Adv. Mater. 2013, 25, 4898–4905. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.J.; Akhter, S. Nanoporous metal organic frameworks as hybrid polymer-metal composites for drug delivery and biomedical applications. Drug Discov. Today 2017, 22, 625–637. [Google Scholar] [CrossRef]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/metal organic framework (MOF) nanocomposites for biomedical applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Merino, S.; Martin, C.; Kostarelos, K.; Prato, M.; Vazquez, E. Nanocomposite hydrogels: 3D polymer-nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Jin, N.; Morin, E.A.; Henn, D.M.; Cao, Y.; Woodcock, J.W.; Tang, S.; He, W.; Zhao, B. Agarose hydrogels embedded with pH-responsive diblock copolymer micelles for triggered release of substances. Biomacromolecules 2013, 14, 2713–2723. [Google Scholar] [CrossRef]
- Xing, L.; Zheng, H.; Cao, Y.; Che, S. Coordination polymer coated mesoporous silica nanoparticles for pH-responsive drug release. Adv. Mater. 2012, 24, 6433–6437. [Google Scholar] [CrossRef]
- Gangrade, A.; Gawali, B.; Jadi, P.K.; Naidu, V.G.; Mandal, B.B. Photo-electro active nanocomposite silk hydrogel for spatiotemporal controlled release of chemotherapeutics: An in vivo approach towards suppressing solid tumor growth. ACS Appl. Mater. Interfaces 2020, 12, 27905–27916. [Google Scholar] [CrossRef] [PubMed]
- Gangrade, A.; Mandal, B.B. Injectable carbon nanotube impregnated silk based multifunctional hydrogel for localized targeted and on-demand anticancer drug delivery. ACS Biomater. Sci. Eng. 2019, 5, 2365–2381. [Google Scholar] [CrossRef]
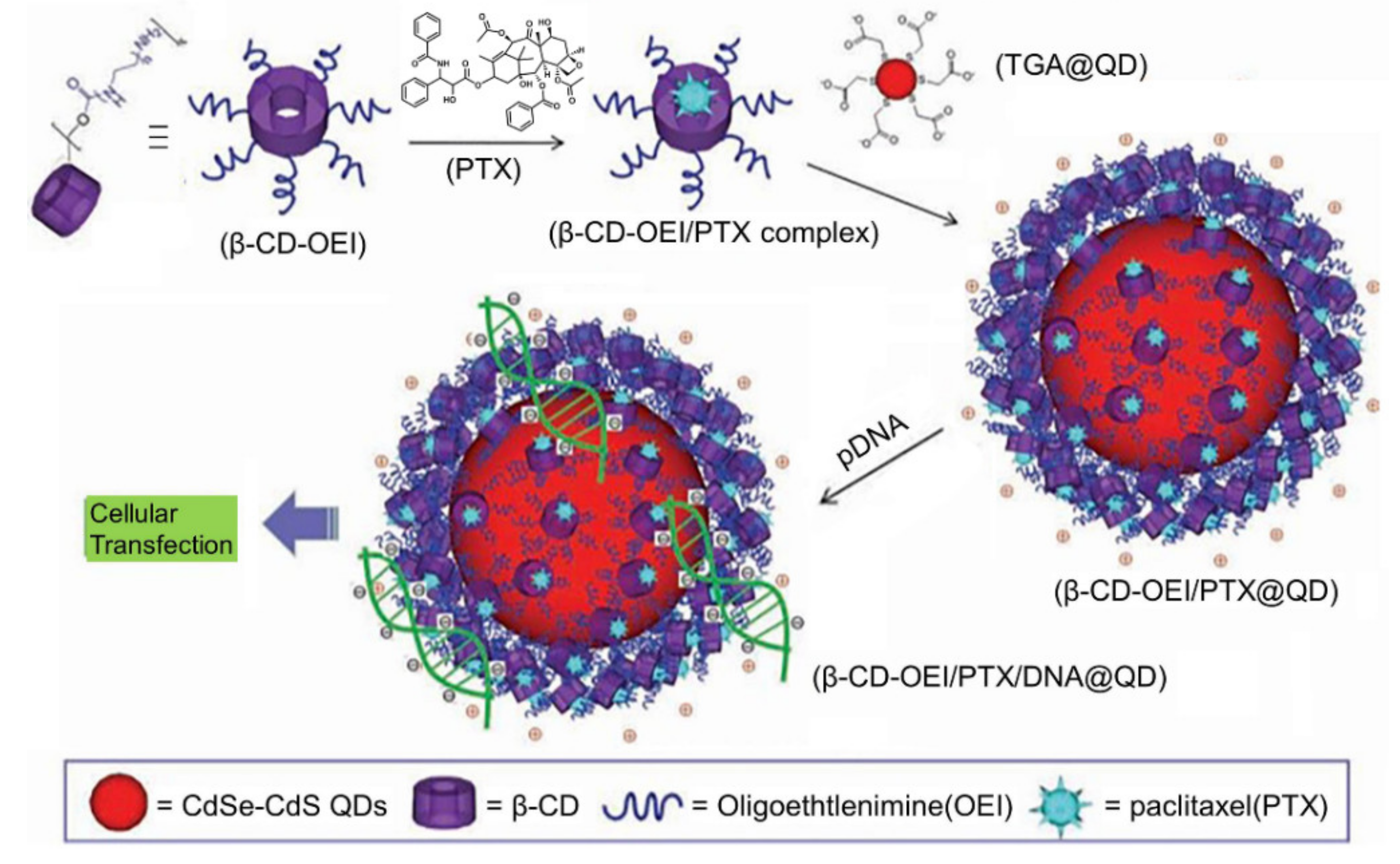
- Jeon, H.; Kim, J.; Lee, Y.M.; Kim, J.; Choi, H.W.; Lee, J.; Park, H.; Kang, Y.; Kim, I.-S.; Lee, B.-H. Poly-paclitaxel/cyclodextrin-SPION nano-assembly for magnetically guided drug delivery system. J. Control Release 2016, 231, 68–76. [Google Scholar] [CrossRef]
- Wang, X.-G.; Dong, Z.-Y.; Cheng, H.; Wan, S.-S.; Chen, W.-H.; Zou, M.-Z.; Huo, J.-W.; Deng, H.-X.; Zhang, X.-Z. A multifunctional metal-organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale 2015, 7, 16061–16070. [Google Scholar] [CrossRef]
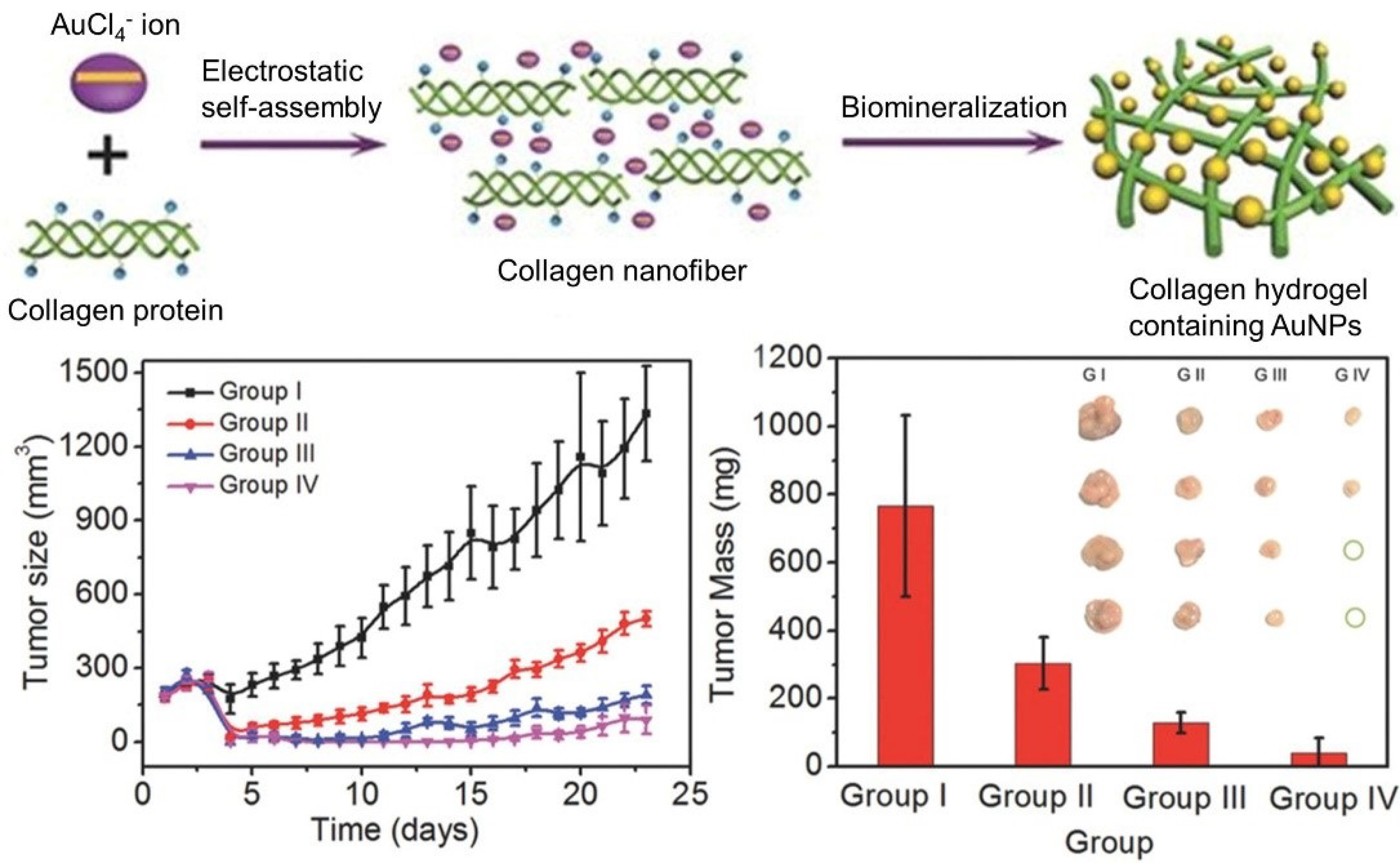
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-H.; Gao, F.-P.; Liu, X.-F.; Zeng, Q.; Guo, S.-S.; Tang, Z.-Y.; Zhao, X.-Z.; Wang, H. Supramolecular gelatin nanoparticles as matrix metalloproteinase responsive cancer cell imaging probes. Chem. Commun. 2013, 49, 4462–4464. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; Li, C.; He, F.; Hou, Z.; Huang, S.; Zhu, H.; Chen, X.; Lin, J. Poly (acrylic acid) modification of Nd3+-sensitized upconversion nanophosphors for highly efficient UCL imaging and pH-responsive drug delivery. Adv. Funct. Mater. 2015, 25, 4717–4729. [Google Scholar] [CrossRef]
- Lu, J.; Ma, S.; Sun, J.; Xia, C.; Liu, C.; Wang, Z.; Zhao, X.; Gao, F.; Gong, Q.; Song, B. Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials 2009, 30, 2919–2928. [Google Scholar] [CrossRef]
- Chen, J.; Shi, M.; Liu, P.; Ko, A.; Zhong, W.; Liao, W.; Xing, M.M. Reducible polyamidoamine-magnetic iron oxide self-assembled nanoparticles for doxorubicin delivery. Biomaterials 2014, 35, 1240–1248. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, W.; Luo, Y.; Wei, Y.; Sun, S.; Chen, J. The performance of composite nanoparticles based on Fe3O4@SiO2/PLGA/PFOB in magnetic resonance imaging and photoacoustic imaging. Sci. Adv. Mater. 2019, 11, 1816–1824. [Google Scholar] [CrossRef]
- An, J.; Yang, X.-Q.; Cheng, K.; Song, X.-L.; Zhang, L.; Li, C.; Zhang, X.-S.; Xuan, Y.; Song, Y.-Y.; Fang, B.-Y.; et al. In Vivo computed tomography/photoacoustic imaging and NIR-triggered chemo-photothermal combined therapy based on a gold nanostar-, mesoporous silica-, and thermosensitive liposome-composited nanoprobe. ACS Appl. Mater. Interfaces 2017, 9, 41748–41759. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Yin, H.; Zhao, F.; Li, J. Multifunctional hybrid nanocarriers consisting of supramolecular polymers and quantum dots for simultaneous dual therapeutics delivery and cellular imaging. Adv. Healthcare Mater. 2013, 2, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.-W.; Lei, Q.; Chen, S.; Qiu, W.-X.; Liu, M.-Y.; Chen, X.; Ding, Y.-X.; Li, P.-H.; Zhang, Q.-Y.; Xu, Z.-S. Supermolecular theranostic capsules for pH-sensitive magnetic resonance imaging and multi-responsive drug delivery. J. Mater. Chem. B 2015, 3, 8499–8507. [Google Scholar] [CrossRef]
- Erol-Taygun, M.; Unalan, I.; Idris, M.I.B.; Mano, J.F.; Boccaccini, A.R. Bioactıve glass-polymer nanocomposites for bone tıssue regeneration applicatıons: A Revıew. Adv. Eng. Mater. 2019, 21, 1900287. [Google Scholar] [CrossRef]
- Wu, C.-J.; Gaharwar, A.K.; Schexnailder, P.J.; Schmidt, G. Development of biomedical polymer-silicate nanocomposites: A materials science perspective. Materials 2010, 3, 2986–3005. [Google Scholar] [CrossRef]
- Hopley, E.L.; Salmasi, S.; Kalaskar, D.M.; Seifalian, A.M. Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Biotechnol. Adv. 2014, 32, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
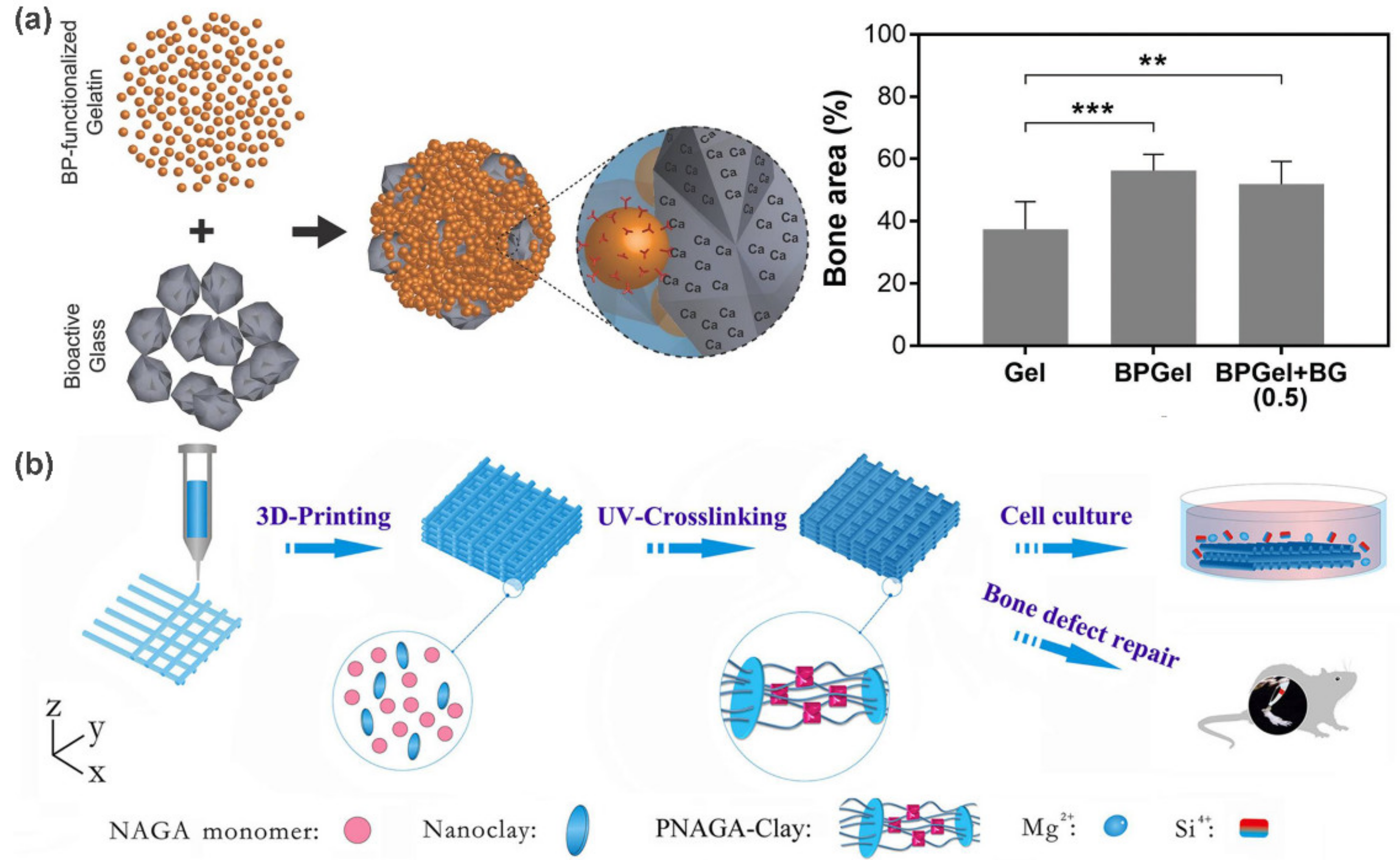
- Diba, M.; Camargo, W.A.; Brindisi, M.; Farbod, K.; Klymov, A.; Schmidt, S.; Harrington, M.J.; Draghi, L.; Boccaccini, A.R.; Jansen, J.A. Composite colloidal gels made of bisphosphonate-functionalized gelatin and bioactive glass particles for regeneration of osteoporotic bone defects. Adv. Funct. Mater. 2017, 27, 1703438. [Google Scholar] [CrossRef]
- Zhao, C.; Qazvini, N.T.; Sadati, M.; Zeng, Z.; Huang, S.; De La Lastra, A.L.; Zhang, L.; Feng, Y.; Liu, W.; Huang, B. A pH-triggered, self-assembled, and bioprintable hybrid hydrogel scaffold for mesenchymal stem cell based bone tissue engineering. ACS Appl. Mater. Interfaces 2019, 11, 8749–8762. [Google Scholar] [CrossRef]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-printed high strength bioactive supramolecular polymer/clay nanocomposite hydrogel scaffold for bone regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Wang, T.; Zhao, B.; Li, J.; Jin, L. Soft graphene nanofibers designed for the acceleration of nerve growth and development. Adv. Mater. 2015, 27, 6462–6468. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yuksekkaya, M.; Wan, K.-t.; et al. Reduced graphene oxide-gelma hybrid hydrogels as scaffolds for cardiac tissue engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef]
- Mehrali, M.; Thakur, A.; Pennisi, C.P.; Talebian, S.; Arpanaei, A.; Nikkhah, M.; Dolatshahi-Pirouz, A. Nanoreinforced hydrogels for tissue engineering: Biomaterials that are compatible with load-bearing and electroactive tissues. Adv. Mater. 2017, 29, 1603612. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, H.; Gao, F.; Xu, Z.; Dai, F.; Liu, W. An injectable supramolecular polymer nanocomposite hydrogel for prevention of breast cancer recurrence with theranostic and mammoplastic functions. Adv. Funct. Mater. 2018, 28, 1801000. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Tan, H.; Pan, C.; Chen, X. Injectable graphene oxide/graphene composite supramolecular hydrogel for delivery of anti-cancer drugs. J. Macromol. Sci. Part A Pure Appl. Chem. 2014, 51, 378–384. [Google Scholar] [CrossRef]
- Sui, K.; Gao, S.; Wu, W.; Xia, Y. Injectable supramolecular hybrid hydrogels formed by MWNT-grafted-poly (ethylene glycol) and alpha-cyclodextrin. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3145–3151. [Google Scholar] [CrossRef]
- Ignat, S.R.; Lazar, A.D.; Selaru, A.; Samoila, I.; Vlasceanu, G.M.; Ionita, M.; Radu, E.; Dinescu, S.; Costache, M. Versatile biomaterial platform enriched with graphene oxide and carbon nanotubes for multiple tissue engineering applications. Int. J. Mol. Sci. 2019, 20, 3868. [Google Scholar] [CrossRef]
- Hitscherich, P.; Aphale, A.; Gordan, R.; Whitaker, R.; Singh, P.; Xie, L.-h.; Patra, P.; Lee, E.J. Electroactive graphene composite scaffolds for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xu, W.; Xin, Y.; Yuan, J.; Ji, Y.; Chu, S.; Liu, J.; Luo, Q. Supramolecular Polymer Nanocomposites for Biomedical Applications. Polymers 2021, 13, 513. https://doi.org/10.3390/polym13040513
Li X, Xu W, Xin Y, Yuan J, Ji Y, Chu S, Liu J, Luo Q. Supramolecular Polymer Nanocomposites for Biomedical Applications. Polymers. 2021; 13(4):513. https://doi.org/10.3390/polym13040513
Chicago/Turabian StyleLi, Xiumei, Wanjia Xu, Yue Xin, Jiawei Yuan, Yuancheng Ji, Shengnan Chu, Junqiu Liu, and Quan Luo. 2021. "Supramolecular Polymer Nanocomposites for Biomedical Applications" Polymers 13, no. 4: 513. https://doi.org/10.3390/polym13040513
APA StyleLi, X., Xu, W., Xin, Y., Yuan, J., Ji, Y., Chu, S., Liu, J., & Luo, Q. (2021). Supramolecular Polymer Nanocomposites for Biomedical Applications. Polymers, 13(4), 513. https://doi.org/10.3390/polym13040513




