Solubilization of Charged Porphyrins in Interpolyelectrolyte Complexes: A Computer Study
Abstract
:1. Introduction
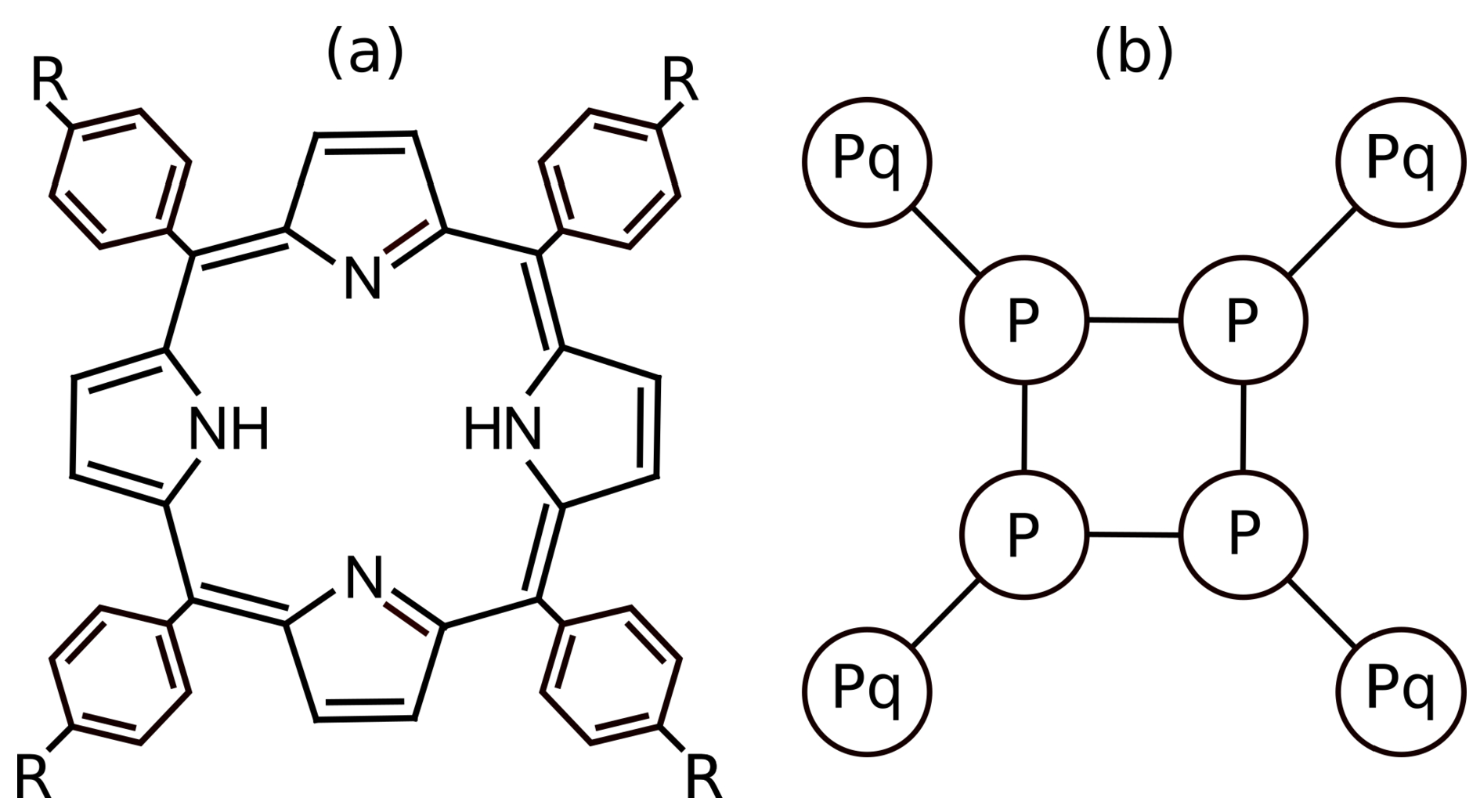
2. Model and Simulation Technique
Parameter Setting
3. Results and Discussion
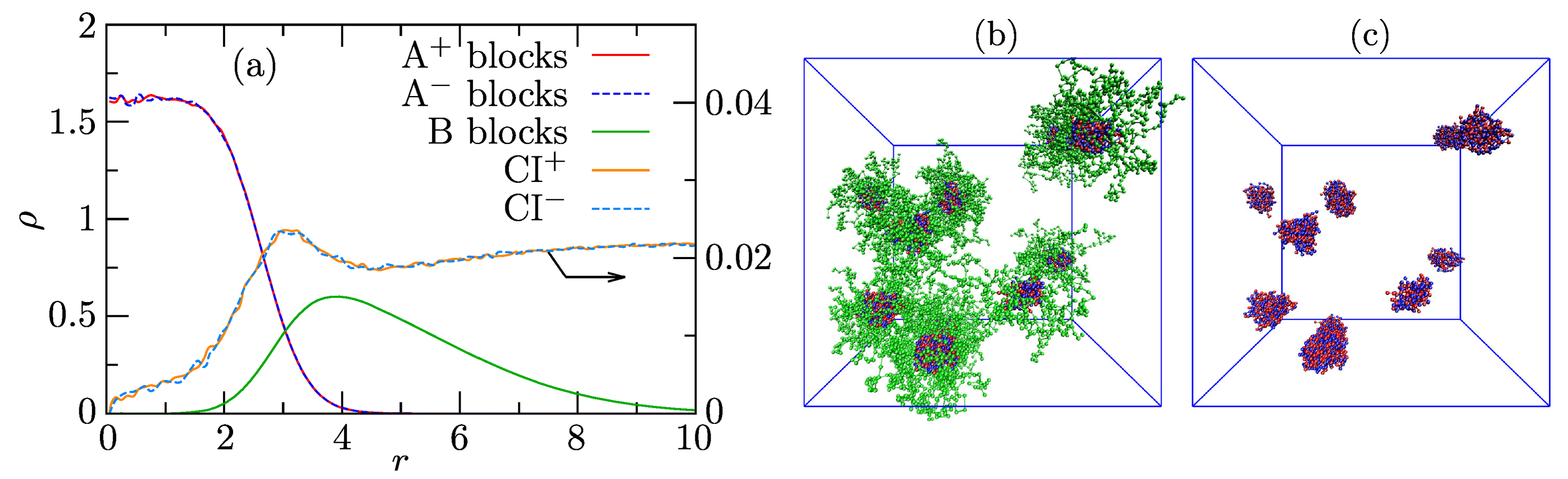
3.1. Electrostatically Stabilized Core-Shell Nanoparticles with IPEC Cores without Solubilized Porphyrin
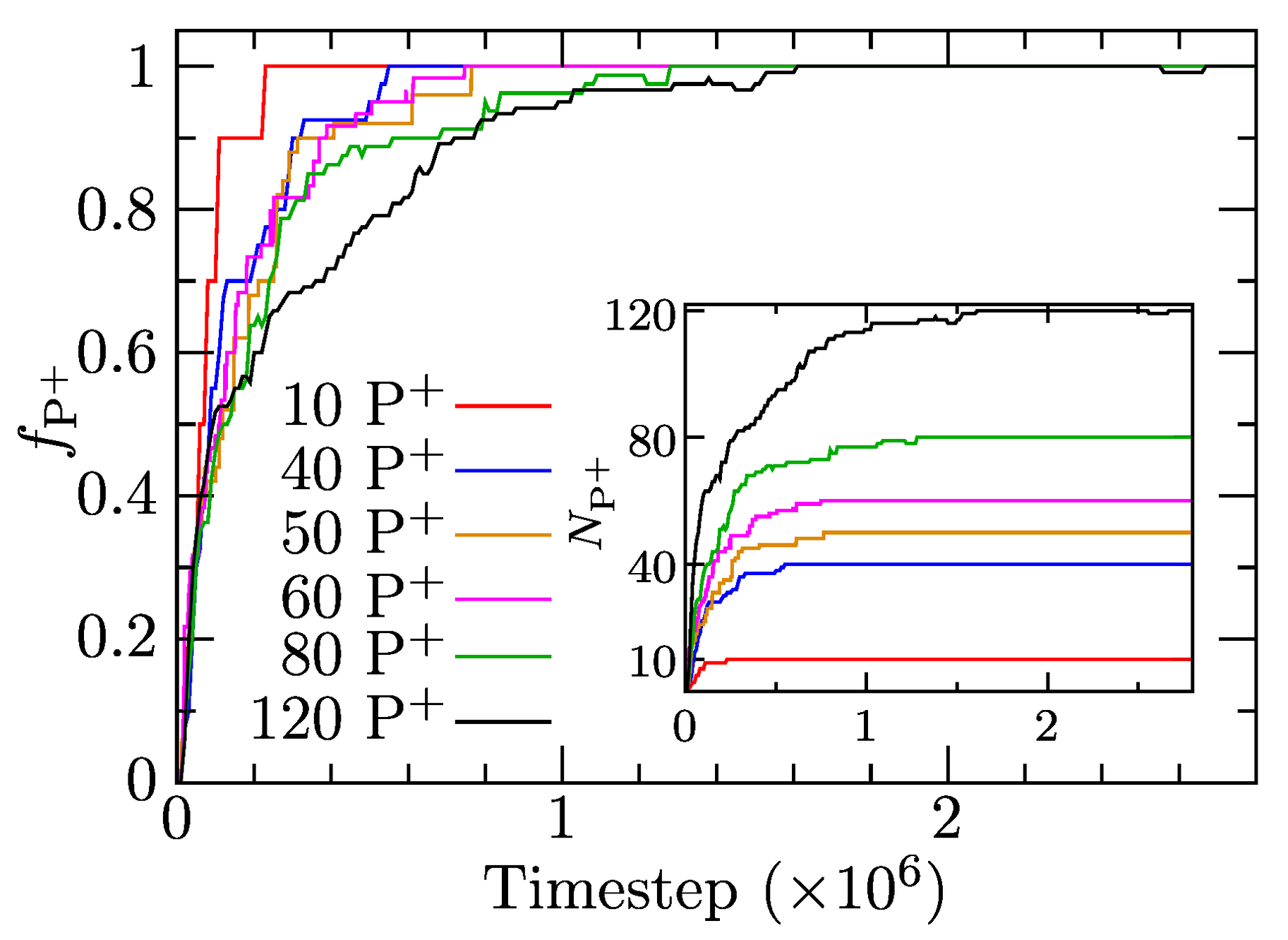
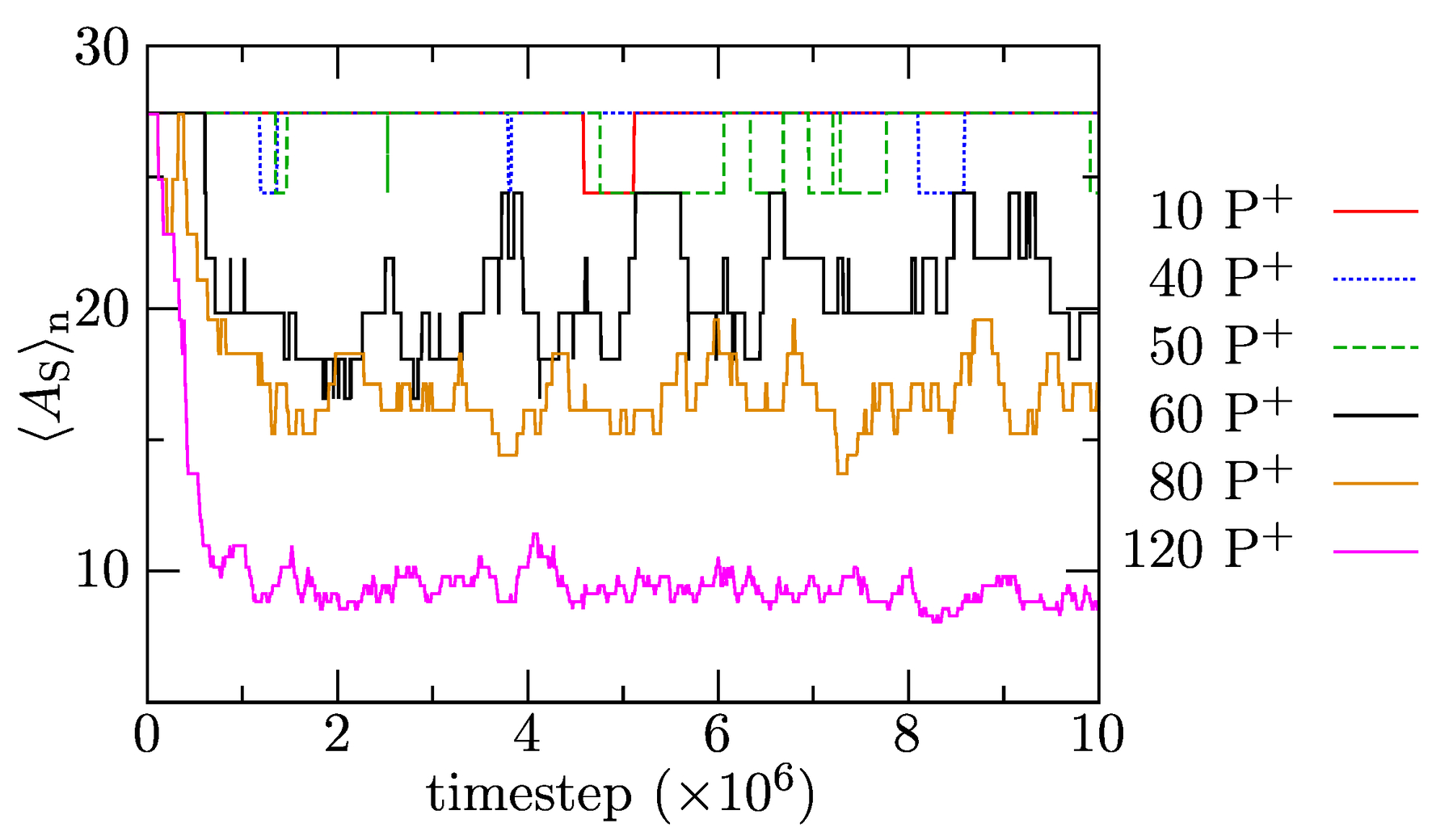
3.2. Solubilization of Cationic Porphyrins into IPEC Cores
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Khan, M.A.; Tariq, M. Emerging applications of porphyrins and metalloporphyrins in biomedicine and diagnostic magnetic resonance imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Song, W.; Rieffel, J.; Lovell, J.F. Emerging applications of porphyrins in photomedicine. Front. Phys. 2015, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, T.J. Photodynamic therapy. In Endoscopic Laser Surgery Handbook; Marcel Dekker Inc.: New York, NY, USA, 1987; p. 424. [Google Scholar]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Jahanbin, T.; Sauriat-Dorizon, H.; Spearman, P.; Benderbous, S.; Korri-Youssoufi, H. Development of Gd (III) porphyrin-conjugated chitosan nanoparticles as contrast agents for magnetic resonance imaging. Mater. Sci. Eng. C 2015, 52, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Manus, L.M.; Strauch, R.C.; Hung, A.H.; Eckermann, A.L.; Meade, T.J. Analytical Methods for Characterizing Magnetic Resonance Probes. Anal. Chem. 2012, 84, 6278–6287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yan, F.; Liu, X.; Wang, P.; Shao, S.; Sun, Y.; Sheng, Z.; Liu, Q.; Lovell, J.F.; Zheng, H. Enhanced drug delivery using sonoactivatable liposomes with membrane-embedded porphyrins. J. Control. Release 2018, 286, 358–368. [Google Scholar] [CrossRef]
- Biesaga, M.; Pyrzyńska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef]
- Leng, F.; Liu, H.; Ding, M.; Lin, Q.P.; Jiang, H.L. Boosting photocatalytic hydrogen production of porphyrinic MOFs: The metal location in metalloporphyrin matters. ACS Catal. 2018, 8, 4583–4590. [Google Scholar] [CrossRef]
- Dini, D.; Calvete, M.J.; Hanack, M. Nonlinear optical materials for the smart filtering of optical radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Osuka, A. Expanded porphyrins: Intriguing structures, electronic properties, and reactivities. Angew. Chem. Int. Ed. 2011, 50, 4342–4373. [Google Scholar] [CrossRef]
- Zucca, P.; Neves, C.; Simões, M.M.; Neves, M.d.G.P.; Cocco, G.; Sanjust, E. Immobilized lignin peroxidase-like metalloporphyrins as reusable catalysts in oxidative bleaching of industrial dyes. Molecules 2016, 21, 964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Gouterman, M.; Wagnière, G.H.; Snyder, L.C. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef] [Green Version]
- Aratani, N.; Osuka, A.; Kim, Y.H.; Jeong, D.H.; Kim, D. Extremely Long, Discrete meso–meso-Coupled Porphyrin Arrays. Angew. Chem. Int. Ed. 2000, 39, 1458–1462. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeong, D.H.; Kim, D.; Jeoung, S.C.; Cho, H.S.; Kim, S.K.; Aratani, N.; Osuka, A. Photophysical Properties of Long Rodlike M eso- Meso-Linked Zinc (II) Porphyrins Investigated by Time-Resolved Laser Spectroscopic Methods. J. Am. Chem. Soc. 2001, 123, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, I.V.; Erdem, S.S.; Pakhomov, S.; Hammer, R.P.; Soper, S.A. Phthalocyanine dimerization-based molecular beacons using near-IR fluorescence. J. Am. Chem. Soc. 2009, 131, 2432–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Stefflova, K.; Niedre, M.J.; Wilson, B.C.; Chance, B.; Glickson, J.D.; Zheng, G. Protease-triggered photosensitizing beacon based on singlet oxygen quenching and activation. J. Am. Chem. Soc. 2004, 126, 11450–11451. [Google Scholar] [CrossRef]
- Procházková, K.; Zelinger, Z.; Lang, K.; Kubát, P. meso-Tetratolylporphyrins substituted by pyridinium groups: Aggregation, photophysical properties and complexation with DNA. J. Phys. Org. Chem. 2004, 17, 890–897. [Google Scholar] [CrossRef]
- Calvete, M.J.; Pinto, S.M.; Pereira, M.M.; Geraldes, C.F. Metal coordinated pyrrole-based macrocycles as contrast agents for magnetic resonance imaging technologies: Synthesis and applications. Coord. Chem. Rev. 2017, 333, 82–107. [Google Scholar] [CrossRef]
- Zhao, L.; Xiang, R.; Zhang, L.; Wu, C.; Ma, R.; An, Y.; Shi, L. Micellization of copolymers via noncovalent interaction with TPPS and aggregation of TPPS. Sci. China Chem. 2011, 54, 343–350. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Ghoroghchian, P.P.; Frail, P.R.; Susumu, K.; Blessington, D.; Brannan, A.K.; Bates, F.S.; Chance, B.; Hammer, D.A.; Therien, M.J. Near-infrared-emissive polymersomes: Self-assembled soft matter for in vivo optical imaging. Proc. Natl. Acad. Sci. USA 2005, 102, 2922–2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, T.D.; Liu, T.W.; Zheng, G. An MRI-sensitive, non-photobleachable porphysome photothermal agent. Angew. Chem. 2014, 126, 7076–7079. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, H.; Yao, S.Q.; Xu, Q.H.; Xu, G.Q. Nanocomposites containing gold nanorods and porphyrin-doped mesoporous silica with dual capability of two-photon imaging and photosensitization. Langmuir 2010, 26, 14937–14942. [Google Scholar] [CrossRef]
- Frühbeißer, S.; Gröhn, F. Porphyrin—Polyelectrolyte Nanoassemblies: The Role of Charge and Building Block Architecture in Self-Assembly. Macromol. Chem. Phys. 2017, 218, 1600526. [Google Scholar] [CrossRef]
- Frühbeißer, S.; Mariani, G.; Gröhn, F. Porphyrin Diacid-Polyelectrolyte Assemblies: Effective Photocatalysts in Solution. Polymers 2016, 8, 180. [Google Scholar] [CrossRef] [Green Version]
- Karayianni, M.; Pispas, S. Self-assembly of amphiphilic block copolymers in selective solvents. In Fluorescence Studies of Polymer Containing Systems; Springer: Berlin/Heidelberg, Germany, 2016; pp. 27–63. [Google Scholar]
- Walther, A.; Muller, A.H. Janus particles: Synthesis, self-assembly, physical properties, and applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef]
- Liu, S.; Armes, S.P. Polymeric surfactants for the new millennium: A pH-responsive, zwitterionic, schizophrenic diblock copolymer. Angew. Chem. Int. Ed. 2002, 41, 1413–1416. [Google Scholar] [CrossRef]
- Berret, J.F.; Vigolo, B.; Eng, R.; Herve, P.; Grillo, I.; Yang, L. Electrostatic self-assembly of oppositely charged copolymers and surfactants: A light, neutron, and X-ray scattering study. Macromolecules 2004, 37, 4922–4930. [Google Scholar] [CrossRef]
- Rumyantsev, A.M.; Zhulina, E.B.; Borisov, O.V. Scaling theory of complex coacervate core micelles. ACS Macro Lett. 2018, 7, 811–816. [Google Scholar] [CrossRef]
- Rumyantsev, A.M.; Zhulina, E.B.; Borisov, O.V. Complex coacervate of weakly charged polyelectrolytes: Diagram of states. Macromolecules 2018, 51, 3788–3801. [Google Scholar] [CrossRef]
- Černochová, Z.; Bogomolova, A.; Borisova, O.V.; Filippov, S.K.; Černoch, P.; Billon, L.; Borisov, O.V.; Štěpánek, P. Thermodynamics of the multi-stage self-assembly of pH-sensitive gradient copolymers in aqueous solutions. Soft Matter 2016, 12, 6788–6798. [Google Scholar] [CrossRef]
- Borisov, O.; Zhulina, E. Theory of self-assembly of triblock ter-polymers in selective solvent towards corona-compartmentalized (Janus) micelles. Polymer 2013, 54, 2043–2048. [Google Scholar] [CrossRef]
- van der Burgh, S.; de Keizer, A.; Stuart, M. Complex coacervation core micelles. Colloidal stability and aggregation mechanism. Langmuir 2004, 20, 1073–1084. [Google Scholar] [CrossRef]
- Voets, I.K. Electrostatically driven assembly of polyelectrolytes. In Fluorescence Studies of Polymer Containing Systems; Springer: Berlin/Heidelberg, Germany, 2016; pp. 65–89. [Google Scholar]
- Uchman, M.; Gradzielski, M.; Angelov, B.; Tošner, Z.; Oh, J.; Chang, T.; Štěpánek, M.; Procházka, K. Thermodynamic and kinetic aspects of coassembly of PEO–PMAA block copolymer and DPCl surfactants into ordered nanoparticles in aqueous solutions studied by ITC, NMR, and time-resolved SAXS techniques. Macromolecules 2013, 46, 2172–2181. [Google Scholar] [CrossRef]
- Uchman, M.; Štěpánek, M.; Prévost, S.; Angelov, B.; Bednár, J.; Appavou, M.S.; Gradzielski, M.; Procházka, K. Coassembly of poly (ethylene oxide)-block-poly (methacrylic acid) and N-dodecylpyridinium chloride in aqueous solutions leading to ordered micellar assemblies within copolymer aggregates. Macromolecules 2012, 45, 6471–6480. [Google Scholar] [CrossRef]
- Matějíček, P.; Uhlík, F.; Limpouchová, Z.; Procházka, K.; Tuzar, Z.; Webber, S.E. Experimental study of hydrophobically modified amphiphilic block copolymer micelles using light scattering and nonradiative excitation energy transfer. Macromolecules 2002, 35, 9487–9496. [Google Scholar] [CrossRef]
- Podhájecká, K.; Štěpánek, M.; Procházka, K.; Brown, W. Hybrid polymeric micelles with hydrophobic cores and mixed polyelectrolyte/nonelectrolyte shells in aqueous media. 2. Studies of the shell behavior. Langmuir 2001, 17, 4245–4250. [Google Scholar] [CrossRef]
- Pleštil, J.; Kříž, J.; Tuzar, Z.; Prochazka, K.; Melnichenko, Y.B.; Wignall, G.; Talingting, M.; Munk, P.; Webber, S. Small-Angle Neutron Scattering Study of Onion-Type Micelles. Macromol. Chem. Phys. 2001, 202, 553–563. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Alakhov, V.Y. Pluronic® block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 1–72. [Google Scholar] [CrossRef]
- Šindelka, K.; Limpouchová, Z.; Lísal, M.; Procházka, K. The electrostatic co-assembly in non-stoichiometric aqueous mixtures of copolymers composed of one neutral water-soluble and one polyelectrolyte (either positively or negatively charged) block: A dissipative particle dynamics study. Phys. Chem. Chem. Phys. 2016, 18, 16137–16151. [Google Scholar] [CrossRef]
- Procházka, K.; Šindelka, K.; Wang, X.; Limpouchová, Z.; Lísal, M. Self-assembly and co-assembly of block polyelectrolytes in aqueous solutions. Dissipative particle dynamics with explicit electrostatics. Mol. Phys. 2016, 114, 3077–3092. [Google Scholar] [CrossRef]
- Šindelka, K.; Limpouchová, Z.; Procházka, K. Computer study of the solubilization of polymer chains in polyelectrolyte complex cores of polymeric nanoparticles in aqueous media. Phys. Chem. Chem. Phys. 2018, 20, 29876–29888. [Google Scholar] [CrossRef] [PubMed]
- Lisal, M.; Šindelka, K.; Sucha, L.; Limpouchova, Z.; Procházka, K. Dissipative particle dynamics simulations of polyelectrolyte self-assemblies. Methods with explicit electrostatics. Polym. Sci. Ser. C 2017, 59, 77–101. [Google Scholar] [CrossRef]
- Uhlík, F.; Limpouchová, Z.; Jelínek, K.; Procházka, K. Polyelectrolyte shells of copolymer micelles in aqueous solutions: A Monte Carlo study. J. Chem. Phys. 2004, 121, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Posel, Z.; Limpouchová, Z.; Šindelka, K.; Lísal, M.; Procházka, K. Dissipative particle dynamics study of the pH-dependent behavior of poly (2-vinylpyridine)-block-poly (ethylene oxide) diblock copolymer in aqueous buffers. Macromolecules 2014, 47, 2503–2514. [Google Scholar] [CrossRef]
- Košovan, P.; Kuldová, J.; Limpouchová, Z.; Procházka, K.; Zhulina, E.B.; Borisov, O.V. Molecular dynamics simulations of a polyelectrolyte star in poor solvent. Soft Matter 2010, 6, 1872–1874. [Google Scholar] [CrossRef]
- Lísal, M.; Limpouchová, Z.; Procházka, K. The self-assembly of copolymers with one hydrophobic and one polyelectrolyte block in aqueous media: A dissipative particle dynamics study. Phys. Chem. Chem. Phys. 2016, 18, 16127–16136. [Google Scholar] [CrossRef] [PubMed]
- Havránková, J.; Limpouchová, Z.; Štěpánek, M.; Procházka, K. Self-Assembly of Heteroarm Star Copolymers–A Monte Carlo Study. Macromol. Theory Simulations 2007, 16, 386–398. [Google Scholar] [CrossRef]
- Uhlík, F.; Limpouchová, Z.; Jel’ınek, K.; Procházka, K. A Monte Carlo study of shells of hydrophobically modified amphiphilic copolymer micelles in polar solvents. J. Chem. Phys. 2003, 118, 11258–11264. [Google Scholar] [CrossRef]
- Limpouchová, Z.; Viduna, D.; Procházka, K. Mixed systems of tethered chains in spherical volumes. A model for cores of mixed copolymer micelles. Macromolecules 1997, 30, 8027–8035. [Google Scholar] [CrossRef]
- Viduna, D.; Limpouchová, Z.; Procházka, K. Conformations of self-avoiding tethered chains and nonradiative energy transfer and migration in dense and constrained systems. A model for cores of polymeric micelles. Macromolecules 1997, 30, 7263–7272. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Espanol, P.; Warren, P. Statistical mechanics of dissipative particle dynamics. EPL (Europhysics Lett. 1995, 30, 191. [Google Scholar] [CrossRef] [Green Version]
- Groot, R.D.; Rabone, K. Mesoscopic simulation of cell membrane damage, morphology change and rupture by nonionic surfactants. Biophys. J. 2001, 81, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Hoogerbrugge, P.; Koelman, J. Simulating microscopic hydrodynamic phenomena with dissipative particle dynamics. EPL (Europhys. Lett.) 1992, 19, 155. [Google Scholar] [CrossRef]
- Mosinger, J.; Lang, K.; Plistil, L.; Jesenská, S.; Hostomský, J.; Zelinger, Z.; Kubát, P. Fluorescent polyurethane nanofabrics: A source of singlet oxygen and oxygen sensing. Langmuir 2010, 26, 10050–10056. [Google Scholar] [CrossRef] [PubMed]
- Kubát, P.; Henke, P.; Berzediová, V.; Štěpánek, M.; Lang, K.; Mosinger, J. Nanoparticles with embedded porphyrin photosensitizers for photooxidation reactions and continuous oxygen sensing. ACS Appl. Mater. Interfaces 2017, 9, 36229–36238. [Google Scholar] [CrossRef] [PubMed]
- Dolanskỳ, J.; Henke, P.; Malá, Z.; Žárská, L.; Kubát, P.; Mosinger, J. Antibacterial nitric oxide-and singlet oxygen-releasing polystyrene nanoparticles responsive to light and temperature triggers. Nanoscale 2018, 10, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Kubát, P.; Henke, P.; Mosinger, J. The effect of iodide and temperature on enhancing antibacterial properties of nanoparticles with an encapsulated photosensitizer. Colloids Surf. Biointerfaces 2019, 176, 334–340. [Google Scholar]
- Kubát, P.; Henke, P.; Raya, R.K.; Miroslav, Š.; Mosinger, J. Polystyrene and Poly (ethylene glycol)-b-Poly (ε-caprolactone) Nanoparticles with Porphyrins: Structure, Size, and Photooxidation Properties. Langmuir 2019, 36, 302–310. [Google Scholar] [CrossRef]
- Espanol, P. Dissipative particle dynamics with energy conservation. EPL (Europhys. Lett.) 1997, 40, 631. [Google Scholar] [CrossRef] [Green Version]
- Šindelka, K.; Limpouchová, Z.; Lísal, M.; Prochǎzka, K. Dissipative particle dynamics study of electrostatic self-assembly in aqueous mixtures of copolymers containing one neutral water-soluble block and one either positively or negatively charged polyelectrolyte block. Macromolecules 2014, 47, 6121–6134. [Google Scholar] [CrossRef]
- O’Sullivan, M.C.; Sprafke, J.K.; Kondratuk, D.V.; Rinfray, C.; Claridge, T.D.; Saywell, A.; Blunt, M.O.; O’Shea, J.N.; Beton, P.H.; Malfois, M.; et al. Vernier templating and synthesis of a 12-porphyrin nano-ring. Nature 2011, 469, 72–75. [Google Scholar] [CrossRef]
- Kondratuk, D.V.; Perdigao, L.M.; O’Sullivan, M.C.; Svatek, S.; Smith, G.; O’Shea, J.N.; Beton, P.H.; Anderson, H.L. Two vernier-templated routes to a 24-porphyrin nanoring. Angew. Chem. 2012, 124, 6800–6803. [Google Scholar] [CrossRef]
- Liu, S.; Kondratuk, D.V.; Rousseaux, S.A.; Gil-Ramírez, G.; O’Sullivan, M.C.; Cremers, J.; Claridge, T.D.; Anderson, H.L. Caterpillar track complexes in template-directed synthesis and correlated molecular motion. Angew. Chem. Int. Ed. 2015, 54, 5355–5359. [Google Scholar] [CrossRef] [Green Version]
- Kubát, P.; Lang, K.; Procházková, K.; Anzenbacher, P. Self-aggregates of cationic meso-tetratolylporphyrins in aqueous solutions. Langmuir 2003, 19, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Kubát, P.; Lang, K.; Zelinger, Z.; Kral, V. Aggregation and photophysical properties of water-soluble sapphyrins. Chem. Phys. Lett. 2004, 395, 82–86. [Google Scholar] [CrossRef]
- Mosinger, J.; Janošková, M.; Lang, K.; Kubát, P. Light-induced aggregation of cationic porphyrins. J. Photochem. Photobiol. Chem. 2006, 181, 283–289. [Google Scholar] [CrossRef]
- Chang, H.Y.; Lin, Y.L.; Sheng, Y.J.; Tsao, H.K. Structural characteristics and fusion pathways of onion-like multilayered polymersome formed by amphiphilic comb-like graft copolymers. Macromolecules 2013, 46, 5644–5656. [Google Scholar] [CrossRef]
- del Rosario Rodríguez-Hidalgo, M.; Soto-Figueroa, C.; Vicente, L. Mesoscopic study of salt-responsive polymeric micelles: Structural inversion mechanisms via sequential addition of inorganic salts. Soft Matter 2013, 9, 5762–5770. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, J. pH-sensitive drug loading/releasing in amphiphilic copolymer PAE–PEG: Integrating molecular dynamics and dissipative particle dynamics simulations. J. Control. Release 2012, 162, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Melchor, M.; Mayoral, E.; Velazquez, M.E.; Alejandre, J. Electrostatic interactions in dissipative particle dynamics using the Ewald sums. J. Chem. Phys. 2006, 125, 224107. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: New York, NY, USA, 2003; Volume 23. [Google Scholar]
- McQuarrie, D.A.; Simon, J.D. Physical Chemistry: A Molecular Approach; University Science Books: Sausalito, CA, USA, 1997; Volume 1. [Google Scholar]
- Chifotides, H.T.; Dunbar, K.R. Anion- π interactions in supramolecular architectures. Accounts Chem. Res. 2013, 46, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E. Understanding substituent effects in noncovalent interactions involving aromatic rings. Accounts Chem. Res. 2013, 46, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Frontera, A.; Quinonero, D.; Deya, P.M. Cation–π and anion–π interactions. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 440–459. [Google Scholar] [CrossRef]






| A | B | P | Pq | CI | S | |
|---|---|---|---|---|---|---|
| A | 25 | |||||
| B | 35 | 25 | ||||
| P | 25 | 35 | 18 | |||
| Pq | 25 | 35 | 25 | 25 | ||
| CI | 27 | 26 | 39 | 39 | 25 | |
| S | 35 | 26 | 39 | 39 | 25 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šindelka, K.; Limpouchová, Z.; Procházka, K. Solubilization of Charged Porphyrins in Interpolyelectrolyte Complexes: A Computer Study. Polymers 2021, 13, 502. https://doi.org/10.3390/polym13040502
Šindelka K, Limpouchová Z, Procházka K. Solubilization of Charged Porphyrins in Interpolyelectrolyte Complexes: A Computer Study. Polymers. 2021; 13(4):502. https://doi.org/10.3390/polym13040502
Chicago/Turabian StyleŠindelka, Karel, Zuzana Limpouchová, and Karel Procházka. 2021. "Solubilization of Charged Porphyrins in Interpolyelectrolyte Complexes: A Computer Study" Polymers 13, no. 4: 502. https://doi.org/10.3390/polym13040502