Reactive Extrusion of Maleic-Anhydride-Grafted Polypropylene by Torque Rheometer and Its Application as Compatibilizer
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Chemical Reaction and Reactive Extrusion Process
2.3. Mechanical Blending of PET and PP with MAH-g-PP
2.4. Process and Physical Characteristics
3. Results and Discussion
3.1. Reactive Extrusion Process
- dynamic viscosity (Pa·s)
- shear stress (N/cm2)
- shear rate (sec-1)
- torque (Nm)
- effective spindle length (m)
- spindle radius (m)
- container radius (m)
- rotational speed (radians/s)
- radial location
3.2. Carbonyl Index of Grafted PP
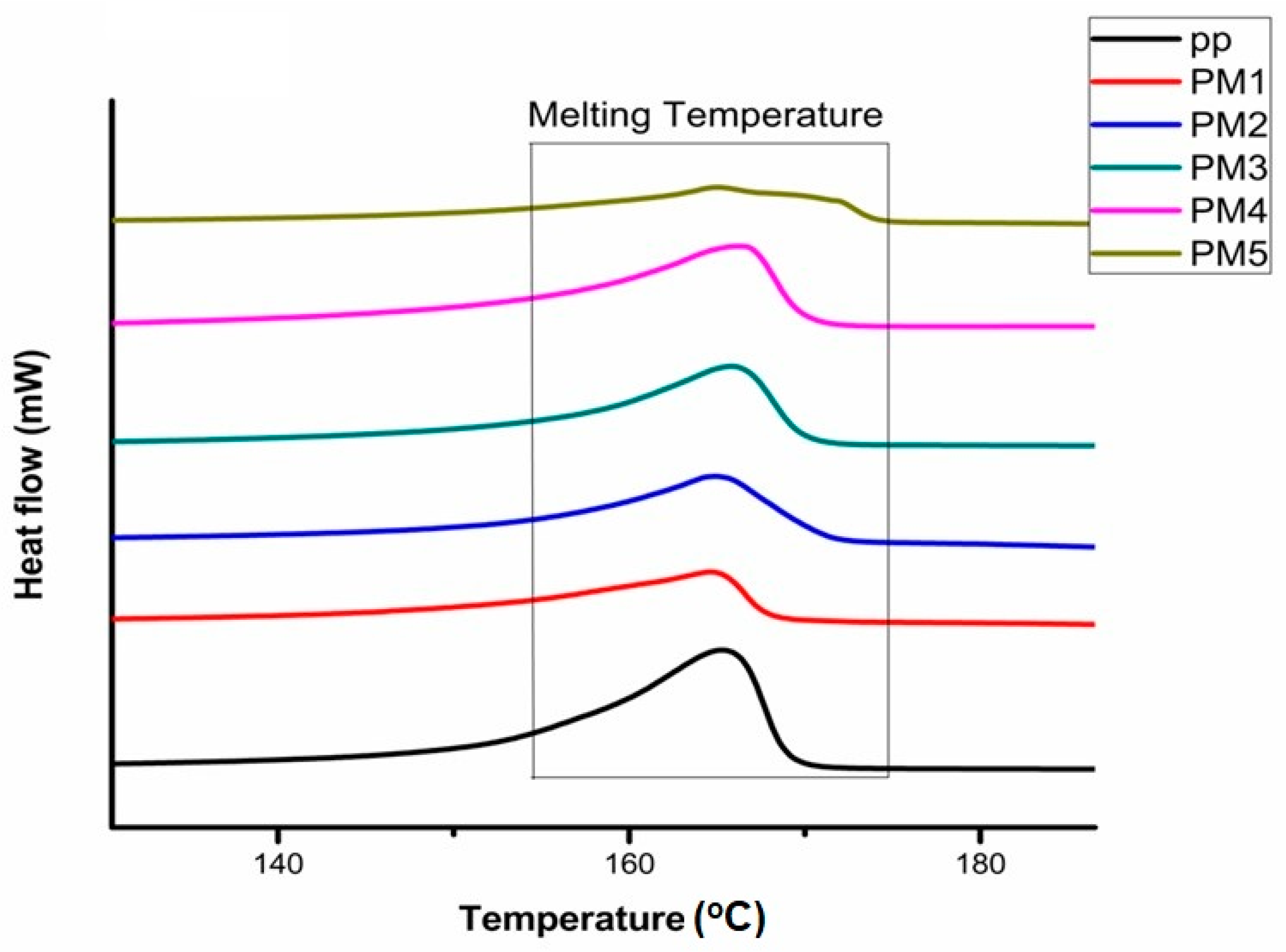
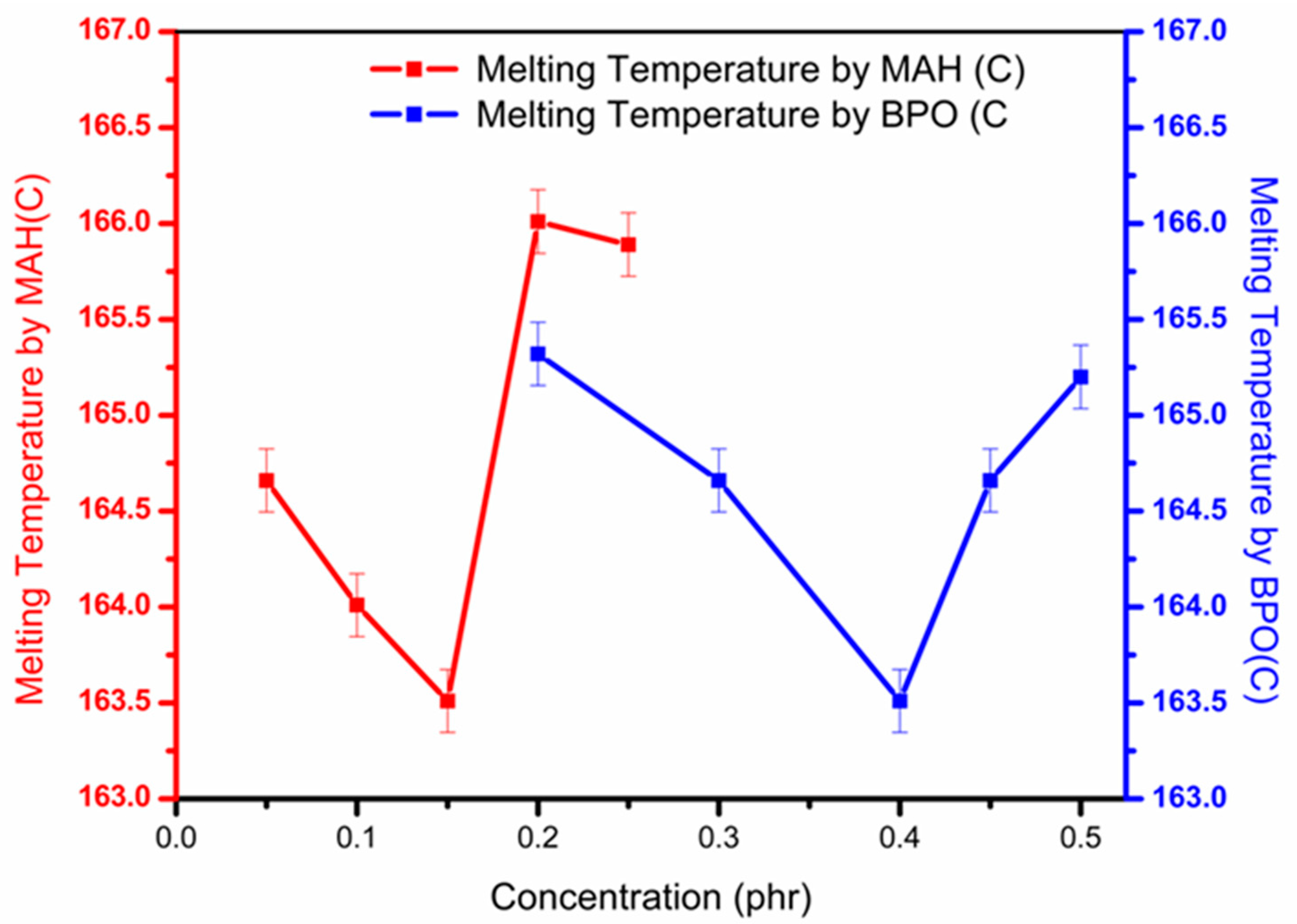
3.3. Crystallinity and Melting Temperature of Grafted Samples
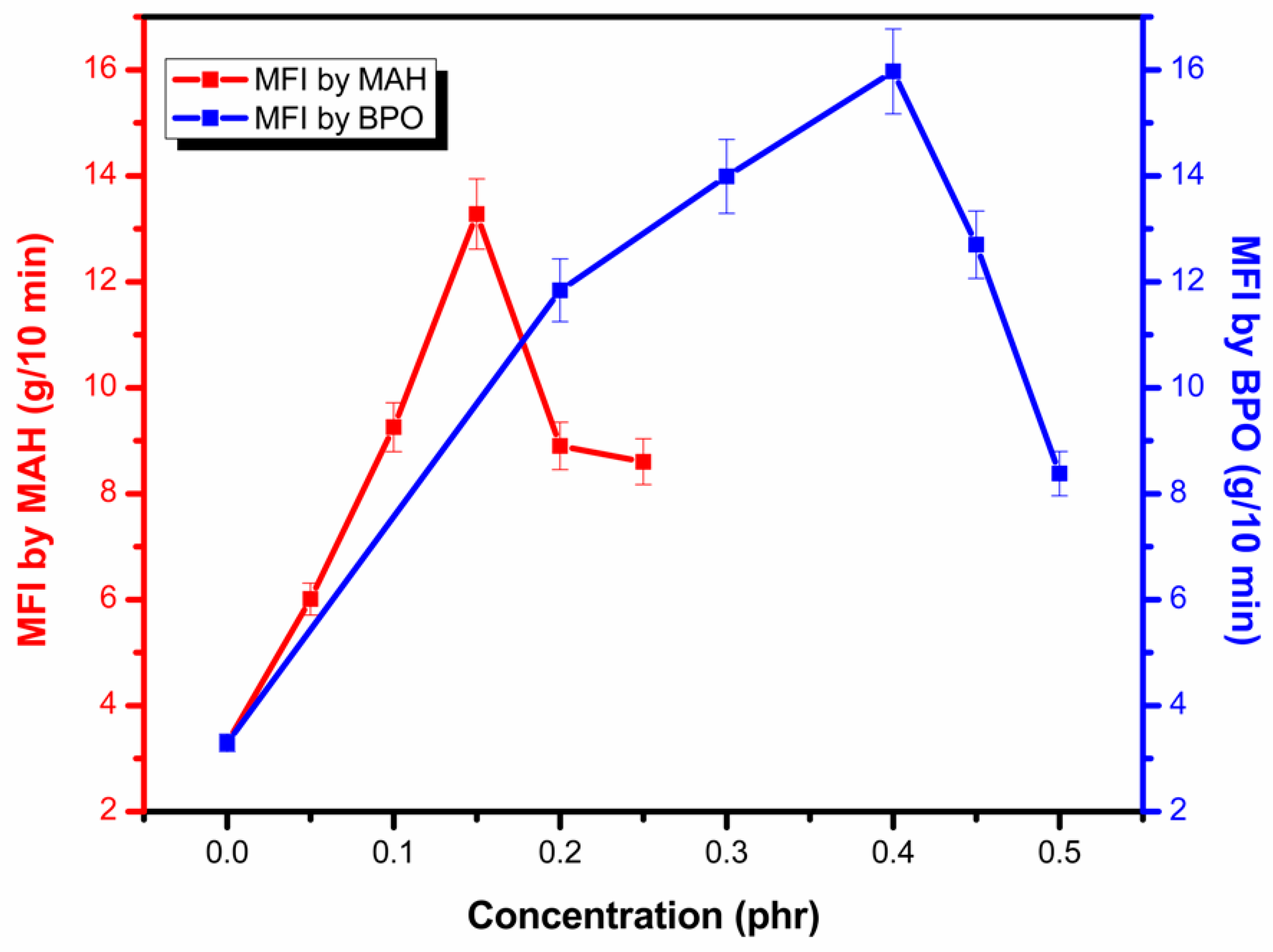
3.4. Melt Flow Index (MFI)
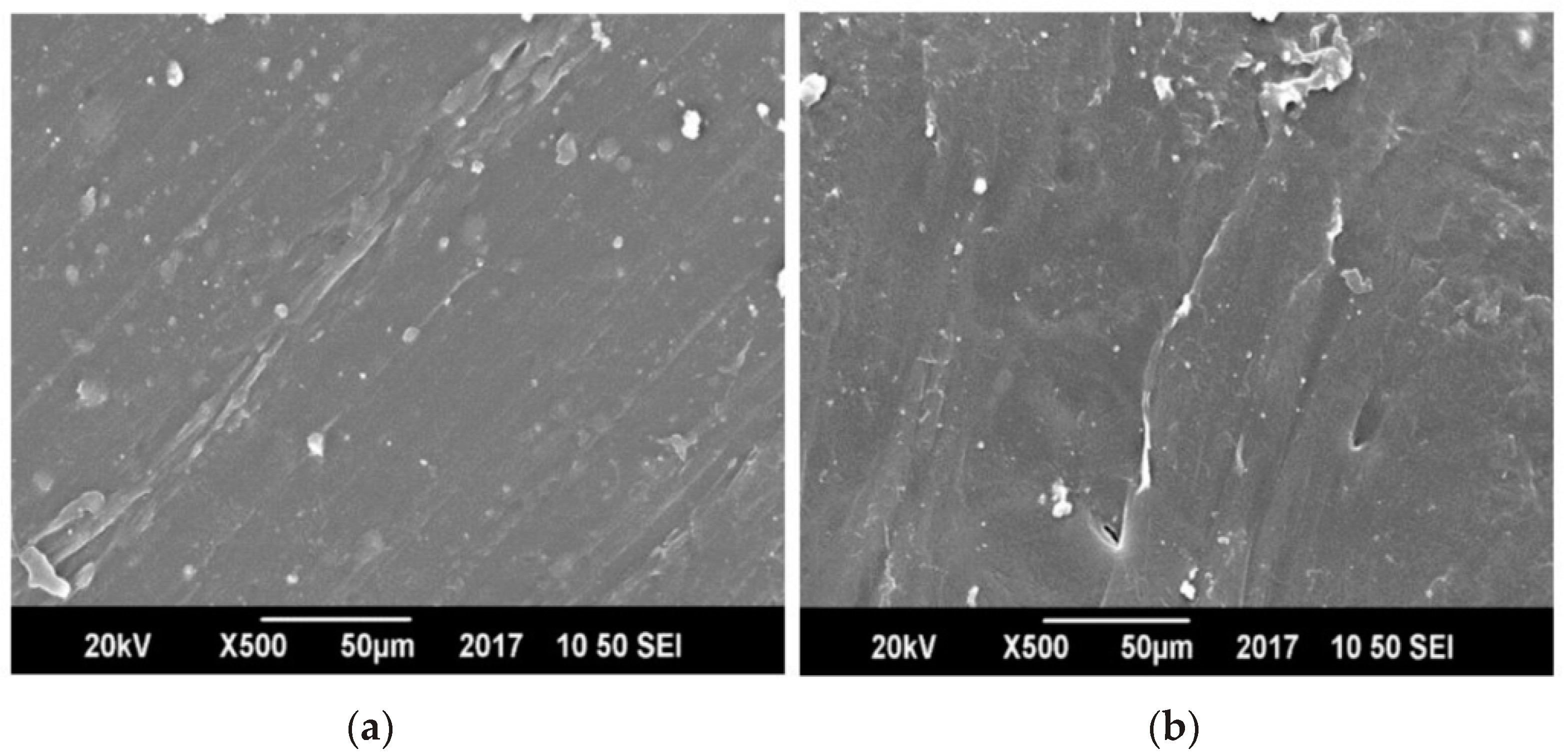
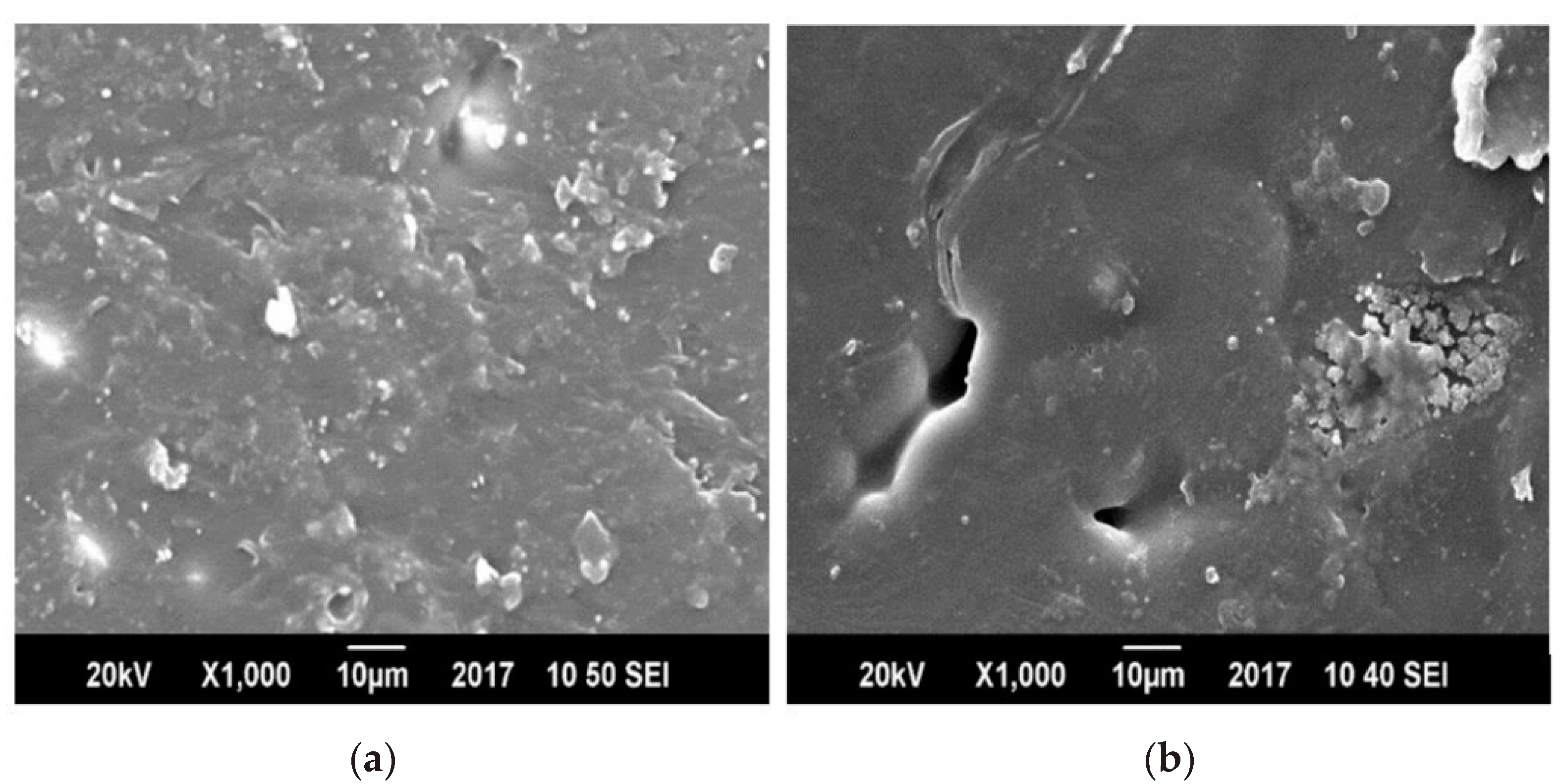
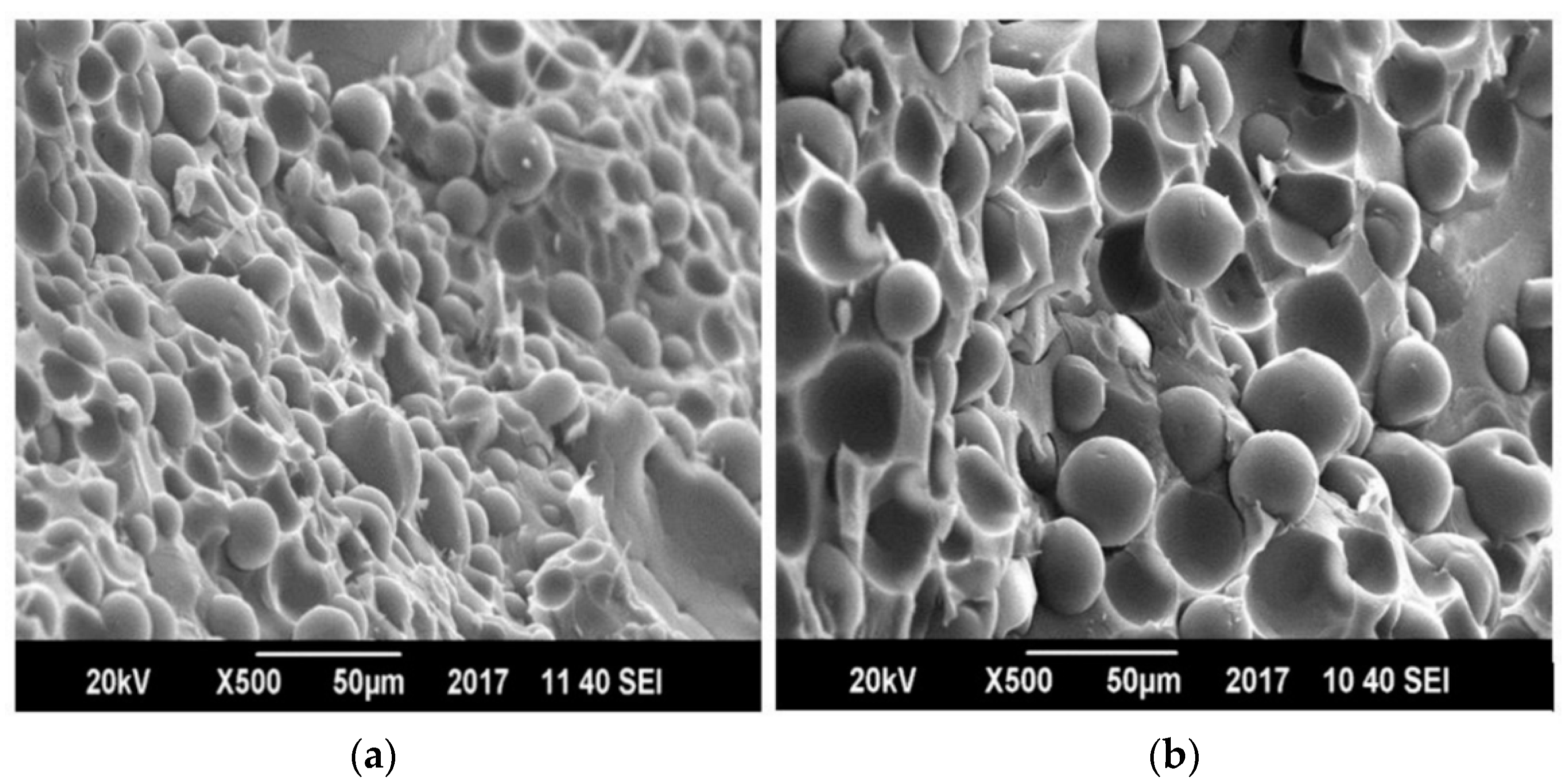
3.5. Morphology by Scanning Electron Microscopy (SEM)
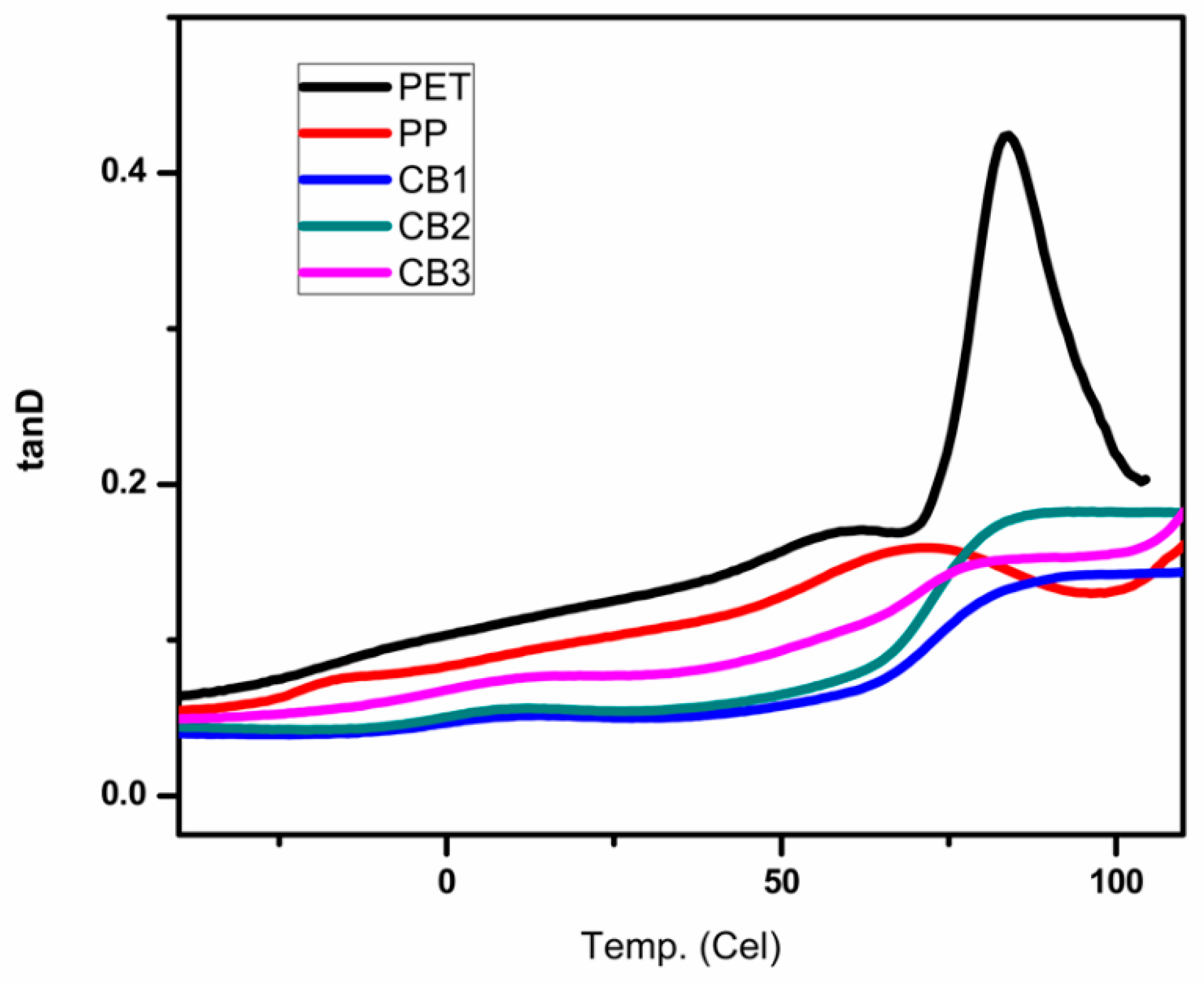
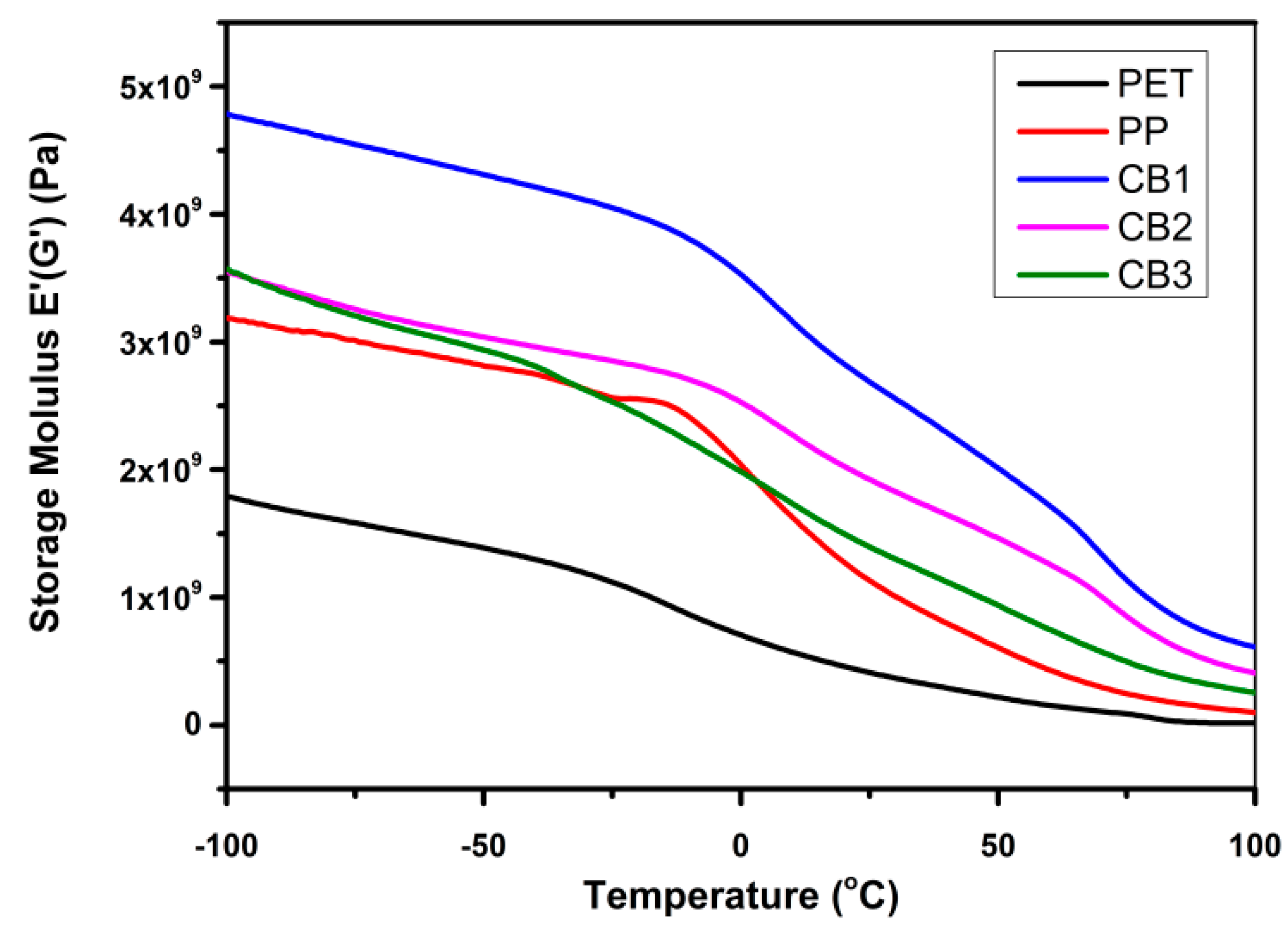
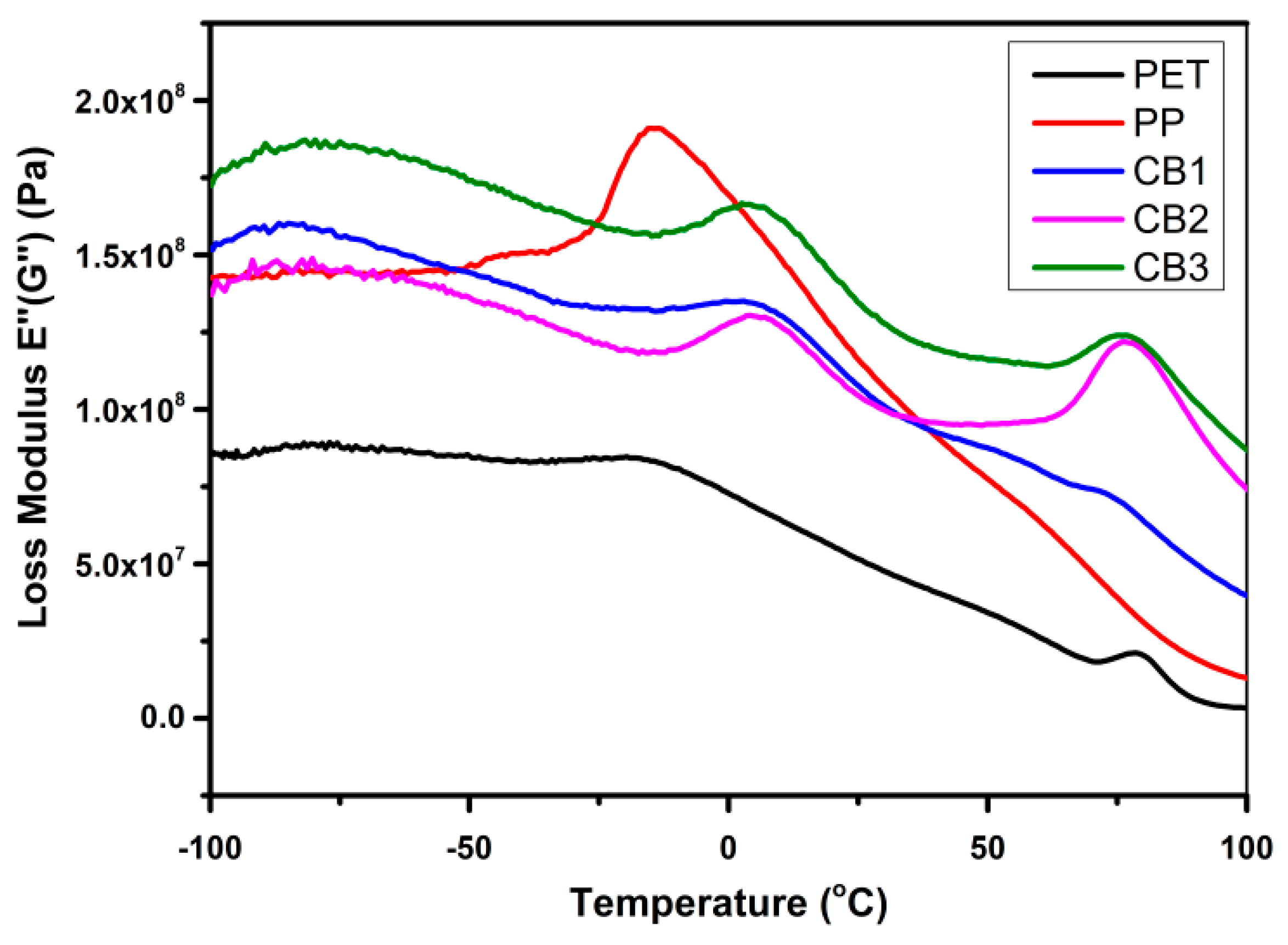
3.6. Dynamic Mechanical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PP | Polypropylene |
| MAH | Maleic anhydride |
| MAH-g-PP | Maleic anhydride grafted polypropylene |
| BPO | Benzoyl peroxide |
| Phr | Parts per hundred |
| DCP | Dicumyl peroxide |
| DSC | Differential scanning calorimetry |
| HDPE | High density polyethylene |
| SEM | Scanning electron microscope |
| DMA | Dynamic mechanical analyzer |
| Mn | Number average molecular weight |
| PET | Polyethylene terephthalate |
| CI | Carbonyl index |
| Tm | Melting temperature |
| FTIR | Fourier-transform infrared spectroscopy |
| Tg | Glass transition temperature |
References
- Karger-Kocsis, J.; Bárány, T. Polypropylene Handbook: Morphology, Blends and Composites; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Naqvi, M.K.; Choudhary, M.S. Chemically Modified Polyolefins and Their Blends. J. Macromol. Sci. Part C 1996, 36, 601–629. [Google Scholar] [CrossRef]
- Isayev, A.I. Encyclopedia of Polymer Blends, Volume 3: Structure; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 3. [Google Scholar]
- Berzin, F.; Flat, J.-J.; Vergnes, B. Grafting of maleic anhydride on polypropylene by reactive extrusion: Effect of maleic anhydride and peroxide concentrations on reaction yield and products characteristics. J. Polym. Eng. 2013, 33, 673–682. [Google Scholar] [CrossRef]
- Bettini, S.H.P.; Agnelli, J.A.M. Grafting of maleic anhydride onto polypropylene by reactive extrusion. J. Appl. Polym. Sci. 2002, 85, 2706–2717. [Google Scholar] [CrossRef]
- Oromiehie, A.; Ebadi-Dehaghani, H.; Mirbagheri, S. Chemical modification of polypropylene by maleic anhydride: Melt grafting, characterization and mechanism. Int. J. Chem. Eng. Appl. 2014, 5, 117. [Google Scholar]
- Galia, A.; De Gregorio, R.; Spadaro, G.; Scialdone, O.; Filardo, G. Grafting of Maleic Anhydride onto Isotactic Polypropylene in the Presence of Supercritical Carbon Dioxide as a Solvent and Swelling Fluid. Macromolecules 2004, 37, 4580–4589. [Google Scholar] [CrossRef]
- Liu, T.; Hu, G.-H.; Tong, G.-S.; Zhao, L.; Cao, G.-P.; Yuan, W.-K. Supercritical carbon dioxide assisted solid-state grafting process of maleic anhydride onto polypropylene. Ind. Eng. Chem. Res. 2005, 44, 4292–4299. [Google Scholar] [CrossRef]
- Pruthtikul, R.; Liewchirakorn, P. Preparation of Polypropylene Graft Maleic Anhydride (PP-g-MA) via Twin Screw Extrusion. Adv. Mater. Res. 2010, 93–94, 451–454. [Google Scholar] [CrossRef]
- Qiu, W.; Endo, T.; Hirotsu, T. A novel technique for preparing of maleic anhydride grafted polyolefins. Eur. Polym. J. 2005, 41, 1979–1984. [Google Scholar] [CrossRef]
- Sathe, S.N.; Rao, G.S.; Devi, S. Grafting of maleic anhydride onto polypropylene: Synthesis and characterization. J. Appl. Polym. Sci. 1994, 53, 239–245. [Google Scholar] [CrossRef]
- Shi, D.; Yang, J.; Yao, Z.; Wang, Y.; Huang, H.; Jing, W.; Yin, J.; Costa, G. Functionalization of isotactic polypropylene with maleic anhydride by reactive extrusion: Mechanism of melt grafting. Polymer 2001, 42, 5549–5557. [Google Scholar] [CrossRef]
- Iqbal, M.; Chuai, C.; Huang, Y.; Che, C. Modification of low-density polyethylene by graft copolymerization with maleic anhydride and blends with polyamide 6. J. Appl. Polym. Sci. 2010, 116, 1558–1565. [Google Scholar] [CrossRef]
- Bualek-Limcharoen, S.; Samran, J.; Amornsakchai, T.; Meesiri, W. Effect of compatibilizers on mechanical properties and morphology of in-situ composite film of thermotropic liquid crystalline polymer/polypropylene. Polym. Eng. Sci. 1999, 39, 312–320. [Google Scholar] [CrossRef]
- Ko, T.-M.; Ning, P. Peroxide-catalyzed swell grafting of maleic anhydride onto polypropylene. Polym. Eng. Sci. 2000, 40, 1589–1595. [Google Scholar] [CrossRef]
- Henry, G.R.; Drooghaag, X.; Rousseaux, D.D.; Sclavons, M.; Devaux, J.; Marchand-Brynaert, J.; Carlier, V. A practical way of grafting maleic anhydride onto polypropylene providing high anhydride contents without sacrificing excessive molar mass. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 2936–2947. [Google Scholar] [CrossRef]
- Li, D.; Han, B.; Liu, Z. Grafting of 2-Hydroxyethyl Methacrylate onto Isotactic Poly(propylene) Using Supercritical CO2 as a Solvent and Swelling Agent. Macromol. Chem. Phys. 2001, 202, 2187–2194. [Google Scholar] [CrossRef]
- Tzoganakis, C. Reactive extrusion of polymers: A review. Adv. Polym. Technol. 1989, 9, 321–330. [Google Scholar] [CrossRef]
- Tang, H.; Dai, W.; Chen, B. A new method for producing high melt strength polypropylene with reactive extrusion. Polym. Eng. Sci. 2008, 48, 1339–1344. [Google Scholar] [CrossRef]
- Achilias, D.; Sideridou, I. Kinetics of the Benzoyl Peroxide/Amine Initiated Free-Radical Polymerization of Dental Dimethacrylate Monomers: Experimental Studies and Mathematical Modeling for TEGDMA and Bis-EMA. Macromolecules 2004, 37, 4254–4265. [Google Scholar] [CrossRef]
- Gaylord, N.G.; Mehta, R.; Kumar, V.; Tazi, M. High density polyethylene-g-maleic anhydride preparation in presence of electron donors. J. Appl. Polym. Sci. 1989, 38, 359–371. [Google Scholar] [CrossRef]
- Ho, R.M.; Su, A.C.; Wu, C.H.; Chen, S.I. Functionalization of polypropylene via melt mixing. Polymer 1993, 34, 3264–3269. [Google Scholar] [CrossRef]
- Luo, W.; Liu, X.; Fu, Y. Melt grafting of maleic anhydride onto polypropylene with assistance of α-methylstyrene. Polym. Eng. Sci. 2012, 52, 814–819. [Google Scholar] [CrossRef]
- Vainio, T.; Hu, G.-H.; Lambla, M.; Seppälä, J.V. Functionalized polypropylene prepared by melt free radical grafting of low volatile oxazoline and its potential in compatibilization of PP/PBT blends. J. Appl. Polym. Sci. 1996, 61, 843–852. [Google Scholar] [CrossRef]
- Chandranupap, P.; Bhattacharya, S.N. Reactive processing of polyolefins with MAH and GMA in the presence of various additives. J. Appl. Polym. Sci. 2000, 78, 2405–2415. [Google Scholar] [CrossRef]
- Li, Y.; Xie, X.-M.; Guo, B.-H. Study on styrene-assisted melt free-radical grafting of maleic anhydride onto polypropylene. Polymer 2001, 42, 3419–3425. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, W.; Zhou, C. Rheokinetic study on homogeneous polymer reactions in melt state under strong flow field. Polymer 2009, 50, 4397–4405. [Google Scholar] [CrossRef]
- Bousmina, M.; Ait-Kadi, A.; Faisant, J.B. Determination of shear rate and viscosity from batch mixer data. J. Rheol. 1999, 43, 415–433. [Google Scholar] [CrossRef]
- Sichina, W.J. DSC as Problem Solving Tool: Measurement of Percent Crystallinity of Thermoplastics; Perkin Elmer Instruments: Waltham, MA, USA, 2000. [Google Scholar]
- Menyhárd, A.; Faludi, G.; Varga, J. β-Crystallisation tendency and structure of polypropylene grafted by maleic anhydride and its blends with isotactic polypropylene. J. Therm. Anal. Calorim. 2008, 93, 937–945. [Google Scholar] [CrossRef]
- Abdul Razak, N.C.; Inuwa, I.M.; Hassan, A.; Samsudin, S.A. Effects of compatibilizers on mechanical properties of PET/PP blend. Compos. Interfaces 2013, 20, 507–515. [Google Scholar] [CrossRef]
- Oyman, Z.O.; Tinçer, T. Melt blending of poly(ethylene terephthalate) with polypropylene in the presence of silane coupling agent. J. Appl. Polym. Sci. 2003, 89, 1039–1048. [Google Scholar] [CrossRef]
- Xanthos, M.; Young, M.W.; Biesenberger, J.A. Polypropylene/polyethylene terephthalate blends compatibilized through functionalization. Polym. Eng. Sci. 1990, 30, 355–365. [Google Scholar] [CrossRef]
- Akbari, M.; Zadhoush, A.; Haghighat, M. PET/PP blending by using PP-g-MA synthesized by solid phase. J. Appl. Polym. Sci. 2007, 104, 3986–3993. [Google Scholar] [CrossRef]
- Mofokeng, J.; Luyt, A.; Tábi, T.; Kovacs, J. Comparison of injection moulded, natural fibre reinforced composites with PP and PLA as matrices. J. Thermoplast. Compos. Mate. 2012, 25, 927–948. [Google Scholar] [CrossRef]
- Thirtha, V.; Lehman, R.; Nosker, T. Morphological effects on glass transition behavior in selected immiscible blends of amorphous and semicrystalline polymers. Polymer 2006, 47, 5392–5401. [Google Scholar] [CrossRef]
- Serhatkulu, T.; Erman, B.; Bahar, I.; Fakirov, S.; Evstatiev, M.; Sapundjieva, D. Dynamic mechanical study of amorphous phases in poly (ethylene terephthalate)/nylon-6 blends. Polymer 1995, 36, 2371–2377. [Google Scholar] [CrossRef]
- Zdrazilova, N.; Hausnerova, B.; Kitano, T.; Saha, P. Rheological behaviour of PP/PET and modified PP/PET blends. II. Dynamic viscoelastic properties. Polym. Polym. Compos. 2004, 12, 433–448. [Google Scholar] [CrossRef]
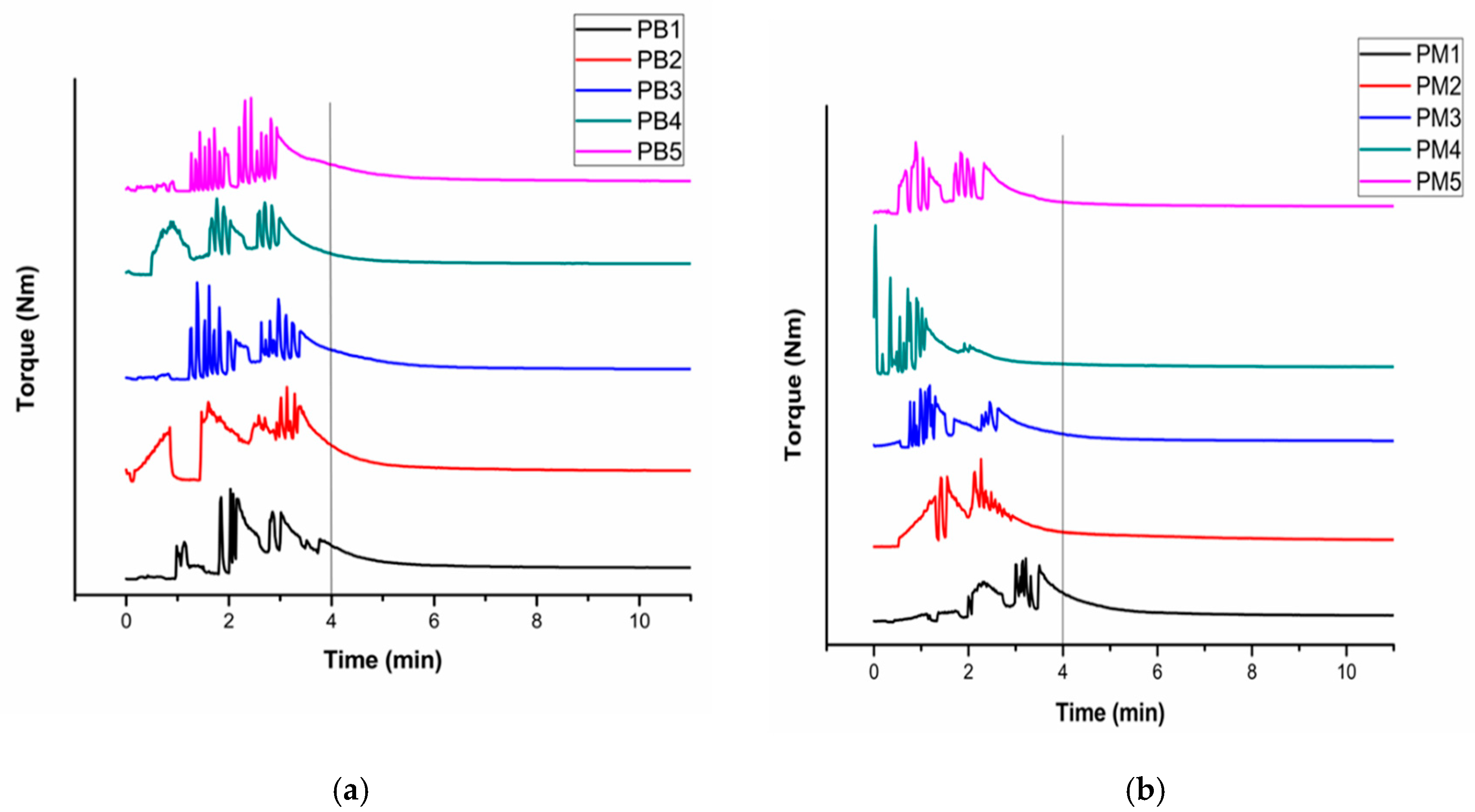
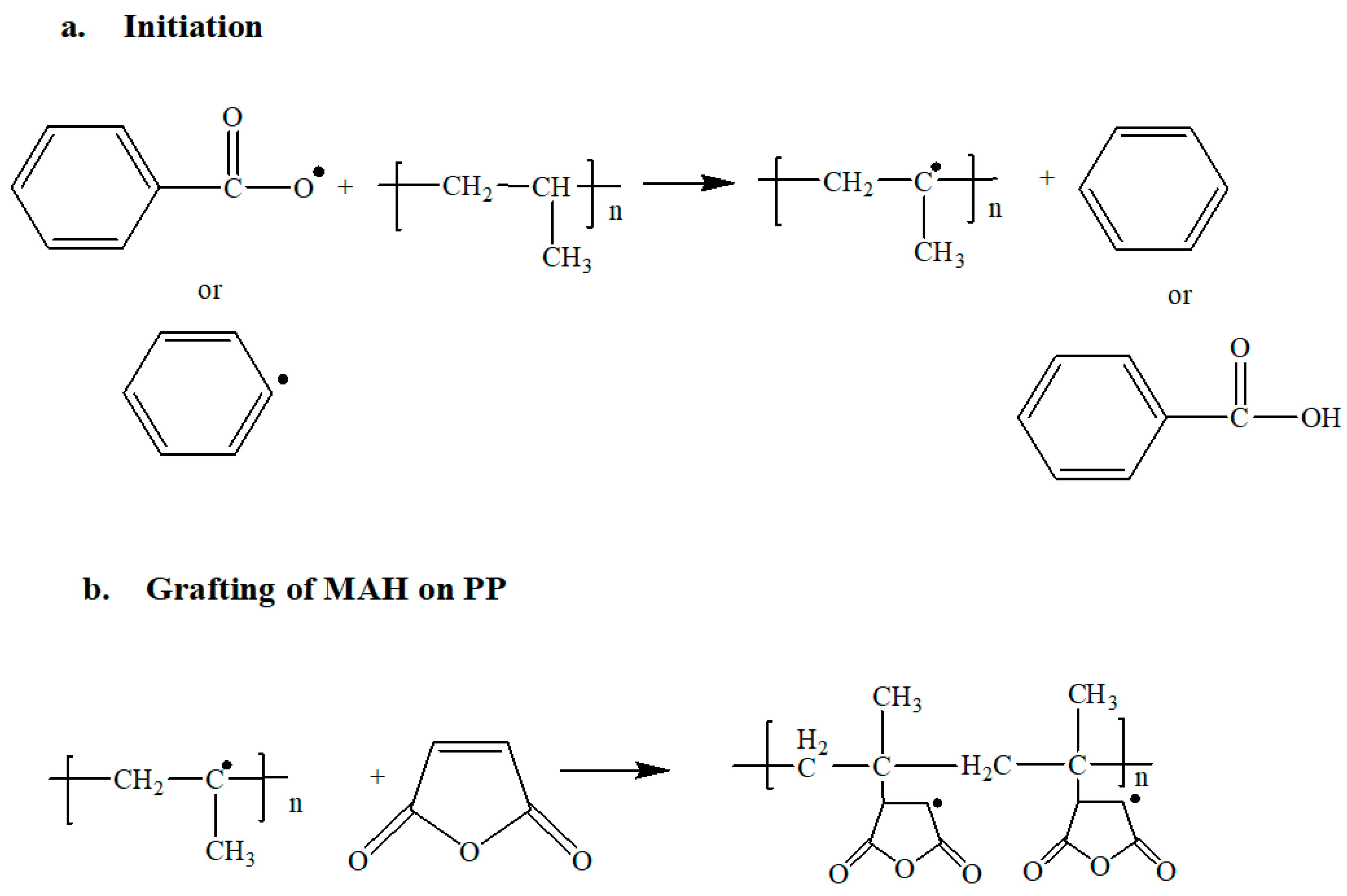
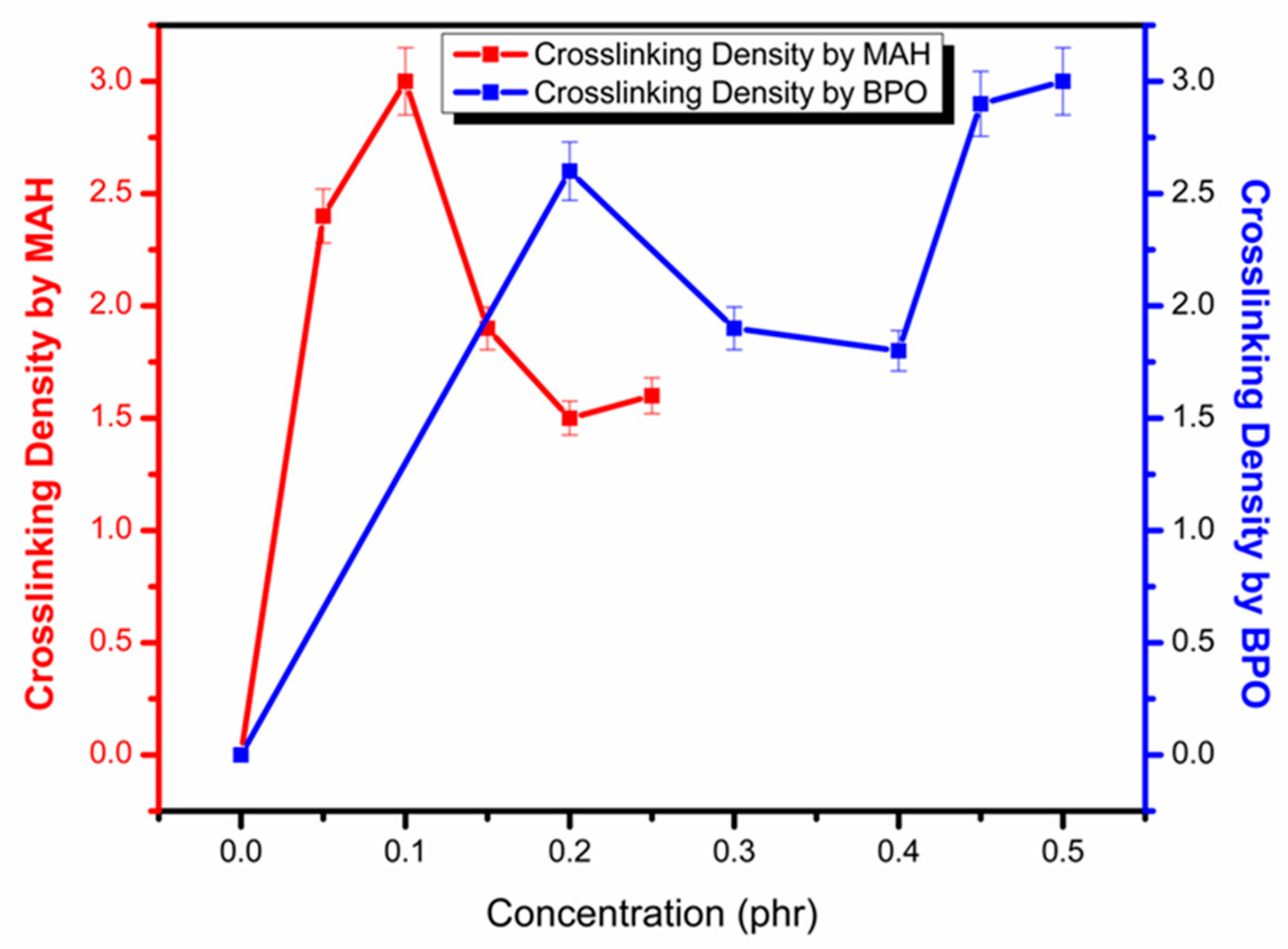
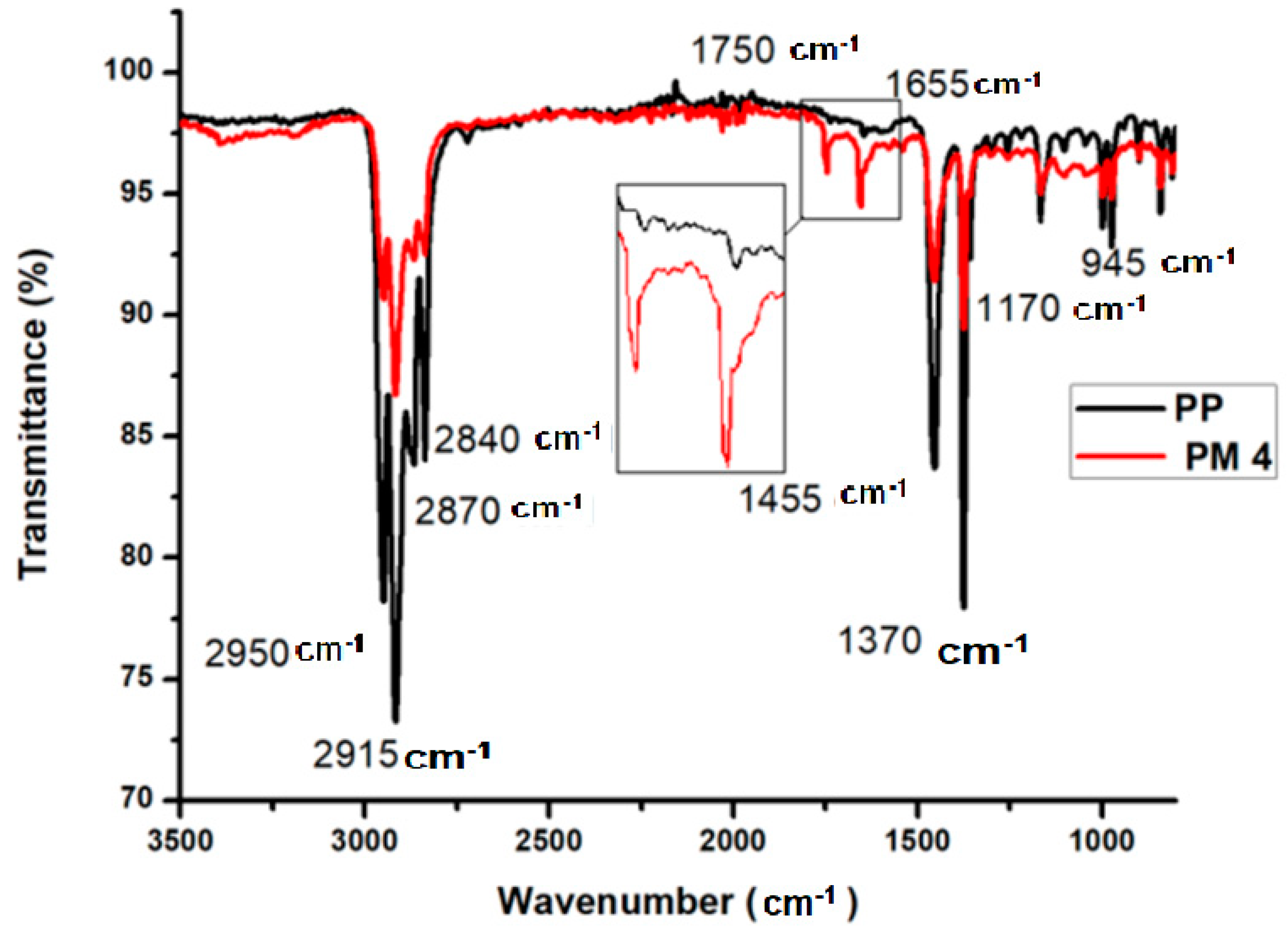


| Sample Name | MAH (phr) | BPO (phr) | Sample Name | MAH (phr) | BPO (phr) |
|---|---|---|---|---|---|
| PM1 | 0.05 | 0.4 | PB1 | 0.15 | 0.2 |
| PM2 | 0.10 | 0.4 | PB2 | 0.15 | 0.3 |
| PM3 | 0.15 | 0.4 | PB3 | 0.15 | 0.4 |
| PM4 | 0.20 | 0.4 | PB4 | 0.15 | 0.45 |
| PM5 | 0.25 | 0.4 | PB5 | 0.15 | 0.5 |
| Samples | Film Grade PET (%) | Isotactic PP (%) | MAH-g-PP (%) |
|---|---|---|---|
| CB 1 | 60% | 39% PP | 1% |
| CB 2 | 60% | 37.5% PP | 2.5% |
| CB 3 | 60% | 35% PP | 5% |
| CB 4 | 60% | 40% PP | - |
| CB 5 | 100% | - | - |
| CB 6 | - | 100% | - |
| Sample Name | CI Value | Standard Deviation (±) | Sample Name | CI Value | Standard Deviation (±) |
|---|---|---|---|---|---|
| PM1 | 0.24 | 0.012 | PB1 | 0.25 | 0.0125 |
| PM2 | 0.37 | 0.0185 | PB2 | 0.27 | 0.0135 |
| PM3 | 0.38 | 0.019 | PB3 | 0.38 | 0.019 |
| PM4 | 0.41 | 0.0205 | PB4 | 0.36 | 0.018 |
| PM5 | 0.40 | 0.02 | PB5 | 0.36 | 0.018 |
| Sample Name | MFI (g/10 min) at 2.16 kg and 190 °C | Standard Deviation (±) | Sample Name | MFI (g/10 min) at 2.16 kg and 190 °C | Standard Deviation (±) |
|---|---|---|---|---|---|
| PP | 3.297 | 0.16485 | |||
| PM1 | 6.011 | 0.30055 | PB1 | 11.84 | 0.592 |
| PM2 | 9.256 | 0.4628 | PB2 | 13.99 | 0.6995 |
| PM3 | 14.28 | 0.664 | PB3 | 15.97 | 0.7985 |
| PM4 | 8.901 | 0.44505 | PB4 | 12.70 | 0.635 |
| PM5 | 8.604 | 0.4302 | PB5 | 8.380 | 0.419 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, A.; Ahmad, N.M.; Abbas, M.A.; Shakir, M.F.; Khaliq, Z.; Rafiq, S.; Ali, Z.; Elaissari, A. Reactive Extrusion of Maleic-Anhydride-Grafted Polypropylene by Torque Rheometer and Its Application as Compatibilizer. Polymers 2021, 13, 495. https://doi.org/10.3390/polym13040495
Tariq A, Ahmad NM, Abbas MA, Shakir MF, Khaliq Z, Rafiq S, Ali Z, Elaissari A. Reactive Extrusion of Maleic-Anhydride-Grafted Polypropylene by Torque Rheometer and Its Application as Compatibilizer. Polymers. 2021; 13(4):495. https://doi.org/10.3390/polym13040495
Chicago/Turabian StyleTariq, Asra, Nasir M. Ahmad, Muhammad Asad Abbas, M Fayzan Shakir, Zubair Khaliq, Sikandar Rafiq, Zulfiqar Ali, and Abdelhamid Elaissari. 2021. "Reactive Extrusion of Maleic-Anhydride-Grafted Polypropylene by Torque Rheometer and Its Application as Compatibilizer" Polymers 13, no. 4: 495. https://doi.org/10.3390/polym13040495
APA StyleTariq, A., Ahmad, N. M., Abbas, M. A., Shakir, M. F., Khaliq, Z., Rafiq, S., Ali, Z., & Elaissari, A. (2021). Reactive Extrusion of Maleic-Anhydride-Grafted Polypropylene by Torque Rheometer and Its Application as Compatibilizer. Polymers, 13(4), 495. https://doi.org/10.3390/polym13040495








